Abstract
The pulmonary endothelium is the target of continuous physiological and pathological stimuli that affect its crucial barrier function. The regulation, defense, and repair of endothelial barrier function require complex biochemical processes. This review examines the role of endothelial phosphorylating enzymes, kinases, a class with profound, interdigitating influences on endothelial permeability and lung function.
Keywords: barrier function, endothelium, kinases, lung, permeability
the endothelium forms a barrier that regulates the exchange of blood fluid, electrolytes, and proteins across the vascular wall and is constantly subjected to dynamic remodeling (187). Deviations of normal endothelial barrier function can lead to, or be caused by, various internal or external stresses, including inflammation, sepsis, diabetes, and atherosclerosis (51). Notably, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are clinical syndromes of noncardiogenic pulmonary edema caused primarily by increased permeability to proteins across the endothelial and epithelial barriers of the lung (21, 135). Microvascular permeability is increased by inflammatory factors, such as histamine, bradykinin, and platelet-activating factor, growth factors, glycation products, cytokines, reactive oxygen species (ROS), and activated leukocytes (150). These mediators induce a plethora of intracellular signaling events that compromise the barrier integrity by modulating the expression of junction proteins, adhesion molecules, or reorganization of the cytoskeleton and focal adhesion complexes (238). Permeability-altering responses are frequently initiated by the binding of agonists on endothelial surface receptors and the consequent activation of signaling molecules, such as kinases, phosphatases, and GTPases, that control protein expression and degradation (224). These molecular cascades act as modulators of cytoskeletal integrity and contractility, leading to fluctuations of endothelial barrier function (137). In this review we focus on the role of kinases in endothelial paracellular permeability.
Protein kinases catalyze the transfer of inorganic phosphate supplied by ATP onto serine, threonine, or tyrosine residues of substrate enzymes. The specificity of protein phosphorylation is determined by the local amino acid sequence that surrounds the residues to be phosphorylated (8).
Protein phosphorylation is opposed by protein dephosphorylation executed by phosphatases. Many protein phosphatases are promiscuous, in that they do not require amino acid-specific sequences to identify substrates for dephosphorylation. However, the specificity of protein phosphatases is often determined by their regulatory subunits, which target phosphatase to the specific substrate (213). Kinases exist in the active or inactive state (142, 270). Their activation occurs by phosphorylation or release of kinase autoinhibition. Upon activation, a kinase can bind to ATP and transfer phosphate. Kinase activity is regulated by Ca2+, Mg2+, and binding to negative regulators or to molecular chaperones, such as heat shock protein (Hsp)-90 (66, 210). An emerging body of evidence suggests that kinase-induced protein phosphorylation is an important regulator of endothelial permeability (Fig. 1).
Fig. 1.
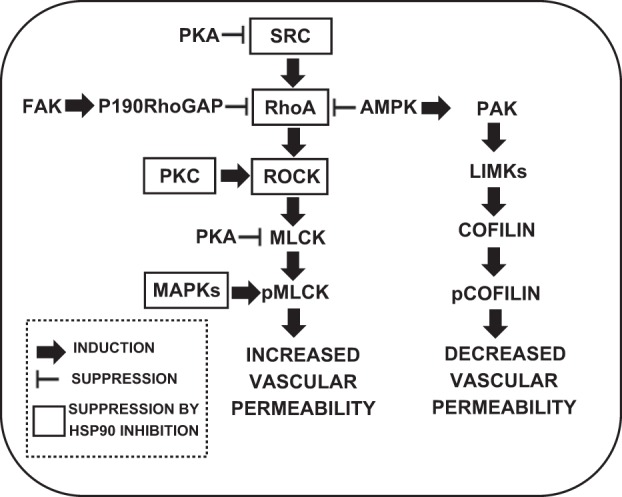
Interaction among major kinase pathways in the pathophysiological control of endothelial barrier function. FAK, focal adhesion kinase; GAP, GTPase-activating protein; AMPK, AMP-activated protein kinase; PAK, p21-activated kinase; ROCK, RhoA-associated protein kinase; LIMK, LIM domain kinase; MLCK, myosin light chain kinase; pMLCK, phosphorylated MLCK; pcofilin, phosphorylated cofilin; MAPK, MAP kinase.
AMP-Activated Protein Kinase
AMP-activated protein kinase (AMPK) was discovered in 1973 to phosphorylate and activate two key enzymes of lipid biosynthesis, acetyl-CoA carboxylase (35) and 3-hydroxy-3-methylglutaryl-CoA reductase (19). Acetyl-CoA carboxylase kinase activity was shown to be activated by 5-AMP (264), whereas 3-hydroxy-3-methylglutaryl-CoA reductase activity was induced by phosphorylation via an upstream kinase (84). It was initially assumed that these were independent events, but, subsequently, Carling et al. discovered that they were functions of a single protein kinase activated by AMP and phosphorylation (34). Since it became clear that this was a kinase with multiple substrates, it was renamed AMP-activated protein kinase after its key activating ligand (164). Mammalian AMPK (28, 119) exists in heterotrimeric complexes consisting of a single catalytic subunit, α, and two accessory subunits, β and γ (49). There are multiple isoforms of each subunit (AMPKα1 and -α2, AMPKβ1 and -β2, and AMPKγ1, -γ2, and -γ3) (85). AMPK regulates metabolic, as well as anti-inflammatory, functions (181, 212).
There is strong evidence that AMPK is involved in the regulation of vascular permeability (109) (Fig. 1) via multiple mechanisms. Activation of AMPK with metformin resealed wounds in LPS-exposed rat pulmonary microvascular endothelial cells; metformin failed to induce barrier repair in AMPK-depleted endothelial cells (151). It was recently reported that N-cadherin coordinates AMPK-mediated lung vascular repair, since disruption of N-cadherin's intracellular domain caused translocation of AMPK away from the membrane and attenuated the AMPK-mediated restoration of barrier function in LPS-treated pulmonary endothelium. AMPK activation reversed the LPS-induced increase in vascular permeability, whereas N-cadherin inhibition inhibited the AMPK-mediated repair (110). Additionally, AMPK inhibits RhoA and RhoA-associated protein kinase (ROCK) activation (247).
AMPK was also shown to exhibit anti-inflammatory activities, at least through inhibition of the Toll-like receptor-4-dependent activation of the transcription factor NF-κB (197, 226). Furthermore, AMPK contributes to the resolution of inflammation by facilitating phagocytosis and clearance of apoptotic cells (182, 274). Additionally, the AMPK-dependent phosphorylation and degradation of FoxO1a attenuate angiopoietin-2 expression and protect against the proinflammatory actions of tumor necrosis factor (TNF)-α (52). It was also reported that AMPK activation reduces vascular permeability and airway inflammation by regulating the hypoxia-inducible factor/vascular endothelial growth factor (VEGF)-A pathway (183).
Myosin Light-Chain Kinase
Myosin light-chain (MLC) kinase (MLCK) is a family of Ca2+/calmodulin (CaM)-dependent protein kinases that phosphorylate the regulatory MLC (MLC2) (210). MLC posttranslational modification is a key molecular cascade that regulates endothelial permeability and barrier function (210) (Fig. 1). MLCK-mediated phosphorylation of MLC2 (5, 227) at Ser19 and, subsequently, at Thr18 induces ATP-dependent actomyosin contraction (105, 127, 169, 210, 242), which increases capillary permeability. Similar to smooth muscle, in vascular endothelium, MLC phosphorylation triggers contraction, resulting in endothelial cell membrane retraction, intercellular gap formation, and barrier compromise (55, 193, 210).
There are distinct isoforms of MLCK (36, 127, 210, 249). The skeletal muscle MLCK is the only tissue-specific MLCK, inasmuch as it is expressed in adult skeletal muscle, but not in cardiac, smooth, or nonmuscular tissues. In contrast, the 130-kDa smooth muscle MLCK is ubiquitous in all adult tissues, including skeletal and cardiac muscle (95). Smooth muscle and nonmuscle MLCK isoforms (smMLCK and nmMLCK, respectively) have wide tissue distribution, and both are expressed in microvascular endothelial cells. They are derived from the same gene (mylk1) on human chromosome 3 and exhibit identical amino acid sequences and protein structures at the COOH terminus, which includes actin-binding, catalytic, inhibitory, CaM-binding, and kinase-related protein domains (64, 127). However, in contrast to smMLCK, nmMLCK also contains a unique 922-amino acid NH2-terminal fragment containing multiple sites for protein-protein interaction (SH2- and SH3-binding domains), as well as potential regulatory phosphorylation sites for important kinases such as protein kinase C (PKC), protein kinase A (PKA), and MAP kinases (MAPKs) (55, 64).
Endothelial (nonmuscle) MLCK is represented by four isoforms, with MLCK1 and MLCK2 being the most abundant (127). Despite structural similarity, these isoforms reveal some differences in their regulation (23, 56). For example, MLCK1 and MLCK3a are phosphorylated by the Src-family kinases (SFKs) at Tyr464 and Tyr471, which are not present in other MLCK isoforms (210). Importantly, Src-dependent MLCK phosphorylation activates MLCK (23), representing an additional modality involved in MLCK regulation. For example, thrombin increases endothelial permeability in a Src/MLCK-dependent manner via MLC-mediated contractile mechanisms in human lung microvascular endothelial cells (HLMVECs) (112). Additionally, in human umbilical vein endothelial cells (HUVECs), the MLCK-dependent MLC phosphorylation has been shown to regulate the permeability of intact isolated postcapillary venules and pulmonary microvessels in response to histamine and thrombin (245, 246, 272). Mylk−/− mice are protected against increased lung vascular permeability and neutrophil adhesion induced by intraperitoneal LPS (246, 263). nmMLCK exerts an important role in VEGF-induced permeability. Activation of the VEGF signaling pathway in bovine pulmonary artery endothelial cells (BPAECs) increases MYLK gene and protein levels. Consequent increased nmMLCK mRNA and protein expression is a result of increased nmMYLK promoter activity, regulated in part by binding of the Sp1 transcription factor. This suggests that MYLK may be an important ARDS candidate gene and a therapeutic target that is highly influenced by excessive VEGF concentrations in the inflamed lung (216).
CaM is a major regulator of MLCK activity. MLCK contains a CaM-binding domain that maintains MLCK in an inactive state when CaM is not bound. In human embryonic kidney (HEK-293) cells, the binding of CaM results in MLCK activation (25). In kidney endothelial cells, CaM binding to MLCK in the presence of Ca2+ is crucial for MLCK activation (64, 243).
Treatment with the protein phosphatase (PP) inhibitor calyculin A also increases MLC phosphorylation in 3T3 cells (225) and coronary venular endothelial cells (130), suggesting a constitutive MLCK activity. Therefore, it is believed that basal endothelial barrier function is maintained by tonic MLCK activity (193).
MLCK activity is inhibited pharmacologically by compounds such as ML-7 and ML-9 in HEK-293 cells (211, 272). ML-7 improves endothelial dysfunction via tight junction regulation in the aorta of a rabbit model of atherosclerosis (41) and attenuates endothelial cell permeability in association with a downregulation of junction proteins via mechanisms involving MLCK-mediated MLC phosphorylation (276).
Whereas MLC phosphorylation depends on the activation of a MLCK, MLC dephosphorylation is executed by MLC phosphatase (MLCP), which consists of the catalytic subunit CS1β (formerly CS1δ), a M20 subunit with unknown function, and the myosin-targeting regulatory subunit MYPT1 (46). MYPT1 is phosphorylated by Rho kinase at Thr496, leading to MLCP inhibition and increased MLC phosphorylation (111). In smooth muscle cells (SMCs), Rho kinase can increase the level of MLC phosphorylation by direct phosphate incorporation at the same residue as does MLCK (11). Several other kinases are suspected in the modulation of MLC phosphorylation (see below), including PKC (28), which phosphorylates not only MLC, but also the specific MLCP inhibitor CPI-17 at Thr38, leading to increased inhibitory potency toward MLCP and PKA, which reduces the phosphorylation of MLC and opposes agonist-induced hyperpermeability in mouse lung endothelial cells (185). That effect is possible due to MLCP association with myosin and MLC dephosphorylation (22).
Nonreceptor Tyrosine Kinases
SFKs are nonreceptor tyrosine kinases that are involved in multiple signaling functions. The prototypical Src isoform viral Src kinase (v-Src) was originally isolated from the cancer-inducing Rous sarcoma virus (30, 186). SFKs include ≥11 isoforms, Blk, Brk, Fgr, Frk, Fyn, Hck, Lck, Lyn, Src, Srm, and Yes (154, 228), with molecular weights ranging from 52 to 62 kDa (99). The isoforms Src, Fyn, and Yes are expressed in various mammalian tissues and are involved in endothelial signaling (178).
SFKs are composed of eight distinct functional regions. From the NH2 to the COOH terminus, these regions include a myristylated site, the SH4 Src homology domain, a unique region, a SH3 domain, a SH2 domain, a linker, the SH1 kinase/catalytic domain, and the regulatory domain. SH3 and SH2 are protein-protein interaction domains. The SH2 domain binds phosphotyrosine motifs in an inter- or intramolecular manner. The SH1 domain is the site of tyrosine kinase activity (98).
The main phosphorylation sites on Src are Tyr416 (SH1 domain) and Tyr527 (regulatory domain). Tyr416 is able to undergo autophosphorylation (194). Tyr527 is phosphorylated and dephosphorylated by many proteins, such as Csk (COOH-terminal Src kinase), SHP-1 (Src-homology 2 domain-containing phosphatase 1), SHP-2, or the protein tyrosine phosphatase PTP1 (102, 275). Both phosphorylation sites play a key role in regulating the activity of SFKs (99).
SFKs mediate microvascular endothelial hyperpermeability through phosphorylation of tyrosine residues on regulatory proteins, including those involved in endothelial cell-cell and cell-matrix adhesions (98, 99, 178, 270). Thus, Src kinase is required for the endothelial hyperpermeability in response to VEGF (58), TNFα (176), or ROS in pulmonary cells (114). Src kinase is also involved in the enhanced hyperpermeability induced by activated neutrophils in human dermal microvascular cells (229). In HLMVECs, Src phosphorylates and activates MLCK, which in turn leads to cytoskeletal actomyosin contraction, actin stress fiber formation, and barrier compromise (23, 99) (Fig. 1).
Hsp90 governs the regulation of v-Src (261). It shifts the conformation of v-Src toward high-activity states that would otherwise be unstable (26). We recently reported that the Hsp90 inhibitor 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) blocks LPS-induced tyrosine phosphorylation of Hsp90 in BPAECs, as well as HLMVECs (17). LPS induces concentration- and time-dependent Src-mediated tyrosine phosphorylation of Hsp90α and Hsp90β in HLMVECs. Mass spectrometry identified Tyr309 as a major site of tyrosine phosphorylation on Hsp90α (Tyr300 of Hsp90β). LPS-induced Hsp90 phosphorylation was prevented by the Hsp90 inhibitor 17-AAG in vitro, as well as in lungs from LPS-treated mice in vivo. Furthermore, 17-AAG prevented LPS-induced Src activation. LPS-induced Hsp90 phosphorylation was also prevented by the Src inhibitor PP2 (17).
In HLMVECs, TNFα causes a SFK-dependent phosphorylation and redistribution of VE-cadherin and gap formation, leading to increased paracellular permeability (9, 99, 144, 176, 257). Furthermore, both focal adhesion kinase (FAK) and paxillin, located in focal adhesion complexes, are Src substrates, and their activities are mainly regulated through phosphorylation by SFKs (190, 228, 262). Both Fyn and Src are associated with FAK and mediate signals initiated by integrin-extracellular matrix (ECM) binding (136). In HUVECs and HEK-293 cells, Src kinases phosphorylate FAK, leading to its binding to αvβ5-integrin and causing an increase in endothelial permeability due to disruption of integrin binding to the ECM (59).
In BPAECs, albumin binding to the gp60 receptor triggers Src activation by phosphorylation (98, 99). Once activated, Src causes phosphorylation of caveolin-1 (at Tyr14) and dynamin-2 (at Tyr597), which in turn results in endocytosis and transcytosis of plasma components (208).
H2O2 increases the activity of Src and other SFKs, including Lck (18, 86, 167), via oxidation at two cysteine residues and through the dephosphorylation of Tyr527 (72, 191). Exposure of pulmonary endothelial cells to H2O2 increases Src activity, leading to endothelial hyperpermeability (114). SFK inhibitors, herbimycin A and PP1, prolong the onset and attenuate the magnitude of the H2O2-induced increase in pulmonary endothelial permeability (114).
VEGF-induced increased vascular permeability requires SFKs (58, 252). Animals that do not express Src or Yes do not elicit VEGF-mediated vascular hyperpermeability (58). VEGF receptor (VEGFR)-1, VE-cadherin, and β-catenin form a complex that exerts a key role in endothelial barrier integrity (124, 230) and is dissociated upon VEGF stimulation (69, 251). Activated Src is recruited to Flk-1 after VEGF stimulation (43), suggesting that Src contributes to the VEGF-induced tyrosine phosphorylation of VE-cadherin and β-catenin in HUVECs (60). VEGF stimulation results in the endocytosis of VE-cadherin and the consequent disruption of the endothelial junctions. In axillary lymph node endothelial cells of mice, β-arrestin aids VE-cadherin endocytosis due to its ability to interact with Src (138) and facilitates the pulmonary Src-dependent phosphorylation of cadherin-catenin complexes (180, 251). Furthermore, in cultured embryonic endothelial cells, Src kinase regulates the VEGF-induced abundance of the FAK-αvβ5-integrin complexes, which are reduced in cells derived from Src knockout (KO) mice (137). These findings indicate that the Src-dependent and VEGF-induced FAK-αvβ5-integrin complex formation is an important regulatory mechanism of vascular permeability (83).
Besides SFKs, pharmacological inhibition of the Abl family of nonreceptor tyrosine kinases by imatinib achieves protective effects in animal models of inflammation. Mice, as well as HLMVECs lacking Abl, exhibit activated Rac1 and Rap1 and attenuated agonist-induced Ca2+ mobilization and actomyosin contraction (42).
Protein Kinase C
PKC is a family of Ser/Thr kinases consisting of classical (α, βI, βII, and γ), novel (δ, ε, η, and θ), and atypical (ι/λ and ζ) isoforms (116). Classical PKC isoforms are activated by binding to Ca2+ and diacylglycerol (DAG), novel PKC isoforms are activated by DAG, and atypical PKC isoforms are not activated by either Ca2+ or DAG. Activated PKC phosphorylates target proteins at Ser/Thr residues (8).
Most studies report that PKCs are mediators of endothelial hyperpermeability (270) through the activation of Ras-GTP, Raf, MAP/ERK kinases (MEKs), ERK1/2, c-Jun NH2-terminal kinases (JNK), and p38 MAPK (Fig. 1). In human and mouse pulmonary endothelial cells, activated PKCs increase actin polymerization (63, 220), induce disassembly of adherens junctions (10, 200), and cause rearrangement of focal adhesions (1).
PKCα mediates endothelial hyperpermeability in response to inflammatory stimulation, as well as during hyperglycemia, ischemia, and angiogenesis (94). PKCβII mediates hyperpermeability in the diabetic kidney and retina (70, 250). In HLMVECs, PKCα phosphorylates p120 catenin at Ser879, an effect that causes VE-cadherin internalization and a consequent junction destabilization (239). In mice and HLMVECs, inhibition of PKCβ and PKCζ decreases endothelial permeability by decreasing Ca2+ levels and impairing MLC-triggered contraction (82, 88, 100, 193). However, it has also been reported that PKCα exerts a protective effect on barrier integrity via RhoA suppression (80, 81), and PKCδ and PKCε may also be barrier-protective (70, 238). Recently, PKCδ KO mice and PKC inhibitors were used to show the involvement of PKCδ in vascular hyperpermeability in ALI (7) in pulmonary cells. The LPS-induced lung injury was more severe in PKCδ KO mice than the corresponding controls. PKCδ inhibition promoted barrier dysfunction in vitro, and administration of a PKCδ-specific inhibitor significantly increased steady-state vascular permeability. PKCδ inhibition increased neutrophil transmigration, indicating that PKCδ inhibition induces structural changes in endothelial cells, allowing extravasation of proteins and neutrophils (7). PKCδ induction has also been shown to decrease endothelial permeability by increasing focal adhesion contacts (88, 224). Furthermore, PKCε may exert a protective role on the heart and brain endothelium (159).
Nitric oxide (NO) has been identified as a potential downstream target of PKC. Endothelial cells exposed to PKC activators exhibit increased levels of NO synthase (NOS) phosphorylation and NO production (57). It has also been suggested that the barrier-weakening effect of PKC is mediated through the endothelial production of NO, suggesting an interactive role of PKC and NOS in the modulation of microvascular barrier function (101). In HLMVECs, PKC-dependent phosphorylation of endothelial NOS at Thr495 regulates endothelial NOS coupling and endothelial barrier function in response to G+ toxins (37).
Focal Adhesion Kinase
FAK is a cytoplasmic protein-tyrosine kinase that facilitates cytoskeletal remodeling, formation, and disassembly of cell adhesion structures and regulates the Rho-family GTPases (231). It facilitates the localization and cyclic activation of guanine nucleotide exchange factors and GTPase-activating proteins (GAPs) (232). Activated FAK recruits c-Src at focal adhesion sites to form a FAK-Src signaling complex. This signaling complex phosphorylates other focal adhesion signaling proteins such as paxillin and p130Cas, thereby activating diverse signaling pathways important in the regulation of cell migration (267).
FAK forms a complex with p120RasGAP and p190RhoGAP, resulting in the localization of p190RhoGAP at nascent focal adhesions. FAK-induced phosphorylation of p190RhoGAP is associated with the inhibition of RhoA at leading-edge focal adhesions. As Rho and Rac activity are antagonistic, Rho inhibition can also indirectly facilitate Rac activation (232).
The NH2-terminal domain of FAK binds to the COOH-terminal domain of integrins or growth factor receptors (184, 259). The COOH terminus of FAK is known as FAK-related nonkinase and is involved in the recruitment of FAK to focal adhesions at the cell membrane in response to phosphorylation (157).
FAK activity is mainly regulated through phosphorylation by SFKs (228). Inhibition of FAK tyrosine phosphorylation prevents focal adhesion formation and cell contraction (201, 202). Shear stress and growth factors activate focal adhesions through FAK tyrosine phosphorylation (3, 20, 79, 217). Overexpression of dominant-negative FAK in endothelial cells inhibits cell contraction induced by FAK activation (203). FAKs contain tyrosine phosphorylation sites that are important for focal adhesion assembly regulation (259). Phosphorylation of Tyr397 permits FAK binding to proteins containing the SH2 domain. FAK recruits SFKs to the focal adhesion complex, which in turn regulates FAK (270). Basal FAK phosphorylation levels are important for stabilizing focal adhesions (45) and help maintain normal physiological pulmonary endothelial permeability (184).
FAK-dependent strengthening of focal adhesions enhances endothelial barrier function under normal physiological conditions (259). In human pulmonary artery endothelial cells, FAK translocation to the focal adhesion complex attenuates the decrease in transendothelial electrical resistance induced by inflammatory agents (152) and is required for enhanced barrier function under hyperosmotic conditions (149). In Chinese hamster ovary cells, FAK binds to p120-catenin and suppresses the activity of the Rho family of GTPases (173, 269). It also prevents oxidant-induced barrier dysfunction by regulating VE-cadherin phosphorylation (224). In mouse pulmonary endothelial cells, conditional deletion of FAK disrupts lung vascular barrier function due to destabilization of RhoA and Rac1 activities (204).
However, expression of dominant-negative FAK prevents adherens junction disruption in response to VEGF or oxidants, indicating that FAK may also disrupt barrier function. In HUVECs, VEGF increases FAK tyrosine phosphorylation and recruitment to focal contacts associated with stress fibers (128). Genetic or pharmacological FAK inhibition prevents VEGF-stimulated permeability downstream of the VEGFR, both in vivo and in vitro. Similarly, in pulmonary cells, FAK phosphorylated at Tyr397, Tyr576, and Tyr925 contributes to thrombin-induced hyperpermeability, whereas FAK phosphorylated at only Tyr576 is associated with cortical actin focal adhesion and barrier strengthening (214, 236).
MAP Kinases
MAPKs mediate various cellular responses to growth factors and cytokines (121). In mammalian cells, the most abundant MAPKs are the extracellular signal-related kinases (ERK1/2), JNK, and p38 protein kinase. MAPK activation modulates the permeability of isolated rat epithelial cell monolayers following cyclic stretch (44). In HUVECs, inhibition of ERK1/2 with PD98059 or inhibition of p38 with SB203580 blocks VEGF-induced hyperpermeability (122, 258). Moreover, in pig coronary venules, ERK1/2 regulates the baseline barrier function and cGMP-induced hyperpermeability in endothelial cells (240, 268).
Many inflammatory agents cause ERK1/2 phosphorylation/activation (61, 115, 244, 254) and induce endothelial cytoskeletal contraction through a p38-related mechanism (75, 122). Advanced glycosylation end products induce ERK1/2 phosphorylation and leakage of retinal microvascular endothelial cells (221). Activation of the ERK1/2 or the p38 pathway can disrupt endothelial barrier function (77) and trigger the H2O2-induced loss of endothelial tight junctions (128). Conversely, ERK1/2 suppression is associated with Rho inhibition (177). Furthermore, there is a direct link among p38 MAPK activation, remodeling of the microtubule network, and endothelial barrier regulation: p38 MAPK inhibition attenuates the nocodazole-induced microtubule depolymerization, actin remodeling, and lung endothelial barrier dysfunction (24).
cAMP- and cGMP-Dependent Protein Kinases
PKA is a cAMP-dependent protein kinase that strengthens pulmonary endothelial barrier integrity (54, 92, 160, 171, 206, 209). It causes MLC dephosphorylation (163), dissociation of F-actin from myosin (125), stabilization of cytoskeletal filaments (91), and strengthening of cell-matrix adhesions (123). In rat aortas, PKA can also inhibit leukocyte adhesion and platelet aggregation (80, 129). In HUVECs, cAMP potentiates VE-cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway (62). Basal levels of cAMP are critical in maintaining normal barrier function under resting conditions, and increased levels of cAMP prevent increases in microvascular permeability (53, 54, 93). In HUVECs, prostaglandin D2-D-type prostanoid (DP) signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway (120) and exendin-4 promotes endothelial barrier enhancement via PKA- and Epac1-dependent Rac1 activation (133).
Selective inhibition of NOS, guanylate cyclase, or PKG suppresses shear stress-induced (271) and VEGF-induced (258) hyperpermeability. Most studies suggest that increases in endothelial cGMP are barrier-protective and prevent oxidant-mediated endothelial barrier dysfunction, possibly via PKG1 (158). However, there is also evidence in pulmonary endothelial cells that activation of the cGMP-dependent pathway is a necessary step in eliciting the hyperpermeability response to various inflammatory mediators (93, 153).
Phosphodiesterases (PDEs) catalyze the breakdown of cAMP and cGMP. Three PDE isoforms, cGMP-stimulated PDE II and IV and cGMP-inhibited PDE III, have been shown to affect cAMP-associated alteration of endothelial permeability (50, 53, 54, 171).
TESKs, LIMKs, and Cofilin
In 1984, it was observed that, when phosphorylated, actin-depolymerizing factor/cofilin (hereafter called cofilin) did not bind to actin, suggesting that this phosphorylation may be the crucial posttranslational modification that dictated the interaction between cofilin and actin. Phosphorylated and dephosphorylated cofilin exist, but the phosphorylated species represents a regulated, inactive form of cofilin (148) that is reactivated by dephosphorylation (147). The single cofilin phosphopeptide was sequenced, and Ser3 was identified as the phosphorylation site.
Cofilin has emerged as one of the protein families playing an essential role in actin dynamics at the plasma membrane during cell protrusion (141). Cofilin is a small (19-kDa) ubiquitous protein that binds to both G- and F-actin; it has a higher affinity for ADP-bound subunits and enhances the rate of monomer dissociation from the pointed end of actin filaments (33, 140). In addition, cofilin can also sever actin filaments and, thus, directly generate free actin barbed ends (51). The depolymerization and severing activities of cofilin are presumably due to its ability to bind cooperatively to F-actin and cause a twist in the actin filament, which promotes the destabilization of actin-actin interactions and, thus, fragmentation of the filament (147). Cofilin functions to depolymerize filaments, so that actin can be recycled for another round of polymerization (14, 97) and can induce polymerization of actin by virtue of its severing activity (71, 162).
The severing and depolymerization activity of cofilin in human and mouse pulmonary endothelial cells can be inhibited by phosphorylation on Ser3, which abolishes its actin-binding activity (6, 161). Four different kinases that appear to be downstream of the Rho-family GTPases phosphorylate cofilin: LIM domain kinases LIMK1 and LIMK2 and testis-specific kinases TESK1 and TESK2 (14, 47, 195, 233). Phosphatases, including type 1, type 2A (12), type 2B (148), type 2C (273), and a novel cofilin phosphatase, slingshot (170), have been implicated in the reactivation of cofilin by dephosphorylation in a variety of cell types. LIMK1 induction and cofilin phosphorylation promote endothelial barrier disruption (78).
p21-Activated Kinase
The p21-activated kinase (PAK) family of Ser/Thr kinases is involved in cytoskeletal reorganization, MAPK signaling, apoptotic signaling, control of phagocyte NADPH oxidase (Nox), and growth factor-induced neurite outgrowth (48, 119). Several mechanisms that induce PAK activity have been reported. Binding of Rac/cdc42 to the CRIB (or PBD) domain near the NH2 terminus of PAK causes autophosphorylation and conformational changes in PAK (119). In rat vascular SMCs, phosphorylation of PAK1 at Thr423 by 3-phosphoinositide-dependent kinase-1 induces activation of PAK1 (118). Several autophosphorylation sites, including Ser199 and Ser204 of PAK1 and Ser192 and Ser197 of PAK2, have been identified (143).
The effects of PAK on endothelial barrier function remain controversial. In HUVECs, PAK is phosphorylated on Ser141 during its activation downstream of the small GTPase Rac. The phosphorylated subfraction is reported to translocate to endothelial cell-cell junctions in response to serum, VEGF, basic fibroblast growth factor, TNFα, histamine, and thrombin (223), and blocking PAK activation in HLMVECs prevented the increase in monolayer permeability in response to these factors. Similarly, inhibition of PAK, in vivo, reduces permeability in atherosclerosis-prone regions, suggesting that matrix-specific PAK activation mediates elevated vascular permeability in atherogenesis (179). In BPAECs, direct phosphorylation of MLC by PAK leads to increased contractility and increased endothelial permeability (222). In rat fat endothelial cells, PAK disrupts endothelial cell-cell junctions by direct phosphorylation and subsequent internalization and degradation of VE-cadherin (67).
On the other hand, in HLMVECs and mouse lungs, activated PAK phosphorylates and inhibits guanine nucleotide exchange factor H1, which leads to decreased ROCK-MLC activity, decreased contractility, and decreased endothelial permeability (237). Furthermore, PAK protects against an increase in permeability in a hypoxia-induced pulmonary hypertension model (189). It was recently shown that endothelial PAK2 deletion leads to increased vascular permeability (188).
ROCK
ROCK is a Ser/Thr kinase that is known as the downstream target of the small GTP-binding protein RhoA (132). ROCK mediates RhoA-induced actin-cytoskeletal changes via the phosphorylation of MYPT1 at Thr496 (81, 117, 218).
Two ROCK isoforms have been identified and have been associated with increases in vascular permeability (Fig. 1). The ROCK1 gene is located on chromosome 18 and encodes a 1,354-amino acid protein (107, 131). The ROCK2 gene is located on chromosome 12 and encodes a 1,388-amino acid protein (145). ROCK1 and ROCK2 share an overall 65% homology in amino acid sequence and 92% homology in their kinase domains (134). ROCK1 is widely expressed in most tissues, except brain and muscle, whereas ROCK2 is most highly expressed in muscle, brain, heart, lung, and placenta (165). Both ROCK1 and ROCK2 are expressed in vascular endothelial cells and SMCs (156, 175, 255).
Angiotensin II and IL-1β upregulate both isoforms of ROCK in human coronary vascular SMCs. This is mediated by PKC and NF-κB (96). In vascular SMCs, silencing of either ROCK isoform leads to an increased protein expression of the other isoform (174).
Although ROCK1 and ROCK2 are ubiquitously expressed and highly homologous, several mechanisms have been reported to differentially regulate ROCK isoform activities. Overexpression of ROCK1 or ROCK2 in mouse lungs and HLMVECs can increase MLC phosphorylation, an effect that is associated with increased pulmonary vascular permeability (265). ROCK2 binds to the MYPT1 subunit of MLCP and plays a predominant role in vascular SMC contractility (248). ROCK2 drives the lysophosphatidic acid-mediated activation of NF-κB (215), and ROCK1 KO mice exert a reduced vascular inflammation and neointima formation after flow cessation-induced vascular injury in the ligated carotid artery (172).
LIMK1 and LIMK2 are well-characterized ROCK substrates, which in turn phosphorylate cofilin in mouse and human lungs. In endothelial cells, it was demonstrated that shear stress, VEGF, and thrombin induce ROCK-dependent LIMK activation and subsequent cofilin phosphorylation, leading to sterol regulatory element-binding protein 2 activation, rearrangements of the actin cytoskeleton, and a decrease in cofilin oligomerization (90).
Hsp90 and Kinases
Hsp90 is required for maintaining the stability and activity of a diverse group of client proteins, including protein kinases, transcription factors, and hormone receptors, that are involved in cell signaling, proliferation, survival, oncogenesis, and cancer progression. Inhibition of Hsp90 alters the Hsp90-client protein complex, leading to reduced activity, misfolding, ubiquitination, and proteasomal degradation of the client proteins, including numerous kinases (31) (Fig. 1). Hsp90 inhibitors are now being actively developed, and >17 agents have entered clinical trials (168). In addition, several laboratories focus on the anti-inflammatory activity of Hsp90 inhibitors in the lung (15, 16).
It was recently demonstrated that Hsp90 regulates Nox activity and is necessary for superoxide production by Nox1, Nox2, Nox3, and Nox5 (38). Hsp90 binds to and regulates Nox protein stability. These actions are opposed by the Hsp90 inhibitors Hsp70 and COOH terminus of heat shock conjugate 70-interacting protein (CHIP), which promote the ubiquitination and degradation of Nox proteins and, thus, reduce ROS production (39). Induction of ROS has been associated with increases in vascular permeability (103). Moreover, the activin receptor-like ALK1 and ALK5 were found to interact with Hsp90, and Hsp90 inhibition suppresses the constitutively active ALK5-induced endothelial hypoxanthine-guanine phosphoribosyltransferase (hyprt) permeability (13). Hsp90 inhibition also prevents and restores LPS-mediated hyperpermeability in HLMVECs. These effects appear to be mediated by inhibition of RhoA signaling, which is an important effector of vascular permeability (89). Furthermore, LPS induces pp60c-Src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung, and this effect was prevented in vivo and in vitro by Hsp90 inhibition (17). More recently, it was shown that the transcription factor p53 is involved in mediation of the barrier-protective effects of Hsp90 inhibitors in HLMVECs and mouse lungs. Hsp90 inhibition induces the upregulation of the wild-type p53, an effect that restores endothelial barrier function by disrupting the LPS-induced RhoA/MLC2 pathway (16).
Redundancy and Interplay Within the Kinase Network
The vascular endothelium is the first barrier confronting the fluid or the inflammatory cells migrating from the lumen to the alveoli. Endothelial barrier dysfunction causes major fluid leak, a hallmark of ALI/ARDS. Effective lung repair requires correction of two critical abnormalities: the hyperfiltration across the endothelial barrier and the edema accumulated in the alveolar spaces. In ALI, failure of endothelial cells to sieve proteins is responsible for the hyperpermeability and the resulting pulmonary edema (21).
Kinases are crucial regulators of vascular barrier integrity, since they are able to translate environmental and pathological stimuli into vascular barrier responses. There is intense cross talk between the different kinases, which in turn forges a plethora of intracellular pathways. For example, Src activates MLC by tyrosine phosphorylation in a Rho GTPase-dependent manner (65). Rho kinase-induced MLC phosphorylation is exerted at the same residue as MLCK (104, 234). In HLMVECs, VEGF-induced hyperpermeability involves partial activation of the transcription factor Sp1, which induces MLCK expression (216). Similarly, the thrombin-induced hyperpermeability in HUVECs is Src/MLCK-dependent (27, 74). In mouse lungs, MLC can be directly phosphorylated by PKC, in contrast to PKA, which reduces MLC phosphorylation (77).
PKA exerts its barrier-strengthening effect not only by dephosphorylating MLC (77, 163, 209), but also via Rac1 activation (133) and Src suppression. In HEK-293, mouse NIH-3T3, and NIH-3T3-A14 cells, G protein-coupled receptors inhibit Src signaling by activating Csk in a cAMP-PKA-dependent manner (2).
In rat epididymal microvascular endothelial cells and HUVECs, Src is crucial for the TNFα-induced endothelial barrier disruption, through VE-cadherin phosphorylation (76, 87) and direct phosphorylation of FAK, leading to disruption of integrin binding to the ECM (253). In HUVECs, Src is also involved in VEGF-induced hyperpermeability via tyrosine phosphorylation of VE-cadherin and β-catenin (4).
In HUVECs, VEGF-induced hyperpermeability is MAPK-dependent, as it is abolished by inhibition of ERK1/2 and p38 (155, 205). However, VEGF stimulation also promotes the rapid endocytosis of VE-cadherin by a PAK-dependent mechanism. PAK-mediated phosphorylation of a highly conserved motif within the intracellular tail of VE-cadherin results in the disassembly of intercellular junctions (69). PAK2 has been shown to phosphorylate MLCK (73).
Thrombin induces ERK activation via PKC-dependent and -independent pathways. The PKC-independent pathway requires the involvement of the Src kinases, while the PKC-dependent mechanism depends on PKCε (146). In HEK-293 cells, PKC regulation of the JNK pathway is triggered via PKCε (29), and in rat primary microglia cultures, PKCα is required in the LPS-induced activation of the p38 MAPK (166). PKC is involved in the MLC2 activation, since in BPAECs, MLC phosphorylation occurs with a stoichiometry similar to that mediated by MLCK (241). Furthermore, in HLMVECs, MLCK is inhibited by PAK (199), while PKC upregulates ROCK, which in turn induces MLC phosphorylation (68).
In mouse lungs, FAK activity can be regulated by SFKs and can synergistically regulate VE-cadherin and vascular permeability (207). Src-mediated phosphorylation of FAK is a pivotal regulator of actin and adhesion dynamics, since it controls cell migration and anchorage-independent growth (253). Moreover, FAK deletion in mouse lungs has been known to disrupt the barrier integrity by RhoA destabilization (204). However, in a rabbit model of atherosclerosis, genetic or pharmacological FAK inhibition in endothelial cells prevented the VEGF-stimulated permeability downstream of the VEGFR or Src tyrosine kinase activation (40). A similar effect, namely, disruption of the inflammatory RhoA/MLC2 pathway, can be exerted by a PAK-dependent mechanism in mouse lungs and coronary vessels (199, 237, 256). PAK was found to be activated by AMPK induction in human pulmonary artery endothelial cells (260). LIMKs are downstream of Rho-family GTPases, PAK and ROCK, so that PAK1 and ROCK phosphorylate LIMKs, increasing LIMK activity. In HeLa and COS-7 cells, LIMK inhibits the actin depolymerization activity of cofilin by phosphorylating the Ser3 residue of cofilin (51). Phosphorylation of cofilin at Tyr68 by Src leads to its degradation through the ubiquitin-proteasome pathway via a redundant pathway (266). Indeed, Rho kinase is reported to possess an intrinsic barrier-protective activity at the cell margins, apart from the well-established barrier-disruptive activity at contractile F-actin stress fibers (235).
Cell shape is a result of dynamic interactions among the cytoskeleton, the cell membrane, the adhesion complexes, and the environment. Certain shapes are required for particular cell behaviors (198). Motility is generated by formation of filopodial and lamellipodial protrusions at the leading edge (LE); at the trailing edge (TE), a propulsive force is induced by high contractility and disassembly of adhesion (126). Rho-family GTPases control cytoskeletal organization to regulate cell shape (108) and then promote localized changes in cell morphology and coordination of shape changes over the entire cell (192).
In cells migrating using cycles of protrusion, adhesion, and retraction, activation of RhoA promotes adhesion disassembly at the TE as well as cell-wide tension that is critical to restriction of protrusion at the LE (113). In some migrating cells, RhoA is activated at the LE together with cell advancement and is spatially segregated from a proximally localized wave of Rac1 and Cdc42 activity (139, 196). Compartmentalized regulation of Rho-family GTPase activity is critical for generation of particular shapes, since it has been shown that migration is inhibited when Rac activation is uniformly distributed throughout the cell (106, 219).
Activation of the Src, MLC, PKC, MAPK, PKC, ROCK, cGMP, and FAK kinases results in increased endothelial barrier dysfunction. On the other hand, activation of PKA, cAMP, TESKs, and LIMKs is associated with strengthening of endothelial barrier integrity. These effects vary among different cell types. The manner by which kinases operate in the vasculature and regulate endothelial permeability to proteins is further complicated, since these short-lived molecules orchestrate multiple interactions and mediate the cross talk among both them and downstream effectors. Moreover, the spectrum of this complexity becomes even greater, if we consider the lack of consensus over the exact subcellular localization of many of these molecules during inflammatory insults or cell movement (32).
Conclusions
Control of pulmonary endothelial barrier function is governed by complex, interdigitating, and occasionally redundant pathways that reflect the crucial importance of the endothelial barrier in normal homeostasis and the critical role of endothelial barrier dysfunction in numerous pathologies. Kinases represent major players within these pathways and exert profound pathophysiological influences on the function of the vascular barrier. Overexpression of many of these regulatory enzymes is induced by inflammatory stimuli and severely affects normal vascular function, leading to physiological abnormalities. An emerging body of studies suggest that inhibition of the activity of certain of these proteins may have important therapeutic effects in clinical practice.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-101902.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.B. prepared the figure; N.B. and A.D.V. drafted the manuscript; N.B., A.D.V., and J.D.C. edited and revised the manuscript; N.B., A.D.V., and J.D.C. approved the final version of the manuscript.
REFERENCES
- 1.Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem 272: 15442–15451, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsen H, Vang T, Tasken K. Protein kinase A intersects SRC signaling in membrane microdomains. J Biol Chem 278: 17170–17177, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861 and migration and anti-apoptosis in endothelial cells. Biochem J 360: 255–264, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam AP. Regulation of endothelial adherens junctions by tyrosine phosphorylation. Mediators Inflamm 2015: 272858, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine-1-phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res 88: 344–351, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem 270: 17582–17587, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Ahn JJ, Jung JP, Park SE, Lee M, Kwon B, Cho HR. Involvement of protein kinase C-δ in vascular permeability in acute lung injury. Immune Netw 15: 206–211, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aitken A. Protein consensus sequence motifs. Methods Mol Biol 211: 465–485, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Alexander JS, Alexander BC, Eppihimer LA, Goodyear N, Haque R, Davis CP, Kalogeris TJ, Carden DL, Zhu YN, Kevil CG. Inflammatory mediators induce sequestration of VE-cadherin in cultured human endothelial cells. Inflammation 24: 99–113, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JS, Jackson SA, Chaney E, Kevil CG, Haselton FR. The role of cadherin endocytosis in endothelial barrier regulation: involvement of protein kinase C and actin-cadherin interactions. Inflammation 22: 419–433, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Ambach A, Saunus J, Konstandin M, Wesselborg S, Meuer SC, Samstag Y. The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur J Immunol 30: 3422–3431, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Antonov AS, Antonova GN, Fujii M, ten Dijke P, Handa V, Catravas JD, Verin AD. Regulation of endothelial barrier function by TGF-β type I receptor ALK5: potential role of contractile mechanisms and heat shock protein 90. J Cell Physiol 227: 759–771, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393: 805–809, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Barabutis N, Catravas JD. Anti-inflammatory activity of Hsp90 inhibitors in the human vasculature. Med Surg Urol 2: e104, 2012. [Google Scholar]
- 16.Barabutis N, Dimitropoulou C, Birmpas C, Joshi A, Thangjam G, Catravas JD.. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 308: L776–L787, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barabutis N, Handa V, Dimitropoulou C, Rafikov R, Snead C, Kumar S, Joshi A, Thangjam G, Fulton D, Black SM, Patel V, Catravas JD. LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am J Physiol Lung Cell Mol Physiol 304: L883–L893, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barchowsky A, Munro SR, Morana SJ, Vincenti MP, Treadwell M. Oxidant-sensitive and phosphorylation-dependent activation of NF-κB and AP-1 in endothelial cells. Am J Physiol Lung Cell Mol Physiol 269: L829–L836, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Beg ZH, Allmann DW, Gibson DM. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and with protein fractions of rat liver cytosol. Biochem Biophys Res Commun 54: 1362–1369, 1973. [DOI] [PubMed] [Google Scholar]
- 20.Berk BC, Corson MA, Peterson TE, Tseng H. Protein kinases as mediators of fluid shear stress stimulated signal transduction in endothelial cells: a hypothesis for calcium-dependent and calcium-independent events activated by flow. J Biomech 28: 1439–1450, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75: 593–615, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Bindewald K, Gunduz D, Hartel F, Peters SC, Rodewald C, Nau S, Schafer M, Neumann J, Piper HM, Noll T. Opposite effect of cAMP signaling in endothelial barriers of different origin. Am J Physiol Cell Physiol 287: C1246–C1255, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60Src. J Biol Chem 276: 8567–8573, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 289: L75–L84, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal DK, Takio K, Edelman AM, Charbonneau H, Titani K, Walsh KA, Krebs EG. Identification of the calmodulin-binding domain of skeletal muscle myosin light chain kinase. Proc Natl Acad Sci USA 82: 3187–3191, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boczek EE, Reefschlager LG, Dehling M, Struller TJ, Hausler E, Seidl A, Kaila VR, Buchner J. Conformational processing of oncogenic v-Src kinase by the molecular chaperone Hsp90. Proc Natl Acad Sci USA 112: E3189–E3198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochem Biokhim 67: 75–84, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res 76: 202–207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandlin I, Eiseler T, Salowsky R, Johannes FJ. Protein kinase Cμ regulation of the JNK pathway is triggered via phosphoinositide-dependent kinase 1 and protein kinase Cε. J Biol Chem 277: 45451–45457, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Brugge JS, Erikson RL. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature 269: 346–348, 1977. [DOI] [PubMed] [Google Scholar]
- 31.Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P. Maximizing the therapeutic potential of HSP90 inhibitors. Mol Cancer Res 13: 1445–1451, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrne KM, Monsefi N, Dawson JC, Degasperi A, Bukowski-Wills JC, Volinsky N, Dobrzynski M, Birtwistle MR, Tsyganov MA, Kiyatkin A, Kida K, Finch AJ, Carragher NO, Kolch W, Nguyen LK, von Kriegsheim A, Kholodenko BN. Bistability in the Rac1, PAK, and RhoA signaling network drives actin cytoskeleton dynamics and cell motility switches. Cell Syst 2: 38–48, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136: 1307–1322, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett 223: 217–222, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem 248: 378–380, 1973. [PubMed] [Google Scholar]
- 36.Chan JY, Takeda M, Briggs LE, Graham ML, Lu JT, Horikoshi N, Weinberg EO, Aoki H, Sato N, Chien KR, Kasahara H. Identification of cardiac-specific myosin light chain kinase. Circ Res 102: 571–580, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Kumar S, Yu Y, Aggarwal S, Gross C, Wang Y, Chakraborty T, Verin AD, Catravas JD, Lucas R, Black SM, Fulton DJ. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+ toxins. PLos One 9: e99823, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxid Redox Signal 14: 2107–2119, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F, Yu Y, Qian J, Wang Y, Cheng B, Dimitropoulou C, Patel V, Chadli A, Rudic RD, Stepp DW, Catravas JD, Fulton DJ. Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arterioscler Thromb Vasc Biol 32: 2989–2999, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. VEGF-induced vascular permeability is mediated by FAK. Dev Cell 22: 146–157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng X, Wang X, Wan Y, Zhou Q, Zhu H, Wang Y. Myosin light chain kinase inhibitor ML7 improves vascular endothelial dysfunction via tight junction regulation in a rabbit model of atherosclerosis. Mol Med Rep 12: 4109–4116, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chislock EM, Pendergast AM. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLos One 8: e85231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou MT, Wang J, Fujita DJ. Src kinase becomes preferentially associated with the VEGFR, KDR/Flk-1, following VEGF stimulation of vascular endothelial cells. BMC Biochem 3: 32, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen TS, Gray Lawrence G, Khasgiwala A, Margulies SS. MAPK activation modulates permeability of isolated rat alveolar epithelial cell monolayers following cyclic stretch. PLos One 5: e10385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell 14: 3065–3081, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Csortos C, Kolosova I, Verin AD. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am J Physiol Lung Cell Mol Physiol 293: L843–L854, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem 276: 32115–32121, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J 17: 754–764, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies SP, Hawley SA, Woods A, Carling D, Haystead TA, Hardie DG. Purification of the AMP-activated protein kinase on ATP-γ-Sepharose and analysis of its subunit structure. Eur J Biochem 223: 351–357, 1994. [DOI] [PubMed] [Google Scholar]
- 50.DeFouw LM, DeFouw DO. Differential phosphodiesterase activity contributes to restrictive endothelial barrier function during angiogenesis. Microvasc Res 62: 263–270, 2001. [DOI] [PubMed] [Google Scholar]
- 51.DesMarais V, Ghosh M, Eddy R, Condeelis J. Cofilin takes the lead. J Cell Sci 118: 19–26, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Dixit M, Bess E, Fisslthaler B, Hartel FV, Noll T, Busse R, Fleming I. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res 77: 160–168, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Draijer R, Atsma DE, van der Laarse A, van Hinsbergh VW.. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res 76: 199–208, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Draijer R, Vaandrager AB, Nolte C, de Jonge HR, Walter U, van Hinsbergh VW. Expression of cGMP-dependent protein kinase I and phosphorylation of its substrate, vasodilator-stimulated phosphoprotein, in human endothelial cells of different origin. Circ Res 77: 897–905, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 279: 24692–24700, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Duran WN, Seyama A, Yoshimura K, Gonzalez DR, Jara PI, Figueroa XF, Boric MP. Stimulation of NO production and of eNOS phosphorylation in the microcirculation in vivo. Microvasc Res 60: 104–111, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin-αvβ5 in vascular endothelial growth factor signaling. J Cell Biol 157: 149–160, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111: 1853–1865, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Fleming I, Fisslthaler B, Busse R. Calcium signaling in endothelial cells involves activation of tyrosine kinases and leads to activation of mitogen-activated protein kinases. Circ Res 76: 522–529, 1995. [DOI] [PubMed] [Google Scholar]
- 62.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol 16: 489–494, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60src. Am J Physiol Lung Cell Mol Physiol 276: L989–L998, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Gaudreault N, Perrin RM, Guo M, Clanton CP, Wu MH, Yuan SY. Counter regulatory effects of PKCβII and PKCδ on coronary endothelial permeability. Arterioscler Thromb Vasc Biol 28: 1527–1533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gavard J. Breaking the VE-cadherin bonds. FEBS Lett 583: 1–6, 2009. [DOI] [PubMed] [Google Scholar]
- 68.Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Gα12/13 and Gα11/q. J Biol Chem 283: 29888–29896, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8: 1223–1234, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319–1331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science 304: 743–746, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol 25: 6391–6403, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem 275: 18366–18374, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol 130: 613–627, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldberg PL, MacNaughton DE, Clements RT, Minnear FL, Vincent PA.. p38 MAPK activation by TGF-β1 increases MLC phosphorylation and endothelial monolayer permeability. Am J Physiol Lung Cell Mol Physiol 282: L146–L154, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D, Malik AB. Evidence of a common mechanism of disassembly of adherens junctions through Gα13 targeting of VE-cadherin. J Exp Med 211: 579–591, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778: 729–756, 2008. [DOI] [PubMed] [Google Scholar]
- 78.Gorovoy M, Han J, Pan H, Welch E, Neamu R, Jia Z, Predescu D, Vogel S, Minshall RD, Ye RD, Malik AB, Voyno-Yasenetskaya T. LIM kinase 1 promotes endothelial barrier disruption and neutrophil infiltration in mouse lungs. Circ Res 105: 549–556, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gozin A, Franzini E, Andrieu V, Da Costa L, Rollet-Labelle E, Pasquier C. Reactive oxygen species activate focal adhesion kinase, paxillin and p130cas tyrosine phosphorylation in endothelial cells. Free Radic Biol Med 25: 1021–1032, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol 55: 662–675, 1994. [PubMed] [Google Scholar]
- 81.Grassie ME, Sutherland C, Ulke-Lemee A, Chappellaz M, Kiss E, Walsh MP, MacDonald JA. Cross-talk between Rho-associated kinase and cyclic nucleotide-dependent kinase signaling pathways in the regulation of smooth muscle myosin light chain phosphatase. J Biol Chem 287: 36356–36369, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grinnell KL, Harrington EO. Interplay between FAK, PKCδ, and p190RhoGAP in the regulation of endothelial barrier function. Microvasc Res 83: 12–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 271: 695–701, 1996. [DOI] [PubMed] [Google Scholar]
- 84.Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr 34: 31–55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes 62: 2164–2172, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hardwick JS, Sefton BM. Activation of the Lck tyrosine protein kinase by hydrogen peroxide requires the phosphorylation of Tyr394. Proc Natl Acad Sci USA 92: 4527–4531, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hardyman MA, Wilkinson E, Martin E, Jayasekera NP, Blume C, Swindle EJ, Gozzard N, Holgate ST, Howarth PH, Davies DE, Collins JE. TNF-α-mediated bronchial barrier disruption and regulation by src-family kinase activation. J Allergy Clin Immunol 132: 665–675, 2013. [DOI] [PubMed] [Google Scholar]
- 88.Harrington EO, Brunelle JL, Shannon CJ, Kim ES, Mennella K, Rounds S. Role of protein kinase C isoforms in rat epididymal microvascular endothelial barrier function. Am J Respir Cell Mol Biol 28: 626–636, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Harrington EO, Newton J, Morin N, Rounds S. Barrier dysfunction and RhoA activation are blunted by homocysteine and adenosine in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 287: L1091–L1097, 2004. [DOI] [PubMed] [Google Scholar]
- 90.Hartmann S, Ridley AJ, Lutz S. The function of rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol 6: 276, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hastie LE, Patton WF, Hechtman HB, Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol 172: 373–381, 1997. [DOI] [PubMed] [Google Scholar]
- 92.He P, Zeng M, Curry FE.. cGMP modulates basal and activated microvessel permeability independently of [Ca2+]i. Am J Physiol Heart Circ Physiol 274: H1865–H1874, 1998. [DOI] [PubMed] [Google Scholar]
- 93.He P, Zeng M, Curry FE. Dominant role of cAMP in regulation of microvessel permeability. Am J Physiol Heart Circ Physiol 278: H1124–H1133, 2000. [DOI] [PubMed] [Google Scholar]
- 94.Hempel A, Lindschau C, Maasch C, Mahn M, Bychkov R, Noll T, Luft FC, Haller H. Calcium antagonists ameliorate ischemia-induced endothelial cell permeability by inhibiting protein kinase C. Circulation 99: 2523–2529, 1999. [DOI] [PubMed] [Google Scholar]
- 95.Herring BP, Dixon S, Gallagher PJ. Smooth muscle myosin light chain kinase expression in cardiac and skeletal muscle. Am J Physiol Cell Physiol 279: C1656–C1664, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hiroki J, Shimokawa H, Higashi M, Morikawa K, Kandabashi T, Kawamura N, Kubota T, Ichiki T, Amano M, Kaibuchi K, Takeshita A. Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J Mol Cell Cardiol 37: 537–546, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell 16: 649–664, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu G, Minshall RD. Regulation of transendothelial permeability by Src kinase. Microvasc Res 77: 21–25, 2009. [DOI] [PubMed] [Google Scholar]
- 99.Hu G, Place AT, Minshall RD. Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules. Chem Biol Interact 171: 177–189, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCα and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol 289: L176–L185, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol Heart Circ Physiol 273: H2442–H2451, 1997. [DOI] [PubMed] [Google Scholar]
- 102.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem 69: 373–398, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res 80: 383–392, 1997. [DOI] [PubMed] [Google Scholar]
- 104.Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem 271: 4733–4740, 1996. [DOI] [PubMed] [Google Scholar]
- 105.Ikebe M, Hartshorne DJ, Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J Biol Chem 261: 36–39, 1986. [PubMed] [Google Scholar]
- 106.Inoue T, Meyer T. Synthetic activation of endogenous PI3K and Rac identifies an AND-gate switch for cell polarization and migration. PLos One 3: e3068, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 15: 1885–1893, 1996. [PMC free article] [PubMed] [Google Scholar]
- 108.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269, 2005. [DOI] [PubMed] [Google Scholar]
- 109.Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol 305: L844–L855, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jian MY, Liu Y, Li Q, Wolkowicz P, Alexeyev M, Zmijewski J, Creighton J. N-cadherin coordinates AMP kinase-mediated lung vascular repair. Am J Physiol Lung Cell Mol Physiol 310: L71–L85, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaneko-Kawano T, Takasu F, Naoki H, Sakumura Y, Ishii S, Ueba T, Eiyama A, Okada A, Kawano Y, Suzuki K. Dynamic regulation of myosin light chain phosphorylation by Rho-kinase. PLos One 7: e39269, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kasa A, Csortos C, Verin AD. Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue Barriers 3: e974448, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, Theriot JA. Mechanism of shape determination in motile cells. Nature 453: 475–480, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kevil CG, Okayama N, Alexander JS. H2O2-mediated permeability. II. Importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol 281: C1940–C1947, 2001. [DOI] [PubMed] [Google Scholar]
- 115.Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H2O2-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol 279: C21–C30, 2000. [DOI] [PubMed] [Google Scholar]
- 116.Kheifets V, Mochly-Rosen D. Insight into intra- and inter-molecular interactions of PKC: design of specific modulators of kinase function. Pharmacol Res 55: 467–476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996. [DOI] [PubMed] [Google Scholar]
- 118.King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM.. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J Biol Chem 275: 41201–41209, 2000. [DOI] [PubMed] [Google Scholar]
- 119.Knaus UG, Bokoch GM. The p21Rac/Cdc42-activated kinases (PAKs). Int J Biochem Cell Biol 30: 857–862, 1998. [DOI] [PubMed] [Google Scholar]
- 120.Kobayashi K, Tsubosaka Y, Hori M, Narumiya S, Ozaki H, Murata T. Prostaglandin D2-DP signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway. Arterioscler Thromb Vasc Biol 33: 565–571, 2013. [DOI] [PubMed] [Google Scholar]
- 121.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869, 2001. [DOI] [PubMed] [Google Scholar]
- 122.Lal BK, Varma S, Pappas PJ, Hobson RW 2nd, Duran WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res 62: 252–262, 2001. [DOI] [PubMed] [Google Scholar]
- 123.Lampugnani MG, Giorgi M, Gaboli M, Dejana E, Marchisio PC. Endothelial cell motility, integrin receptor clustering, and microfilament organization are inhibited by agents that increase intracellular cAMP. Lab Invest 63: 521–531, 1990. [PubMed] [Google Scholar]
- 124.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 13: 1175–1189, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol Cell Physiol 260: C1052–C1059, 1991. [DOI] [PubMed] [Google Scholar]
- 126.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 84: 359–369, 1996. [DOI] [PubMed] [Google Scholar]
- 127.Lazar V, Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK). Genomics 57: 256–267, 1999. [DOI] [PubMed] [Google Scholar]
- 128.Le Boeuf F, Houle F, Sussman M, Huot J. Phosphorylation of focal adhesion kinase (FAK) on Ser732 is induced by Rho-dependent kinase and is essential for proline-rich tyrosine kinase-2-mediated phosphorylation of FAK on Tyr407 in response to vascular endothelial growth factor. Mol Biol Cell 17: 3508–3520, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90α impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem 282: 9364–9371, 2007. [DOI] [PubMed] [Google Scholar]
- 130.Leopoldt D, Yee HF Jr, Rozengurt E. Calyculin-A induces focal adhesion assembly and tyrosine phosphorylation of p125Fak, p130Cas, and paxillin in Swiss 3T3 cells. J Cell Physiol 188: 106–119, 2001. [DOI] [PubMed] [Google Scholar]
- 131.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 16: 5313–5327, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 270: 29051–29054, 1995. [DOI] [PubMed] [Google Scholar]
- 133.Li AQ, Zhao L, Zhou TF, Zhang MQ, Qin XM. Exendin-4 promotes endothelial barrier enhancement via PKA- and Epac1-dependent Rac1 activation. Am J Physiol Cell Physiol 308: C164–C175, 2015. [DOI] [PubMed] [Google Scholar]
- 134.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol 50: 17–24, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Looney MR, Bhattacharya J. Live imaging of the lung. Annu Rev Physiol 76: 431–445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lowell CA, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev 10: 1845–1857, 1996. [DOI] [PubMed] [Google Scholar]
- 137.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 267: L223–L241, 1994. [DOI] [PubMed] [Google Scholar]
- 138.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ.. β-Arrestin-dependent formation of β2-adrenergic receptor-Src protein kinase complexes. Science 283: 655–661, 1999. [DOI] [PubMed] [Google Scholar]
- 139.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature 461: 99–103, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maciver SK. How ADF/cofilin depolymerizes actin filaments. Curr Opin Cell Biol 10: 140–144, 1998. [DOI] [PubMed] [Google Scholar]
- 141.Maciver SK, Hussey PJ. The ADF/cofilin family: actin-remodeling proteins. Genome Biol 3: reviews3007, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 298: 1912–1934, 2002. [DOI] [PubMed] [Google Scholar]
- 143.Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol 17: 1129–1143, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mark KS, Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-α exposure. Life Sci 64: 1941–1953, 1999. [DOI] [PubMed] [Google Scholar]
- 145.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15: 2208–2216, 1996. [PMC free article] [PubMed] [Google Scholar]
- 146.Maulon L, Mari B, Bertolotto C, Ricci JE, Luciano F, Belhacene N, Deckert M, Baier G, Auberger P. Differential requirements for ERK1/2 and P38 MAPK activation by thrombin in T cells. Role of P59Fyn and PKCε. Oncogene 20: 1964–1972, 2001. [DOI] [PubMed] [Google Scholar]
- 147.McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J Cell Biol 138: 771–781, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton 39: 172–190, 1998. [DOI] [PubMed] [Google Scholar]
- 149.Mehta D. Focal adhesion kinase regulation of endothelial barrier function, apoptosis, and neovascularization. Microvasc Res 83: 1–2, 2012. [DOI] [PubMed] [Google Scholar]
- 150.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 151.Mehta D, Ravindran K, Kuebler WM. Novel regulators of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 307: L924–L935, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol 539: 779–789, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Meyer DJ Jr, Huxley VH. Capillary hydraulic conductivity is elevated by cGMP-dependent vasodilators. Circ Res 70: 382–391, 1992. [DOI] [PubMed] [Google Scholar]
- 154.Meyn MA 3rd, Smithgall TE. Small molecule inhibitors of Lck: the search for specificity within a kinase family. Mini Rev Med Chem 8: 628–637, 2008. [DOI] [PubMed] [Google Scholar]
- 155.Miao Z, Dong Y, Fang W, Shang D, Liu D, Zhang K, Li B, Chen YH. VEGF increases paracellular permeability in brain endothelial cells via upregulation of EphA2. Anat Rec 297: 964–972, 2014. [DOI] [PubMed] [Google Scholar]
- 156.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation 110: 3708–3714, 2004. [DOI] [PubMed] [Google Scholar]
- 157.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6: 56–68, 2005. [DOI] [PubMed] [Google Scholar]
- 158.Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, Stephens RS, Verin AD, Tuder RM, Pearse DB. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290: L919–L930, 2006. [DOI] [PubMed] [Google Scholar]
- 159.Monti M, Donnini S, Giachetti A, Mochly-Rosen D, Ziche M.. δPKC inhibition or εPKC activation repairs endothelial vascular dysfunction by regulating eNOS post-translational modification. J Mol Cell Cardiol 48: 746–756, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morel NM, Petruzzo PP, Hechtman HB, Shepro D. Inflammatory agonists that increase microvascular permeability in vivo stimulate cultured pulmonary microvessel endothelial cell contraction. Inflammation 14: 571–583, 1990. [DOI] [PubMed] [Google Scholar]
- 161.Moriyama K, Iida K, Yahara I. Phosphorylation of Ser3 of cofilin regulates its essential function on actin. Genes Cells 1: 73–86, 1996. [DOI] [PubMed] [Google Scholar]
- 162.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol 166: 697–708, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moy AB, Shasby SS, Scott BD, Shasby DM. The effect of histamine and cyclic adenosine monophosphate on myosin light chain phosphorylation in human umbilical vein endothelial cells. J Clin Invest 92: 1198–1206, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Munday MR, Campbell DG, Carling D, Hardie DG. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem 175: 331–338, 1988. [DOI] [PubMed] [Google Scholar]
- 165.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 392: 189–193, 1996. [DOI] [PubMed] [Google Scholar]
- 166.Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Protein kinase C-α requirement in the activation of p38 mitogen-activated protein kinase, which is linked to the induction of tumor necrosis factor-α in lipopolysaccharide-stimulated microglia. Neurochem Int 44: 205–214, 2004. [DOI] [PubMed] [Google Scholar]
- 167.Nakamura K, Hori T, Sato N, Sugie K, Kawakami T, Yodoi J. Redox regulation of a src family protein tyrosine kinase p56lck in T cells. Oncogene 8: 3133–3139, 1993. [PubMed] [Google Scholar]
- 168.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18: 64–76, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nepiyushchikh ZV, Chakraborty S, Wang W, Davis MJ, Zawieja DC, Muthuchamy M. Differential effects of myosin light chain kinase inhibition on contractility, force development and myosin light chain 20 phosphorylation of rat cervical and thoracic duct lymphatics. J Physiol 589: 5415–5429, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell 108: 233–246, 2002. [DOI] [PubMed] [Google Scholar]
- 171.Noel PE, Fletcher JR, Thompson WJ. Rolipram and isoproterenol reverse platelet activating factor-induced increases in pulmonary microvascular permeability and vascular resistance. J Surg Res 59: 159–164, 1995. [DOI] [PubMed] [Google Scholar]
- 172.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest 118: 1632–1644, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Noren NK, Liu BP, Burridge K, Kreft B.. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 150: 567–580, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci 67: 3823–3836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nuno DW, Harrod JS, Lamping KG. Sex-dependent differences in Rho activation contribute to contractile dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 297: H1469–H1477, 2009. [DOI] [PubMed] [Google Scholar]
- 176.Nwariaku FE, Liu Z, Zhu X, Turnage RH, Sarosi GA, Terada LS. Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery 132: 180–185, 2002. [DOI] [PubMed] [Google Scholar]
- 177.Nwariaku FE, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol (1985) 95: 1889–1895, 2003. [DOI] [PubMed] [Google Scholar]
- 178.Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol 291: L129–L141, 2006. [DOI] [PubMed] [Google Scholar]
- 179.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol 176: 719–727, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol 25: 389–402, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park DW, Jiang S, Liu Y, Siegal GP, Inoki K, Abraham E, Zmijewski JW. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L735–L745, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Park DW, Jiang S, Tadie JM, Stigler WS, Gao Y, Deshane J, Abraham E, Zmijewski JW. Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol Med 19: 387–398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Park SJ, Lee KS, Kim SR, Chae HJ, Yoo WH, Kim DI, Jeon MS, Lee YC. AMPK activation reduces vascular permeability and airway inflammation by regulating HIF/VEGFA pathway in a murine model of toluene diisocyanate-induced asthma. Inflamm Res 61: 1069–1083, 2012. [DOI] [PubMed] [Google Scholar]
- 184.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 116: 1409–1416, 2003. [DOI] [PubMed] [Google Scholar]
- 185.Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 7: 287–308, 2000. [DOI] [PubMed] [Google Scholar]
- 186.Purchio AF, Erikson E, Brugge JS, Erikson RL. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci USA 75: 1567–1571, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qiao RL, Bhattacharya J. Segmental barrier properties of the pulmonary microvascular bed. J Appl Physiol (1985) 71: 2152–2159, 1991. [DOI] [PubMed] [Google Scholar]
- 188.Radu M, Lyle K, Hoeflich KP, Villamar-Cruz O, Koeppen H, Chernoff J.. p21-activated kinase 2 regulates endothelial development and function through the Bmk1/Erk5 pathway. Mol Cell Biol 35: 3990–4005, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer 14: 13–25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rankin S, Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. Bell-shaped dose response and cross-talk with bombesin. J Biol Chem 269: 704–710, 1994. [PubMed] [Google Scholar]
- 191.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312: 1882–1883, 2006. [DOI] [PubMed] [Google Scholar]
- 192.Ridley AJ. Life at the leading edge. Cell 145: 1012–1022, 2011. [DOI] [PubMed] [Google Scholar]
- 193.Rigor RR, Shen Q, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase signaling in endothelial barrier dysfunction. Med Res Rev 33: 911–933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roskoski R., Jr. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun 331: 1–14, 2005. [DOI] [PubMed] [Google Scholar]
- 195.Rosok O, Pedeutour F, Ree AH, Aasheim HC. Identification and characterization of TESK2, a novel member of the LIMK/TESK family of protein kinases, predominantly expressed in testis. Genomics 61: 44–54, 1999. [DOI] [PubMed] [Google Scholar]
- 196.Rounds S, Lu Q, Harrington EO, Newton J, Casserly B. Pulmonary endothelial cell signaling and function. Trans Am Clin Climatol Assoc 119: 155–167, 2008. [PMC free article] [PubMed] [Google Scholar]
- 197.Russe OQ, Moser CV, Kynast KL, King TS, Olbrich K, Grosch S, Geisslinger G, Niederberger E. LPS inhibits caspase 3-dependent apoptosis in RAW264.7 macrophages induced by the AMPK activator AICAR. Biochem Biophys Res Commun 447: 520–525, 2014. [DOI] [PubMed] [Google Scholar]
- 198.Sailem H, Bousgouni V, Cooper S, Bakal C. Cross-talk between Rho and Rac GTPases drives deterministic exploration of cellular shape space and morphological heterogeneity. Open Biol 4: 130132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283: 2083–2085, 1999. [DOI] [PubMed] [Google Scholar]
- 200.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca2+ signalling and PKCα activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol 533: 433–445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 1540: 1–21, 2001. [DOI] [PubMed] [Google Scholar]
- 202.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 71: 435–478, 1999. [DOI] [PubMed] [Google Scholar]
- 203.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 14: 92–101, 2004. [DOI] [PubMed] [Google Scholar]
- 204.Schmidt TT, Tauseef M, Yue L, Bonini MG, Gothert J, Shen TL, Guan JL, Predescu S, Sadikot R, Mehta D. Conditional deletion of FAK in mice endothelium disrupts lung vascular barrier function due to destabilization of RhoA and Rac1 activities. Am J Physiol Lung Cell Mol Physiol 305: L291–L300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S.. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation 111: 1184–1191, 2005. [DOI] [PubMed] [Google Scholar]
- 206.Seibert AF, Thompson WJ, Taylor A, Wilborn WH, Barnard J, Haynes J. Reversal of increased microvascular permeability associated with ischemia-reperfusion: role of cAMP. J Appl Physiol (1985) 72: 389–395, 1992. [DOI] [PubMed] [Google Scholar]
- 207.Serrels A, Canel M, Brunton VG, Frame MC. Src/FAK-mediated regulation of E-cadherin as a mechanism for controlling collective cell movement: insights from in vivo imaging. Cell Adh Migr 5: 360–365, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem 279: 20392–20400, 2004. [DOI] [PubMed] [Google Scholar]
- 209.Sheldon R, Moy A, Lindsley K, Shasby S, Shasby DM. Role of myosin light-chain phosphorylation in endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol 265: L606–L612, 1993. [DOI] [PubMed] [Google Scholar]
- 210.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 87: 272–280, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shi J, Takahashi S, Jin XH, Li YQ, Ito Y, Mori Y, Inoue R. Myosin light chain kinase-independent inhibition by ML-9 of murine TRPC6 channels expressed in HEK293 cells. Br J Pharmacol 152: 122–131, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shi X, Sun H, Zhou D, Xi H, Shan L. Arctigenin attenuates lipopolysaccharide-induced acute lung injury in rats. Inflammation 38: 623–631, 2015. [DOI] [PubMed] [Google Scholar]
- 213.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell 139: 468–484, 2009. [DOI] [PubMed] [Google Scholar]
- 214.Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J 17: 2240–2249, 2003. [DOI] [PubMed] [Google Scholar]
- 215.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-κB p65. J Biol Chem 285: 12536–12542, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shimizu Y, Camp SM, Sun X, Zhou T, Wang T, Garcia JG. Sp1-mediated nonmuscle myosin light chain kinase expression and enhanced activity in vascular endothelial growth factor-induced vascular permeability. Pulm Circ 5: 707–715, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Soldi R, Sanavio F, Aglietta M, Primo L, Defilippi P, Marchisio PC, Bussolino F. Platelet-activating factor (PAF) induces the early tyrosine phosphorylation of focal adhesion kinase (p125FAK) in human endothelial cells. Oncogene 13: 515–525, 1996. [PubMed] [Google Scholar]
- 218.Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun 269: 652–659, 2000. [DOI] [PubMed] [Google Scholar]
- 219.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 160: 375–385, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stasek JE Jr, Patterson CE, Garcia JG. Protein kinase C phosphorylates caldesmon77 and vimentin and enhances albumin permeability across cultured bovine pulmonary artery endothelial cell monolayers. J Cell Physiol 153: 62–75, 1992. [DOI] [PubMed] [Google Scholar]
- 221.Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation end products: clinical effects and molecular mechanisms. Mol Metab 3: 94–108, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: β-PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell 18: 2346–2355, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stockton RA, Schaefer E, Schwartz MA.. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem 279: 46621–46630, 2004. [DOI] [PubMed] [Google Scholar]
- 224.Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ 4: 535–551, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Suzuki A, Itoh T. Effects of calyculin A on tension and myosin phosphorylation in skinned smooth muscle of the rabbit mesenteric artery. Br J Pharmacol 109: 703–712, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tadie JM, Bae HB, Deshane JS, Bell CP, Lazarowski ER, Chaplin DD, Thannickal VJ, Abraham E, Zmijewski JW. Toll-like receptor 4 engagement inhibits adenosine 5′-monophosphate-activated protein kinase activation through a high mobility group box 1 protein-dependent mechanism. Mol Med 18: 659–668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Takashima S. Phosphorylation of myosin regulatory light chain by myosin light chain kinase and muscle contraction. Circ J 73: 208–213, 2009. [DOI] [PubMed] [Google Scholar]
- 228.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609, 1997. [DOI] [PubMed] [Google Scholar]
- 229.Tinsley JH, Ustinova EE, Xu W, Yuan SY. Src-dependent, neutrophil-mediated vascular hyperpermeability and β-catenin modification. Am J Physiol Cell Physiol 283: C1745–C1751, 2002. [DOI] [PubMed] [Google Scholar]
- 230.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem 272: 25968–25975, 1997. [DOI] [PubMed] [Google Scholar]
- 231.Tomar A, Lawson C, Ghassemian M, Schlaepfer DD. Cortactin as a target for FAK in the regulation of focal adhesion dynamics. PLos One 7: e44041, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol 21: 676–683, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Toshima J, Toshima JY, Suzuki M, Noda T, Mizuno K. Cell-type-specific expression of a TESK1 promoter-linked lacZ gene in transgenic mice. Biochem Biophys Res Commun 286: 566–573, 2001. [DOI] [PubMed] [Google Scholar]
- 234.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol 150: 797–806, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol 27: 2332–2339, 2007. [DOI] [PubMed] [Google Scholar]
- 236.van Nieuw Amerongen GP, Natarajan K, Yin G, Hoefen RJ, Osawa M, Haendeler J, Ridley AJ, Fujiwara K, van Hinsbergh VW, Berk BC. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res 94: 1041–1049, 2004. [DOI] [PubMed] [Google Scholar]
- 237.van Nieuw Amerongen GP, van Hinsbergh VW. Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler Thromb Vasc Biol 21: 300–311, 2001. [DOI] [PubMed] [Google Scholar]
- 238.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008. [DOI] [PubMed] [Google Scholar]
- 239.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. PKCα activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res 111: 739–749, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Varma S, Breslin JW, Lal BK, Pappas PJ, Hobson RW 2nd, Duran WN.. p42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res 63: 172–178, 2002. [DOI] [PubMed] [Google Scholar]
- 241.Venema RC, Raynor RL, Noland TA Jr, Kuo JF. Role of protein kinase C in the phosphorylation of cardiac myosin light chain 2. Biochem J 294: 401–406, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Verin AD, Gilbert-McClain LI, Patterson CE, Garcia JG. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol 19: 767–776, 1998. [DOI] [PubMed] [Google Scholar]
- 243.Verin AD, Lazar V, Torry RJ, Labarrere CA, Patterson CE, Garcia JG. Expression of a novel high molecular-weight myosin light chain kinase in endothelium. Am J Respir Cell Mol Biol 19: 758–766, 1998. [DOI] [PubMed] [Google Scholar]
- 244.Verin AD, Liu F, Bogatcheva N, Borbiev T, Hershenson MB, Wang P, Garcia JG. Role of ras-dependent ERK activation in phorbol ester-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 279: L360–L370, 2000. [DOI] [PubMed] [Google Scholar]
- 245.Vogel SM, Gao X, Mehta D, Ye RD, John TA, Andrade-Gordon P, Tiruppathi C, Malik AB. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol Genomics 4: 137–145, 2000. [DOI] [PubMed] [Google Scholar]
- 246.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA 100: 6233–6238, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang S, Liang B, Viollet B, Zou MH. Inhibition of the AMP-activated protein kinase-α2 accentuates agonist-induced vascular smooth muscle contraction and high blood pressure in mice. Hypertension 57: 1010–1017, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res 104: 531–540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Watterson DM, Schavocky JP, Guo L, Weiss C, Chlenski A, Shirinsky VP, Van Eldik LJ, Haiech J. Analysis of the kinase-related protein gene found at human chromosome 3q21 in a multi-gene cluster: organization, expression, alternative splicing, and polymorphic marker. J Cell Biochem 75: 481–491, 1999. [PubMed] [Google Scholar]
- 250.Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications. Diabet Med 18: 945–959, 2001. [DOI] [PubMed] [Google Scholar]
- 251.Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, Burstein D, Doukas J, Soll R, Losordo D, Cheresh D. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest 113: 885–894, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437: 497–504, 2005. [DOI] [PubMed] [Google Scholar]
- 253.Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO. SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol 24: 8113–8133, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wheeler-Jones CP, Pearson JD. Thrombin and histamine phosphorylate the 42 kDa mitogen-activated protein kinase in HUVEC. Biochem Soc Trans 23: 203S, 1995. [DOI] [PubMed] [Google Scholar]
- 255.Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol 138: 757–766, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wirth A, Schroeter M, Kock-Hauser C, Manser E, Chalovich JM, De Lanerolle P, Pfitzer G. Inhibition of contraction and myosin light chain phosphorylation in guinea-pig smooth muscle by p21-activated kinase 1. J Physiol 549: 489–500, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wong RK, Baldwin AL, Heimark RL. Cadherin-5 redistribution at sites of TNF-α and IFN-γ-induced permeability in mesenteric venules. Am J Physiol Heart Circ Physiol 276: H736–H748, 1999. [DOI] [PubMed] [Google Scholar]
- 258.Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol Heart Circ Physiol 271: H2735–H2739, 1996. [DOI] [PubMed] [Google Scholar]
- 259.Wu MH. Endothelial focal adhesions and barrier function. J Physiol 569: 359–366, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol 182: 1021–1030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Xu Y, Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci USA 90: 7074–7078, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yamada H, Saito F, Fukuta-Ohi H, Zhong D, Hase A, Arai K, Okuyama A, Maekawa R, Shimizu T, Matsumura K. Processing of β-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum Mol Genet 10: 1563–1569, 2001. [DOI] [PubMed] [Google Scholar]
- 263.Yanase M, Ikeda H, Ogata I, Matsui A, Noiri E, Tomiya T, Arai M, Inoue Y, Tejima K, Nagashima K, Nishikawa T, Shibata M, Ikebe M, Rojkind M, Fujiwara K. Functional diversity between Rho-kinase- and MLCK-mediated cytoskeletal actions in a myofibroblast-like hepatic stellate cell line. Biochem Biophys Res Commun 305: 223–228, 2003. [DOI] [PubMed] [Google Scholar]
- 264.Yeh LA, Lee KH, Kim KH. Regulation of rat liver acetyl-CoA carboxylase. Regulation of phosphorylation and inactivation of acetyl-CoA carboxylase by the adenylate energy charge. J Biol Chem 255: 2308–2314, 1980. [PubMed] [Google Scholar]
- 265.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170: 443–453, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yoo Y, Ho HJ, Wang C, Guan JL. Tyrosine phosphorylation of cofilin at Y68 by v-Src leads to its degradation through ubiquitin-proteasome pathway. Oncogene 29: 263–272, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yu JA, Deakin NO, Turner CE. Emerging role of paxillin-PKL in regulation of cell adhesion, polarity and migration. Cell Adh Migr 4: 342–347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yu P, Hatakeyama T, Aramoto H, Miyata T, Shigematsu H, Nagawa H, Hobson RW, Duran WN. Mitogen-activated protein kinases regulate platelet-activating factor-induced hyperpermeability. Microcirculation 12: 637–643, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. TRPC channels as STIM1-regulated SOCs. Channels (Austin) 3: 221–225, 2009. [DOI] [PubMed] [Google Scholar]
- 270.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol 39: 213–223, 2002. [DOI] [PubMed] [Google Scholar]
- 271.Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol Heart Circ Physiol 263: H641–H646, 1992. [DOI] [PubMed] [Google Scholar]
- 272.Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: modulation of basal and agonist-stimulated venular permeability. Am J Physiol Heart Circ Physiol 272: H1437–H1443, 1997. [DOI] [PubMed] [Google Scholar]
- 273.Zhan Q, Bamburg JR, Badwey JA. Products of phosphoinositide specific phospholipase C can trigger dephosphorylation of cofilin in chemoattractant stimulated neutrophils. Cell Motil Cytoskeleton 54: 1–15, 2003. [DOI] [PubMed] [Google Scholar]
- 274.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L497–L504, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zheng XM, Resnick RJ, Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPα. EMBO J 19: 964–978, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhu HQ, Wang XB, Han JX, Hu ZP, Wang Y, Zhou Q, Gui SY, Wang Y. Myosin light chain kinase inhibitor attenuates atherosclerosis and permeability via reduced endothelial tight junction in rabbits. Int J Cardiol 168: 5042–5043, 2013. [DOI] [PubMed] [Google Scholar]


