Abstract
Addiction is mediated in large part by pathological motivation for rewarding, addictive substances, and alcohol-use disorders (AUDs) continue to extract a very high physical and economic toll on society. Compulsive alcohol drinking, where intake continues despite negative consequences, is considered a particular obstacle during treatment of AUDs. Aversion-resistant drives for alcohol have been modeled in rodents, where animals continue to consume even when alcohol is adulterated with the bitter tastant quinine, or is paired with another aversive consequence. Here, we describe a two-bottle choice paradigm where C57BL/6 mice first had 24-h access to 15% alcohol or water. Afterward, they drank quinine-free alcohol (alcohol-only) or alcohol with quinine (100 μM), in a limited daily access (LDA) two-bottle-choice paradigm (2 h/day, 5 days/week, starting 3 h into the dark cycle), and achieved nearly binge-level blood alcohol concentrations. Interestingly, a single, initial 24-h experience with alcohol-only enhanced subsequent quinine-resistant drinking. In contrast, mice that drank alcohol–quinine in the 24-h session showed significantly reduced alcohol–quinine intake and preference during the subsequent LDA sessions, relative to mice that drank alcohol-only in the initial 24-h session and alcohol–quinine in LDA sessions. Thus, mice could find the concentration of quinine we used aversive, but were able to disregard the quinine after a single alcohol-only drinking session. Finally, mice had low intake and preference for quinine in water, both before and after weeks of alcohol-drinking sessions, suggesting that quinine resistance was not a consequence of increased quinine preference after weeks of drinking of alcohol–quinine. Together, we demonstrate that a single alcohol-only session was sufficient to enable subsequent aversion-resistant consumption in C57BL/6 mice, which did not reflect changes in quinine taste palatability. Given the rapid development of quinine-resistant alcohol drinking patterns, this model provides a simple, quick, and robust method for uncovering the mechanisms that promote aversion-resistant consumption.
Introduction
Addiction to alcohol or other abused substances is characterized by increased and often pathological motivation to seek and consume the substance (Koob and Volkow, 2010, Larimer et al., 1999 and Sinha, 2009). Despite decades of research, alcohol-use disorders (AUDs) remain a major problem globally, and continue to extract a very high personal, social, and economic toll (Blincoe et al., 2002, Bouchery et al., 2011, CDC, 2014, Harwood et al., 1998, Hingson et al., 2005, Mokdad et al., 2004, SAMHSA., 2015 and Sacks et al., 2013), in part due to the lack of effective clinical treatments (Spanagel, 2009 and World Health Organization, 2014). Developing novel, effective treatments to counteract alcohol abuse in humans would be greatly aided by understanding the neural and behavioral mechanisms that underlie and mediate excessive drinking.
In this regard, it is clear that compulsive drives for alcohol, where drinking persists despite knowledge of associated negative consequences, are considered a major obstacle when treating AUDs (Anton, 2000, Anton et al., 1996, Hopf and Lesscher, 2014, Koob and Volkow, 2010, Larimer et al., 1999, Modell et al., 1992, Naqvi et al., 2014, Sinha, 2009 and Tiffany and Conklin, 2000). Thus, there is considerable interest in understanding the circuitry and molecular mechanisms that promote this consequence-resistant, compulsion-like alcohol intake. Voluntary alcohol intake models, where animals drink despite pairing alcohol with an aversive consequence (e.g., foot shock or bitter-tasting quinine), offer opportunities to study preclinical aversion-resistant drinking in a manner that may reflect some compulsive aspects of human AUDs (Hopf and Lesscher, 2014, Hopf et al., 2010, Lesscher et al., 2010, Loi et al., 2010, Marchant et al., 2013, Spanagel, 2009 and Vengeliene et al., 2009). Unfortunately, the brain mechanisms that directly regulate aversion-resistant drinking have been understudied until very recently (Barbier et al., 2015, Lesscher et al., 2012, Seif et al., 2013, Seif et al., 2015, Vendruscolo et al., 2012 and Warnault et al., 2016). A major barrier to studying aversion-resistant drinking is the lack of simple, robust models.
Here, our studies describe a drinking paradigm in C57BL/6 mice, adapted from Lesscher et al. (2010), where mice consistently engaged in alcohol drinking despite pairing alcohol with the aversive consequence of bitter-tasting quinine. In particular, our results strongly suggest that a single alcohol-only drinking experience was sufficient to promote future quinine-resistant alcohol consumption and preference in mice, indicating a rapid development of quinine-resistant intake. This model will facilitate future studies to better determine how various factors are responsible for driving compulsion-like, aversion-resistant alcohol drinking.
Methods
Animals
Male C57BL/6 mice, 7–8 weeks of age, were purchased from Jackson Laboratories. Subjects were single-housed on a 12:12-h light:dark cycle with lights off at 10:00 AM. Food and water were available ad libitum. All procedures followed the Guide for Care and Use of Laboratory Animals provided by the National Institutes of Health, and approval of the institutional animal care and use committee of UCSF.
Materials
15% alcohol was prepared from a 95% stock. Quinine hydrochloride dihydrate (Sigma–Aldrich, Q1125) was used to prepare the 100-μM quinine (40-mg/L) adulterated alcohol.
Limited daily access (LDA) two-bottle choice drinking
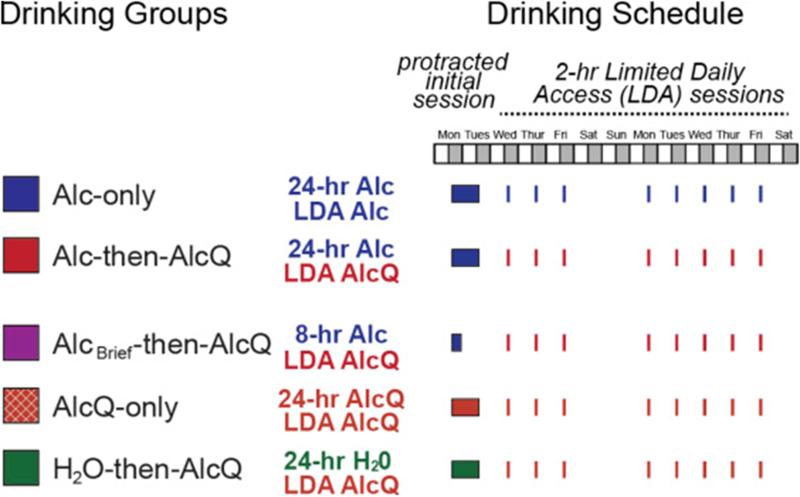
We adapted the methods of Lesscher et al. (2010), which showed quinine-resistant alcohol drinking after 2 weeks of limited access intake (Fig. 1 and Table 1). Mice were first acclimated to housing conditions for ~2 weeks. In the major experimental groups (Alc-only and Alc-then-AlcQ, see Fig. 1 and Table 1), mice first had one 24-h session of alcohol-only drinking under two-bottle choice (one bottle with 15% alcohol in water, one bottle with water), and then a 24-h withdrawal period. A 24-h period was chosen since it is utilized in other widely used models of intermittent-access alcohol drinking (e.g., Hwa et al., 2016 and Warnault et al., 2016). Thereafter, mice drank under a Limited Daily Access (LDA) paradigm, with 2 h/day, 5 days/week of two-bottle choice starting ~3 h into the dark cycle each day. To test for quinine-resistant drinking, we compared mice that drank alcohol without quinine (Alc-only, blue columns) during LDA sessions, with mice drinking alcohol–quinine (100 μM, 40 mg/L) (Alc-then-AlcQ, red column) during LDA sessions. In order to control for side preference, the bottle placements of the solutions were alternated between each drinking session.
Fig. 1.
Diagram showing the different experimental groups and their drinking schedules. Mice had one initial protracted session of voluntary, two-bottle-choice alcohol intake (24 h for most groups), followed by limited daily access (LDA) drinking for 2 h/day, 5 days/week thereafter. See Table 1 and Methods section for full details.
Table 1.
Experimental groups for Fig. 1, Fig. 2 and Fig. 3. During the initial 24-h access or brief 8-h alcohol access sessions and subsequent LDA sessions, mice drank under two-bottle choice access: one bottle had 15% alcohol (with or without quinine) in water, while the other had water only. See Methods section for further details.
| Experimental group | n for Fig. 1, Fig. 2 and Fig. 3 | Column color in Fig. 1, Fig. 2 and Fig. 3 | Substance consumed in the protracted initial session | Substance consumed in LDA sessions |
|---|---|---|---|---|
| Alc-only | 40 | Blue | 24-h alcohol-only | Alcohol-only |
| Alc-then-AlcQ | 29 | Red | 24-h alcohol-only | Alcohol + quinine |
| AlcBrief-then-AlcQ | 15 | Purple | 8-h alcohol-only | Alcohol + quinine |
| AlcQ-only | 12 | Red-brown (with white hatching) | 24-h alcohol + quinine | Alcohol + quinine |
| H2O-then-AlcQ | 12 | Green | 24-h water-only | Alcohol + quinine |
To better understand the impact of drinking quinine-free alcohol on subsequent Alc-then-AlcQ drinking, additional groups of mice were examined. Some mice (AlcQ-only, orange hatched columns) received 15% alcohol with 100-μM quinine under two-bottle choice during both the initial 24-h session and the subsequent LDA sessions. Other mice (H2O-then-AlcQ; green columns) received water-only during the 24-h drinking session, and then drank alcohol + quinine during the LDA sessions (Fig. 1 and Table 1). To rule out the possibility that higher levels of alcohol intake during the initial drinking session were related to development of quinine resistance, additional mice only had initial access to alcohol-only for 8 h; these mice then drank alcohol + quinine during LDA sessions (AlcBrief-then-AlcQ; purple columns) (Fig. 1 and Table 1).
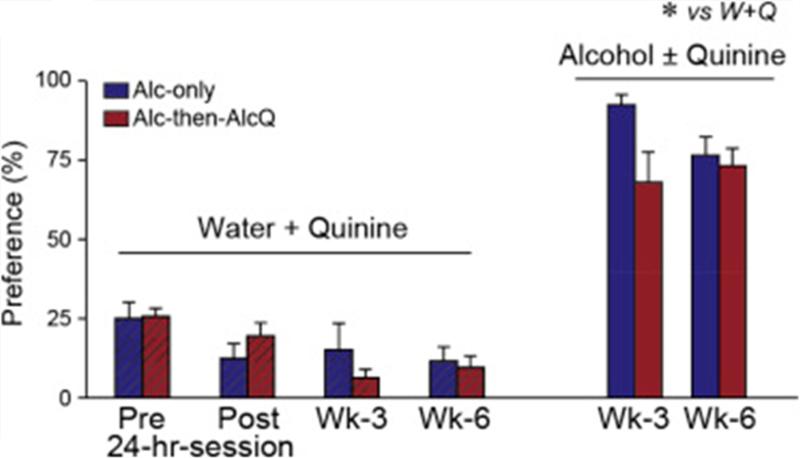
In addition, it is possible that the quinine-resistant drinking actually reflected the development of a preference for quinine across weeks of drinking. Thus, to test whether there were changes in aversion or preference for quinine over time, another set of mice were given two-bottle choice for water with or without 100-μM quinine before and after the 24-h drinking session, and also during the third and sixth weeks of LDA drinking. Water–quinine (W + Q) intake within a 2-h period was tested on two successive days and averaged. For W + Q testing at LDA time points, W + Q access was examined at the same time and in lieu of access to drinking alcohol. For W + Q testing before and after the 24-h drinking session, the pre-24-h W + Q occurred starting the same time as alcohol drinking would, and was averaged across two days. W + Q drinking after the 24-h session was determined for only a single day, the alcohol withdrawal day.
Blood alcohol concentrations
BACs were measured in mice after 2 weeks of Alc-only LDA drinking, using previously described methods (Weiss et al., 1993 and Zapata et al., 2006). Briefly, immediately following the conclusion of the 2-h drinking session, trunk blood (after rapid cervical dislocation and decapitation) was collected in heparinized capillary tubes. Serum was extracted using 3.4% perchloric acid and centrifuged for 5 min at 2000 rpm. The supernatant was subjected to the nicotinamide dinucleotide redox (NAD+/NADH) assay, brought about by alcohol in the presence of alcohol dehydrogenase. For 11 mice drinking an average alcohol level of 3.3 ± 0.4 g/kg/2 h, the average BAC was 31.6 ± 11.3 mg%. Another set of Alc-only and Alc-then-AlcQ (n = 9/group) were used to measure BACs after 30 min of drinking. The average alcohol intake was 1.6 ± 0.2 g/kg/30 min (Alc-only) and 1.5 ± 0.3 g/kg/30 min (Alc-then-AlcQ), with no difference in alcohol intake at 30 min for the two groups (t[1,16] = 0.22, p = 0.83). In addition, BAC levels after these 30-min sessions were 85.1 ± 15.8 mg% (Alc-only) and 69.6 ± 13.8 mg% (Alc-then-AlcQ), and there were no group differences in BAC levels (t[1,16] = 0.74, p = 0.47). These data suggest that mice drank a majority of alcohol during the initial part of the 2-h session, and that measurement of BACs at the end of the 2-h LDA session underestimated the peak BACs that these animals achieved.
Data analysis
After each drinking session, we measured the weight of liquid consumed to determine the intake level of alcohol (g/kg-body weight) or quinine-adulterated water (mL/kg-body weight), as well as the preference ratio (volume of intake/total volume of fluid intake). All data were statistically analyzed by SPSS Statistics (IBM Corporation, ibm.com). Alcohol intake levels and preference during the 24-h drinking session were calculated and compared across groups using a one-way ANOVA, followed by a Tukey's post hoc test. For LDA drinking, we first calculated the average intake and preference across the second week of intake for each animal. Results were then compared across groups with a one-way ANOVA, followed by Tukey's post hoc test. For the water–quinine experiment, intake and preference for each solution were compared across time using a two-way ANOVA with repeated measures (group × time) followed by Tukey's post hoc test or t test. Kruskal–Wallis test for normality was performed using GraphPad Prism (v5.0).
Results
Prior exposure to unadulterated alcohol enhances aversion-resistant drinking
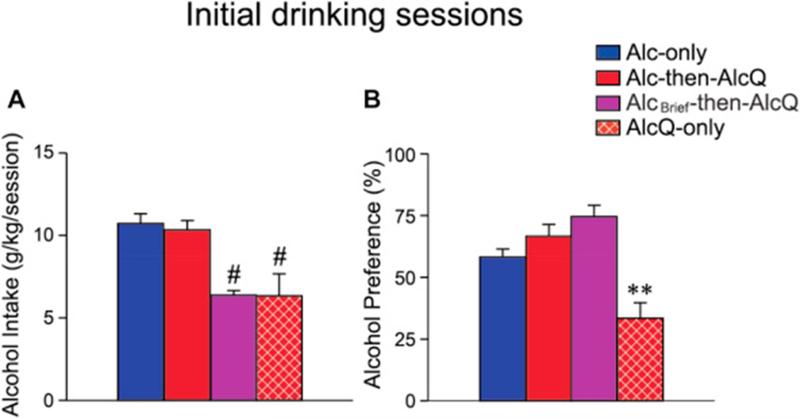
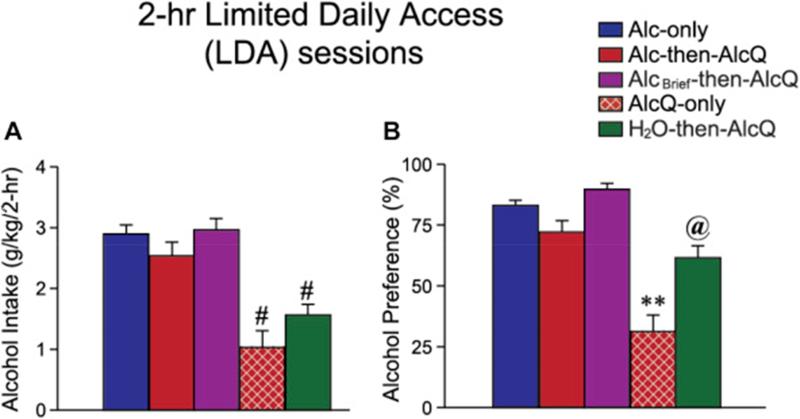
As shown in Fig. 1 and Table 1, mice first drank different substances during an initial protracted intake period (24 h of consumption in the majority of experimental groups). Subsequently, mice drank alcohol ± quinine intake in limited daily access (LDA) sessions for 2 h/day, 5 days/week (see Methods section for details). For analysis of LDA consumption, intake for each mouse was averaged across all drinking days during the second week of LDA. Week 1 LDA intake is shown in Supplemental Fig. 1; intake in the first week was somewhat more variable, since this was the first exposure for many animals to quinine in alcohol. One-way ANOVAs showed significant differences in alcohol ± quinine intake across experimental groups during both the initial, protracted intake sessions (Fig. 2; drinking: F[3,92] = 9.972, p < 0.001; preference: F[3,92] = 9.132, p < 0.001; analysis did not include water intake in H2O-then-AlcQ mice) and during Week 2 of LDA (Fig. 3; drinking: F[4,103] = 12.020, p < 0.001; preference: F[4,103] = 22.352, p < 0.001).
Fig. 2.
Mice given access to alcohol–quinine in the initial 24-h session showed significantly reduced intake and preference during that session. Alcohol intake (A) and preference (B) in different experimental groups during the initial 24-h session. Blue columns: Alc-only mice, n = 40; red columns: Alc-then-AlcQ mice, n = 29; red-brown with white-hatched columns: AlcQ-only mice, n = 12; purple columns: AlcBrief-then-AlcQ mice, n = 15; green columns: H2O-then-AlcQ mice, n = 12. Data are shown as mean ± SEM. **p < 0.01 versus all other groups; #p < 0.01 between indicated group and both Alc-only and Alc-then-AlcQ mice. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Mice given access to alcohol-only during the initial, protracted intake session showed aversion-resistance during subsequent LDA intake. Alcohol intake (A) and preference (B) in different experimental groups during Week 2 of LDA intake. Sample size for each experimental group is given in the legend of Fig. 2. Data are shown as mean ± SEM. #p < 0.05 between indicated group and Alc-only, Alc-then-AlcQ and AlcBrief-then-AlcQ mice; **p < 0.01 versus all other groups; @p < 0.05 between indicated group and Alc-only, AlcQ-only, and AlcBrief-then-AlcQ mice.
We were particularly interested in determining whether drinking of different substances during the initial protracted intake period would influence alcohol ± quinine intake in subsequent LDA sessions. In particular, we were interested in the possibility that some mice would exhibit aversion-resistant alcohol consumption, where drinking persisted despite pairing with aversive consequences (in this case, quinine adulteration). Thus, we first compared intake in mice that drank alcohol-only in the initial, protracted-access session, and then consumed either alcohol-only (Alc-only mice, n = 40, blue columns) or alcohol-quinine (Alc-then-AlcQ mice, n = 29, red columns) during the LDA sessions. Importantly, LDA intake and preference were very similar in Alc-only and Alc-then-AlcQ mice (Fig. 3, p > 0.1). Since these mice showed similar LDA alcohol drinking with and without quinine adulteration, these results suggest that Alc-then-AlcQ mice showed aversion-resistant alcohol consumption, where alcohol intake persisted despite pairing with the adverse consequence of bitter-tasting quinine.
It is possible that mice did not find the concentration of quinine that we used aversive. Thus, we examined drinking levels in mice that consumed alcohol–quinine during both the initial 24-h session and subsequent LDA sessions (AlcQ-only; n = 12; red-brown with white-hatched columns; Fig. 1 and Table 1). Interestingly, drinking levels and preference in AlcQ-only mice during the initial 24-h session were significantly reduced relative to Alc-only and Alc-then-AlcQ mice (Fig. 2A and B, all p < 0.01). In addition, during the second week of LDA intake, drinking level and preference were significantly reduced in AlcQ-only mice relative to Alc-only and Alc-then-AlcQ mice (Fig. 3A and B, all p < 0.05). These results are important for two reasons. First, they indicate that mice can find quinine in alcohol aversive, indicated by greatly reduced intake and preference. Second, these results suggest that a single session of alcohol-only drinking was sufficient to enable subsequent aversion-resistant intake. In particular, both Alc-then-AlcQ and AlcQ-only mice drank alcohol–quinine during LDA sessions, with the only difference being the solution consumed during the initial protracted session (alcohol-only in Alc-then-AlcQ mice, and alcohol–quinine in AlcQ-only mice). Since LDA alcohol–quinine drinking levels were much greater in Alc-then-AlcQ mice relative to AlcQ-only mice (Fig. 3A), these results strongly suggest that it was the initial alcohol-only drinking sessions that conferred aversion-resistance in Alc-then-AlcQ mice.
AlcQ-only mice drank significantly less alcohol in the initial, protracted session relative to Alc-then-AlcQ mice (Fig. 2A, p < 0.01). Thus, the lack of quinine resistance in subsequent LDA sessions in AlcQ-only mice could reflect the lower alcohol exposure in the initial consumption session. We therefore examined LDA intake in an additional control group which had only 8 h of initial access to alcohol-only rather than 24 h (AlcBrief-then-AlcQ; n = 15; purple columns). AlcBrief-then-AlcQ mice exhibited similar alcohol intake levels in the initial drinking period as AlcQ-only mice (Fig. 2A, p > 0.1) and significantly lower initial intake than Alc-only and Alc-then-AlcQ mice (Fig. 2A, both p < 0.05). However, alcohol–quinine intake in LDA sessions was significantly greater in AlcBrief-then-AlcQ mice relative to AlcQ-only mice (Fig. 3A, p < 0.05), and was very similar to intake levels in Alc-then-AlcQ mice (Fig. 3A, p > 0.1). Thus, either 24- or 8-h access to drinking alcohol-only was sufficient to enable subsequent aversion-resistant intake. In addition, the lower level of initial alcohol consumption in AlcQ-only mice was not sufficient to explain the very low alcohol–quinine drinking levels in subsequent LDA sessions.
Finally, if a single initial period of alcohol-only drinking was necessary for development of aversion resistance in subsequent intake sessions, then mice drinking water-only during the initial protected intake session (H2O-then-AlcQ; n = 12; green columns) should exhibit greatly reduced alcohol–quinine intake during LDA sessions. In fact, LDA alcohol–quinine intake was significantly reduced in H2O-then-AlcQ mice relative to Alc-then-AlcQ mice and AlcBrief-then-AlcQ mice (Fig. 3A, both p < 0.05), and was not different from AlcQ-only mice (Fig. 3A, p > 0.1). These results further support the possibility that the initial alcohol-only drinking experience was necessary for subsequent aversion-resistant intake.
Alcohol intake, with or without quinine, was not associated with development of quinine preference
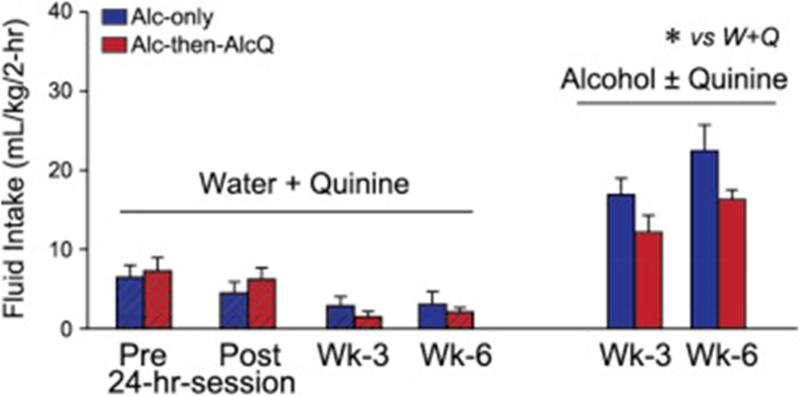
Willingness to drink alcohol despite adulteration with quinine in Alc-then-AlcQ mice might reflect a developed preference for the taste of quinine, since it has been repeatedly paired with alcohol intake. Thus, we examined whether Alc-only and Alc-then-AlcQ mice (n = 9–10 per group) mice were willing to drink water–quinine versus water under two-bottle choice. Overall, water–quinine intake levels were much lower than alcohol intake levels (Fig. 4; F[1,30] = 5.24, p = 0.03), as were preferences (Fig. 5; F[1,30] = 4.46, p = 0.04). Also, we examined water–quinine drinking before and after the 24-h alcohol session, as well as after 3 and 6 weeks of LDA consumption. Water–quinine intake (F[3,45] = 6.50, p = 0.003) and preference (F[3,45] = 4.98, p = 0.005) actually decreased across time (Fig. 4 and Fig. 5), indicating that mice did not develop a preference for quinine taste across the weeks of alcohol drinking. In addition, a twoway ANOVA with repeated measures (group × time) found no differences between Alc-only and Alc-then-AlcQ mice in water–quinine intake (Fig. 4; Interaction: F[3,48] = 0.79, p = 0.46; Group: F[1,16] = 0.001, p = 0.98; Time: F[3,48] = 7.31, p = 0.001) and preference (Fig. 5; Interaction: F[3,45] = 1.12, p = 0.35; Group: F[1,15] = 0.04, p = 0.84; Time: F[3,45] = 4.98, p = 0.005) at any of the different time points. Together with the results above, these findings suggest that quinine-resistant drinking in Alc-then-AlcQ mice did not reflect increased tolerance or a change in taste palatability for the otherwise aversive quinine.
Fig. 4.
Aversion-resistant drinking of quinine-adulterated alcohol was not associated with altered intake of water–quinine. Alc-only and AlcQ-only mice had much higher intake of alcohol ± quinine versus water–quinine. Data are shown as mean ± SEM. *p < 0.05 water versus alcohol.
Fig. 5.
Aversion-resistant drinking of quinine-adulterated alcohol was not associated with altered preference or aversion to water–quinine. No difference in water–quinine preference between Alc-only and Alc-then-AlcQ mice. Data are shown as mean ± SEM. *p < 0.05 water versus alcohol.
Use of quinine adulteration to assess aversion-resistant drinking
After long-term intermittent alcohol intake in rats, the level of intake of alcohol-only and alcohol–quinine is not different for experiments with typical group sample sizes (Seif et al., 2013 and Seif et al., 2015). However, by combining results from >100 rats, there is, in fact, a slight (~15%) but significant decrease in alcohol drinking with quinine adulteration (Hopf et al., 2010 and Seif et al., 2013; F.W.H. unpublished results). Importantly, however, the effect of quinine adulteration in these intermittent-drinking rats is much less than observed in control rats, e.g., where rats drinking under a continuous-access paradigm show a >60% decrease in alcohol intake with the same concentration of quinine in alcohol (Hopf et al., 2010). Thus, intermittent-drinking animals are considered to exhibit aversion-resistant intake relative to control groups.
To examine whether a similar pattern is observed in mice during the second week of LDA drinking (where intake across all days of Week 2 LDA was averaged for each mouse), we combined results from the animals shown in Fig. 2 and Fig. 3 with additional data from mice used for experiments for other manuscripts (but where results at 2 weeks of intake were not included in the submission). Across this much larger sample of C57BL/6 mice, the level of LDA alcohol drinking in Alc-then-AlcQ mice was in fact slightly (~15%), but significantly, lower than LDA intake levels in Alc-only mice (Alc-only: 2.96 ± 0.10 g/kg-body weight, n = 102; Alc-then-AlcQ: 2.47 ± 0.12 g/kg-body weight, n = 92; t[1,192] = 3.04, p = 0.03). However, the impact of quinine adulteration in Alc-then-AlcQ mice was much less than the larger quinine reduction of intake in control groups here (i.e., the ~60% decrease in intake levels in both AlcQ-only and H2O-then-AlcQ mice relative to Alc-then-AlcQ mice). Thus, Alc-then-AlcQ drinking led to the development of aversion-resistant alcohol drinking, relative to mice that did not have an initial session of drinking alcohol-only.
Several studies of aversion-resistant intake for cocaine and alcohol suggest that there may be two populations of animals, one that is aversion-resistant and one that is aversion-sensitive (Cooper et al., 2007, Deroche-Gamonet et al., 2004 and Pelloux et al., 2015; see Discussion section). Thus, we examined the distribution of drinking levels in these larger cohorts of mice drinking data, and used a Kruskal–Wallis test to assess whether the distributions deviated significantly from a normal distribution. However, we found that the distribution of Week 2 LDA drinking in both Alc-only mice and Alc-then-AlcQ mice did not deviate from a normal distribution (Alc-only: Kruskal–Wallis statistic = 0.063; Alc-then-AlcQ: Kruskal–Wallis statistic = 0.087; both p > 0.1), suggesting that the drinking data could not be distinguished from a single normally distributed population in both groups.
Discussion
Our results demonstrate that outbred C57BL/6 mice rapidly developed aversion-resistant patterns of alcohol consumption. Remarkably, only a single, protracted voluntary alcohol drinking session was sufficient to enable future quinine-resistant intake. This differs from rats, where several months of intermittent alcohol intake is required for developing aversion-resistant alcohol drinking (see below). Quinine-resistant drinking in mice likely was not due to inability to sense quinine, since mice drinking alcohol–quinine only in all sessions showed greatly reduced alcohol consumption in both the initial 24-h session and during LDA drinking sessions. Mice that drank water-only in the initial 24-h period also exhibited much lower alcohol–quinine intake by Week 2 of LDA. Furthermore, mice that drank alcohol-only for only 8 h in the initial session also exhibited high alcohol–quinine levels, suggesting that the lower level of 24-h intake in AlcQ-only mice was not per se responsible for the subsequent low levels of alcohol–quinine drinking. In addition, water–quinine intake was much lower than alcohol ± quinine intake across the weeks of drinking, and not different between the Alc-only and Alc-then-AlcQ groups. This suggests that the quinine concentration we used was strongly aversive, and that mice in the Alc-then-AlcQ group did not develop a quinine taste preference after weeks of alcohol drinking. Finally, mice exhibited nearly binge-level BACs after 30 min of access to alcohol, suggesting that binge-like peak BACs were achieved during the LDA drinking sessions. Taken together, our results indicate that C57BL/6 mice quickly developed compulsion-like, quinine-resistant drinking of quinine-adulterated alcohol, which did not reflect changes in quinine palatability.
The rapid development of aversion-resistant alcohol drinking in C57BL/6 mice is in strong contrast to rats, where several months of intermittent alcohol intake are required for Wistar rats to develop quinine-resistant drinking patterns (Hopf and Lesscher, 2014, Hopf et al., 2010, Loi et al., 2010, Seif et al., 2013, Seif et al., 2015 and Spoelder et al., 2015). Although there are many species differences that might explain these dissimilarities, one clear finding is that C57BL/6 mice drink high levels of alcohol from the onset, with binge-like levels of alcohol intake even during a first, 4-h drinking session (Mulligan et al., 2011). In contrast, outbred rat lines under intermittent alcohol access show low levels of initial intake, but then increase consumption across the first month of drinking (Carnicella et al., 2014, Hopf and Lesscher, 2014, Hopf et al., 2010 and Simms et al., 2008). Thus, higher levels of voluntary intake may be related to more rapid development of aversion-resistant consumptions patterns. Also, previous findings from Lesscher et al., 2010 and Lesscher et al., 2012), upon which our model is based, found that quinine-resistant alcohol drinking in C57BL/6 mice was evident after 2 weeks of intake, and, interestingly, mice in that study initially drank low levels of alcohol, which increased across the first week of intake. There are several differences between our model and that of Lesscher and colleagues, including single (here) versus group housing, and drinking in home cages versus separate drinking chambers. Thus, additional experiments will be required to address the contribution of intake level, species, and other factors to the rate of development of quinine-resistant drinking.
We also note that, across a large sample of mice, LDA alcohol drinking in Alc-then-AlcQ mice was slightly (~15%) but significantly lower than LDA levels in Alc-only mice, which might conflict with the idea that drinking in Alc-then-AlcQ mice reflects aversion-resistant consumption. However, our previous studies of quinine-resistant alcohol drinking in a very large sample of intermittent-access, alcohol-drinking rats used alcohol adulterated with 30 mg/L of quinine, and alcohol–quinine intake was also slightly (~15%) but significantly reduced relative to drinking of alcohol-only (Hopf et al., 2010 and Seif et al., 2013; F.W.H. unpublished results). Importantly, our results also indicate that quinine in alcohol can lead to a much greater reduction in drinking levels, where AlcQ-only mice, which have alcohol + quinine from the onset, showed a ~60% decrease in LDA alcohol intake during Week 2 (Fig. 3). Similarly, mice that drank water in the initial protracted intake session also showed significantly lower alcohol–quinine drinking levels during LDA sessions (Fig. 3). In agreement, quinine strongly reduces the more moderate alcohol consumption in rats drinking under continuous access, a control group for the intermittent-access rats that developed aversion-resistant drinking after ~3 months of drinking (Hopf et al., 2010). In addition, alcohol-preferring rats that drank alcohol for 1.5 months under intermittent access showed only small sensitivity to quinine, while preferring rats drinking under continuous-access showed a >60% reduction in alcohol intake by quinine (Loi et al., 2010). Thus, we believe the quinine concentration used here represented a robust and practical compromise to allow study of quinine-resistant drinking patterns relative to control drinkers (see also Barbier et al., 2015, Lesscher et al., 2010, Lesscher et al., 2012, Seif et al., 2013, Seif et al., 2015, Vendruscolo et al., 2012 and Warnault et al., 2016).
Some studies have suggested the presence of separate populations of aversion-resistant and -sensitive animals, but we did not find evidence for this possibility. As described in greater detail in Hopf and Lesscher (2014), the relative proportion of animals exhibiting aversion resistance and sensitivity can depend on the level of aversion. For example, some studies have observed defined populations of footshock-resistant and -sensitive rats for alcohol (Seif et al., 2013) and cocaine (Cooper et al., 2007, Deroche-Gamonet et al., 2004 and Pelloux et al., 2015). In contrast, other studies have shown that sufficiently high shock intensity will suppress intake in all animals (Cooper et al., 2007 and Seif et al., 2013), and shock-paired cues suppress cocaine intake in all animals tested (Vanderschuren & Everitt, 2004). Similarly, we have shown in rats that some concentrations of quinine in alcohol are tolerated by all rats, while higher concentrations lead some rats to exhibit lower aversion-resistant intake (Hopf et al., 2010). Thus, while animals certainly show variability in the resistance to aversion during intoxicant intake, it seems plausible that there is not a specific population that is resistant or sensitive per se, but that there is individual variability in the level of resistance or sensitivity, and that intermediate levels of aversion will lead to the appearance of more and less resistant aversion-resistant intoxicant intake.
Quinine-resistant drinking examined here could reflect that the concentration of quinine we utilized was not aversive, or that alcohol drinking increased the preference for quinine. However, AlcQ-only mice, that drank alcohol–quinine in both the initial 24-h session and the LDA sessions, showed greatly reduced intake levels and preference for alcohol–quinine, suggesting that mice can find this concentration of quinine aversive when added to alcohol. In addition, intake levels and preference for the same concentration of quinine in water was low (see also Bachmanov et al., 1996a, Bachmanov et al., 1996b and Tordoff et al., 2002) and actually decreased across the weeks of alcohol drinking. Thus, mice did not develop a preference for the taste of quinine across weeks of alcohol drinking, which concurs with other studies where quinine-resistant drinking in mice and rats did not reflect changes in taste palatability (Barbier et al., 2015, Lesscher et al., 2010, Lesscher et al., 2012, Seif et al., 2013, Seif et al., 2015, Vendruscolo et al., 2012 and Warnault et al., 2016). Together, these findings indicate that C57BL/6 mice have the ability to find quinine aversive when added to alcohol, but they no longer do so once they have had a single session of drinking alcohol-only.
We have interpreted the willingness of Alc-then-AlcQ mice to drink quinine-adulterated alcohol as evidence of development of aversion resistance, in part because AlcQ-only mice that drank alcohol–quinine in all drinking sessions had much lower intake levels and preference. However, another possibility is that AlcQ-only mice developed a conditioned taste aversion to quinine in alcohol (Eckardt, 1975) during the first protracted intake session, which then persisted across subsequent LDA intake sessions. While we cannot completely rule out this possibility, we consider it unlikely for several reasons. First, we note that mice drinking alcohol-only and alcohol–quinine both showed very low preference for quinine in water, and with a significant decline in preference across weeks of alcohol drinking. Thus, we can at least say that mice did not exhibit a change in preference for quinine per se. In addition, mice that drank water during the initial 24-h access period also showed greatly reduced intake and preference of alcohol–quinine during 2-week LDA drinking, and at levels that were similar to AlcQ-only mice. Thus, mice showed similarly reduced quinine-alcohol drinking in the second week of LDA sessions whether or not they had a protracted initial experience with alcohol–quinine, making it unlikely that reduced alcohol–quinine intake in AlcQ-only mice reflected development of a conditioned taste aversion during the initial 24-h drinking session.
In conclusion, our findings establish an alcohol-drinking model in mice where, surprisingly, a single session of alcohol-only drinking was sufficient to enable subsequent aversion-resistant consumption, without developing a preference for quinine over time. The drinking paradigm described here is thus a quick and simple drinking model, which will be useful for future quinine-resistant drinking experiments, including for defining the minimum alcohol intake required for development of aversion-resistant drinking, and for identifying the molecules and circuits that drive development and expression of compulsion-like, aversion-resistant drinking.
Supplementary Material
Acknowledgments
We thank Drs. Dorit Ron and Robert Messing for their critical review of this manuscript. Supported by NIAAAP50 AA017072.
References
- Anton RF. Obsessive-compulsive aspects of craving: Development of the obsessive compulsive drinking scale. Addiction. 2000;95(Suppl. 2):S211–S217. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Archives of General Psychiatry. 1996;53:225–231. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: A genetic analysis. Behavior Genetics. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcoholism: Clinical and Experimental Research. 1996;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Tapocik JD, Juergens N, Pitcairn C, Borich A, Schank JR, et al. DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavior and plasticity. The Journal of Neuroscience. 2015;35:6153–6164. doi: 10.1523/JNEUROSCI.4571-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blincoe L, Seay A, Zaloshnja E, Miller T, Romano E, Luchter S, et al. The economic impact of motor vehicle crashes. (Report) U.S. Department of Transportation; Washington, DC: 2002. [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventive Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Excessive drinking costs U.S. $223.5 billion. (Report) Centers for Disease Control; Atlanta, GA: 2014. [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ. The role of orosensory stimuli from ethanol and blood-alcohol levels in producing conditioned taste aversion in the rat. Psychopharmacologia. 1975;44:267–271. doi: 10.1007/BF00428905. [DOI] [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Developments in Alcoholism. 1998;14:307–330. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M, Wechsler H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: Changes from 1998 to 2001. Annual Review of Public Health. 2005;26:259–279. doi: 10.1146/annurev.publhealth.26.021304.144652. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcoholism: Clinical and Experimental Research. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake Alcohol. 2014;48:253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA. Social stress-escalated intermittent alcohol drinking: Modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology. 2016;233:681–690. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt's cognitive-behavioral model. Alcohol Research & Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Houthuijzen JM, Groot Koerkamp MJ, Holstege FC, Vanderschuren LJ. Amygdala 14-3-3ζ as a novel modulator of escalating alcohol intake in mice. PLoS One. 2012;7:e37999. doi: 10.1371/journal.pone.0037999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, van Kerkhof LW, Vanderschuren LJ. Inflexible and indifferent alcohol drinking in male mice. Alcoholism: Clinical and Experimental Research. 2010;34:1219–1225. doi: 10.1111/j.1530-0277.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, et al. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcoholism: Clinical and Experimental Research. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Current Opinion in Neurobiology. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell JG, Glaser FB, Mountz JM, Schmaltz S, Cyr L. Obsessive and compulsive characteristics of alcohol abuse and dependence: Quantification by a newly developed questionnaire. Alcoholism: Clinical and Experimental Research. 1992;16:266–271. doi: 10.1111/j.1530-0277.1992.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Rhodes JS, Crabbe JC, Mayfield RD, Harris RA, Ponomarev I. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2011;35:659–670. doi: 10.1111/j.1530-0277.2010.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: A critical neural substrate for craving and drug seeking under conflict and risk. Annals of the New York Academy of Sciences. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ. Differential vulnerability to the punishment of cocaine related behaviours: Effects of locus of punishment, cocaine taking history and alternative reinforcer availability. Psychopharmacology. 2015;232:125–134. doi: 10.1007/s00213-014-3648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Roeber J, Bouchery EE, Gonzales K, Chaloupka FJ, Brewer RD. State costs of excessive alcohol consumption, 2006. American Journal of Preventive Medicine. 2013;45:474–485. doi: 10.1016/j.amepre.2013.06.004. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Risk and protective factors and initiation of substance use: Results from the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration; USA: 2015. [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature Neuroscience. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, et al. d-Serine and d-cycloserine reduce compulsive alcohol intake in rats. Neuropsychopharmacology. 2015;40:2357–2367. doi: 10.1038/npp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: Implications for addiction treatment development. Addiction Biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiological Reviews. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Hesseling P, Baars AM, Lozeman-van 't Klooster JG, Rotte MD, Vanderschuren LJ, et al. Individual variation in alcohol intake predicts reinforcement, motivation, and compulsive alcohol use in rats. Alcoholism: Clinical and Experimental Research. 2015;39:2427–2437. doi: 10.1111/acer.12891. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl. 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: Implications for large-scale phenotyping projects. The Journal of Nutrition. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr., Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. The Journal of Neuroscience. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addiction Biology. 2009;14:384–396. doi: 10.1111/j.1369-1600.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, et al. The BDNF valine 68 to methionine polymorphism increases compulsive alcohol drinking in mice that is reversed by tropomyosin receptor kinase B activation. Biological Psychiatry. 2016;79:463–473. doi: 10.1016/j.biopsych.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. The Journal of Pharmacology and Experimental Therapeutics. 1993;267:250–258. [PubMed] [Google Scholar]
- World Health Organization . Global status report on alcohol and health-2014. World Health Organization; 2014. [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.