Abstract
Deep vein thrombosis (DVT) resolves via a sterile inflammatory response. Defining the inflammatory response of DVT may allow for new therapies that do not involve anticoagulation. Previously, we have shown that Toll-like receptor 9 (Tlr9) gene deleted mice had impaired venous thrombosis (VT) resolution. Here, we further characterize the role of Tlr9 signaling and sterile inflammation in chronic VT and vein wall responses. First, we found a human precedent exists with Tlr9+ cells present in chronic post thrombotic intraluminal tissue. Second, in a stasis VT mouse model, endogenous danger signal mediators of uric acid, HMGB-1, and neutrophil extracellular traps marker of citrullinated histone-3 (and extracellular DNA) were greater in Tlr9−/− thrombi as compared with WT, corresponding with larger VT at 8 and 21d. Fewer M1 type (CCR2+) monocyte/macrophages (MØ) were present in Tlr9−/− thrombi than WT controls at 8d, suggesting an impaired inflammatory cell influx. Using bone marrow derived monocyte (BMMØ) cell culture, we found decreased fibrinolytic gene expression with exposure to several endogenous danger signals. Next, adoptive transfer of cultured Tlr9+/+ BMMØ to Tlr9−/− mice normalized VT resolution at 8d. Lastly, although the VT size was larger at 21d in Tlr9−/− mice and correlated with decreased endothelial antigen markers, no difference in fibrosis was found. These data suggest that Tlr9 signaling in MØ is critical for later VT resolution, is associated with necrosis clearance, but does not affect later vein wall fibrosis. These findings provide insight into the Tlr9 MØ mechanisms of sterile inflammation in this disease process.
Introduction
Deep vein thrombosis (DVT) is a major clinical problem with an estimated incidence of more than 900,000 cases per year in the U.S.1 Complications include pulmonary embolism, which often presents as sudden death, and post-thrombotic syndrome (PTS), a chronic condition with symptoms of limb pain, swelling, and ulceration in severe cases.2 The primary treatment for DVT is anticoagulation. Although effective, anticoagulation significantly increases risk of bleeding and is contraindicated in many patients.3 New therapies for DVT, and in particular PTS, that work by non-anticoagulant mechanisms could dramatically improve the treatment of this disease.
DVT resolution is an inflammatory process that resembles sterile wound healing.4 Multiple thrombus factors may contribute to this inflammatory resolution response, including cell-breakdown products. Endogenous “danger signals”, such as nucleic acids,5 uric acid,6 and high mobility group box-1 (HMGB-1),7 are known to be released from damaged and dying cells and to trigger an innate immune response. Recent data suggests that neutrophils (PMN) release neutrophil extracellular traps (NETs) that promote VT,8, 9 and may modulate leukocyte actions. Late VT resolution is dependent on the monocyte/macrophage (MØ) for fibrinolysis, neovascularization, and matrix remodeling.4, 10 These cells may be pro-inflammatory, so called M1 type (i.e. cysteine-cysteine receptor 2 +; CCR2+),11, 12 or M2, healing type.13 M1 type MØ are important for necrosis clearance, and to allow secondary inflammation resolution.14 Prior work has shown impaired VT resolution in CCR2−/− mice,15 although the effect on the late vein wall responses are unknown.
Vein wall injury is dependent on inflammatory cell influx, matrix metalloproteinases, and the thrombogenic injury,4, 16–18 but mechanisms of vein wall fibrosis have only been partially characterized. Phenotypically, several markers are suggestive of fibrotic injury in the venous system, including gain of mesenchymal markers such as alpha-smooth muscle antigen (αSMA) and fibroblast specific protein-1(FSP-1), and loss of endothelial markers such as VE Cadherin and CD31.17
Many endogenous danger signals including NETs are recognized by pattern recognition receptors,19 including Toll-like receptors.20 Toll-like receptor (Tlr) 9 recognizes host DNA in the setting of sterile inflammation.21 Previous work has shown that mice deficient in Tlr9 have impaired resolution of experimental venous thrombosis (VT) and altered markers of sterile inflammation.22 Furthermore, administering an exogenous Tlr9 ligand increased VT early resolution in wild type (WT) mice. However, the cell types involved in this process were not defined, a non-stasis VT may respond differently,23 and the vein wall response in Tlr9−/− mice has not been investigated.
In this study, we tested the hypotheses that endogenous danger signals are present in and contribute to sterile inflammation in late experimental VT in a Tlr9 dependent fashion. We show citrullinated histones (Cit-H3), uric acid, and HMGB-1 are present in experimental stasis VT, and that loss of Tlr9 signaling increases their concentration, while blunting the sterile inflammatory response. The effect of Tlr9−/− is model dependent, and midterm stasis thrombus resolution is directly dependent on intact MØ Tlr9 signaling. Lastly, Tlr9 mediated activities are less important in late vein wall fibrotic injury than other MØ activities.
Materials and Methods
Reagents
Oligodeoxynucleotide (ODN) 1826 and ODN 1826 control were purchased from Invivogen (San Diego, CA). Monosodium urate (MSU) crystals were prepared by incubating supersaturated uric acid solutions (4 – 5 mg/ml) in 0.1M borate (pH 8.5) at room temperature for >48 h, followed by washing with ethanol and acetone.6 MSU crystals were resuspended by sonicating in sterile PBS and vortexed immediately prior to use. RNA for stimulation of cell cultures was prepared by pooling RNA extracted from multiple murine bone marrow-derived macrophage (BMMØ) cultures. Necrotic cells were prepared by subjecting murine BMMØ cultures to three to five cycles of freezing at −80°C and thawing at 37°C. SYTOX Green was purchased from Invitrogen (Carlsbad, CA).
Mice
Male Balb/c (WT) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Male Tlr9−/− mice on Balb/c background were purchased from S. Akira (Coley Pharmaceutical, Wellesley, MA). CCR2−/− on a Balb/c genetic background were also used for select experiments.15 All mice that underwent survival surgeries were used between 7 and 12 weeks of age (20 – 30 g). Mice underwent general anesthesia with isoflorane/O2 for all surgical procedures and all animal experiments were approved by the Animal Use Committee at the University of Michigan.
Stasis model of VT
WT and Tlr9−/− mice underwent surgical ligation of the inferior vena cava (IVC) and cauderization of visible contributing vessels below the renal veins, producing full stasis and subsequent VT formation. This model is well-characterized and consistently produces VT.15, 16, 22, 24, 25 IVC and thrombus were harvested at 8 and 21 days after induction of stasis for tissue analysis. Thrombus and IVC were left intact for histology or carefully separated for the other measurements.
Non-stasis model of VT
To examine the robustness of experimental results across models of VT, an endothelial injury model of VT17, 26 was used to establish thrombus in the setting of continued blood flow. WT and Tlr9−/− mice underwent intraluminal electrolytic injury to their IVC. Briefly, a 25 gauge needle was inserted into the IVC and put in contact with the anterior IVC wall between the renal veins and iliac bifurcation. A current of 250 μAmp was applied for 15 min, consistently producing non-occlusive, mural thrombi. Vein wall and thrombi were harvested at 8d and 21d.
Histological Analysis/Immunohistochemical Staining
The following were stained on paraffin embedded tissue sections (thickness 5 μm) for: CCR2, (1:200; Abcam, Cambridge, MA), FSP-1 (1:500; Millipore, Temcula, CA), Smooth Muscle Myosin Heavy Chain (SMMHC, 1:50; Abcam) and Tlr9 (InvivoGen, San Diego, CA). For CCR2+, FSP-1, SMMHC cell counts, positive cells were counted and totaled in 5 high power fields (hpf, 1000X) radially around the IVC wall or thrombus.15, 25 Trichrome staining in the human sections was done as described.22 Vein wall collagen content was determined using our previously described Sirius Red method.18, 27
To visualize NET in thrombus sections, we stained for cit-H3 (1:500, Abcam) and co-stained with extracellular DNA, labeled with 1uM SYTOX (Invitrogen, Grand Island, NY) at room temperature. Slides were cover slipped with ProLong Gold with DAPI mounting medium (Invitrogen). Pictures were taken using a Nikon Eclipse E400 microscope 1000x equipped with a Nikon Digital Sight DS-U3 camera using the DAPI (nuclei), FITC (SYTOX) and Texas Red (Cit-H3) channels.
Generation of bone marrow-derived macrophages (BMMØ)
Bone marrow was harvested from WT and Tlr9−/− mice by flushing their femurs and tibias with cold RPMI 1640 (Mediatech, Herndon, VA). For generation of BMMØ, bone marrow cells were cultured in L929 cell-conditioned medium as previously described.28, 29 Six days after initial culture, cells were transferred to multi-well plates with cell densities of 1 × 106 cells/mL. After overnight rest, cells were stimulated as indicated.
Cells were collected for RNA isolation at 2, 4, or 8 hours after stimulation, and cell-free supernatant was collected at 24 hours for protein analysis. Dose response curves were generated by stimulating WT BMMØ with monosodium urate (MSU; the biologically active form of uric acid), a suspension of necrotic cells, or purified RNA with analysis of IL-1β gene expression. In pilot studies we found that MSU or RNA added to final concentrations of 300 μg/mL and 1 μg/mL, respectively, significantly increased IL-1α gene expression compared with no stimulation (P < 0.05, n = 3). Addition of 5 × 105 necrotic cells in 0.5 mL media to 1 × 106 cells in 1.0 mL media, hereafter referred to as a 50% addition, significantly decreased IL-1α gene expression compared with no stimulation (P < 0.05, n = 3). These were the smallest doses to demonstrate significant changes in IL-1β gene expression, and were therefore used in later experiments.
Antigen and enzyme activity analysis
Thrombus was homogenized and sonicated in 1 mL of protease inhibitor buffer (Roche, Basel, Switzerland). Interleukin-1 (IL)-1 alpha, beta, and IL-18, antigen levels were quantified by commercially available ELISAs (R&D Systems, Minneapolis. MN). Uric acid concentration was determined using the Quantichrom Uric Acid Assay (Bioassay Systems, Hayward, CA). Caspase-1 and -3 activities were determined using commercially available colorimetric kits (R&D Systems). All measurements were normalized to total protein determined by Pierce BCA assay (ThermoScientific, Waltham, MA).
Quantitative Real-Time Polymerase Chain Reaction
RNA was isolated from BMMΦ using the RNeasy Mini Kit (Qiagen, Frederick, MD) and reverse transcribed with oligo dT primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Real-time quantitative PCR analysis was performed using the 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). The following gene expression assays were purchased from SABiosciences (Frederick, MD): IL-1α, Urokinase Plasminogen activator(uPA), Matrix Metalloproteinase 9 (MMP9), Tissue Factor (TF) and Glutaraldehyde Phosphate Dehydrogenase (GAPDH; Applied Biosystems, Grand Island, NY) was used for loading control. Gene expression data are presented as relative expression compared to non-stimulated control, calculated using 2−ΔΔCT. At least 3 independent experiments were performed to verify results.
Western Immunoblotting
Thrombus at 8d or vein wall thrombus at 21d was homogenized as described.17, 30 Antigen qualification including cit-H3 (1:500; Abcam), Elastase (1:1000, Abcam), Cathepsin G (1:20,000; Novus Biologicals, Littleton, CO), Tissue Factor (TF; 1:1000, Santa Cruz, Dallas, TX), CD31(1:1000, Santa Cruz), Fibronectin (FN; 1:1000), VE-cadherin(1:1000), and alpha Smooth Muscle Actin (αSMA; 1:10000) (all from Abcam) and B-Actin (1/50,000; Santa Cruz,) as described.17, 30 Primary antibodies were diluted in TBST, added to the membrane, and incubated at 4°C overnight while gently shaking. For normalization of proteins on the western blots, the membranes were stripped and probed with anti-B-actin antibodies conjugated with HRP (Santa Cruz). The membranes were developed with the West-Pico ECL kit (Pierce, Rockford, IL). Densitometry was performed using Image J, image processing and analysis in Java.
Adoptive Transfer
BMMØ were cultured as above until the point when they were ready to be replated (day 6). At this point, cells were collected, pelleted, and then resuspended in PBS without Ca/Mg at a concentration of 5 × 106 cells/150 μL. The cells were kept on ice and injected the same day, intravenously and immediately before mice underwent IVC ligation. The thrombus was harvested and weighed at 8 days. This is a typical dose for effective adoptive transfer.
Statistical analysis
Data was analyzed and graphed using the Prism 4.0 software program for Windows (GraphPad Software, San Diego, CA). Statistical significance was calculated using two-tailed t test and defined as P<0.05. qRT-PCR data was log transformed for better normality prior to statistical analysis. Data are presented as mean ± SD.
Results
Human post thrombotic veins have Tlr9+ and CCR2+ staining cells present
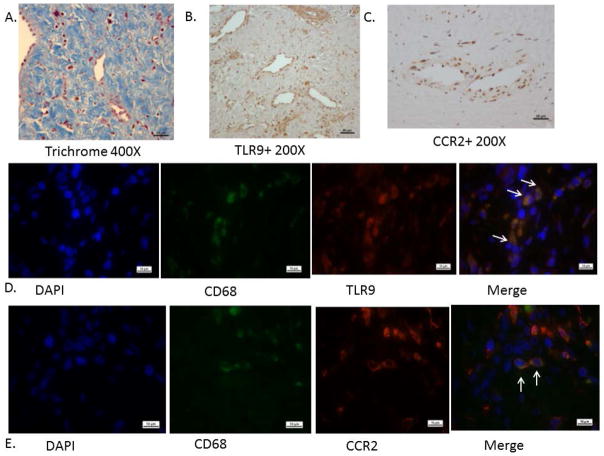
The intraluminal tissue of post thrombotic veins is generally unavailable as surgery is not often performed for patients with PTS. However, with access to these specimens,17 we stained common femoral vein sections for Tlr9+ cells and proinflammatory M1 MØ marker, CCR2 (Fig 1). We found these mononuclear cells were present throughout the intraluminal tissue, suggesting a role in the long-term fibrotic resolution response in humans (n = 3). Similar numbers of Tlr9+/+ and CCR2+ cells were present in the scar tissue, and in similar regions of the fibrotic tissue. Co-localization by immunofluorescent staining of CCR2/CD68 showed that 55–77% colocolization and Tlr9/CD68 showed 100 % colocalization asmacrophage type cells (n = 3) (Fig 1). The segments of chronic femoral thrombosis were all highly fibrotic, as shown with trichrome staining. All patients had their DVT > 1 year, but less than 7 years. All patients had been treated with standard anticoagulant therapy, and underwent common femoral endophlebectomy producing the tissue specimen as described.31
Figure 1.
Human post thrombotic vein wall sections: A) Trichrome staining showing dense fibrosis. B) Anti-Tlr9 staining; note numerous mononuclear cells associated with neo vessel channels. C) Anti-CCR2+ staining also present in the scar matrix. D) Immunoflourescent imaging of co-localized CD68 with TLR9 @ 1000X. E) Immunoflourescent imaging of co-localized CD68 with CCR2 @ 1000X. Representative of 2 – 3 sample sections.
Murine Tlr9−/− thrombi contain more necrotic cell debris markers than controls
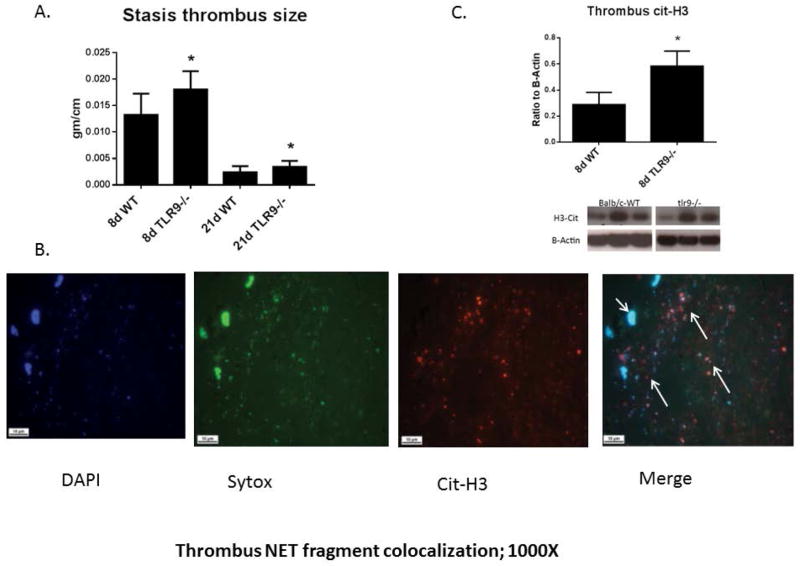
In experimental models of VT resolution, thrombus size (measured by mass per length) is the sum of coagulation and fibrinolysis over time.15, 22, 25 We reconfirmed VT size was larger in Tlr9−/− mice as compared with WT at 8d, and now confirmed larger VT at 21d (Fig 2a). Given the increased VT size in Tlr9−/− mice, we assessed several pro-thrombotic and necrosis markers. Neutrophils play an important role in early VT resolution32 and have been shown to release procoagulant NETs in experimental VT in primates and mice.8, 9, 33 We found NET like structures34 were present in 8d murine VT (SYTOX extracellular DNA co-localized with cit-H3) (Fig. 2b). As these fragments were not able to be accurately quantified histologically and are not PMN associated NETs, we used cit-H3 levels by immunoblotting, and found increased levels in Tlr9−/− as compared with WT (Fig. 2c). Given that these differences could reflect simply a difference in PMN numbers, we assessed Ly6G+ staining. We found no significant difference in 8d thrombus Ly6G+ PMN in Tlr9−/− as compared with WT (41+/− 4 vs. 31 +/− 3 cells/5 hpf; n = 4 – 5; p = .10).
Figure 2.
A) Thrombus size was greater in Tlr9−/− as compared with WT mice (n = 8 – 12); B) Thrombus NET fragments are shown by immunofluorescence with SYTOX (green), DAPI (blue) and cit-H3 (red) at 8d; 1000X. Long arrows denote extracellular NETs, while short arrow denotes a cell nucleus. Representative of n = 3. C) Cit-H3 was elevated in Tlr9−/− as compared with WT by immunoblotting. n = 5 – 6; *p <.05, by t-test.
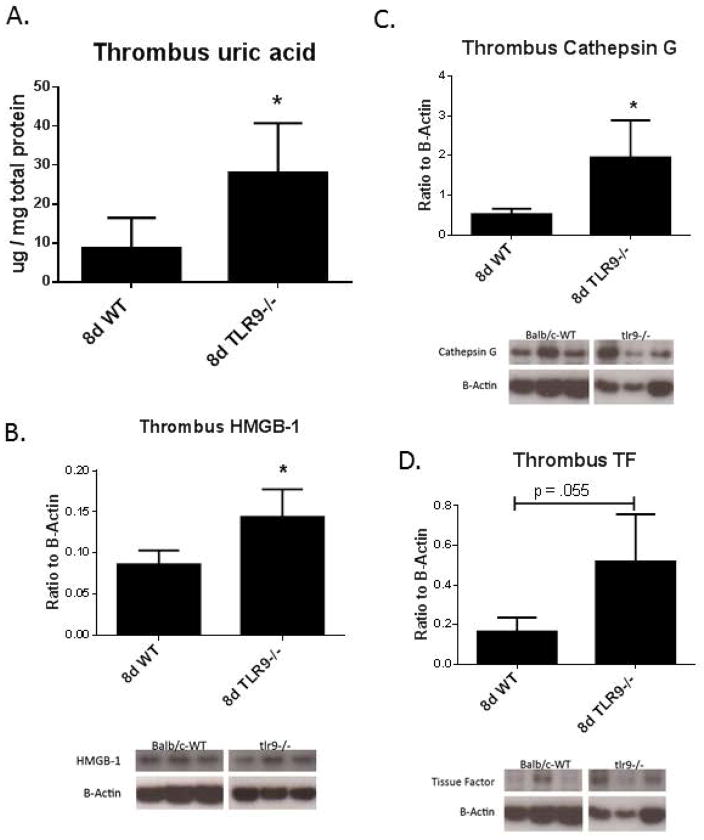
Sterile inflammation is also induced when cells lyse and release endogenous danger signals, such as uric acid and HMGB-1.6, 7 Uric acid concentration was increased in the Tlr9−/− thrombi at 8d as compared to WT controls (Fig. 3a). Of note, baseline plasma uric acid concentration was 1.5 ± 0.2 μg/mg protein with no difference between WT and Tlr9−/− mice (n = 2 – 3). HMGB-1 concentration was increased in Tlr9−/− VT at 8d as compared to WT controls (Fig. 3b) by immunoblotting. We also assessed two other markers related to thrombosis, cathepsin G and TF,35 and found these were increased in Tlr9−/− thrombi as compared with WT, all at 8d (Fig. 3d, e).
Figure 3.
A) Thrombus uric acid concentration was increased in Tlr9−/− mice at 8 d compared with WT controls; B) Thrombus HMGB-1 concentration was increased in Tlr9−/− thrombi at 8 d compared to WT controls. Markers of cellular thrombotic processes were all elevated in 8d Tlr9−/− as compared with WT by immunoblotting, including; C) Cathepsin G; and D) TF (all n = 5 – 7), * = p<.05 by t-Test. β-actin used as loading control.
To determine whether apoptosis was altered in Tlr9−/− mice, we assessed thrombus apoptotic processes by two critical enzymes, caspase-1 and -3. No differences in caspase levels were observed at 8 or 21d between groups (not shown). Consistently, direct TUNEL staining of the thrombus sections showed no significant difference in Tlr9−/− as compared with WT at 8d (43 +/− 20 vs. 23 +/− 5 cells/5hpf; n = 4, p = .18) or at 21d (6.5 +/− 1 vs. 5.2 +/− 1 cells/5hpf; n = 4 p = .64).
Endogenous danger signals and Tlr9 activation alter the procoagulant/fibrinolytic response of BMMØ in vitro
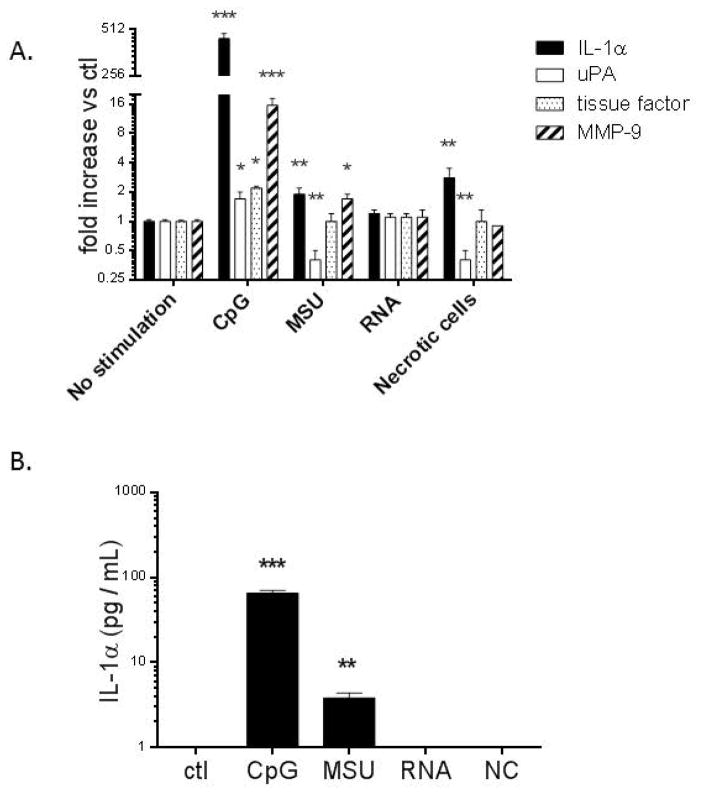
As uric acid, necrotic cells, and extracellular RNA were potential instigators of sterile inflammation in VT and may promote thrombosis, we investigated the effect of these endogenous danger signals on WT BMMØ fibrinolytic/coagulation gene expression in vitro at 8 hours post exposure (Fig. 4a). Prior work suggested that activation of Tlr9 with exogenous ODN increased VT resolution.22 The Tlr9 agonist ODN 1826 (CpG) was associated with increased IL-1a, uPA, TF and MMP9 gene expression. MSU was associated with increased IL-1a and MMP9, but reduced uPA. The necrotic cell homogenate was associated with increased IL-1a but decreased uPA. Conversely, stimulation with RNA had no effect on gene expression. Only CpG and MSU were associated with increased IL-1α protein expression (at 24 hours post exposure), while control, RNA, and necrotic cell homogenate had undetectable levels (Fig. 4b). Of note, MØ stimulated with non- Tlr9 activating CpG control and stimulation of Tlr9−/− cells with CpG showed no difference in IL-1α gene expression as compared with non-stimulated WT cells (not shown).
Figure 4.
BMMØ cell culture gene expression results at 8 hours after exposure. A) WT BMMØ was stimulated with either ODN 1826 (CpG), MSU 300 μg/mL, or control media (ctl); or RNA 1 μg/mL. necrotic cells (50%). Data shown are from at least 3 independent experiments; B) BMMØ protein levels of IL-1α under same conditions at 24 hours post exposure as in (A). n = 3 – 4. *** = P < 0.001, ** = P < 0.01, * = P < 0.05, compared to non-stimulated control. uPA = urokinase plasminogen activator; MMP = matrix metalloproteinase, RA = relative activity.
Midterm VT is dependent on intact MØ Tlr9 signaling
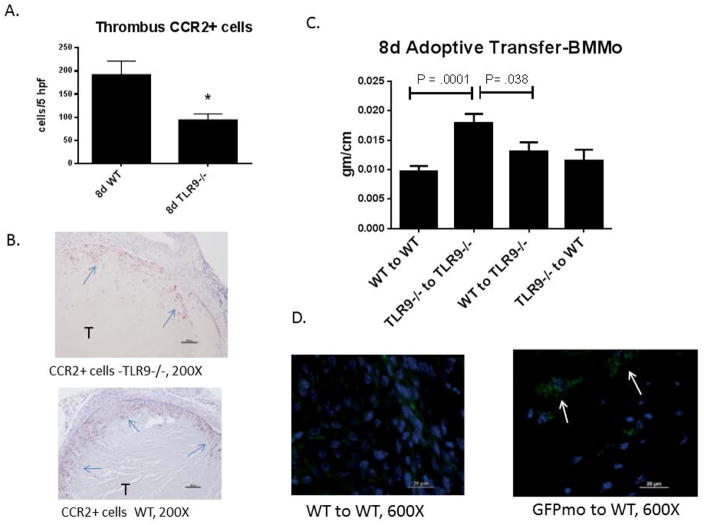
Monocytes/macrophages are critical for processing sterile necrosis,5 play an important role in experimental VT resolution,4, 15, 32 and express Tlr9.21 Given the impaired late sterile inflammatory processes in Tlr9−/− mice, we assessed intra-thrombus M1 type MØ (CCR2+) cells.11, 12 Fewer CCR2+ cells were present in the Tlr9−/− thrombi as compared with WT at 8d (Fig. 5a – b). Co-staining of CCR2+ with F4/80 was documented in VT in both Tlr9−/− and WT (Supplemental Figure 1).
Figure 5.
A) Fewer CCR2+ were observed in the Tlr9−/− thrombi as compared with WT at 8d (n = 5 – 7). B) Immunohistology of VT showing numerous CCR2+ cells in WT, with fewer cells in Tlr9−/− mice (arrows denote cells; T = thrombus); C) Adoptive transfer of BMMØ with 8 d harvest to assess the VT resolution phenotype. Transfer of Tlr9+/+ (WT) BMMØ to Tlr9−/− mice restored the WT VT resolution phenotype (n = 6) but no difference was found with Tlr9−/− to WT, and Tlr9−/− to Tlr9−/− VT were larger than all other conditions, by one way ANOVA with Bonferoni correction. D) Example of GFP-labeled BMMØ into WT showing intra-thrombus straining (arrows) (n =3).
We have previously shown that Tlr9−/− mice have impaired VT resolution22 (and Fig 2a). To determine whether loss of Tlr9 signaling in MØ was responsible for the observed impairment in VT resolution in Tlr9−/− mice, we employed an adoptive transfer strategy. Infusion of Tlr9+/+ BMMØ intravenously into Tlr9−/− mice restored the WT thrombus resolution phenotype with a 28% reduction in thrombus size at the 8d time point, whereas Tlr9−/− to Tlr9−/− remained significantly larger than WT (Fig. 5c). Of note, using a separate set of mice with BMMØ fluorescent labeled cells (GFP), approximately 15% of MØ were positively stained in the thrombus after one injection (n = 3; Fig. 5d), confirming their influx into the thrombus at 8 days.
As DVT in humans are often exposed to active blood flow around the thrombus,36 we next determined if a similar impairment in VT resolution was observed in a non-stasis VT model, comparing Tlr9−/− with WT mice.26 Not surprisingly, non-stasis induced thrombi were roughly half the size of stasis-induced thrombi, but consistent with the stasis model, Tlr9−/− mice had ~50% increased VT size as compared with WT at 8d (7.9 +/− 1.2 vs. 4.4 +/− .4 mg/mm; n = 4 – 6, p = .045), but not at 21d (4.3 +/− .5 vs. 4.4 +/− 1 mg/mm; n = 5, P = .8).
Mesenchymal and endothelial vein wall markers are altered in Tlr9−/− mice, but without an effect on collagen deposition
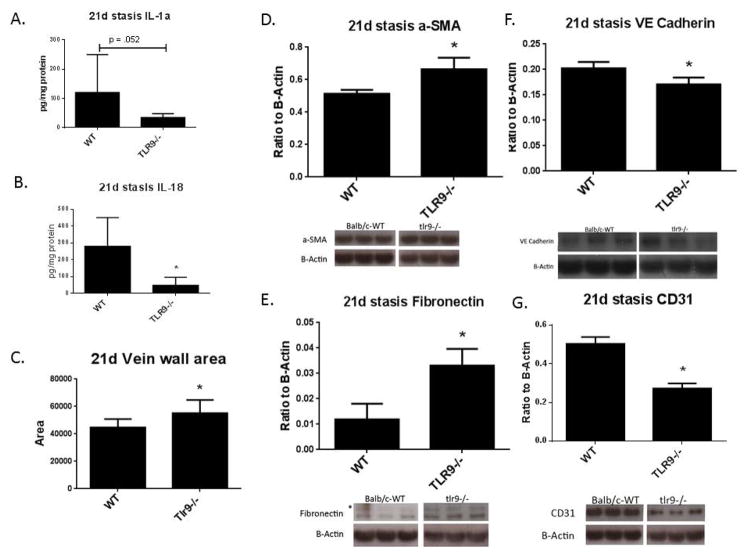
Given the impaired resolution of the stasis VT in Tlr9−/− mice, we next assessed the vein wall inflammatory and fibrotic response, corresponding to mid and late timepoints of VT resolution.17, 18, 37 We first assessed 8 and 21d vein wall cytokine levels. While no significant difference was found at 8d, we found reduced IL-1α and IL-18 in Tlr9−/− vein walls as compared with WT at 21d (Fig. 6a, b). Consistent with what we found in the thrombus at 8d, we also found fewer CCR2+ cells in the vein wall at 8 days, with fewer in Tlr9−/− as compared with WT (3.6+/− .2 vs. 7.8+/−; n = 5, p = .005). Moreover, no difference in vein wall collagen content was observed at 8d post thrombosis (data not shown).
Figure 6.
Decreased vein wall proinflammatory cytokines were observed in Tlr9−/− compared with WT at 21d. A). IL-1α and B) IL-18 (n = 4 – 6). C) Vein wall area was increased in Tlr9−/−as compared with WT. Area in pixels. (n = 7). Fibrotic markers of the vein wall as measured by Western immunoblotting. C) αSMA, and D) Fibronectin were increased in Tlr9−/− compared with WT, while E) VE-cadherin, and F) CD31 were reduced (n = 5 – 7) *p <.05 by t-Test.
Similarly there was no difference in vein wall collagen content in the Tlr9−/− mice as compared with WT by Sirius red histological analysis (7.5 +/− 1.1 vs. 8.1 +/− 0.7; % area; n = 7, P = NS). However, an increase of ~19% in vein wall thickening was found in Tlr9−/− as compared with WT (Fig 6c). We found that fibrotic response markers were altered in the 21d vein wall in Tlr9−/− mice as compared with WT mice. Increased alpha-SMA and FN, and decreased VE-cadherin and CD31 was found in the post thrombotic vein walls at 21d, consistent with impaired endothelization (Fig. 6d – g). Less CD31 staining was present on the luminal areas in Tlr9−/− as compared with WT (Supplemental Figure 2). Myofibroblasts may contribute to the vein wall post thrombotic phenotype.17 Consistent with a lack of increased fibrosis in the Tlr9−/− vein walls, no significant differences in FSP-1+ or SMMHC+ cell counts in the vein wall were found (not shown). However, less CCR2+ cells were present in the Tlr9−/− vein wall as compared with WT (6 +/− vs. 2 +/− .5 cells/5 hpf; n = 5; p = .008). The residual WT was also compared for collagen content, (9.1 +/− 2% area; n = 7, p = .82) and FSP1 + cell counts (6 +/− .5 vs. 4.3 +/−, n = 6 − 7 p = .11).
Given that CCR2+ cells were fewer in the Tlr9−/− thrombus, we assessed the vein wall fibrotic response at 21 days in CCR2−/− mice by Sirius red histological analysis as a comparator to Tlr9−/− vein wall response.17, 18 Unlike the Tlr9−/− mice, a marked decrease in fibrosis was present in CCR2−/− mice as compared with WT (Supplemental Figure 3).
Discussion
Venous thrombosis resolution is a sterile inflammatory process, mediated by leukocytes, chemokines, and fibrinolytic factors.4 We have previously shown that loss of Tlr9 signaling results in impaired VT resolution,22 but the potential endogenous ligands were not fully delineated, the MØ responses were not defined, only the stasis model was examined and the vein wall response not defined. Herein, we show that: 1) Tlr9+ cells are present in resolving late human specimens of vein scar; 2) Cellular necrosis products are greater in Tlr9−/− thrombi than controls; 3) Tlr9 ligands increase MØ pro-fibrinolytic function; 4) Midterm VT resolution is dependent on MØ with intact Tlr9 signaling; 5) Late vein wall fibrosis is not Tlr9 dependent, but is CCR2 dependent.
A novel finding in this study was the presence of Tlr9+ mononuclear cells in samples of human post thrombotic vein tissue. These cells were adjacent to the neovascular channels in the collagen rich tissue. A similar histomorphology of mononuclear CCR2+ cells were also present. These descriptive data suggest that Tlr9+ cells are involved with chronic vein wall remodeling after DVT. Given the chronicity and differences in ages, we are not able to define their role as pathologic or physiologic. Interestingly, a prior study showed that patients with acute DVT had decreased circulating monocyte Tlr9 gene expression, further supporting a role in DVT pathogenesis.38
NETs have been shown to be procoagulant8, 35 and are present in experimental VT in baboons and mice.33, 39 Furthermore, NETs have been shown to induce a sterile inflammatory response through Tlr9 signaling, as shown in models of systemic lupus erythematosus.20, 40, 41 We found evidence that NETs were increased in Tlr9−/− VT as compared with WT, based on increased cit-H3 in Tlr9−/− VT, and showed co-localization of extracellular DNA with cit-H3 histologically. These data suggest that intact Tlr9 signaling is important for NET clearance, and the elevated NET structures may be due to a greater production of NETs in early thrombogenesis. It is also possible that NETS continue to be generated in Tlr9−/− mice, but we believe this is less likely as few PMNs are present in the thrombus after 4 days and similar markers of Ly6G+ cells were present at 8 d in Tlr9−/− and WT. However, cathepsin G reflects PMN activation35 with increased levels in Tlr9−/− mice, and may also stimulate platelet mediated activities.42 Histones such as cit-H3, released from NETs may also be directly pro-thrombotic,43, 44 and in the stasis setting may accumulate and account for the increased VT size. The histologic sections suggest this may be the case, but the experiments herein don’t directly answer the isolated role of cit-H3.
We postulate that impaired sterile processing in Tlr9−/− mice occurs as significantly increased uric acid and HMGB-1 at 8d post-thrombosis was found. Uric acid is well established as a cell breakdown product, capable of inciting inflammation.5 The nuclear protein HMGB-1 is released by lysing cells, but can be secreted by MØ as a cytokine.7 The 8d post-thrombotic time point also corresponds with the maximum infiltration of MØ in VT resolution.10, 15 These necrosis factors may also directly contribute to the pro-thrombotic phenotypes of the Tlr9−/− mice as the 8d VT were significantly larger. We are not able to determine in real-time whether increased thrombogenesis or impaired resolution is occurring at this point, but by consensus, thrombogenesis is thought to occur at or before 48 hours.23, 45 Interestingly, we found no difference in markers of apoptosis in the Tlr9−/− mice, suggesting this process in VT is not Tlr9 dependent at these later time points.
Monocyte/macrophages are critical mediators of VT resolution, and our data suggest their activities are in part Tlr9 dependent.4, 15 First, we found that Tlr9+/+ MØ adoptively transferred into Tlr9−/− mice restored the Tlr9+/+ VT resolution phenotype, although not fully, suggesting residual effects of the remaining Tlr9−/− leukocytes. This suggests that loss of Tlr9 signaling in MØ was directly responsible for impaired midterm VT resolution. This is not entirely surprising, as MØ may have their maximal effect on later (>4 d) VT resolution,4, 10, 15 and direct most post inflammatory healing responses.13 We also showed significantly fewer M1 type MØ (CCR2+) were present in Tlr9−/− VT at midterm which may have accounted for impaired resolution. Second, the lack of normal resolution in 8d Tlr9−/− mice may be due to impaired fibrinolytic function of the MØ as suggested by in vitro analysis. Specifically, we found that a suspension of necrotic sterile cells affected the in vitro coagulant/fibrinolytic phenotype of the wild type BMMØ with less uPA gene expression. MSU, the biologically active form of uric acid, also induced a mixed phenotype in BMMØ (decreased uPA gene expression) and increased gene expression for MMP-9, an enzyme known to play a role in vein wall remodeling and fibrinolysis.16, 24, 46 Thus, in the setting of increased cell necrosis products with Tlr9 deletion, MØ coagulation/profibrinolytic functions may be impaired in vivo. Lastly, we also demonstrated that Tlr9 activation by the CpG agonist directly affects the coagulant/fibrinolytic behavior of MØ in vitro, promoting a pro-lytic phenotype by gene expression evaluation, and may explain our previous findings of smaller VT in WT mice treated with the Tlr9 agonist.22 However, we acknowledge that protein or activity levels were not determined.
The primary difference between the non-stasis and stasis models of VT we used is that the non-stasis induced thrombus is continuously exposed to active peri-thrombus blood flow while the stasis induced thrombus is not. These two models are replicative of what is believed to occur in human DVT, with areas of stasis and non-stasis.38, 47 We found the non-stasis VT resolution in Tlr9−/− mice modestly impaired as compared to WT, and only at 8d, based on thrombus size. This suggests the mechanism of thrombogenic injury affects Tlr9 mediated activities, and Tlr9 signaling may be less important in late 21d non-stasis VT.
Experimentally, thrombus size does not correlate with ultimate vein wall fibrotic injury response.17, 48 Consistently, while the VT size was larger at 21d in the Tlr9−/− mice, no difference in fibrosis was found. That is, while vein wall endothelial markers were decreased (correlating with luminal endothelial location) and mesenchymal markers were increased, the vein wall collagen content was similar at 8 and 21. Moreover, there was no alteration in vein wall FSP-1 cell numbers, the latter of which correlates with fibrosis.17 This contrasts with vein wall response in CCR2−/− mice in stasis VT, with markedly less collagen, suggesting a decreased fibrotic injury response. These data suggest that Tlr9 signaling in MØ (and possibly other cells) likely affects different cellular functions than does CCR2 signaling in the context of vein wall injury. While we have no way to assess for vein wall physiological function, the decreased endothelial and increased mesenchymal markers may portend a greater pro-thrombotic milieu at the blood-vein wall interface, and may explain the larger VT at 21d in the Tlr9−/− mice as well as the greater vein wall thickening. The lack of increased fibrotic injury in Tlr9−/− mice may also be related to decreased IL-1α and IL-18 at 21d, as protection from vein wall fibrosis has been observed in other settings.18 This finding is consistent with the known Tlr9 signaling actions that induce IL-1 cytokine family transcription.49
This study demonstrates that there is a strong link between endogenous danger signals, sterile inflammation, and Tlr9 signaling in experimental late VT resolution. Future work will define if Tlr9 agonism can accelerate late VT resolution and improve late vein wall healing. Treatment of DVT by immunomodulation may be a promising approach, as clinical trials of therapies that act by manipulation of Tlr9 signaling are currently underway in a variety of other diseases.49
Supplementary Material
Supplemental Figure 1: Co-localization of F4/80 (green) with CCR2 (red) shows positive cell staining; nuclei are blue (DAPI) (n = 3 sample sections).
Supplemental Figure 2: Comparison of 21 day thrombosed IVC section with CD31 staining. More ablumenal surface staining was present in WT as compared with Tlr9−/− mice (n = 3 samples sections).
Supplemental Figure 3. Late fibrosis response in CCR2−/− mice. A) Markedly less vein wall collagen is present in CCR2−/− mice as compared with WT; n = 5 – 7, * = P < .05. B, C) Representative histologic sections are shown, with less dense collagen in CCR2−/− as compared with WT at 21d with Sirus Red staining.
Acknowledgments
This work was supported by NIH HL092129 and HL31237 and a Howard Hughes Medical Institute Medical Student Research Fellowship.
We thank Beau Carson, Matt Schaller, and Sumanta Mukherjee for fruitful discussions and advice about cell culture experiments; Laura Maurer for assistance with experiments; and Pam Lincoln and Kelli Rule for managing the mouse colonies.
Footnotes
There are no conflicts-of-interest to report for any authors of this work.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500–509. doi: 10.1016/j.jvs.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, Murad MH American College of Chest P. Prevention of vte in nonsurgical patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 5.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 7.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein hmgb1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 8.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A. Leukocytes and the natural history of deep vein thrombosis: Current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31:506–512. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory m1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 13.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen GY, Nunez G. Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, Varma M, Dewyer NA, Deatrick KB, Upchurch GR, Jr, Wakefield TW, Hogaboam C, Kunkel SL. Targeted deletion of ccr2 impairs deep vein thombosis resolution in a mouse model. J Immunol. 2006;177:3388–3397. doi: 10.4049/jimmunol.177.5.3388. [DOI] [PubMed] [Google Scholar]
- 16.Henke PK, Varma MR, Moaveni DK, Dewyer NA, Moore AJ, Lynch EM, Longo C, Deatrick CB, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb Haemost. 2007;98:1045–1055. [PubMed] [Google Scholar]
- 17.Laser A, Elfline M, Luke C, Slack D, Shah A, Sood V, Deatrick B, McEvoy B, Ostra C, Comerota A, Kunkel S, Hogaboam C, Henke PK. Deletion of cysteine-cysteine receptor 7 promotes fibrotic injury in experimental post-thrombotic vein wall remodeling. Arterioscler Thromb Vasc Biol. 2014;34:377–385. doi: 10.1161/ATVBAHA.113.302428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deatrick KB, Luke CE, Elfline MA, Sood V, Baldwin J, Upchurch GR, Jr, Jaffer FA, Wakefield TW, Henke PK. The effect of matrix metalloproteinase 2 and matrix metalloproteinase 2/9 deletion in experimental post-thrombotic vein wall remodeling. J Vasc Surg. 2013;58:1375–1384. e1372. doi: 10.1016/j.jvs.2012.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 20.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Henke PK, Mitsuya M, Luke CE, Elfline MA, Baldwin JF, Deatrick KB, Diaz JA, Sood V, Upchurch GR, Wakefield TW, Hogaboam C, Kunkel SL. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler Thromb Vasc Biol. 2011;31:43–49. doi: 10.1161/ATVBAHA.110.216317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz JA, Obi AT, Myers DD, Jr, Wrobleski SK, Henke PK, Mackman N, Wakefield TW. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:556–562. doi: 10.1161/ATVBAHA.111.244608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, Pearce CG, Upchurch GR, Wakefield TW, Henke PK. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42:140–148. doi: 10.1016/j.jvs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte cxcr2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24:1130–1137. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 26.Diaz JA, Hawley AE, Alvarado CM, Berguer AM, Baker NK, Wrobleski SK, Wakefield TW, Lucchesi BR, Myers DD., Jr Thrombogenesis with continuous blood flow in the inferior vena cava. A novel mouse model. Thromb Haemost. 2010;104:366–375. doi: 10.1160/TH09-09-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deatrick KB, Obi A, Luke CE, Elfline MA, Sood V, Upchurch GR, Jr, Jaffer F, Wakefield TW, Henke PK. Matrix metalloproteinase-9 deletion is associated with decreased mid-term vein wall fibrosis in experimental stasis dvt. Thromb Res. 2013;132:360–366. doi: 10.1016/j.thromres.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito T, Schaller M, Hogaboam CM, Standiford TJ, Sandor M, Lukacs NW, Chensue SW, Kunkel SL. Tlr9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through dc-derived notch ligand delta-like 4. J Clin Invest. 2009;119:33–46. doi: 10.1172/JCI35647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi AD, Raymond T, Coelho AL, Kunkel SL, Hogaboam CM. A systemic granulomatous response to schistosoma mansoni eggs alters responsiveness of bone-marrow-derived macrophages to toll-like receptor agonists. J Leukoc Biol. 2008;83:314–324. doi: 10.1189/jlb.1007689. [DOI] [PubMed] [Google Scholar]
- 30.Sood V, Luke CE, Deatrick KB, Baldwin J, Miller EM, Elfline M, Upchurch GR, Jr, Wakefield TW, Henke PK. Urokinase plasminogen activator independent early experimental thrombus resolution: Mmp2 as an alternative mechanism. Thromb Haemost. 104:1174–1183. doi: 10.1160/TH10-03-0184. [DOI] [PubMed] [Google Scholar]
- 31.Comerota AJ, Grewal NK, Thakur S, Assi Z. Endovenectomy of the common femoral vein and intraoperative iliac vein recanalization for chronic iliofemoral venous occlusion. J Vasc Surg. 2010;52:243–247. doi: 10.1016/j.jvs.2010.02.260. [DOI] [PubMed] [Google Scholar]
- 32.Varma MR, Varga AJ, Knipp BS, Sukheepod P, Upchurch GR, Kunkel SL, Wakefield TW, Henke PK. Neutropenia impairs venous thrombosis resolution in the rat. J Vasc Surg. 2003;38:1090–1098. doi: 10.1016/s0741-5214(03)00431-2. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pannucci CJ, Laird S, Dimick JB, Campbell DA, Henke PK. A validated risk model to predict 90-day vte events in postsurgical patients. Chest. 2014;145:567–573. doi: 10.1378/chest.13-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 36.Meissner MH, Caps MT, Bergelin RO, Manzo RA, Strandness DE., Jr Propagation, rethrombosis and new thrombus formation after acute deep venous thrombosis. J Vasc Surg. 1995;22:558–567. doi: 10.1016/s0741-5214(95)70038-2. [DOI] [PubMed] [Google Scholar]
- 37.Obi AT, Diaz JA, Ballard-Lipka NL, Roelofs KJ, Farris DM, Lawrence DA, Wakefield TW, Henke PK. Plasminogen activator-1 overexpression decreases experimental postthrombotic vein wall fibrosis by a non-vitronectin-dependent mechanism. J Thromb Haemost. 2014;12:1353–1363. doi: 10.1111/jth.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deatrick KB, Elfline M, Baker N, Luke CE, Blackburn S, Stabler C, Wakefield TW, Henke PK. Postthrombotic vein wall remodeling: Preliminary observations. J Vasc Surg. 2011;53:139–146. doi: 10.1016/j.jvs.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brill A, Fuchs TA, Savchenko A, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2011 doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type i ifn production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via tlr7 and tlr9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faraday N, Schunke K, Saleem S, Fu J, Wang B, Zhang J, Morrell C, Dore S. Cathepsin g-dependent modulation of platelet thrombus formation in vivo by blood neutrophils. PloS one. 2013;8:e71447. doi: 10.1371/journal.pone.0071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 44.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein c activation. J Thromb Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 45.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewyer NA, Sood V, Lynch EM, Luke CE, Upchurch GR, Jr, Wakefield TW, Kunkel S, Henke PK. Plasmin inhibition increases mmp-9 activity and decreases vein wall stiffness during venous thrombosis resolution. J Surg Res. 2007;142:357–363. doi: 10.1016/j.jss.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meissner MH, Manzo RA, Bergelin RO, Markel A, Strandness DE., Jr Deep venous insufficiency: The relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18:596–605. discussion 606-598. [PubMed] [Google Scholar]
- 48.Baldwin JF, Sood V, Elfline MA, Luke CE, Dewyer NA, Diaz JA, Myers DD, Wakefield T, Henke PK. The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis. Journal of vascular surgery. 2012;56:1089–1097. doi: 10.1016/j.jvs.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on tlr9 and the nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Co-localization of F4/80 (green) with CCR2 (red) shows positive cell staining; nuclei are blue (DAPI) (n = 3 sample sections).
Supplemental Figure 2: Comparison of 21 day thrombosed IVC section with CD31 staining. More ablumenal surface staining was present in WT as compared with Tlr9−/− mice (n = 3 samples sections).
Supplemental Figure 3. Late fibrosis response in CCR2−/− mice. A) Markedly less vein wall collagen is present in CCR2−/− mice as compared with WT; n = 5 – 7, * = P < .05. B, C) Representative histologic sections are shown, with less dense collagen in CCR2−/− as compared with WT at 21d with Sirus Red staining.