Abstract
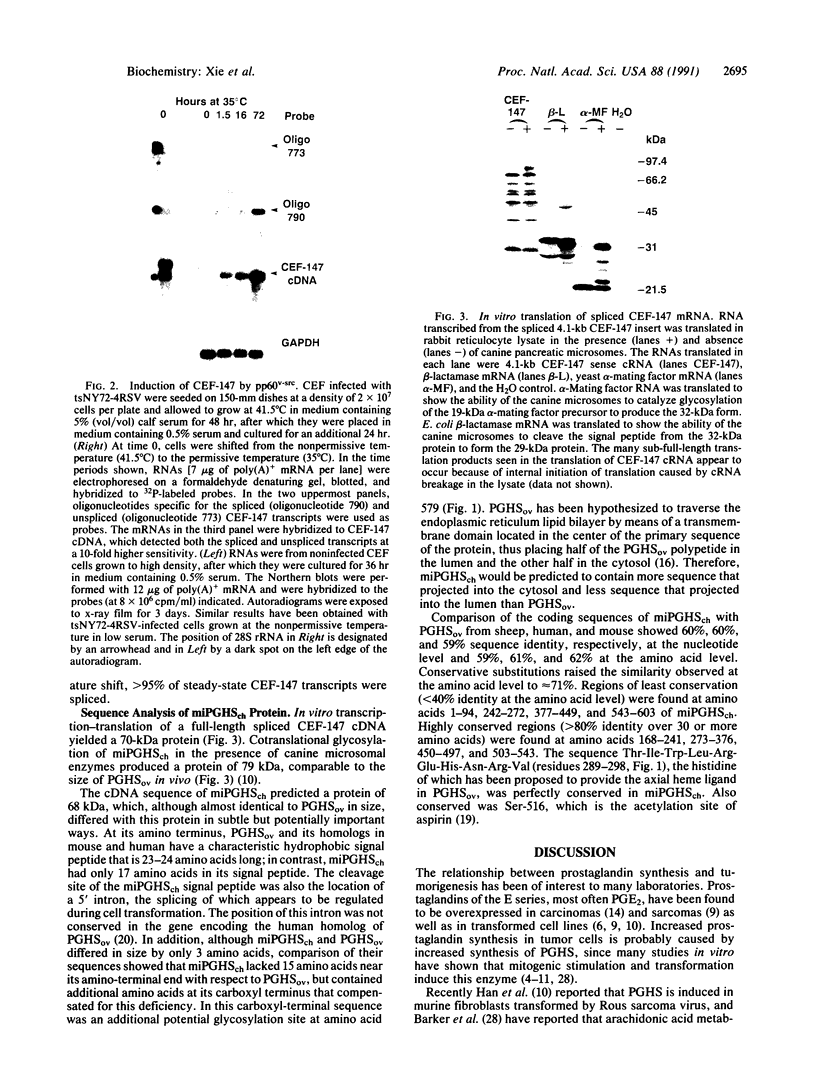
Rous sarcoma virus was shown to induce in chicken embryo fibroblasts (CEF) a 4.1-kilobase mRNA (designated CEF-147) encoding a 603-amino acid protein. Analysis of the protein sequence showed that it shared 59% amino acid identity with sheep prostaglandin G/H synthase, the enzyme that catalyzes the rate-limiting steps in the production of prostaglandins. Significant differences, at both the protein and mRNA levels, existed between the src oncogene product-inducible prostaglandin synthase and the protein isolated and cloned from sheep seminal vesicle, suggesting that the src-inducible prostaglandin synthase may be a new form of the enzyme. A distinguishing feature of src-inducible prostaglandin synthase mRNA is its low abundance in nonproliferating chicken embryo fibroblasts and its relatively high abundance in src-transformed cells. Additionally, the majority of the src-inducible prostaglandin synthase RNA present in nonproliferating cells was found to be nonfunctional because of the presence of an unspliced intron that separated the signal peptide from the remainder of the protein. Upon mitogenic stimulation, this intron was removed, resulting in the induction of fully-spliced CEF-147 mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan A., Cramer S., Bandyopadhyay G. K., Imagawa W., Yang J., Elias J., Beattie C. W., Das Gupta T. K., Nandi S. Differential proliferative response to linoleate in cultures of epithelial cells from normal human breast and fibroadenomas. Cancer Res. 1989 Feb 15;49(4):857–862. [PubMed] [Google Scholar]
- Barker K., Aderem A., Hanafusa H. Modulation of arachidonic acid metabolism by Rous sarcoma virus. J Virol. 1989 Jul;63(7):2929–2935. doi: 10.1128/jvi.63.7.2929-2935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard P. A., Alcorta D., Simmons D. L., Luk K. C., Erikson R. L. Constitutive expression of a gene encoding a polypeptide homologous to biologically active human platelet protein in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6715–6719. doi: 10.1073/pnas.84.19.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld S., Zaltsman Y. A., Krispin M., Zakut H., Zor U., Kohen F. Antitumor effects of inhibitors of arachidonic acid cascade on experimentally induced intestinal tumors. Dis Colon Rectum. 1987 Jan;30(1):43–46. doi: 10.1007/BF02556922. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M. L., Korte K., MacDonald P. C. Epidermal growth factor stimulation of prostaglandin E2 biosynthesis in amnion cells. Induction of prostaglandin H2 synthase. J Biol Chem. 1988 Jun 5;263(16):7846–7854. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt D. L., el-Harith E. A., Kraemer S. A., Andrews M. J., Yao E. F., Armstrong R. L., Smith W. L. The aspirin and heme-binding sites of ovine and murine prostaglandin endoperoxide synthases. J Biol Chem. 1990 Mar 25;265(9):5192–5198. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Han J. W., Sadowski H., Young D. A., Macara I. G. Persistent induction of cyclooxygenase in p60v-src-transformed 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1990 May;87(9):3373–3377. doi: 10.1073/pnas.87.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka M., Oshima T., Yokota K., Yamamoto S., Kumegawa M. Possible induction of fatty acid cyclooxygenase in mouse osteoblastic cells (MC3T3-E1) by cAMP. Biochim Biophys Acta. 1988 Dec 9;972(3):339–346. doi: 10.1016/0167-4889(88)90210-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Parhar R. S. Cure of B16F10 melanoma lung metastasis in mice by chronic indomethacin therapy combined with repeated rounds of interleukin 2: characteristics of killer cells generated in situ. Cancer Res. 1988 Mar 1;48(5):1072–1079. [PubMed] [Google Scholar]
- Maier J. A., Hla T., Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [PubMed] [Google Scholar]
- Merlie J. P., Fagan D., Mudd J., Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [PubMed] [Google Scholar]
- Parker J., Daniel L. W., Waite M. Evidence of protein kinase C involvement in phorbol diester-stimulated arachidonic acid release and prostaglandin synthesis. J Biol Chem. 1987 Apr 15;262(11):5385–5393. [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Rosen G. D., Birkenmeier T. M., Raz A., Holtzman M. J. Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1358–1365. doi: 10.1016/0006-291x(89)91819-6. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano S., Stoeckle M. Y., Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1987 May 8;49(3):321–328. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Topley N., Floege J., Wessel K., Hass R., Radeke H. H., Kaever V., Resch K. Prostaglandin E2 production is synergistically increased in cultured human glomerular mesangial cells by combinations of IL-1 and tumor necrosis factor-alpha 1. J Immunol. 1989 Sep 15;143(6):1989–1995. [PubMed] [Google Scholar]
- Yokota K., Kusaka M., Ohshima T., Yamamoto S., Kurihara N., Yoshino T., Kumegawa M. Stimulation of prostaglandin E2 synthesis in cloned osteoblastic cells of mouse (MC3T3-E1) by epidermal growth factor. J Biol Chem. 1986 Nov 25;261(33):15410–15415. [PubMed] [Google Scholar]
- Yokoyama C., Takai T., Tanabe T. Primary structure of sheep prostaglandin endoperoxide synthase deduced from cDNA sequence. FEBS Lett. 1988 Apr 25;231(2):347–351. doi: 10.1016/0014-5793(88)80847-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama C., Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophys Res Commun. 1989 Dec 15;165(2):888–894. doi: 10.1016/s0006-291x(89)80049-x. [DOI] [PubMed] [Google Scholar]
- Young M. R., Knies S. Prostaglandin E production by Lewis lung carcinoma: mechanism for tumor establishment in vivo. J Natl Cancer Inst. 1984 Apr;72(4):919–922. [PubMed] [Google Scholar]