Abstract
Thalamic oscillators contribute to both normal rhythms associated with sleep and anesthesia and abnormal, hypersynchronous oscillations that manifest behaviorally as absence seizures. In this review, we highlight new findings that refine thalamic contributions to cortical rhythms and suggest that thalamic oscillators may be subject to both local and global control. We describe endogenous thalamic mechanisms that limit network synchrony and discuss how these protective brakes might be restored to prevent absence seizures. Finally, we describe how intrinsic and circuit-level specializations among thalamocortical loops may determine their involvement in widespread oscillations and render subsets of thalamic nuclei especially vulnerable to pathological synchrony.
Introduction
Although the anatomical organizational principles of the thalamus are well established (Pinault, 2004; Jones, 2007; Sherman, 2007), recent discoveries highlight new operational principles, especially regarding dynamic changes in thalamocortical networks relevant to sleep, attention, and seizures. To properly frame these exciting new findings that address network function, it is useful to review its major anatomical structures and their relationships.
The dorsal thalamus houses multiple excitatory thalamocortical (TC) relay nuclei that provide the major ascending input to cortex; these relays project to distinct cortical areas and are associated with specific sensory, limbic, motor, and executive pathways (Jones, 2007). The thalamic reticular nucleus (RT, Fig. 1) is a thin sheet of GABAergic neurons that envelops the lateral aspect of the dorsal thalamus along its dorsoventral and rostrocaudal axes; it provides both feed-forward and feedback inhibition to excitatory TC relay neurons within the varied thalamic nuclei (Fig. 1, i1) (Pinault, 2004). In some species, local thalamic interneurons provide inhibition to sculpt TC responses to sensory stimuli (Hirsch et al., 2015). The thalamocortical oscillations discussed in this review are common features of sleep and epilepsy among mammals, including rodents in which most TC nuclei lack local interneurons, This cross-species commonality suggests that interneurons play relatively minor roles in these rhythmic activities, at least in their gross electrographic and behavioral signatures, and thus will be omitted from circuit models discussed here. Layer 6 corticothalamic (CT) neurons provide feedback to the input TC nucleus, synapsing with both RT (Fig. 1, e2) and TC (Fig. 1, e3) to complete an oscillatory thalamocortical loop (Sherman, 2007). Layer 5 CT neurons provide strong feedforward excitation to adjacent TC nuclei as part of an ascending cortico-thalamo-cortical loop but do not synapse with RT (Groh et al., 2008; Sherman, 2007); this pathway will be addressed in the context of thalamic oscillations in the final section of this review. Layer 6 CT-RT activation results in feedforward inhibition of TC cells (Jones, 2007; Sherman, 2007). In addition to their primary cortical projection, TC neurons also project to RT (Fig. 1, e1), driving largely reciprocal GABAergic feedback inhibition. Brainstem and other arousal centers innervate the thalamus to dynamically modulate the transmission of ascending inputs to cortex (McCormick, 1992; Jones, 2007).
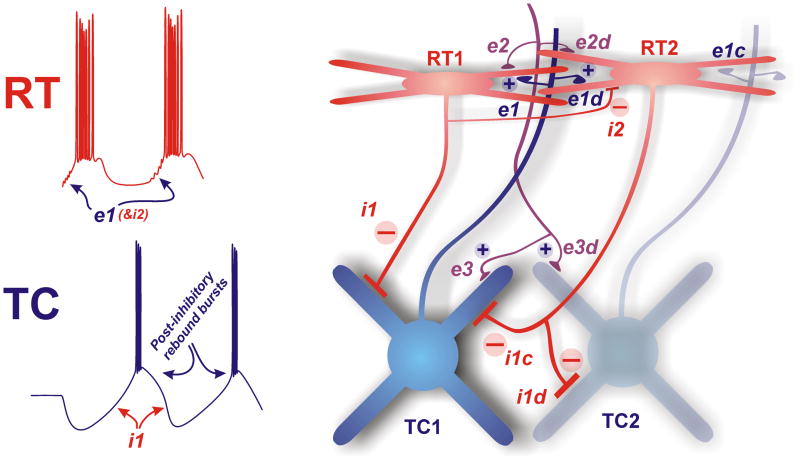
Figure 1. Elements of the thalamic rhythm generator.
Thalamocortical cells (TC, blue) make excitatory projections to related cortical areas, and emit axon collaterals that make excitatory synapses (e1) onto inhibitory neurons in reticular thalamus (RT, red). These connections are divergent (e1d) in that individual TC cells contact more than one RT cell and convergent (e1c) in that multiple TC cells contact each RT cell. RT cells, in turn, provide feedback inhibition back to TC cells (i1) that is convergent (i1c) and divergent (i1d). The intracellular records on the left show how i1 activation leads to generation of post-inhibitory rebound bursts (lower trace) that produce recurrent excitation of RT cells through synapse e1 (upper trace) that perpetuates the intrathalamic rhythm. In addition, RT cells contact each other through gap junctions (not shown) and chemical inhibitory synapses (i2); the latter will suppress thalamic rhythmicity. Excitatory feedback from the cortex projects to both RT (e2) and TC (e3), each with convergence and divergence. Each of these synapses is implicated in promoting, synchronizing, driving, and regulating thalamocortical oscillations. The individual compositions and functions of each major synapse are provided in Figure 3.
Tight connectivity with cortex enables the thalamus to participate in global oscillations, reflections of coordinated neural activity detectable by scalp EEG electrodes. The frequency and amplitude of these oscillations correlate with arousal level and are thought to represent different modes of neural processing (Steriade, 2000; Buzsaki, 2006). During sleep or under anesthesia, the thalamus autonomously generate two different rhythms: delta and spindle oscillations. TC neurons are intrinsic pacemakers, capable of firing periodic bursts of action potentials at 1–2 Hz that when synchronized generate delta oscillations within thalamus (McCormick and Pape, 1990; Soltesz et al., 1991; Nunez et al., 1992). Reciprocal synaptic connectivity between TC and RT enables the thalamus to generate 7–14Hz spindle oscillations (Fig. 1, left), which persist even after removal of cortical inputs in vivo (Morison and Bassett, 1945; Steriade et al., 1985). Similarly, isolated thalamic in vitro preparations generate persistent spindle-related activity (Huguenard and Prince, 1994a; von Krosigk et al., 1993; Warren et al., 1994). Spindles propagate bidirectionally between thalamus and cortex (Contreras and Steriade, 1996; Contreras et al., 1996) and are reflected as global spindle oscillations during stage 2 non-REM sleep (De Gennaro and Ferrara, 2003).
In absence epilepsy, changes to the thalamocortical network enable it to enter a hypersynchronous state, generating high amplitude global oscillations with a stereotyped spike-and-wave signature that repeats at 3–8Hz, depending on species (Steriade et al., 1993; Crunelli and Leresche, 2002; Noebels, 2003; Noebels et al., 2012). These spike-wave discharges (SWD) occur in concert with abrupt cessation of movement and loss of consciousness, seemingly interrupting cognitive and motor outputs of the thalamocortical network. Together, SWD and the corresponding behavioral arrest comprise an absence seizure.
We begin this review by summarizing intrinsic and synaptic properties of thalamic networks that enable both normal and hypersynchronous oscillations, with emphasis on recent work that redefines their relationships to global rhythms, sleep, and seizures. These studies find that thalamocortical oscillations are not always global events (Barthó et al., 2014; Lewis et al., 2015; Nir et al., 2011; Vyazovskiy et al., 2011), meaning that individual thalamic oscillators may be dynamically regulated to enable thalamocortical rhythms at multiple spatial scales.
In the second section, we describe how intrinsic properties of thalamic neurons, synaptic strengths, and connectivity patterns within the network limit synchrony while permitting normal oscillations, and how their disruption enhances thalamic synchronization and enables SWDs. Accumulating evidence shows that any of several subtle, targeted changes to thalamic oscillatory machinery can induce thalamic hypersynchrony and absence seizures (Christian et al., 2013; Cope et al., 2009; Ernst et al., 2009; Huntsman et al., 1999; Paz et al., 2011). We also explore an interesting intersection between oscillation synchrony and attention at the CT-RT synapse (Ahrens et al., 2015; Paz et al., 2011), which may help explain attentional deficits associated with absence epilepsy (Masur et al., 2013). Importantly, these protective brakes represent targets for epilepsy treatments to limit synchrony within the thalamocortical network.
In the final section, we explore specializations across TC nuclei that may determine the oscillatory abilities of each thalamocortical loop as a function of behavioral state. Local enhancement and focal starting points of traditionally global oscillations suggest underlying differences between thalamocortical loops (Andrillon et al., 2011; Meeren et al., 2002; Tenney et al., 2013). In this context, we also ask whether nucleus-specific specializations may bias subcircuits within the thalamocortical loop toward hypersynchronization and global propagation of oscillations.
Thalamic contributions to widespread oscillations
Thalamic delta: intrinsic generators in TC neurons
In TC neurons, modest hyperpolarization to just 5–10 mV below a normal resting potential of −60 mV results in a switch to burst firing mode, in which depolarizing stimuli each evoke a burst of action potentials (>100 MHz) supported by a prolonged low threshold spike (LTSs, 20–70 ms) (Llinás and Jahnsen, 1982). Further TC membrane potential hyperpolarization to ~−80 mV leads to autonomous rhythm generation characterized by periodic LTSs at frequencies of 1–2 Hz that persist in isolated thalamic slices under complete pharmacological synaptic blockade (McCormick and Pape, 1990a; Soltesz et al., 1991). This intrinsic oscillation results from an interaction between two currents: the low-threshold calcium current, It, which enables the LTS, and a hyperpolarization-activated current, Ih (McCormick and Pape, 1990a; Soltesz et al., 1991). This push-pull oscillation results from reciprocal activation of the two currents. Simply put, Ih activation causes TC depolarization and It activation that leads to an LTS. Each LTS results in Ih inactivation that allows for a post-LTS hyperpolarization, which both deinactivates It and promotes subsequent activation of Ih, ultimately leading to another LTS. This intrinsic pacemaker activity provides the basis for the thalamic delta oscillation whose internal frequency depends on the activation/deactivation kinetics of Ih. Diverse brainstem neuromodulatory systems converge on the thalamus, where they provide coordinated input that regulates the oscillatory properties of thalamic neurons as a function of behavioral state (McCormick, 1992; Varela, 2014). Neuromodulatory systems partially determine the firing mode and oscillatory properties of TC neurons either by setting the membrane potential (McCormick and Prince, 1987; Mooney et al., 2004) or, in the case of norepinephrine and serotonin in VB thalamus, by enhancing Ih to selectively suppress burst firing (McCormick and Pape, 1990b; Monckton and McCormick, 2002).
Thalamic contributions to slow wave sleep
Oscillations in the slow and delta range (0.5–4Hz) dominate during deep slow wave sleep, but the role of intrinsic TC delta rhythmicity in global delta oscillations remains unclear. During sleep, the cortex generates a 0.3–1 Hz rhythm termed the slow oscillation, which consists of alternating periods of high activity (Up states) and silence (Down states) (Steriade et al., 1993a; Timofeev et al., 2000; Crunelli and Hughes, 2010). This cortical rhythm serves as a natural trigger for TC synchronization. Throughout an Up state, corticothalamic output dominates in the CT-RT-TC pathway, driving widespread coordinated RT-mediated hyperpolarization across the TC population that evokes phase-locked rebound bursting just after Up state onset (Contreras and Steriade, 1995; Timofeev and Steriade, 1996). Once entrained, clock-like thalamic delta oscillations are presumed to drive cortical counterparts (Timofeev and Steriade, 1996); however, the delta component of slow wave sleep could also derive from persistent intracortical activity during the Up state, which may mask thalamic input. In vivo disconnection experiments either of isolated cortical regions or the entire cortical hemisphere describe a periodic oscillations varying in frequency from <0.1 to 4 Hz depending on the preparation (Kellaway et al., 1966; Timofeev et al., 2000). Large bilateral thalamic lesions only modestly reduce EEG delta power in freely moving rats, arguing against a major thalamic contribution to cortical delta (Fuller et al., 2011). Notably, Carracedo et al. elicited delta oscillations in isolated cortical slices by lowering cholinergic and dopaminergic tone (2013), indicating that thalamic connectivity is not strictly required for such activities. In these slices, layer 5 intrinsically bursting pyramidal cells interacted with tonic, GABAB-mediated inhibition to generate a 1–2 Hz rhythm throughout the cortical network. Together, these data support that intracortical circuits can generate delta oscillations, even without thalamic input.
Recent findings do suggest new roles for thalamic delta in global oscillations. Blocking thalamic input to cortex, either by surgical disconnection or pharmacological inactivation, lengthens the period of the cortical slow oscillation (David et al., 2013; Lemieux et al., 2014). Furthermore, repetitive optogenetic activation of VB thalamus evokes periodic putative burst firing resembling thalamic delta; up to 1.5Hz stimulation entrains the cortical slow oscillation, suggesting that these two oscillators may synchronize one another (David et al., 2013). Inducing tonic mode firing in a subregion of RT with optogenetic stimulation simultaneously increases EEG delta power in local, anatomically connected cortical areas and decreases arousal level (Lewis et al., 2015). Increasing stimulus power increased delta power across a larger cortical region, potentially due to recruitment of a larger RT population. Within thalamus, tonic RT activation reduces overall firing rates in TC but promotes putative burst firing that is phase-locked to cortical slow waves, consistent with either enhancement of thalamic delta oscillations or incomplete thalamic inactivation. Importantly, tonic RT firing evokes tonic hyperpolarization in TC neurons (Herd et al., 2013), which could either enable thalamic delta or silence thalamic output depending on its strength. In either case, targeted TC hyperpolarization evokes a spatially restricted version of global sleep-related oscillations.
This spatially restricted, RT-evoked delta power boost resembles endogenous local slow wave enhancement observed in humans and rodents. In humans, slow wave power is amplified during sleep in task-related regions after learning (Huber et al., 2004), suggesting a local, functionally relevant, component to sleep. Further, slow waves become more localized during late-stage slow wave sleep (Nir et al., 2011), suggesting dynamic coordination between modular slow wave generators. In awake rats, isolated slow waves become more prevalent with sleep deprivation and have been shown to interfere with task performance; these local oscillations have been hypothesized to reflect increasing need for sleep (Vyazovskiy et al., 2011). Tonic TC hyperpolarization, either through tonic RT activation or another controlling source can generate isolated slow waves, even during wakefulness. In addition, RT’s topographic relationship with TC relays makes it an attractive potential control point for local slow wave sleep, which could be dynamically controlled to increase delta power over a continuum of spatial scales according to behavioral need.
Spindle oscillations: an intra-thalamic oscillation
Strong recurrent connectivity between TC and RT enable thalamic spindle oscillations (Huguenard and Prince, 1994a; Warren et al., 1994; Bal et al., 1995a,b; Kim et al., 1995). These 7–15Hz oscillations last between 1 and 3 seconds and are separated by a 5–20s refractory period; they persist even after removal of cortical input (Morison and Bassett, 1945; Steriade et al., 1985). In field potential and EEG recordings, oscillation amplitude first waxes and then wanes, as neurons are gradually recruited and later drop out as the spindle progresses. During a spindle oscillation, groups of synchronized RT neurons transiently inhibit TC cells via GABAA and GABAB receptors (i1, Fig. 1, 3A)(Steriade et al., 1993; von Krosigk et al., 1993; Huguenard and Prince, 1994; Warren et al., 1994; Jacobsen et al., 2001). Upon release from inhibition, TC neurons fire post-inhibitory rebound bursts (Fig. 1, lower left) that re-excite RT neurons (e1, Fig. 1, 3C), initiating another RT burst (Fig. 1, upper left) and the next cycle of the oscillation. Divergent projections from RT neurons to multiple TC cells (i1d, Fig. 1) along with divergent TC output back to RT (e1d, Fig. 1) amplify and spread the signal across a larger and larger intra-thalamic network. Through these divergent projections, spindle-like activity propagates laterally across the network in vitro (Kim et al., 1995; Destexhe et al., 1996a; Golomb et al., 1996). However, in vivo, coordinating cortical feedback overrides intrinsic thalamic spindle propagation, leading to rapid synchronization in the thalamus (Contreras and Steriade, 1996; Contreras et al., 1996).
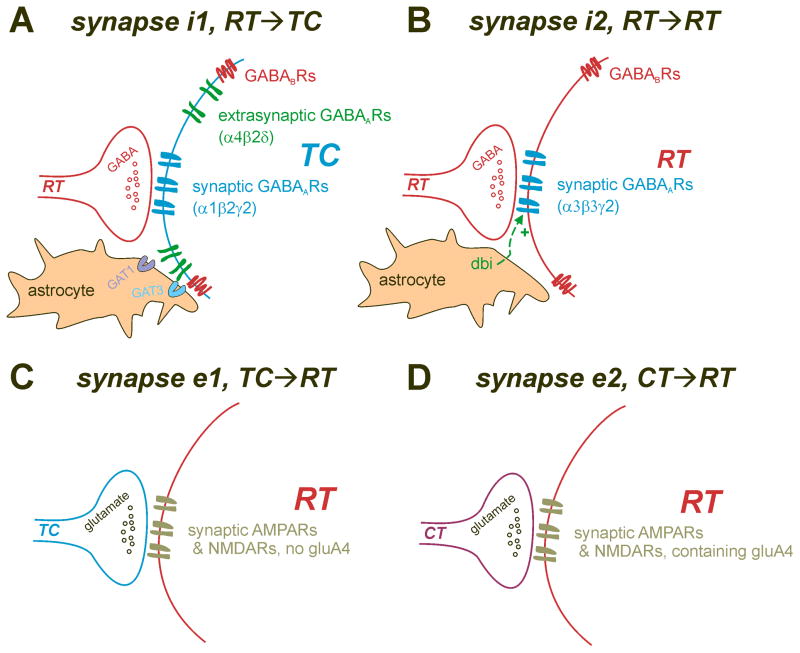
Figure 3. Synaptic elements in thalamic circuit that regulate synchrony and oscillations.
A. RT →TC, synapse i1. This inhibitory synapse is responsible for the phasic inhibition that drives post-inhibitory rebound firing in TC cells. GABA is released to activate α1β2γ2 GABAARs within the synapse, and can spillover to activate extrasynaptic α4β2δ GABAARs and GABAB receptors with slower kinetics. Spillover to extrasynaptic receptors is tightly regulated by GABA uptake via astrocytic GAT1 and GAT3. B. RT →RT, synapse i2. Chemical inhibitory signaling between RT cells is largely dependent on α3β3γ2 GABAARs, although a weak GABAB component is also present. Astrocytes in RT appear to release an endogenous benzodiazepine site ligand derived from benzodiazepine binding inhibitor (DBI) producing a constitutive positive allosteric modulation of RT GABAARs, that suppresses synchrony in the network. C. RT →TC, synapse e1. This synapse provides excitatory feedback within the thalamic loop to reinforce spindle oscillations and SWD. Although gluA4 is the major AMPA receptor subunit in RT, it does not appear to contribute to excitation at this synapse, as it’s synaptic strength is unchanged by deletion of gria4, which encodes gluA4. D. CT →RT, synapse e2. This synapse provides feedback from the cortex that can reinforce global CTC oscillations. Inactivation of gria4 weakens this synapse, leading to underexcitation of RT, and failure to produce cortical feed-forward inhibition (synapse i1) with resultant hyperexcitation at the CT→TC synapse, e3 (not shown).
Because the propagation latency from TC to RT is relatively brief (<10–20 ms, (Bal et al., 1995b)) kinetics of RT burst firing and TC responses to resultant IPSPs determine spindle oscillation frequency. In RT neurons, It is carried by Cav3.3, and to a lesser extent, Cav3.2 T-type channels, which have slower kinetics than Cav3.1 expressed by TC neurons (Astori et al., 2011; Klöckner et al., 1999; Talley et al., 1999). Dendritic location of these channels amplifies It to enable prolonged bursts with a characteristic accelerando-decelerando firing pattern in RT neurons (Fig. 2A, top row) (Domich et al., 1986; Destexhe et al., 1996b; Crandall et al., 2010). Burst duration controls the strength and time course of TC synaptic hyperpolarization and thus the precise timing of rebound LTS in TC cells during a spindle (Huguenard and Prince, 1992). Calcium-dependent SK potassium channels contribute to a prominent burst afterhyperpolarization following each RT LTS (Avanzini et al., 1989), limiting bursts to a precise duration (Bal and Mccormick, 1993; Debarbieux et al., 1998; Cueni et al., 2008; Kleiman-Weiner et al., 2009). RT burst firing triggers GABAA-mediated TC IPSPs (70–100 ms) (i1, Fig. 1, 3A), which determine the latency to post-inhibitory rebound LTS. Thus, increasing RT burst duration increases and prolongs inhibitory output and slows the oscillation (Bal et al., 1995b; Kleiman-Weiner et al., 2009), as does pharmacological enhancement of the post-synaptic inhibitory response (Jacobsen et al., 2001).
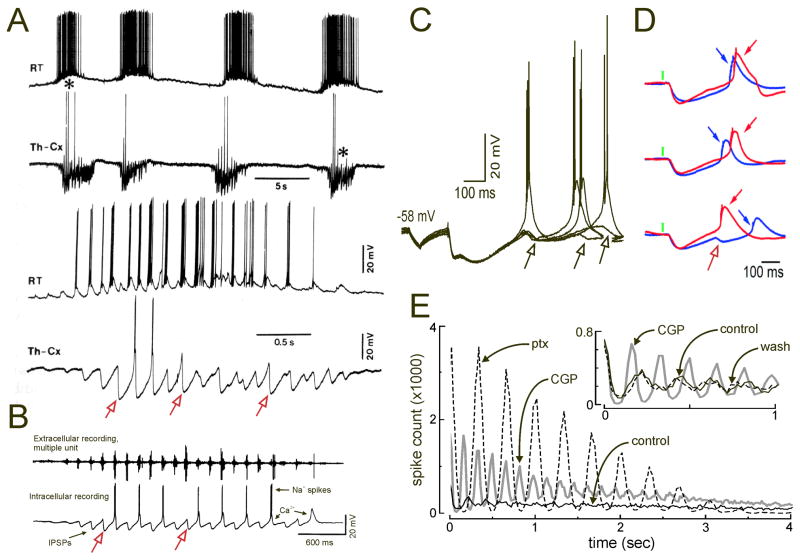
Figure 2. RT driven synaptic inhibition both drives spindle oscillations and desynchronizes them in vivo and in vitro.
A. Intracellular recordings from RT and TC (Th-Cx) cells during spontaneous spindle responses in felines. Upper two traces show sequences of 4 consecutive spindles, with the first, marked with asterisk, expanded in lower two traces. Note that RT cells fire on most cycles of the spindle sequence, while TC cells fire on many fewer cycles. Rhythmic IPSPs in TC cells lead to occasional rebound LTS responses, especially with the largest IPSPs, yet some large IPSPs that appear to be sufficient to do so, are instead followed by a subsequent IPSP that serves to veto the LTS. Some of these vetoed events are marked by open red arrows. B. Recordings from ferret LGN slices with spontaneous spindle like sequences evident in multi-unit extracellular recordings (upper trace) show similar periodic IPSPs (lower trace), some with rebound LTSs (Ca2+) that drive Na+ spikes, and other with clear vetoed events (red open arrows). C. Intracellular recordings from ventrobasal TC neurons in rat slices during evoked spindle-like oscillations. Multiple overlaid sequential responses are shown. Responses were evoked by extracellular stimulation of the internal capsule, which activates excitatory synapses (e2, e3) onto RT cells cause them to fire and produce inhibitory responses and rebound LTSs in the recorded TC cell. Note that sequential sweeps with the equivalent stimulus yielded LTSs with highly variable latencies. In many cases, a second IPSP arrives (open arrows) that delays or vetoes the LTS. D. Intracellular recordings from two (red and blue) nearby mouse TC neurons during 3 sequential optogenetic activations (green bar) of cholinergic inputs to RT. Note that sometime the blue TC cell leads the red cell (upper two traces), but that occasionally a second IPSP arrives (bottom trace) and vetoes (open red arrow) the early LTS leading to a response delayed by hundreds of ms. E. Reduced heterogeneity of TC IPSPs in the isolated thalamic network increases network synchrony. Autocorrelograms for TC cell multiunit spikes in rat VB-RT slices under various oscillatory conditions. The baseline autocorrelogram (black trace) shows very little structure and synchrony. Addition of the GABAB antagonist CGP35348 (grey trace) increases network synchrony (increase in peak to valley ratio of autocorrelogram), and similar increases in synchrony are produced by the GABAA antagonist picrotoxin (ptx). Thus, increasing IPSP homogeneity by abbreviating them to only contain GABAA responses sped and synchronized the oscillation (inset), or by lengthening them to only contain slower GABAB responses slowed and synchronized the oscillation. A. Modified from (Steriade and and Deschenes, 1988), B. Modified from (von Krosigk et al., 1993), C. modified from (Huguenard and Prince, 1994a), D. Modified from (Pita-Almenar et al., 2014), E. modified from (Jacobsen et al., 2001).
Intrathalamic rhythmicity is also promoted in part by intrinsic resonance of RT neurons at spindle frequencies, yet the role of such resonance on spindle generation remains unclear. RT cells can intrinsically oscillate within spindle range (7–12 Hz) for up to 1 second upon release from hyperpolarization in vitro (Avanzini et al., 1992; Bal and McCormick, 1993). This intrinsic oscillation mainly depends on push-pull interactions between T-channels and small conductance calcium-activated potassium (SK) channels (Kohler et al., 1996). The push-pull oscillation is promoted by a persistent depolarizing drive from a kinetically slower Ca2+-activated non-specific cationic current (Bal and McCormick, 1993). Surgical isolation of RT from relay thalamus and cortex in vivo abolishes global spindle oscillations, yet focal spindle oscillations persist in the disconnected RT (Steriade et al., 1987). While this finding may reflect intrinsic RT rhythmicity, any remaining input from lateral TC could form a residual intrathalamic network that generates the observed oscillations. In addition, intracellular recordings from TC neurons during spindles show that a few cycles of subthreshold rhythmic hyperpolarization can precede rebound LTSs (Steriade and and Deschenes, 1988; Timofeev et al., 2001). While such rhythmic hyperpolarizations reflect RT-mediated inhibition, whether these arise from intrinsic RT rhythmicity or spindle oscillation propagation from an adjacent intrathalamic network remains unknown. Contrary to the intrinsic RT generator hypothesis, in vitro, recurrent excitation (e1, Fig. 1) is required to generate spindle-like oscillations in vitro (Bal et al., 1995a,b; Jacobsen et al., 2001; Kim et al., 1995; Warren et al., 1994), suggesting that active excitatory feed back is necessary. In vitro studies have further shown that the output from individual TC and RT cells can be very potent drivers of activity (Bal et al., 1995a,b; Gentet and Ulrich, 2003), indicating that interactions among small numbers of TC/RT neurons may be sufficient for spindle initiation and that sparse but critical TC participation at early spindle stages may have been underreported. Thus, while intrinsic RT oscillations may facilitate or entrain intra-thalamic oscillations, intact reciprocal connections between RT and TC appear to be required for full expression of spindles.
Membrane potential strongly influences whether a TC neuron will entrain a spindle or delta oscillation. TC neurons only participate in spindle oscillations when their membrane potentials are held within a narrow range (−60 to −65mV), which promotes excitation-evoked burst firing in RT and rebound LTS in TC (Bal et al., 1995a, 1995b). Notably, this degree of hyperpolarization is not strong enough to enable intrinsic TC delta oscillations (Nunez et al., 1992). During a transition into slow wave sleep, changes in neuromodulatory tone gradually lower resting membrane potentials across the thalamus (Hirsch et al., 1983), biasing thalamic oscillations to spindles, followed by delta oscillations.
Competing theories advocate different features of the intrathalamic network that limit spindle duration and prevalence. Activity dependent TC depolarization resulting from progressive Ih activation over the course of a single spindle could ultimately block rebound LTSs towards the end of each spindle (Bal and McCormick, 1996; Lüthi and McCormick, 1999). Similar activity-dependent hyperpolarization in RT neurons may promote their late drop out of the spindle network event (Bal et al., 1995b; Kim and Mccormick, 1998), consistent with the observation that initial recruitment of many RT neurons predicts a shorter spindle duration (Barthó et al., 2014). These two mechanisms limit the number of cycles that thalamic components can maintain during an oscillation; alternatively, inputs that desynchronize the intra-thalamic network may limit spindle duration. Recordings both in vivo and in vitro highlight network heterogeneity that limits spindle synchronicity (Fig. 2). Notably, individual spindle related IPSPs in TC cells can either promote or prevent rebound LTS generation in a winner-take-all competition. These network responses have significant cellular and trial-to-trial variability, with, for example, various TC cells receiving heterogeneous IPSPs (Fig. 2C,D) (Huguenard and Prince, 1994a; Pita-Almenar et al., 2014). This leads to a situation in which some TC cells will be the first to respond with rebound LTSs. These result in rapid re-excitation of RT, followed by a second round of RT-dependent inhibition that can veto incipient TC output (Fig. 2. open arrows throughout) and sparsify the network spindle. Normalization of IPSPs, e.g. through pharmacological means that promote homogeneous IPSPs across the TC population, decreases sparseness and synchronizes the network (e.g., Fig. 2E) (Jacobsen et al., 2001). In addition, divergent TC-RT projections (e1d, Fig.1) tend to desynchronize adjacent RT neurons and ultimately destabilize the intra-thalamic network (Pita-Almenar et al., 2014). Extrinsic influences will also affect the thalamic spindle network. For example, increasingly desynchronized cortical feedback over the course of spindle oscillations may gradually interrupt spindles (Bonjean et al., 2011; Timofeev et al., 2001). These circuit-based destabilizing mechanisms would interact with cell-intrinsic mechanisms, including those described above, to shape the overall profile of the oscillation.
Local and global components of spindle oscillations
Careful analysis of human spindle oscillations reveals two spindle-range oscillations with distinct frequencies and spatial restrictions across cortex. Centrotemporal areas exhibit faster (13–15 Hz) spindles compared to slower (10–13 Hz) spindles observed in frontal cortices (Anderer et al., 2001; Andrillon et al., 2011). Rodent spindles exhibit a similar spatial dichotomy, with distinct spindles restricted to anterior and posterior cortices (Kim et al., 2015; Terrier and Gottesmann, 1978). Unlike human spindle oscillations, anterior and posterior spindle frequency differs by a modest 1Hz in rats (Terrier and Gottesmann, 1978), and so far such differences have not been observed in mice (Kim et al., 2015). In humans, spindle oscillations propagate in a posterior-anterior direction, with fast centrotemporal spindles leading their slower frontal counterparts. Notably, the cortical slow oscillation – the supposed trigger for global spindle initiation – travels in an opposite, anterior posterior direction (Nir et al., 2011; Sheroziya and Timofeev, 2014). Only centrotemporal spindles appear to be triggered by the onset of slow oscillation Up states as predicted by earlier studies (Andrillon et al., 2011; Mölle and Bergmann, 2011). Frontal spindles break this rule; instead, they occur near transitions into Down states. Furthermore, fast spindles are prominent in both stage 2 and 3 non-REM sleep, whereas slow spindles are preferentially expressed during stage 3 (Mölle and Bergmann, 2011); thus the two oscillations also appear to be subject to differential state-dependent control. Reports conflict on whether fast or slow spindles are most closely tied to memory consolidation (Mölle and Bergmann, 2011; Lustenberger et al., 2014), raising the possibility that these two widespread oscillations may also be independently amplified according to behavioral demand.
While it is possible that the two spindle types derive from different oscillatory sources, a parsimonious explanation for their disparate topographies and frequencies is that the thalamic nuclei driving the two cortical regions may oscillate at different frequencies. Variations between thalamic subnetworks could result in different spindle oscillation frequencies. For example, RT neurons with fewer SK channels would have weaker post-burst afterhyperpolarization, which would lengthen RT bursts and, consequently, the spindle oscillation period (Bal and Mccormick, 1993; Debarbieux et al., 1998; Cueni et al., 2008; Kleiman-Weiner et al., 2009).
Spatially restricted spindle oscillations have also been observed in human subjects and evoked in rodent models. In humans, local spindles occur during slow wave sleep but are not tied to ongoing slow oscillations (Andrillon et al., 2011), implying that another, perhaps subcortical source may initiate spindles in a subregion of thalamus. In contrast to slow oscillations, spindles become more synchronized over the course of slow wave sleep, further dissociating the two oscillations. In mice, targeted optogenetic stimulation of RT with single, brief light pulses elicited hyperpolarization followed by a rebound burst in TC cells and sometimes evoked a spindle oscillation across the thalamocortical network (Halassa et al., 2011; Barthó et al., 2014). Spindles could only be evoked in sleeping mice, demonstrating that thalamic rhythmogenesis in these preparations cannot overcome state-dependent neuromodulatory control. This could be due to the narrow range of membrane potentials that permits spindle oscillations in TC neurons (Nunez et al., 1992). Local RT stimulation changes oscillation dynamics within a restricted corticothalamic network, observed at only a subset of thalamic (Barthó et al., 2014) electrodes. In contrast to tonic RT activation, which enhances slow wave power, a single activating pulse can evoke a local spindle oscillation. RT thus represents a multifunctional control point for local sleep-related oscillations, which could be directed by tonic and phasic excitatory sources to enhance slow wave and spindle activity, respectively. In addition to enabling local cortical oscillations, dynamic subregion-specific control of RT could also differentially engage thalamic processing to aid behavioral performance, such as by priming relevant modality-specific pathways during sensorimotor tasks.
Relevance of spindle oscillations to sleep architecture
Human subjects with more prevalent spindles are resistant to noise- and stress-induced sleep disruptions, suggesting that these oscillations could reflect a mechanism that promotes slow wave sleep and, as a result, may reduce sensitivity to external interruptions (Dang-Vu et al., 2010, 2015). Patterned RT stimulation that mimics activation during spindle oscillations reliably evokes cortical spindle oscillations (Kim et al., 2012; Ni et al., 2016). When presented periodically during sleep, spindle-like RT stimulation increases the overall duration of slow wave sleep. Similarly, single pulse optogenetic activation of cholinergic inputs to RT evoked cortical spindle oscillations, and repetition during sleep increased slow wave sleep duration (Ni et al., 2016). It should be noted that these studies did not test whether evoked cortical spindles were local or global, so it is unclear what spatial scale of spindle entrainment is most important to prolong slow wave sleep. Nevertheless, these studies show that brief, periodic activation of RT can determine spindle prevalence during slow wave sleep. Furthermore, these studies suggest an intriguing hypothesis: spindle oscillations may directly influence subsequent global state to prolong slow wave sleep.
Absence seizures: abnormal synchronization across thalamus and cortex
The thalamocortical network is also capable of generating high-amplitude global oscillations characterized by a spike-wave EEG signature that repeats at 3–8 Hz, depending on species, and lasts 2–30 seconds (Crunelli and Leresche, 2002; Meeren et al., 2002). These spike-wave discharges (SWD) rapidly generalize across both cerebral hemispheres, corresponding with abrupt cessation of movement and apparent loss of consciousness, although some sensory-evoked neural activity persists (Chipaux et al., 2013). Absence seizures are the defining feature of both childhood and juvenile absence epilepsy, and they are also common in juvenile myoclonic epilepsy (Duncan, 1997). All three disorders are genetically determined, yet in most cases, it is impossible to determine which mutations directly contribute to seizures (Maheshwari and Noebels, 2014). During SWD in rats, RT and TC are simultaneously excited by synchronous, transient cortical input (Danober et al., 1998; Pinault, 2003; Polack and Charpier, 2006). CT-RT excitation (e2, Fig. 1, 3B) tends to override that of CT-TC (e3, Fig. 1, 3D); as a result, TC cells are briefly hyperpolarized by strong CT stimulation as the direct CT-TC excitation appears to be shunted by a more powerful feedforward CT-RT-TC inhibition (Golshani et al., 2001)(e2 and i1, Fig. 1). A subsequent rebound LTS in TC propagates back to RT (e1, Fig. 1) and cortex, triggering the next cycle of the oscillation. Notably, rodent SWDs are faster (6–10Hz) than those of humans (3–6Hz), which may represent a key difference between the two network oscillators (Crunelli and Leresche, 2002; Meeren et al., 2002).
While faithful TC-CT relay is required for an intact oscillatory circuit (Meeren et al., 2009), the necessity of rebound LTSs in TC during SWD is still contested based on data from disconnection experiments with in vivo anesthetized preparations. It should be noted that these preparations allow cortex and thalamus to each generate SWD in isolation and thus synchronize different circuits from the intact thalamocortical network required for absence seizures (see below). In vivo sharp electrode recordings from cats and rats during SWD document rhythmic TC IPSPs with occasional rebound spikes but no LTSs (Steriade and Contreras, 1995; Pinault, 2003); however, the lack of rebound LTS may reflect methodological issues. The membrane leak incurred by the sharp electrode will shunt membrane hyperpolarization of each IPSP, reducing the likelihood of sufficient T channel deinactivation to enable rebound bursting. Consistent with this potential explanation, TC neurons with the highest recorded input resistance are more likely to fire rebound spikes (Pinault, 2003). Single-unit extracellular recordings and multiunit activity reliably document rhythmic, high frequency barrages of TC spikes phase-locked to each SWD cycle (Steriade and Conteras, 1995; Pinault et al., 2001; Pinault, 2003,), which corroborates reliable TC LTS observed during hypersynchronous oscillations in vitro (Huguenard and Prince, 1994b; Warren et al., 1994; Bal et al., 1995a,b; Blumenfeld and McCormick, 2000; Jacobsen et al., 2001). Together, these data support the idea that rebound LTS from TC are relayed to cortex to reinitiate SWD in vivo.
Researchers have long sought a hidden generator of SWD – a vulnerable link in the thalamocortical network susceptible to hypersynchrony and capable of rapid propagation. In anesthetized cats, direct cortical application of GABAA agonists penicillin or bicuculline generates an SWD-like EEG signature in the treated area, even after large thalamic lesions (Fisher and Prince, 1977; Steriade and Contreras, 1998). Furthermore, conjugated estrogens applied directly to surgically isolated cortical slabs in anesthetized cats yield a similar EEG pattern (Marcus and Watson, 1964). In contrast, application of the GABAA agonist bicuculline to isolated thalamic slices synchronizes and slows spindle-like oscillatory activity to 3–4 Hz, resembling SWD (von Krosigk et al,. 1993; Huguenard and Prince, 1994b; Jacobsen et al., 2001). In vivo, injection of bicuculline into the thalamus evokes SWD-like oscillations in anesthetized rats but not in cats (Castro-Alamancos, 1999; Steriade and Contreras, 1998). While cortical and thalamic circuits each appear capable of generating an SWD EEG signature, it remains unclear whether either one in isolation actually recreates the neural activity patterns that evoke loss of consciousness observed during absence seizures.
Genetic rodent models of absence epilepsy exhibit spontaneous SWD during periods of quiet wakefulness and light sleep, allowing simultaneous observation of behavior and neural activity without anesthesia (Danober et al., 1998; Coenen and Van Luijtelaar, 2003). In the Wag/Rij (Wistar albino Glaxo/Rijswijk) model, unilateral lesions of somatosensory thalamus and RT abolish SWDs bilaterally, demonstrating that the thalamus is required for the global oscillation in these animals (Meeren et al., 2009). Multisite cortical field potential recordings show that SWDs propagate in a stereotyped pattern across cortex, originating consistently from a focal area within somatosensory cortex in both Wag/rij and GAERS (genetic absence epilepsy rats from Strausbourg) (Meeren et al., 2002; Polack et al., 2007). Simultaneous thalamic recordings reveal early SWD onset selectively within somatosensory thalamus that is reciprocally connected with the cortical focus (Meeren et al., 2002). More thorough sampling within the apparent focal network of Wag/rij rats shows that similar oscillations occur first between cortical layer 6, which provides CT feedback, and the posterior thalamic nucleus, a higher order somatosensory TC relay (Lüttjohann and van Luijtelaar, 2012). Interestingly, targeted treatment of the focal cortical network is sufficient for seizure control, as ethosuximide infusion into the cortical focus (see below), but not adjacent cortical areas, rapidly blocks seizures in both Wag/rij and GAERS (Manning et al., 2004; Aker et al., 2010). It therefore appears that a restricted thalamocortical network initiates global SWDs; however, given the distributed nature of the network it is not feasible to rule out that an unsampled area might actually initiate the seizure.
In human patients, absence seizures tend to begin in frontal cortical areas when mapped using high-density EEG and MEG, consistent with an underlying focal generator (Holmes et al., 2004; Westmijse et al., 2009). Even in these cases, activity rapidly generalizes throughout the cortex in less than one second – within one or two cycles of SWD. Absence seizures detected using MEG and analyzed to extrapolate signals from deep sources report early activity in both frontal cortex and thalamus (Tenney et al., 2013). Precise onset location and propagation pattern vary from patient to patient, which may reflect the diverse, and often unexplained, genetic causes of absence epilepsy (Maheshwari and Noebels, 2014). Consistent early activation in frontal cortex and thalamus (Tenney et al., 2013) suggests that human absence seizures may also originate from a focal network origin despite heterogeneous genetic causes.
In the next section, we consider several different mechanisms that limit synchronization within the thalamocortical network whose differential expression across thalamus might render certain loops vulnerable to synchronization, resulting in a latent focal network. Several of these synchrony-limiting features suggest ways that genetic variations associated with absence epilepsy may promote synchrony within thalamus. When combined with a broadly expressed weakly synchronizing genetic mutation, the latent focal network would be most likely to generate absence seizures. Conversely, modest enhancement of a protective mechanism could prevent absence seizure generation in the focal network while minimizing unwanted effects in other thalamic regions. These protective brakes thus may specify the focal network, explain genetic causes of absence seizures, and, importantly, suggest treatments that selectively block absence seizures.
Protective thalamic mechanisms limit hypersynchrony
Balancing intrinsic currents to determine synchrony
Convergent evidence shows that reductions in It lead to destabilization of TC rebound LTSs and protection against SWD. Ethosuximide, a popular and effective anti-absence drug, modestly reduces It in both TC and RT neurons (Coulter et al., 1989a; Huguenard and Prince, 1994a). Ethosuximide elevates the threshold for burst firing across the thalamus, increases burst latency in RT neurons, and blocks pharmacologically induced hypersynchronous oscillations in vitro (Coulter et al., 1989a; Huguenard and Prince, 1994a). Importantly, ethosuximide increases variability in rebound LTS timing and reduces LTS probability, effectively desynchronizing thalamic output (Fig. 2C). While ethosuximide also modulates persistent Na+ currents and Ca2+-activated K+ currents (Leresche et al., 1998), its reduction of It is responsible for its desynchronizing effect, as more specific It blockers similarly impair TC excitability and block epileptogenesis both in vitro and in vivo (Broicher et al., 2007; Porcello et al., 2003; Tringham et al., 2012). In mice lacking Cav3.1, which is expressed in TC and cortex but not RT, TC cells no longer fire rebound bursts in vitro (Kim et al., 2001); further, Cav3.1−/− mice are resistant to absence seizure induction by the GABAB agonist baclofen (Kim et al., 2001). Conversely, transgenic overexpression of Cav3.1, enhances It in both TC and cortical neurons and generates spontaneous SWD in vivo (Ernst et al., 2009). In this case, strengthening It could exert the opposite effect of ethosuximide, both increasing rebound LTS probability and synchronizing LTS timing across thalamus. Genetic mutations in T-type Ca2+ channels Cav3.1 and Cav3.2 have been linked to human absence seizures (Chen et al., 2003; Singh et al., 2007), suggesting that some patients may have an imbalance in It, which promotes hypersynchrony similar to that shown by animal models.
Notably, the three T-type calcium channels that conduct It are expressed differentially across thalamus and cortex, so manipulations like ethosuximide treatment and global knockouts that broadly target It likely act across the thalamocortical network (Talley et al., 1999; Notomi and Shigemoto, 2004). Injecting ethosuximide into the cortical focus but not adjacent cortical regions of Wag/rij and GAERS animals blocks spontaneous SWD (Manning et al., 2004; Aker et al., 2010), whereas thalamic ethosuximide infusion only partially blocks spontaneous SWD (Richards et al., 2003), which may reflect an undescribed It-dependent cortical SWD generator or incomplete infusion into the target area. Ethosuximide’s well characterized reduction of It and corresponding anti-oscillatory action in thalamus in vitro (Coulter et al., 1989b, 1989c; Huguenard and Prince, 1994a) suggest that systemic ethosuximide treatment blocks absence seizures at least in part by destabilizing thalamic output. Manipulations that selectively manipulate T-channel expression in thalamus, such as virally-mediated knockdown or overexpression, could determine its relative contribution to global It imbalance associated with absence seizures.
Extrasynaptic inhibition at the RT-TC synapse (i1)
At the RT-TC synapse (i1, Fig. 1, 3A), GABA can activate three different types of receptors (Figure 1A,B), which together shape post-inhibitory rebound LTS and, as a result, oscillation strength and frequency. Synaptic GABAA receptors (GABAARs) drive fast, phasic inhibition in TC neurons (Brickley et al., 1999; Pirker et al., 2000). Virally-mediated deletion of synaptic GABAARs from somatosensory TC nuclei blocks fast IPSCs but does not prevent burst firing or overtly perturb global rhythms, ruling synaptic receptors out as a major contributors to SWD (Rovó et al., 2014). Extrasynaptic GABAARs and GABAB Rs drive slow phasic and tonic inhibition and are thought to be activated by GABA spillover during periods of intense synaptic release, such as RT burst firing (Cope et al., 2005; Jacobsen et al., 2001; Jia et al., 2005; Kulik et al., 2002). Deletion of the extrasynaptic GABAA-δ subunit reduces tonic inhibition in mouse TC neurons and protects against SWD induction by GABAB agonist γ-butyrobolactone (Cope et al., 2009). Knockout of the extrasynaptic GABAA-α4 subunit, which forms an extrasynaptic receptor with the δ subunit in TC neurons, selectively reduces burst-evoked IPSC duration and tonic inhibition in TC cells (Herd et al., 2013). Furthermore, extrasynaptic GABAA-α4-containing receptors enable tonic RT firing to set TC membrane potentials. Paired recordings of RT and TC cells showed that tonic RT firing influences the excitability and even the firing mode of TC neurons by regulating their membrane potential. Both the shortened burst-evoked IPSCs and reduced activity-dependent tonic inhibition observed in GABAA-α4−/− TC neurons may therefore contribute to the anti-epileptic effect observed in the GABAA-δ−/−. Because RT neurons fire bursts during SWD (Pinault, 2003), limiting slow phasic inhibition may play a major role in protecting against synchrony by blocking extrasynaptic GABAARs. Either tonic depolarization or reduced IPSP duration by extrasynaptic GABAAR blockade might prevent TC neurons from reliably reaching the threshold for rebound LTS, reducing overall network synchrony.
Astrocytes regulate GABA spillover and limit extrasynaptic GABA receptor activation at RT-TC synapses. In the thalamus, GABA transporters GAT-1 and GAT-3 are selectively expressed by astrocytes, with GAT-1 localized more proximate to synapses than GAT-3 (De Biasi et al., 1998; Beenhakker and Huguenard, 2010). Deletion of GAT-1 (GAT-1−/−) or thalamic injection of a GAT-1 antagonist induces SWD in mice in vivo, likely by preventing GABA uptake and thus enhancing activation of extrasynaptic GABAARs in TC (Cope et al., 2009). Enhanced extrasynaptic GABAAR function could tonically hyperpolarize TC membrane potentials and prolong burst-evoked IPSCs (Cope et al., 2009; Herd et al., 2013), which would prime TC neurons for rebound LTS, potentially resulting in enhanced synchrony. Speeding rebound LTS does not necessarily enhance synchrony, as increasing IPSC duration, amplitude, or membrane hyperpolarization can actually desynchronize a the network by revealing masked heterogeneity in intrinsic LTS-determining currents among TC neurons (Sohal et al., 2006). However, if GAT-1 blockade and subsequent enhancement of extrasynaptic GABAAR transmission is mild enough to merely increase the proportion of TC neurons firing rebound LTS on each cycle, it could explain SWD after GAT-1 deletion.
GAT-1 and GAT-3 also differentially modulate GABAB-mediated inhibitory currents. GAT-3 blockade strongly prolongs and amplifies GABAB IPSCs, whereas GAT-1 blockade only yields a moderate enhancement (Beenhakker and Huguenard, 2010). GAT-3 profoundly limits sustained GABAB-mediated inhibition by preventing spillover that can lead to recruitment of GABAB receptors that exist in extensive numbers far from the synapse. GABAB activation in dorsal thalamus promotes hypersynchronous oscillations, as locally delivered GABAB antagonists protect against spontaneous SWD, whereas GABAB agonists enable them (Liu et al., 1992; Vergnes et al., 1997). In mouse thalamic slices, GAT-1−/− and pharmacological blockade of GAT-1 or GAT-3 all enhance tonic inhibition (Cope et al., 2009), which could contribute to synchrony by hyperpolarizing TC cells and priming them for burst firing. By restricting spillover at the RT-TC synapse, astrocytes limit activation of extrasynaptic GABA receptors and resultant slow phasic and tonic inhibition, which imparts variability in rebound LTS and ultimately desynchronizes the network.
Corticothalamic feed-forward inhibition
As described earlier, coordinated corticothalamic input can evoke thalamic spindle and delta oscillations, with the dominant CT-RT pathway (e2, Fig. 1, 3D) driving rebound burst firing in TC. Surprisingly, in some cases blockade of the CT-RT pathway can promote hypersynchrony. Optogenetic circuit mapping in Glua4 knockout mice (Gria4−/−) found that synaptic strength is reduced selectively at CT-RT (e2, Fig. 1, 3D) synapses, while TC-RT (e1, Fig. 1, 3C) and CT-TC (e3, Fig. 1) synapses remain intact (Paz et al., 2011), corresponding to strong expression of Glua4 in RT compared to TC (Petralia and Wenthold, 1992). In vivo, Gria4−/− exhibit SWD and behavioral arrest characteristic of absence seizures (Beyer et al., 2008; Paz et al., 2011). Because CT input no longer drives robust feed-forward RT-mediated inhibition, direct, reliable CT activation of TC is unmasked(Paz et al., 2011). With CT-RT (e2, Fig. 1, 3D) synapses intact in wt animals, strong feed-forward inhibition only permits sparse activation of TC cells, thus maintaining a desynchronized state. By contrast, greater synchronous activation of TC cells by dominant CT excitation in gria4−/− (e3, Fig. 1) leads to excessive activation of cortex and RT. Release of TC cells from RT inhibition then presumably coincides with recurrent CT-TC excitation to potentiate the next cycle of the oscillation. Reducing efficacy of a single synapse type in the thalamocortical network thus yields runaway TC output that builds to generate hypersynchronous oscillations.
Conversely, deleting the receptor tyrosine kinase ErbB4 from somatostatin neurons in RT (Som-ErbB4−/−) strengthens the CT-RT synapse (e2, Fig. 1, 3D), which can be reversed by impairing thalamic GluA4 signaling selectively within the thalamus (Ahrens et al., 2015). These mice would presumably be resistant to SWD induction, but this has not yet been tested. Strengthening the synapse in the Som-ErbB4−/− model enhances sensory discrimination within a single sensory modality but impairs cross-modality performance, corresponding to enhanced feedback inhibition from cortex to thalamic relays. Thus, manipulating the strength of this pathway simultaneously determines oscillatory properties of the thalamocortical network and sensory-evoked behavior.
Implicit and explicit links between SWD and attention
In childhood absence epilepsy, 35% of patients experience impaired attention, generally described as an inability to maintain focus during tasks (Masur et al., 2013). At first glance, absence seizures could disrupt attention simply by interrupting normal cognitive processing with bouts of unconsciousness. However, attentional deficits are still reported even when seizures are well controlled by medication. Ethosuximide reduces seizures without improving attention, while treatment with valproic acid actually increases attentional deficits while minimizing seizures (Masur et al., 2013). Thus, in some CAE patients, ethosuximide prevents SWD without correcting an underlying pathology that simultaneously impairs attention and promotes SWD. Recent studies have shown that RT helps direct attention for task-related behaviors (McAlonan et al., 2008; Halassa et al., 2014), and that CT-RT signaling (e2, Fig. 1, 3D) can determine performance on attention-based tasks (Ahrens et al., 2015; Wimmer et al., 2015). The CT-RT synapse is dynamically regulated in a frequency-dependent manner, only transiently invoking feed-forward inhibition from RT in the face of prolonged CT input (Crandall et al., 2015; Mease et al., 2014). As a result, prolonged CT stimulation reduces sensory adaptation and enhances performance in sensory discrimination tasks (Mease et al., 2014). Selectively weakening this attention-directing synapse generates spontaneous SWD (Paz et al., 2011). The CT-RT synapse is therefore an example of a site where genetic changes impact both attention and network synchrony, highlighting the dynamic, state-dependent nature of the thalamocortical network. In an alert state, when slow thalamocortical oscillations are not favored, this synapse instead sharpens encoding of relevant sensory stimuli over time to enhance attentional performance.
Intra-RT inhibition
Inhibitory synapses between neurons RT neurons (i2, Fig. 1, 3B) enable lateral inhibition within the nucleus that may desynchronize RT output. RT axons branch to extend delicate collaterals within RT in addition to their major arbors in TC nuclei (Scheibel and Scheibel, 1966; Yen et al., 1985; Cox et al., 1996). RT IPSPs can be evoked by local application or laser uncaging of glutamate within RT, demonstrating that RT activation is the source of RT inhibition in these reduced preparations (Sanchez-Vives and McCormick, 1997; Shu and McCormick, 2002; Deleuze and Huguenard, 2006; Lam et al., 2006). Notably, several recent studies in mice have failed to find chemical inhibitory synapses between RT neurons, casting doubt on their importance to thalamocortical synchrony. Paired recordings in mouse thalamic slices found only occasional inhibitory synapses between RT neurons (Landisman et al., 2002; Parker et al., 2009). Furthermore, strong synchronous optogenetic stimulation of CT axons failed to evoke disynaptic IPSCs in RT neurons (Cruikshank et al., 2010). A recent study cites age as a contributing factor, observing detectable intra-RT inhibition only in slices from juvenile animals (Hou et al., 2016). However, antidromic stimulation of RT axons in VB designed to specifically activate RT dependent inhibition does evoke IPSCs in RT neurons (Sun et al., 2011). Similarly, glutamate uncaging studies performed in rat thalamus consistently observe evoked IPSPs in 60% of RT neurons tested (Deleuze and Huguenard, 2006; Lam et al., 2006). While the reasons for this discrepancy, especially in mice, remains to be elucidated, failure to observe intra-RT synapses may be due to differences in species, tissue preparation, or slice plane. For example, coronal slices best preserve these inhibitory synapses and inhibitory intra-RT sources are more distributed than excitatory, electrical synaptic inputs (Deleuze and Huguenard, 2006). Additionally, intra-RT synapses are most readily observed in immature tissue (Hou et al., 2016; Parker et al., 2009), in which slicing induced tissue damage is lessened.
Disrupting inhibitory input to RT neurons results in abnormal synchrony across thalamus and cortex, supporting an active role for intra-RT inhibition (i2, Fig 1, 3B) in regulation of SWD. The GABAA-β3 receptor subunit is expressed in RT but not in first order TC relays (Wisden et al., 1992; Pirker et al., 2000). Single-nucleotide polymorphisms that reduce GABAA-β3 expression are associated with childhood absence epilepsy (Urak et al., 2006; Tandaka et al., 2012), and GABAA-β3 knockout mice (GABAA-β3−/−) have a complex epilepsy phenotype that includes absence seizures (Homanics et al., 1997). In thalamic slices from GABAA-β3−/− mice, GABAA-mediated IPSPs are reduced selectively within RT (Huntsman et al., 1999). Thalamic slices from GABAA-β3−/− mice also exhibited hypersynchronous, epileptiform oscillations in vitro. Thus, targeted removal of the inhibitory brake on rhythmic RT firing entrains thalamic network to epileptiform oscillations. Convergent evidence comes from knockout of the GABAA-α3 receptor subunit, which is found in RT but not TC. Unexpectedly, GABAA-α3 knockout results in a selective compensatory enhancement of inhibition in RT neurons. This protects against hypersynchronous thalamic oscillations in vitro and pentylene tetrazol induced seizures in vivo (Schofield et al., 2009). While RT receives inhibitory input from external sources, such as substantia nigra and globus pallidus, intra-RT inhibition provides the most parsimonious model for desynchronization because of its necessity to the oscillatory thalamocortical loop. With intact intra-RT inhibition, synchronized excitation of RT neurons would cause RT neurons to inhibit others within the network. Because inhibitory contacts are likely to be sparse and somewhat distributed (Destexhe et al., 1996a; Deleuze and Huguenard, 2006; Lam et al., 2006), RT neurons across the network would receive varying levels of inhibition at different times, causing RT neurons to fire with unequal delays, or to fail entirely, on the next cycle of the oscillation. GABAA-mediated RT IPSPs can be relatively long-lasting (200ms) (Zhang et al., 1997), adding to the resulting temporal offset between connected RT neurons. Desynchronized RT output would reach distinct but overlapping groups of TC neurons (Pinault and Deschenes, 1998; Pita-Almenar et al., 2014), producing different IPSC profiles in nearby TC neurons that would desynchronize TC rebound LTS and, as a result, the ongoing oscillation.
Recent findings suggest that weakening intra-RT inhibition can enable SWD. The benzodiazepine clonazepam, a positive allosteric modulator of GABAARs, reduces GABAB-mediated components of TC IPSCs in thalamic slices, apparently by reducing output of RT (Huguenard and Prince, 1994b; Sohal and Huguenard, 2003). Thus, while clonazepam is a broad spectrum benzodiazepine that enhances GABAA mediated inhibition at both RT-RT and RT-TC synapses, its net effect on the thalamic network is to destabilize oscillations by enhancing intra-RT connectivity, which protects against hypersynchrony. Genetic blockade of the benzodiazepine binding site on RT-specific GABAAR-α3 generates spontaneous SWD in vivo (Christian et al., 2013), suggesting that an endogenous benzodiazepine normally enhances RT inhibition to limit synchrony. Similar SWD results after genetic deletion of diazepam binding inhibitor, a putative endozepine acting on the same benzodiazepine binding site as clonazepam. In vitro, both of these genetic manipulations shorten IPSCs in RT compared to controls, demonstrating that the endozepine, like clonazepam, constitutively enhances inhibition in RT. Further, blocking this inhibition promotes hypersynchrony (Christian et al., 2013). Notably, astrocytes may be the source of thalamic endozepine, as metabolical poisoning of glial cells with fluorocitrate shortened GABA responses in a similar manner to genetic endozepine deletion (Christian and Huguenard, 2013). Thus, astrocytes enhance RT inhibition to destabilize the network, presumably via constitutive boosting of intra-RT inhibition.
Divergent TC-RT connectivity
Divergent connections between RT and TC (i1d, e1d, Fig. 1) underlie propagation of spindle oscillations across thalamus (Destexhe et al., 1996a; Pinault and Deschenes, 1998). Recent work shows that an asymmetrical divergence also helps destabilize the network and limits the duration of evoked spindle-like oscillations in vitro. Brief, simultaneous activation of RT neuron via cholinergic inputs evokes a transient spindle-like oscillation in the intra-thalamic network (Pita-Almenar et al., 2014). Initially, simultaneous inhibition from RT evokes synchronous IPSPs but asynchronous rebound LTS in neighboring TC neurons (Fig. 2D). Mapping the microcircuit with paired recordings, Pita-Almenar et al., find that neighboring TC neurons receive inputs from the same RT neuron (i1d, Fig. 1), but neighboring RT neurons receive sparse non-overlapping TC input (e1d, Fig. 1)(Pita-Almenar et al., 2014). Blocking polysynaptic excitation from TC to RT with AMPA/NMDA antagonists reduces rebound LTS jitter by 50% and also increases its probability and speed, demonstrating that this divergent local circuitry desynchronizes network activity. Although the RT-TC pathway is relatively convergent, non-identical synaptic relationships between individual RT-TC pairs result in subtle differences in IPSP profile, which can dramatically impact rebound LTS timing and success (Sohal et al., 2006) and may further desynchronize the network. This microcircuit-scale divergent pathway propagates asynchronous activity across distributed RT neurons to interrupt ongoing oscillations. However, if TC neurons are primed for rebound LTS, subtle differences in RT-TC input patterns may be less effective in destabilizing the network. In absence epilepsy models, it appears that synchronizing factors can override destabilizing effects of the divergent network while taking advantage of its ability to propagate oscillations throughout the thalamus. Gradual network-endowed desynchronization might still contribute to seizure termination, but future study and, perhaps, new circuit rewiring methods would be required to test this hypothesis.
Specializations among thalamocortical loops
Observations of local spindle oscillations and focal networks that drive global oscillations imply that thalamocortical networks may be dynamically and somewhat independently controlled. While most TC neurons are at least capable of participating in spindle oscillations (Steriade and Deschenes, 1984), the anterior thalamus does not generate spindles in cats (Mulle et al., 1985). Further, spindle oscillations have been recorded in rodent anterior thalamus (Tsanov et al., 2011), and activity in anterior-innervating RT is negatively correlated to cortical spindle power and increases uniformly with arousal (Halassa et al., 2014). In rodents, all TC nuclei recorded thus far are phase-locked to SWD, albeit with varying relative phase (Inoue et al., 1993; Paz et al., 2007; Gorji et al., 2011). In absence epilepsy, focal subnetworks may be particularly susceptible to hypersynchronization and global propagation of oscillations. In addition, the principles governing TC oscillations derive mainly from detailed studies of first order thalamic nuclei. Higher order thalamic nuclei engage different cortical circuitry and receive additional strong excitation from cortical layer 5 and inhibition from outside RT and thus may not simply follow rules based on first order nuclei.
Limited evidence suggests that the higher order intralaminar and midline thalamic nuclei may be uniquely able to evoke an absence-like state. In awake, freely moving cats, 10–30Hz stimulation of intralaminar and midline thalamic nuclei evoked global SWD oscillations and absence-like behavior – immobility and lack of responsiveness to external cues (Hunter and Jasper, 1949). In some cases, SWDs persisted after stimulation, suggesting that coordinated activity in thalamus could entrain the thalamocortical network into an absence seizure. This intriguing experiment has only recently been repeated in a mouse model using optogenetic stimulation of higher order thalamus centered on the intralaminar thalamus (Liu et al., 2015). Low frequency stimulation (10Hz) evoked 10Hz cortical oscillations with pronounced spikes similar to rodent SWD and absence-like behavior, whereas high frequency stimulation (40–100Hz) evoked low amplitude, high frequency EEG oscillations consistent with wakefulness and aroused animals from sleep into a waking state (Liu et al., 2015). Unlike the original report in cats, these optogenetically SWD-like oscillations in anesthetized mice stopped abruptly upon stimulus removal, and thus did not fully recapitulate the state change observed with electrical stimulation in cats. Future studies testing optogenetic entrainment of global oscillations across TC nuclei determine whether the intralaminar and midline nuclei are preferentially recruited to initiate SWD in rodents, as they are in cats (Hunter and Jasper, 1949).
Intrinsic properties and neuromodulatory response profiles vary across thalamic nuclei and within RT, demonstrating the potential for specializations that enable differential oscillatory control. In somatosensory thalamus, neurons of the higher order posterior thalamic nucleus fire rebound bursts with slower intra-burst frequency, have higher thresholds for tonic firing, and fire tonically at a lower rate than neurons in first order VB (Landisman and Connors, 2007). With corticothalamic stimulation, posterior thalamic EPSPs were less likely to undergo short-term facilitation, and their IPSPs were more likely to have slow, sustained components reflective of extrasynaptic inhibition. In visual thalamus, neurons of the higher order pulvinar nucleus are more likely to fire a series of rebound bursts instead of a single one, suggesting that they might be more prone to rhythmic bursting, and, as a result, incorporation into oscillations (Wei et al., 2011). Finally, a recent report finds that neurons of the higher order centromedian thalamic nucleus exhibit very little Ih and an elevated tonic firing threshold (Jhangiani-Jashanmal et al., 2016). Effects of neuromodulators differ across thalamic nuclei (Varela, 2014). For example, serotonin hyperpolarizes some higher order nuclei instead of enhancing Ih as it does in first-order relays (Monckton and McCormick, 2002; Varela and Sherman, 2009). Further, In the rat auditory system, acetylcholine hyperpolarizes neurons in higher order thalamus but depolarizes them in first order thalamus (Mooney et al., 2004). Each of these features contribute to TC firing mode and oscillatory capacity. Their differential expression might enable subsets of nuclei to oscillate under other conditions than those observed in first order thalamic relays. In a waking state, these types of specializations may enable a subset of TC nuclei to generate rhythmic bursts characteristic of sleep-related oscillations, as observed in subsets of higher order nuclei during quiet wakefulness (Ramcharan et al., 2005; M Steriade et al., 1993b). They may also bias the intralaminar and midline toward SWD initiation (Hunter and Jasper, 1949; Liu et al., 2015). Unfortunately, available findings are too limited to build a coherent model of state-dependent oscillatory behavior for any of the higher order thalamic nuclei, especially when combined with known differential innervation of thalamus by neuromodulatory systems and other state-related brainstem inputs (Varela, 2014).
In RT, functional subdivisions exhibit distinct burst firing properties. While most neurons in ventral RT fire typical bursts, those in dorsal RT fire impoverished bursts (35%) or single spikes (56%) (Lee et al., 2007). Neurons in the dorsal quadrant of RT project to a mixed first order and higher order collection of visual, anterior limbic, and auditory thalamic nuclei (Pinault and Deschenes, 1998). Because RT burst duration partially determines spindle oscillation period, this difference may enable distinct spindle oscillation frequencies in dorsal and ventral RT-TC networks. It may even bias TC nuclei receiving input from atypically bursting dorsal RT against participating in thalamocortical oscillations. Combined with additional study, these findings may help explain the difference in spindle frequency between anterior and posterior thalamocortical loops observed in rats (Terrier and Gottesmann, 1978), or the finding that selective ablation of caudal RT promotes seizures in rat models of absence epilepsy (Meeren et al., 2009).
While it has yet to be fully tested for most thalamic nuclei, the higher order posterior thalamic nucleus participates in a similar thalamocortical loop to that described for first order nuclei. The posterior nucleus provides strong excitation to the input layers of secondary somatosensory cortex (Lee and Sherman, 2008; Theyel et al., 2010). It also receives layer 6 CT feedback from this cortical area that bifurcates to provide direct excitation and feed-forward layer 6 CT-RT inhibition, and it is reciprocally connected with RT (Lévesque et al., 1996; Pinault and Deschenes, 1998). In addition to this thalamocortical loop, the posterior nucleus and other higher order nuclei receive strong extra-thalamic excitatory and inhibitory inputs that may impact their participation in oscillations. The zona incerta, anterior pretectal nucleus, and substantia nigra pars reticulata project selectively to higher order thalamic nuclei, where they exert strong, non-depressing inhibition that blocks sensory-evoked responses (Trageser and Keller, 2004; Bokor et al., 2005; Lavallée et al., 2005). Cortical input can transiently disinhibit higher order nuclei by inhibiting output within the zona incerta (Barthó et al., 2007; Urbain and Deschênes, 2007). In neurons of the posterior thalamic nucleus, a higher order somatosensory relay, the anterior pretectal nucleus evokes IPSCs that are larger, slower, and less prone to depression than RT-evoked IPSCs (Wanaverbecq et al., 2008), which led to the hypothesis that these extra-RT inhibitory sources dominate higher order TC nuclei. Of these extra-RT inhibitory sources, zona incerta firing is entrained to the anesthesia-induced slow oscillation (Barthó et al., 2007) and phase-locked to SWD (Barthó et al., 2007; Shaw et al., 2013), demonstrating recruitment by ongoing global oscillations. Aside from these studies, state-dependent firing in these inhibitory sources is largely unstudied, making it difficult to predict their impact on sleep-related oscillations in higher order nuclei.
Some higher order thalamic nuclei also receive strong but rapidly depressing excitation from cortical layer 5, which may initially override excitation from cortical layer 6 (Groh et al., 2008; Reichova and Sherman, 2004). Layer 5 CT neurons extend branched axons that innervate multiple targets including higher order TC nuclei but not RT. In primary sensory and motor cortices, CT layer 5 neurons target both the posterior thalamic nucleus and zona incerta (Lévesque et al., 1996), to provide strong excitation followed shortly by disinhibition, opening a window for sensory-evoked responses (Urbain and Deschênes, 2007). The relative timing of layer 5 CT input could either enhance or prevent oscillatory behavior in higher order TC neurons. If layer 5 and 6 CT input occur simultaneously, layer 5 input may override the layer 6 CT-RT pathway and provide simultaneous excitation that promotes a positive feedback loop between CT and TC, as observed after CT-RT block in the Gria4−/− (Paz et al., 2011). Alternatively, layer 5 CT inputs could merely permit oscillations generated by the layer 6-driven thalamocortical loop by opening a window of TC disinhibition coinciding with release with RT inhibition.
Finally, oscillatory models derived from first order relays presume that TC excitation activates a cortical network whose relevant outputs are L6 CT feedback and feed-forward propagation to adjacent TC networks. First order and higher order TC nuclei were first distinguished based on their distinct cortical projection patterns, which reflect activation of different cortical networks. For example, the posterior thalamic nucleus targets both input layers of secondary somatosensory cortex and superficial and deep layers of motor and somatosensory cortices (Ohno et al., 2011). In primary somatosensory cortex, first order and higher order somatosensory nuclei engage distinct intracortical circuits (Bureau et al., 2006), which may be differently suitable to oscillation and generalization. Higher order intralaminar and midline thalamic nuclei can innervate input cortical layers in several patches, and their projections to superficial cortical layer 1 can extend across multiple cortical areas (Deschenes et al., 1996; Van der Werf et al., 2002).
Stimulation of higher order thalamic afferents in layer 1 of frontal cortices evokes strong excitation with weak short-term depression in both layer 2/3 pyramidal cells and layer 1 inhibitory interneurons, preferentially those of the late-spiking class (Cruikshank et al., 2012). Late-spiking interneurons in layer 1 exert distributed inhibition across multiple cortical columns (Jiang et al., 2013). In addition, most higher order nuclei project to cortical layer 5, which extends horizontal axons across multiple cortical columns (Schubert et al., 2006) and can robustly propagate slow oscillations horizontally across cortex in vitro (Wester and Contreras, 2012). The net cortical output in response to higher order TC excitation is therefore difficult to interpret; in either case, higher order TC inputs appear to engage widespread cortical networks. This generalizing effect may be amplified by projections to multiple cortical areas arising from a single TC nucleus or subset of nuclei. Subsets of higher order nuclei, including the intralaminar and midline thalamus, may therefore be uniquely poised to transmit synchronous oscillations broadly across cortex by virtue of their projection to cortical layers 1 and 5.
Concluding remarks
Recent findings are now beginning to place well-studied thalamic oscillators in a broader network context. Studies in unanesthetized, freely moving animals have revealed both local components of global, sleep-related oscillations and neural activity responsible for loss of consciousness and corresponding SWD characteristic of absence epilepsy. Further studies in unanesthetized animals could reveal differential control of TC oscillatory modes as a function of global state, as well as the relative ability of thalamic subregions to initiate and propagate hypersynchronous oscillations. Furthermore, these types of studies could show how strong, potentially controlling, inhibitory and excitatory sources contribute to oscillations in higher order thalamic nuclei.
These methods have been used to directly test how spindle dynamics contribute to sleep architecture (Kim et al., 2012; Ni et al., 2016). These exciting findings suggest that spatially restricted RT activation might endogenously synchronize local oscillations, but the dynamic controllers that drive RT-mediated local spindles and slow waves and determine their spatial propagation remain unknown. Furthermore, a better understanding of these dynamics could enable investigators to relate more nuanced features of spindles and slow waves to memory consolidation, sleep structure, and neuropsychiatric disease.
We have described several protective brakes that prevent thalamic oscillatory circuits from synchronizing by preserving heterogeneous thalamic output. Absence seizures appear to be initiated by a focal subnetwork running “without the brakes,” that when combined with a dynamic or genetic change that further promotes synchrony can, under certain conditions, enable hypersynchronous oscillations. Further study of these protective mechanisms in a more intact network could reveal dynamic controllers that enable occasional, state-dependent absence seizures and determine which thalamic nuclei are most susceptible to hypersynchronization. These types of studies could also show how neural and glial protective mechanisms contribute to thalamic sensory processing and behavior independent of their role in thalamocortical synchrony, an important consideration in the context of treatment and underlying pathology.
This review by Fogerson and Huguenard highlights how local and global regulators of thalamocortical oscillations control sleep and epilepsy. It further describes how dynamic loss of circuit features that normally stabilize thalamic output leads to network hypersynchronization and absence seizures.
Acknowledgments
We would like to thank Jordan Sorokin for careful reading and helpful commentary on the manuscript. This work is supported by CURE and the NINDS grants NS034774 and NS090911.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ahrens S, Jaramillo S, Yu K, Ghosh S, Hwang GR, Paik R, Lai C, He M, Huang ZJ, Li B. ErbB4 regulation of a thalamic reticular nucleus circuit for sensory selection. Nat Neurosci. 2015;18:104–111. doi: 10.1038/nn.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker RG, Tezcan K, Çarçak N, Sakalli E, Akin D, Onat FY. Localized cortical injections of ethosuximide suppress spike-and-wave activity and reduce the resistance to kindling in genetic absence epilepsy rats (GAERS) Epilepsy Res. 2010;89:7–16. doi: 10.1016/j.eplepsyres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Anderer P, Klösch G, Gruber G, Trenker E, Pascual-Marqui RD, Zeitlhofer J, Barbanoj MJ, Rappelsberger P, Saletu B. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001;103:581–592. doi: 10.1016/s0306-4522(01)00028-8. [DOI] [PubMed] [Google Scholar]
- Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, Fried I. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–17834. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Lüthi A. The CaV3. 3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci. 2011;108:13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini G, De Curtis M, Marescaux C, Panzica F, Spreafico R, Vergnes M. Generalized Non-Convulsive Epilepsy: Focus on GABA-B Receptors. Springer; 1992. Role of the thalamic reticular nucleus in the generation of rhythmic thalamo-cortical activities subserving spike and waves; pp. 85–95. [DOI] [PubMed] [Google Scholar]
- Avanzini G, De Curtis M, Panzica F, Spreafico R. Intrinsic properties of nucleus reticularis thalami neurones of the rat studied in vitro. J Physiol. 1989;416:111. doi: 10.1113/jphysiol.1989.sp017752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- Bal T, Mccormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993;468:669. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995a;483:641. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol. 1995b;483:665. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Slézia A, Mátyás F, Faradzs-Zade L, Ulbert I, Harris KD, Acsády L. Ongoing network state controls the length of sleep spindles via inhibitory activity. Neuron. 2014;82:1367–1379. doi: 10.1016/j.neuron.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Slézia A, Varga V, Bokor H, Pinault D, Buzsáki G, Acsády L. Cortical control of zona incerta. J Neurosci. 2007;27:1670–1681. doi: 10.1523/JNEUROSCI.3768-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Huguenard JR. Astrocytes as gatekeepers of GABAB receptor function. J Neurosci. 2010;30:15262–15276. doi: 10.1523/JNEUROSCI.3243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer B, Deleuze C, Letts VA, Mahaffey CL, Boumil RM, Lew TA, Huguenard JR, Frankel WN. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Hum Mol Genet. 2008;17:1738–1749. doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokor H, Frère SGA, Eyre MD, Slézia A, Ulbert I, Lüthi A, Acsády L. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Bonjean M, Baker T, Lemieux M, Timofeev I, Sejnowski T, Bazhenov M. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31:9124–9134. doi: 10.1523/JNEUROSCI.0077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broicher T, Seidenbecher T, Meuth P, Munsch T, Meuth SG, Kanyshkova T, Pape HC, Budde T. T-current related effects of antiepileptic drugs and a Ca 2+ channel antagonist on thalamic relay and local circuit interneurons in a rat model of absence epilepsy. Neuropharmacology. 2007;53:431–446. doi: 10.1016/j.neuropharm.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 2006;4:e382. doi: 10.1371/journal.pbio.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. Oxford University Press; 2006. [Google Scholar]
- Carracedo LM, Kjeldsen H, Cunnington L, Jenkins A, Schofield I, Cunningham MO, Davies CH, Traub RD, Whittington MA. A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms. J Neurosci. 2013;33:10750–10761. doi: 10.1523/JNEUROSCI.0735-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Neocortical synchronized oscillations induced by thalamic disinhibition in vivo. J Neurosci Off J Soc Neurosci. 1999;19:RC27–RC27. doi: 10.1523/JNEUROSCI.19-18-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54:239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- Chipaux M, Vercueil L, Kaminska A, Mahon S, Charpier S. Persistence of cortical sensory processing during absence seizures in human and an animal model: evidence from EEG and intracellular recordings. PLoS One. 2013;8:e58180. doi: 10.1371/journal.pone.0058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Herbert AG, Holt RL, Peng K, Sherwood KD, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Endogenous positive allosteric modulation of GABA A receptors by diazepam binding inhibitor. Neuron. 2013;78:1063–1074. doi: 10.1016/j.neuron.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Huguenard JR. Astrocytes potentiate GABAergic transmission in the thalamic reticular nucleus via endozepine signaling. Proc Natl Acad Sci. 2013;110:20278–20283. doi: 10.1073/pnas.1318031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen AML, Van Luijtelaar E. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science (80-) 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Synchronization of low-frequency rhythms in corticothalamic networks. Neuroscience. 1996;76:11–24. doi: 10.1016/s0306-4522(96)00393-4. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490:159. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lörincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989a;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989b;414:587. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Specific petit mal anticonvulsants reduce calcium currents in thalamic neurons. Neurosci Lett. 1989c;98:74–78. doi: 10.1016/0304-3940(89)90376-5. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Heterogeneous axonal arborizations of rat thalamic reticular neurons in the ventrobasal nucleus. J Comp Neurol. 1996;366:416–430. doi: 10.1002/(SICI)1096-9861(19960311)366:3<416::AID-CNE4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Crandall SR, Cruikshank SJ, Connors BW. A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron. 2015;86:768–782. doi: 10.1016/j.neuron.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall SR, Govindaiah G, Cox CL. Low-threshold Ca2+ current amplifies distal dendritic signaling in thalamic reticular neurons. J Neurosci. 2010;30:15419–15429. doi: 10.1523/JNEUROSCI.3636-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M, Connors BW. Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci. 2012;32:17813–17823. doi: 10.1523/JNEUROSCI.3231-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (< 1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Cueni L, Canepari M, Luján R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Lüthi A. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010;20:R626–R627. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Salimi A, Boucetta S, Wenzel K, O’Byrne J, Brandewinder M, Berthomier C, Gouin J-P. Sleep spindles predict stress-related increases in sleep disturbances. Front Hum Neurosci. 2015:9. doi: 10.3389/fnhum.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, Renger JJ, Lambert RC, Leresche N, Crunelli V. Essential thalamic contribution to slow waves of natural sleep. J Neurosci. 2013;33:19599–19610. doi: 10.1523/JNEUROSCI.3169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi S, Vitellaro-Zuccarello L, Brecha NC. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus. A light and electron-microscopic immunolocalization. Neuroscience. 1998;83:815–828. doi: 10.1016/s0306-4522(97)00414-4. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of I AHP in reticularis neurons. J Neurophysiol. 1998;79:2911–2918. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J Neurosci. 2006;26:8633–8645. doi: 10.1523/JNEUROSCI.2333-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Parent A. Striatal and cortical projections of single neurons from the central lateral thalamic nucleus in the rat. Neuroscience. 1996;72:679–687. doi: 10.1016/0306-4522(96)00001-2. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Bal T, McCormick DA, Sejnowski TJ. Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices. J Neurophysiol. 1996a;76:2049–2070. doi: 10.1152/jn.1996.76.3.2049. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M, Sejnowski TJ, Huguenard JR. In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. J Neurosci. 1996b;16:169–185. doi: 10.1523/JNEUROSCI.16-01-00169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically projecting and reticularis neurones. J Physiol. 1986;379:429. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS. Idiopathic generalized epilepsies with typical absences. J Neurol. 1997;244:403–411. doi: 10.1007/s004150050113. [DOI] [PubMed] [Google Scholar]
- Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating $α$1G-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29:1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Prince DA. Spike-wave rhythms in cat cortex induced by parenteral penicillin. I Electroencephalographic features. Electroencephalogr Clin Neurophysiol. 1977;42:608–624. doi: 10.1016/0013-4694(77)90279-6. [DOI] [PubMed] [Google Scholar]
- Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Ulrich D. Strong, reliable and precise synaptic connections between thalamic relay cells and neurones of the nucleus reticularis in juvenile rats. J Physiol. 2003;546:801–811. doi: 10.1113/jphysiol.2002.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb D, Wang XJ, Rinzel J. Propagation of spindle waves in a thalamic slice model. J Neurophysiol. 1996;75:750–769. doi: 10.1152/jn.1996.75.2.750. [DOI] [PubMed] [Google Scholar]
- Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4-subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorji A, Mittag C, Shahabi P, Seidenbecher T, Pape HC. Seizure-related activity of intralaminar thalamic neurons in a genetic model of absence epilepsy. Neurobiol Dis. 2011;43:266–274. doi: 10.1016/j.nbd.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Groh A, de Kock CPJ, Wimmer VC, Sakmann B, Kuner T. Driver or coincidence detector: modal switch of a corticothalamic giant synapse controlled by spontaneous activity and short-term depression. J Neurosci. 2008;28:9652–9663. doi: 10.1523/JNEUROSCI.1554-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, Wang F, Brown EN, Wilson MA. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158:808–821. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Brown AR, Lambert JJ, Belelli D. Extrasynaptic GABAA receptors couple presynaptic activity to postsynaptic inhibition in the somatosensory thalamus. J Neurosci. 2013;33:14850–14868. doi: 10.1523/JNEUROSCI.1174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Wang X, Sommer FT, Martinez LM. How Inhibitory Circuits in the Thalamus Serve Vision. Annu Rev Neurosci. 2015;38:309–329. doi: 10.1146/annurev-neuro-071013-014229. [DOI] [PubMed] [Google Scholar]
- Hirsch JC, Fourment A, Marc ME. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res. 1983;259:308–312. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45:1568–1579. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CEM, Korpi ER, Mäkelä R, et al. Mice devoid of $γ$-aminobutyrate type A receptor $β$3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Smith AG, Zhang ZW. Lack of intrinsic GABAergic connections in the thalamic reticular nucleus of the mouse. J Neurosci. 2016;36:7246–7252. doi: 10.1523/JNEUROSCI.0607-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci. 1994a;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Clonazepam suppresses GABAB-mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J Neurophysiol. 1994b;71:2576–2581. doi: 10.1152/jn.1994.71.6.2576. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca (2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12:3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Jasper HH. Effects of thalamic stimulation in unanaesthetised animals: the arrest reaction and petit mal-like seizures, activation patterns and generalized convulsions. Electroencephalogr Clin Neurophysiol. 1949;1:305–324. doi: 10.1016/0013-4694(49)90043-7. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science (80- ) 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Inoue M, Duysens J, Vossen JMH, Coenen AML. Thalamic multiple-unit activity underlying spike-wave discharges in anesthetized rats. Brain Res. 1993;612:35–40. doi: 10.1016/0006-8993(93)91641-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen RB, Ulrich D, Huguenard JR. GABAB and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol. 2001;86:1365–1375. doi: 10.1152/jn.2001.86.3.1365. [DOI] [PubMed] [Google Scholar]
- Jhangiani-Jashanmal I, Yamamoto R, Gungor NZ, Pare D. Electroresponsive properties of rat central medial thalamic neurons. J Neurophysiol. 2016 doi: 10.1152/jn.00982.2015. jn–00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2. Cambridge Unviersity Press; Cambridge, England: 2007. [Google Scholar]
- Kellaway P, Gol A, Proler M. Electrical activity of the isolated cerebral hemisphere and isolated thalamus. Exp Neurol. 1966;14:281–304. doi: 10.1016/0014-4886(66)90115-4. [DOI] [PubMed] [Google Scholar]
- Kim A, Latchoumane C, Lee S, Kim GB, Cheong E, Augustine GJ, Shin HS. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc Natl Acad Sci. 2012;109:20673–20678. doi: 10.1073/pnas.1217897109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Hwang E, Lee M, Sung H, Choi JH. Characterization of topographically specific sleep spindles in mice. Sleep. 2015;38:85–96. doi: 10.5665/sleep.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking $α$ 1G T-type Ca 2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- Kim U, Bal T, McCormick DA. Spindle waves are propagating synchronized oscillations in the ferret LGNd in vitro. J Neurophysiol. 1995;74:1301–1323. doi: 10.1152/jn.1995.74.3.1301. [DOI] [PubMed] [Google Scholar]
- Kim U, Mccormick DA. Functional and ionic properties of a slow afterhyperpolarization in ferret perigeniculate neurons in vitro. J Neurophysiol. 1998;80:1222–1235. doi: 10.1152/jn.1998.80.3.1222. [DOI] [PubMed] [Google Scholar]
- Kleiman-Weiner M, Beenhakker MP, Segal WA, Huguenard JR. Synergistic roles of GABAA receptors and SK channels in regulating thalamocortical oscillations. J Neurophysiol. 2009;102:203–213. doi: 10.1152/jn.91158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned α1 subunits, α1G, α1H and α1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci. 1999;11:4171–4178. doi: 10.1046/j.1460-9568.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM. Small-conductance, calcium-activated potassium channels from mammalian brain. Science (80- ) 1996;273:1709. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R. Distinct localization of GABAB receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- Lam YW, Nelson CS, Sherman SM. Mapping of the functional interconnections between thalamic reticular neurons using photostimulation. J Neurophysiol. 2006;96:2593–2600. doi: 10.1152/jn.00555.2006. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. VPM and PoM nuclei of the rat somatosensory thalamus: intrinsic neuronal properties and corticothalamic feedback. Cereb Cortex. 2007;17:2853–2865. doi: 10.1093/cercor/bhm025. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallée P, Urbain N, Dufresne C, Bokor H, Acsády L, Deschênes M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci. 2005;25:7489–7498. doi: 10.1523/JNEUROSCI.2301-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first-and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008;100:317–326. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Govindaiah G, Cox CL. Heterogeneity of firing properties among rat thalamic reticular nucleus neurons. J Physiol. 2007;582:195–208. doi: 10.1113/jphysiol.2007.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M, Chen JY, Lonjers P, Bazhenov M, Timofeev I. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci. 2014;34:5689–5703. doi: 10.1523/JNEUROSCI.1156-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leresche N, Parri HR, Erdemli G, Guyon A, Turner JP, Williams SR, Asprodini E, Crunelli V. On the action of the anti-absence drug ethosuximide in the rat and cat thalamus. J Neurosci. 1998;18:4842–4853. doi: 10.1523/JNEUROSCI.18-13-04842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Gagnon S, Parent A, Deschênes M. Axonal arborizations of corticostriatal and corticothalamic fibers arising from the second somatosensory area in the rat. Cereb cortex. 1996;6:759–770. doi: 10.1093/cercor/6.6.759. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Voigts J, Flores FJ, Schmitt LI, Wilson MA, Halassa MM, Brown EN. Thalamic reticular nucleus induces fast and local modulation of arousal state. Elife. 2015;4:e08760. doi: 10.7554/eLife.08760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lee HJ, Weitz AJ, Fang Z, Lin P, Choy M, Fisher R, Pinskiy V, Tolpygo A, Mitra P, et al. Frequency-selective control of cortical and subcortical networks by central thalamus. Elife. 2015;4:e09215. doi: 10.7554/eLife.09215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Vergnes M, Depaulis A, Marescaux C. Involvement of intrathalamic GABA B neurotransmission in the control of absence seizures in the rat. Neuroscience. 1992;48:87–93. doi: 10.1016/0306-4522(92)90340-8. [DOI] [PubMed] [Google Scholar]
- Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. 1982 doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Wehrle F, Tüshaus L, Achermann P, Huber R. The Multidimensional Aspects of Sleep Spindles and Their Relationship to Word-Pair Memory Consolidation. Sleep. 2014;38:1093–1103. doi: 10.5665/sleep.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. Modulation of a pacemaker current through Ca2+-induced stimulation of cAMP production. Nat Neurosci. 1999;2:634–641. doi: 10.1038/10189. [DOI] [PubMed] [Google Scholar]
- Lüttjohann A, van Luijtelaar G. The dynamics of cortico-thalamo-cortical interactions at the transition from pre-ictal to ictal LFPs in absence epilepsy. Neurobiol Dis. 2012;47:49–60. doi: 10.1016/j.nbd.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Noebels JL. Monogenic models of absence epilepsy: windows into the complex balance between inhibition and excitation in thalamocortical microcircuits. Prog Brain Res. 2014;213:223–252. doi: 10.1016/B978-0-444-63326-2.00012-0. [DOI] [PubMed] [Google Scholar]
- Manning JPA, Richards DA, Leresche N, Crunelli V, Bowery NG. Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience. 2004;123:5–9. doi: 10.1016/j.neuroscience.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Marcus EM, Watson CW. Reproduction in isolated cortex of cat brain of bilateral synchronous and symmetrical spike and slow wave pattern resembling petit mal. Trans Am Neurol Assoc. 1964;89:221. [PubMed] [Google Scholar]
- Masur D, Shinnar S, Cnaan A, Shinnar RC, Clark P, Wang J, Weiss EF, Hirtz DG, Glauser TA, Group CAES, et al. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology. 2013;81:1572–1580. doi: 10.1212/WNL.0b013e3182a9f3ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990a;431:291. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990b;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987;392:147. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease RA, Krieger P, Groh A. Cortical control of adaptation and sensory relay mode in the thalamus. Proc Natl Acad Sci. 2014;111:6798–6803. doi: 10.1073/pnas.1318665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren HKM, Pijn JPM, Van Luijtelaar ELJM, Coenen AML, da Silva FHL. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren HKM, Veening JG, Möderscheim TAE, Coenen AML, van Luijtelaar G. Thalamic lesions in a genetic rat model of absence epilepsy: dissociation between spike-wave discharges and sleep spindles. Exp Neurol. 2009;217:25–37. doi: 10.1016/j.expneurol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Mölle M, Bergmann TO, Marshall l, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engage-ment in memory processing. Sleep. 2011;34:1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monckton JE, McCormick DA. Neuromodulatory role of serotonin in the ferret thalamus. J Neurophysiol. 2002;87:2124–2136. doi: 10.1152/jn.00650.2001. [DOI] [PubMed] [Google Scholar]
- Mooney DM, Zhang L, Basile C, Senatorov VV, Ngsee J, Omar A, Hu B. Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc Natl Acad Sci. 2004;101:320–324. doi: 10.1073/pnas.0304445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison RS, Bassett DL. Electrical activity of the thalamus and basal ganglia in decorticate cats. J Neurophysiol. 1945;8:309–314. [Google Scholar]
- Mulle C, Steriade M, Deschênes M. Absence of spindle oscillations in the cat anterior thalamic nuclei. Brain Res. 1985;334:169–171. doi: 10.1016/0006-8993(85)90581-5. [DOI] [PubMed] [Google Scholar]
- Ni KM, Hou XJ, Yang CH, Dong P, Li Y, Zhang Y, Jiang P, Berg DK, Duan S, Li XM. Selectively driving cholinergic fibers optically in the thalamic reticular nucleus promotes sleep. Elife. 2016;5:e10382. doi: 10.7554/eLife.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels J, Avoli M, Rogawski M, Olsen R, Delgado-Escueta A. Jasper’s Basic Mechanisms of the Epilepsies. Oxford University Press; 2012. [Google Scholar]
- Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Nunez A, Amzica F, Steriade M, et al. Intrinsic and synaptically generated delta (1–4 Hz) rhythms in dorsal lateral geniculate neurons and their modulation by light-induced fast (30–70 Hz) events. Neuroscience. 1992;51:269–284. doi: 10.1016/0306-4522(92)90314-r. [DOI] [PubMed] [Google Scholar]
- Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, Fujiyama F, Sonomura T, Uemura M, Sugiyama K, Kaneko T. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb cortex. 2011 doi: 10.1093/cercor/bhr356. bhr356. [DOI] [PubMed] [Google Scholar]
- Parker PRL, Cruikshank SJ, Connors BW. Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology. J Neurosci. 2009;29:9761–9770. doi: 10.1523/JNEUROSCI.4568-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Bryant AS, Peng K, Fenno L, Yizhar O, Frankel WN, Deisseroth K, Huguenard JR. A new mode of corticothalamic transmission revealed in the Gria4−/−model of absence epilepsy. Nat Neurosci. 2011;14:1167–1173. doi: 10.1038/nn.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Chavez M, Saillet S, Deniau JM, Charpier S. Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J Neurosci. 2007;27:929–941. doi: 10.1523/JNEUROSCI.4677-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Pinault D. Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5–9 Hz oscillations. J Physiol. 2003;552:881–905. doi: 10.1113/jphysiol.2003.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, Deschenes M. Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J Comp Neurol. 1998;391:180–203. doi: 10.1002/(sici)1096-9861(19980209)391:2<180::aid-cne3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Pinault D, Vergnes M, Marescaux C. Medium-voltage 5–9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons. Neuroscience. 2001;105:181–201. doi: 10.1016/s0306-4522(01)00182-8. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA A receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pita-Almenar JD, Yu D, Lu HC, Beierlein M. Mechanisms underlying desynchronization of cholinergic-evoked thalamic network activity. J Neurosci. 2014;34:14463–14474. doi: 10.1523/JNEUROSCI.2321-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Charpier S. Intracellular activity of cortical and thalamic neurones during high-voltage rhythmic spike discharge in Long-Evans rats in vivo. J Physiol. 2006;571:461–476. doi: 10.1113/jphysiol.2005.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcello DM, Smith SD, Huguenard JR. Actions of U-92032, a T-Type Ca2+ Channel Antagonist, Support a Functional Linkage Between I T and Slow Intrathalamic Rhythms. J Neurophysiol. 2003;89:177–185. doi: 10.1152/jn.00667.2002. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci U S A. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Richards DA, Manning JPA, Barnes D, Rombola L, Bowery NG, Caccia S, Leresche N, Crunelli V. Targeting thalamic nuclei is not sufficient for the full anti-absence action of ethosuximide in a rat model of absence epilepsy. Epilepsy Res. 2003;54:97–107. doi: 10.1016/s0920-1211(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Rovó Z, Mátyás F, Barthó P, Slézia A, Lecci S, Pellegrini C, Astori S, Dávid C, Hangya B, Lüthi A, et al. Phasic, nonsynaptic GABA-a receptor-mediated inhibition entrains thalamocortical oscillations. J Neurosci. 2014;34:7137–7147. doi: 10.1523/JNEUROSCI.4386-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Functional properties of perigeniculate inhibition of dorsal lateral geniculate nucleus thalamocortical neurons in vitro. J Neurosci. 1997;17:8880–8893. doi: 10.1523/JNEUROSCI.17-22-08880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. The organization of the nucleus reticularis thalami: a Golgi study. Brain Res. 1966;1:43–62. doi: 10.1016/0006-8993(66)90104-1. [DOI] [PubMed] [Google Scholar]
- Schofield CM, Kleiman-Weiner M, Rudolph U, Huguenard JR. A gain in GABAA receptor synaptic strength in thalamus reduces oscillatory activity and absence seizures. Proc Natl Acad Sci. 2009;106:7630–7635. doi: 10.1073/pnas.0811326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kötter R, Luhmann HJ, Staiger JF. Morphology, electrophysiology and functional input connectivity of pyramidal neurons characterizes a genuine layer Va in the primary somatosensory cortex. Cereb cortex. 2006;16:223–236. doi: 10.1093/cercor/bhi100. [DOI] [PubMed] [Google Scholar]
- Shaw FZ, Liao YF, Chen RF, Huang YH, Lin RCS. The zona incerta modulates spontaneous spike-wave discharges in the rat. J Neurophysiol. 2013;109:2505–2516. doi: 10.1152/jn.00750.2011. [DOI] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheroziya M, Timofeev I. Global intracellular slow-wave dynamics of the thalamocortical system. J Neurosci. 2014;34:8875–8893. doi: 10.1523/JNEUROSCI.4460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, McCormick DA. Inhibitory interactions between ferret thalamic reticular neurons. J Neurophysiol. 2002;87:2571–2576. doi: 10.1152/jn.00850.2001. [DOI] [PubMed] [Google Scholar]
- Singh B, Monteil A, Bidaud I, Sugimoto Y, Suzuki T, Hamano S, Oguni H, Osawa M, Alonso ME, Delgado-Escueta AV, et al. Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Hum Mutat. 2007;28:524. doi: 10.1002/humu.9491. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Huguenard JR. Inhibitory interconnections control burst pattern and emergent network synchrony in reticular thalamus. J Neurosci. 2003;23:8978–8988. doi: 10.1523/JNEUROSCI.23-26-08978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Intrinsic and synaptic dynamics interact to generate emergent patterns of rhythmic bursting in thalamocortical neurons. J Neurosci. 2006;26:4247–4255. doi: 10.1523/JNEUROSCI.3812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991;441:175. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschenes M. Intrathalamic and brainstem-thalamic networks involved in resting and alert states. In: Bentivoglio M, Spreafico R, editors. Cellular Thalamic Mechanisms. Elsevier; Amsterdam: 1988. pp. 51–76. [Google Scholar]
- Steriade M, Contreras D. Spike-wave complexes and fast components of cortically generated seizures. I Role of neocortex and thalamus. J Neurophysiol. 1998;80:1439–1455. doi: 10.1152/jn.1998.80.3.1439. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci. 1995;15:623–642. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Dossi RC, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993a;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Curro R, Contreras D, et al. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (~40 Hz) spike-bursts at ~1000 Hz during waking and rapid eye movement sleep. Neuroscience. 1993b;56:1–9. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res Rev. 1984;8:1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science (80- ) 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sun YG, Wu CS, Lu HC, Beierlein M. Target-dependent control of synaptic inhibition by endocannabinoids in the thalamus. J Neurosci. 2011;31:9222–9230. doi: 10.1523/JNEUROSCI.0531-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Bailey JN, Bai D, Ishikawa-Brush Y, Delgado-Escueta AV, Olsen RW. Effects on promoter activity of common SNPs in 5′ region of GABRB3 exon 1A. Epilepsia. 2012;53:1450–1456. doi: 10.1111/j.1528-1167.2012.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney JR, Fujiwara H, Horn PS, Jacobson SE, Glauser TA, Rose DF. Focal corticothalamic sources during generalized absence seizures: a MEG study. Epilepsy Res. 2013;106:113–122. doi: 10.1016/j.eplepsyres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Terrier G, Gottesmann CL. Study of cortical spindles during sleep in the rat. Brain Res Bull. 1978;3:701–706. doi: 10.1016/0361-9230(78)90021-7. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Bazhenov M, Sejnowski TJ, Steriade M. Contribution of intrinsic and synaptic factors in the desynchronization of thalamic oscillatory activity. Thalamus Relat Syst. 2001;1:53–69. [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol. 1996;76:4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci. 2004;24:8911–8915. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tringham E, Powell KL, Cain SM, Kuplast K, Mezeyova J, Weerapura M, Eduljee C, Jiang X, Smith P, Morrison JL, et al. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci Transl Med. 2012;4:121ra19–121ra19. doi: 10.1126/scitranslmed.3003120. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Wright N, Vann SD, Reilly R, Erichsen JT, Aggleton JP, O’Mara SM. Oscillatory entrainment of thalamic neurons by theta rhythm in freely moving rats. J Neurophysiol. 2011;105:4–17. doi: 10.1152/jn.00771.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urak L, Feucht M, Fathi N, Hornik K, Fuchs K. A GABRB3 promoter haplotype associated with childhood absence epilepsy impairs transcriptional activity. Hum Mol Genet. 2006;15:2533–2541. doi: 10.1093/hmg/ddl174. [DOI] [PubMed] [Google Scholar]
- Urbain N, Deschênes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Varela C. Thalamic neuromodulation and its implications for executive networks. Front Neural Circuits. 2014;8 doi: 10.3389/fncir.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to serotonergic activation between first and higher order thalamic nuclei. Cereb Cortex. 2009;19:1776–1786. doi: 10.1093/cercor/bhn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes M, Boehrer A, Simler S, Bernasconi R, Marescaux C. Opposite effects of GABA B receptor antagonists on absences and convulsive seizures. Eur J Pharmacol. 1997;332:245–255. doi: 10.1016/s0014-2999(97)01085-6. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science (80- ) 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanaverbecq N, Bodor ÁL, Bokor H, Slézia A, Lüthi A, Acsády L. Contrasting the functional properties of GABAergic axon terminals with single and multiple synapses in the thalamus. J Neurosci. 2008;28:11848–11861. doi: 10.1523/JNEUROSCI.3183-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RA, Agmon A, Jones EG. Oscillatory synaptic interactions between ventroposterior and reticular neurons in mouse thalamus in vitro. J Neurophysiol. 1994;72:1993–2003. doi: 10.1152/jn.1994.72.4.1993. [DOI] [PubMed] [Google Scholar]
- Wei H, Bonjean M, Petry HM, Sejnowski TJ, Bickford ME. Thalamic burst firing propensity: a comparison of the dorsal lateral geniculate and pulvinar nuclei in the tree shrew. J Neurosci. 2011;31:17287–17299. doi: 10.1523/JNEUROSCI.6431-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester JC, Contreras D. Columnar interactions determine horizontal propagation of recurrent network activity in neocortex. J Neurosci. 2012;32:5454–5471. doi: 10.1523/JNEUROSCI.5006-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmijse I, Ossenblok P, Gunning B, Van Luijtelaar G. Onset and propagation of spike and slow wave discharges in human absence epilepsy: a MEG study. Epilepsia. 2009;50:2538–2548. doi: 10.1111/j.1528-1167.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature. 2015;526:705–709. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CT, Conley M, Hendry SH, Jones EG. The morphology of physiologically identified GABAergic neurons in the somatic sensory part of the thalamic reticular nucleus in the cat. J Neurosci. 1985;5:2254–2268. doi: 10.1523/JNEUROSCI.05-08-02254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Huguenard JR, Prince DA. GABAA receptor-mediated Cl− currents in rat thalamic reticular and relay neurons. J Neurophysiol. 1997;78:2280–2286. doi: 10.1152/jn.1997.78.5.2280. [DOI] [PubMed] [Google Scholar]