Abstract
Although the specific events dictating systemic lupus erythematosus (SLE) pathology remain unclear, abundant evidence indicates a critical role for dysregulated cytokine signaling in disease progression. Notably, the suppressor of cytokine signaling (SOCS) family of intracellular proteins, in particular the kinase inhibitory region (KIR) bearing SOCS1 and SOCS3, play a critical role in regulating cytokine signaling. To assess a relationship between SOCS1/SOCS3 expression and SLE, the goals of this study were to: 1) evaluate the time kinetics of SOCS1/SOCS3 message and protein expression based on SLE associated stimulations, 2) compare levels of SOCS1 and SOCS3 present in SLE patients and healthy controls by message and protein, 3) relate SOCS1/SOCS3 expression to inflammatory markers in SLE patients, and 4) correlate SOCS1/SOCS3 levels to current treatments. We found that SOCS1 and SOCS3 were most abundant in murine splenic samples at 48 hours subsequent to stimulation by anti-CD3, LPS, or interferon gamma. In addition, significant reductions in SOCS1 and SOCS3 were present within PMBC’s of SLE patients compared to controls by both mRNA and protein expression. We also found that decreased levels of SOCS1 in SLE patients were correlated to enhanced levels of inflammatory markers and up-regulated expression of MHC class II. Finally, we show that patients receiving steroid treatment possessed higher levels SOCS1 compared to SLE patient counterparts, and that steroid administration to human PBMCs up-regulated SOCS1 message in a dose and time dependent manner. Together, these results suggest that therapeutic strategies focused on SOCS1 signaling may have efficacy in the treatment of SLE.
Keywords: Autoimmunity, lupus, tolerance, signal transduction, kinase, Janus kinase/signal transducers and activators of transcription (Jak/STAT), biomarkers
Background
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with clinically heterogeneous manifestations. Although the cause of SLE is unknown, studies show that dysregulated cytokine signaling plays a significant role in SLE onset and progression.[1] In addition to excessive type I and type II interferon (IFN) signaling, lupus patients often present with excessive IL6 [2,3]. The Janus kinase/signal transducers and activators of transcription (Jak/STAT) pathway is critically involved in transducing the inflammatory programming mediated by lupus associated cytokines, subsequent to cognate extracellular receptor binding [4,5]. After the formation of the ligand/receptor complex, Jaks become activated and then activate STAT molecules. The activated STAT molecules nuclear translocate and mediate transcription of inflammatory factors. Notably, in addition to the generation of inflammatory mediators, STAT molecules also mediate up-regulation of suppressor of cytokine signaling (SOCS) molecules. SOCS proteins serve as negative feedback inhibitors of cytokine responsiveness [6,7]. Significantly, several rodent studies have implicated a relationship between SOCS protein deficiencies and lupus-like pathologies [1,8-11], although the SOCS protein expression kinetics in relation to SLE associated stimulations are poorly understood.
SOCS1 and SOCS3 are known to play a critical role in the regulation of lupus-associated stimulatory pathways such as toll like receptor, T cell receptor, and cytokine signaling [12,13]. Rodent studies have demonstrated a reciprocal relationship between SOCS1 and SOCS3 expression within T-cells and macrophages [13-16], however the SOCS1/SOCS3 relationship in the context of human lupus is not known. Although previous studies focusing on SOCS1 in SLE patients have expanded our understanding in the area, these studies generally focused on message levels, and are conflicting [17-20]. Notably, SOCS3 protein levels in SLE patients have not been examined. In this study, we assess SOCS1 and SOCS3 levels in response to various stimulations, and within SLE patients and healthy controls. We also compare SOCS1 and SOCS3 levels to SLEDAI levels and markers of inflammation within patients. We found that SOCS1 and SOCS3 levels were remarkably reduced in both message and protein levels within SLE patients, compared to controls, and correlated well to increased levels of inflammation. In addition, we found that a number of SLE patients possessed an inverted activated STAT1/SOCS1 ratio that was correlated to significantly enhanced levels of MHC class II up-regulation, thus potentially connecting a lupus promoting mechanism to SOCS deficiency.
Methods
Patients and Clinical samples
The University of Florida (UF) Institutional scientific research and review board (IRB) on human subjects approved the study protocol, in accordance to the ethical principles established by the Declaration of Helsinki. Written informed consents were obtained from all patients and controls in the study. In all, 34 SLE patients (Table-1) and 11 healthy controls were enrolled in the present study. All SLE patients fulfilled the 1982 American College of Rheumatology (ACR) revised criteria for SLE [21]. The patient’s age ranged from 22 to 73 years while the healthy control ages ranged from 27 to 55 years. Healthy controls (N=11) were recruited from the outpatient clinics at the participating institutions. All patients underwent baseline investigations as per clinical requirements. Disease activity was assessed by the SLE disease activity index (SLEDAI). For the present data SLE Patients with SLEDIA value <2 was considered as inactive and patients with ≥2 were categorized under active SLEDIA patients [22] Table-1).
Table-1.
Information on SLE patients included in the study.
| SLE Patients | Age/Gender | SLEDAI | Prednisone (mg) | Plaquenil (mg) |
|---|---|---|---|---|
| 1 | 27/F | 2 | 0 | 0 |
| 2 | 36/F | 2 | 0 | (100 or 300) |
| 3 | 63/F | 4 | 20 | 400 |
| 4 | 53/F | 10 | 20 | 0 |
| 5 | 69/M | 2 | 25 | 200 |
| 6 | 73/F | 2 | 0 | 0 |
| 7 | 31/F | 6 | 0 | 400 |
| 8 | 38/F | ND | 0 | 400 |
| 9 | 28/F | 0 | 0 | 400 |
| 10 | 45/F | 4 | 10 | 400 |
| 11 | 55/F | 10 | 5 | 400 |
| 12 | 62/F | ND | 0 | 400 |
| 13 | 55/F | ND | 0 | 200 |
| 14 | 59/F | 0 | 0 | 400 |
| 15 | 22/F | ND | 20 | 400 |
| 16 | 41/F | 2 | 10 | 400 |
| 17 | 23/F | ND | 5 | 400 |
| 18 | 58/F | 0 | 0 | 0 |
| 19 | 32/F | 0 | 0 | 100 mg Quinacrine |
| 20 | 67/F | 0 | 2.5 | 400 |
| 21 | 39/F | 7 | 0 | 400 |
| 22 | 61/M | ND | 8 | 0 |
| 23 | 61/F | ND | 5 | 400 |
| 24 | 46/F | 0 | 0 | 100 mg Quinacrine |
| 25 | 66/F | 0 | 0 | 200 |
| 26 | 51/F | 4 | 10 | 400 |
| 27 | 66/F | ND | 0 | 400 |
| 28 | 44/F | ND | 4 | 400 |
| 29 | 28/F | 4 | 40 | 400 |
| 30 | 56/F | 2 | 0 | 200 |
| 31 | 44/F | 0 | 0 | 200 |
| 32 | 64/F | 0 | 5 | 400 |
| 33 | 29/F | ND | ND | ND |
| 34 | 18/F | ND | ND | ND |
Statement for Animal Use
C57BL/6 mice were purchased from the St. Jude Animal facility (Memphis, TN) and mated in the University of Florida Cancer and Genetics Animal Facility. Mice were maintained in sterile micro-isolators under specific pathogen free conditions at the University of Florida Cancer and Genetics Animal Facility. The University of Florida Institutional Animal Care and Use Committee (IACUC) approved all animal protocols and experiments were performed in strict accordance to the approved protocols.
Preparation of Peripheral blood mononuclear cells (PBMCs) and total RNA extraction
PBMCs were purified from heparinized blood by Ficoll-Hypaque gradient centrifugation. Briefly, whole blood was diluted with PBS (1:1) and gently layered on Ficoll (Lymphocyte Separation Medium; Cellgro, Manassas, VA). Cells were centrifuged continuously at 400xg for 20 minutes. PBMCs were collected from the interface layer and washed 3 times with PBS. After isolation, PBMCs were lysed with RNA lysis buffer (Promega) and stored at -80° C until RNA extraction and qPCR protocols were performed.
In vitro cell culture
C57BL/6 mouse harvested splenocytes were suspended in RPMI-1640 (Gibco Laboratories, Grand Island, NY) containing 10% FBS, penicillin (100U/ml) and streptomycin (100 ug/ml). The splenocytes were treated in-vitro with LPS (5000 ug/ml, Sigma-Aldrich) and IFNγ (500 U, R & D Systems, Inc.) at the time points 6, 12, 24 and 48hr in the presence or absence of anti-CD3 (4 ug/ml) (Leinco Technologies, Inc.). Incubations were carried out at 37°C in 5% CO2 and 95% air. Subsequent to incubation, cellular pellets were harvested and prepared for analysis by western blot analysis and quantitative PCR.
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was performed on PBMCs isolated from 33 SLE patients, 11 healthy controls, or in-vitro treated C57Bl/6 splenocytes to determine the transcript levels of SOCS1, SOCS3 genes in PBMCs, relative to GAPDH. In brief, cDNA synthesis was performed using the iQ cDNA synthesis kit (Bio-Rad; Hercules, CA). The qPCR was subsequently performed using the iQ SYBR Green Super mix (Bio-Rad) and gene-specific primers for SOCS1 and SOCS3. The real time RT-PCR reaction was performed on CFX connect Tm-Real time system. The PCR for the target gene and GAPDH was duplicated twice for each sample and the results were corrected for GAPDH expression as an internal control. The data was further analyzed using CFX Manager Tm software version 3.0. (Bio-Rad technologies. Inc.,).
Western blot analysis
Frozen PBMC cell pellets from 32 SLE Patients, 6 Healthy controls, or in vitro treated C57BL/6 splenocytes were thawed and lysed in RIPA lysis buffer (Santa Cruz Technologies, Inc.,) followed by protein estimation through BSA 2.0mg/ml standard curve preparation with Pierce protein assay reagents (Thermo Scientific Product # 23209). Equal quantities of proteins were loaded and separated in 4%-12% SDS PAGE gel (Bio-Rad Laboratories, Hercules, CA), and separated proteins were transferred to nitrocellulose membrane (Bio-Rad Laboratories). The membranes were then blocked and incubated with polyclonal anti-rabbit antibodies specific to the KIR regions of SOCS1 and SOCS3 (a kind gift from Dr. Howard Johnson, UF), anti-pSTAT1, anti-pERK1/2, anti-pAKT (Ser473), total STAT1, total ERK1/2, (Santa Cruz) and total AKT (Cell signaling) respectively. Protein visualization was obtained subsequent to the addition of the appropriate secondary antibodies conjugated to HRP followed by utilization of the ECL detection system according to the manufacturer’s instruction (Pierce; Thermo Scientifics). GAPDH was used as a loading control. The results were calculated as densitometry arbitrary units using the ImageJ online software.
Statistical analysis
The software Graph Pad Prism v.5 was utilized to determine statistical significance. Data were presented as mean ± standard error mean (SEM) unless otherwise denoted. The Student t-test was used for non-paired samples with a 95% confidence limit. Simple linear regressions and Pearson’s chi-square test were used to measure the correlation among different parameters with a 95% confidence limit. P-value at p<0.05 were considered to be statically significant.
Results
Antibody Validation and Establishment of SOCS1 and SOCS3 Up-regulation Kinetics
Previous research has shown that SOCS1 and SOCS3 levels were up regulated, directly or indirectly, by several cellular stimulations associated with SLE including type 1 interferons, type 2 interferon (IFNγ), lipopolysaccharide, and TCR stimulation [23-26]. Two factors contributing to the capacity of SOCS1 and SOCS3 to limit immune response are 1) the time in which up-regulation occurs subsequent to signal input and 2) the duration of the SOCS protein up-regulation. In order to assess the expression kinetics of SOCS1 and SOCS3 in primary immune cells subsequent to varied external stimuli, we treated murine splenocytes with LPS, IFNγ, or TCR stimulatory anti-CD3. We then assessed changes in the expression of SOCS1 and SOCS3 protein levels at 6, 12, 24 and 48 hours by western blotting. Whereas basal levels of SOCS1 were clearly present in untreated samples, SOCS3 protein levels were less prominent in the absence of external stimulation (Fig 1 A, B). Consistent with previous studies [27-33] two SOCS1 specific bands are readily present in murine splenocyte samples at a molecular weight ranging from 24 kDA to 37kDa. Changes in SOCS1 protein levels in response to LPS or CD3 stimulation were modest at 6 hours, but then steadily increased to a two-fold margin by 48 hours as denoted by densitometic numeric values displayed below the representative bands (Figure 1 A, B). TCR mediated increases in SOCS3 were also maximal at 24 to 48 hours. IFNγ mediated up-regulation of SOCS1 and SOCS3 protein levels were most evident at the earlier 6 and 12 hour time points, followed by a return to levels comparable to untreated at 24 hours. Given that it has been shown that SOCS1 expression is differentially regulated at transcriptional and posttranscriptional levels [34], we next assessed SOCS1 message expression subsequent to IFNγ, LPS, and TCR stimulation at the same time points used to assess changes in protein levels. Up-regulation of SOCS1 mRNA levels presented similarly to protein expression, with abundant up-regulation by IFNγ at early time points while LPS mediated SOCS1 mRNA expression was most evident at 24 and 48 hours (Figure 1C). Together these data show that IFNγ, TCR stimulation, and LPS differentially up-regulate SOCS1 and SOCS3 protein expression.
Figure-1. Antibody Validation and Establishment of SOCS1 and SOCS3 Up-regulation Kinetics.
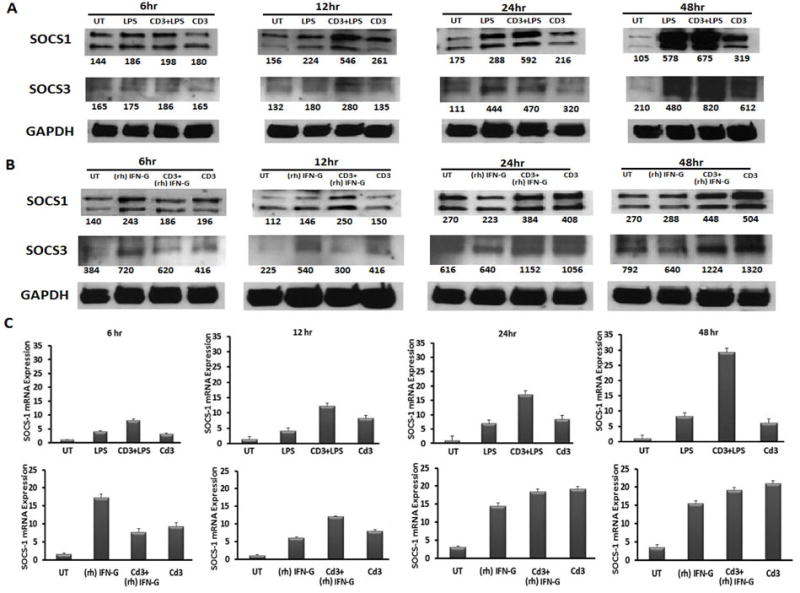
Splenocytes, isolated from C57BL/6 mice, were harvested and treated in-vitro with LPS (5000 ug/ml) or IFNγ(500 U) at the time points 6, 12, 24 and 48hr in the presence or absence of anti-CD3 (4 ug/ml). Subsequent to simulation, splenocyte cell pellets were collected and prepared for western blot analysis or quantitative PCR. Figures represent (A) LPS treated (B) recombinant (rh) IFNγ treated cell lysates screened for SOCS1, SOCS3 and GAPDH respectively. (C) The graphs represent the SOCS1 mRNA expression for respective treatments of LPS and (rh) IFNγ.
Reduced mRNA and Protein levels of SOCS1 and SOCS3 in SLE patients
In order to assess whether deficiencies in regulatory proteins could possibly contribute to the well-established cytokine signaling defects present in SLE patients, we first measured SOCS1 and SOCS3 mRNA expression in SLE patients in relation to healthy controls. As can be seen in Figure 2 (A and B), the mean SOCS1 message levels in PBMC isolated from SLE patients were two-fold lower than that of controls (p<0.01). Although not statistically significant, SOCS3 message levels were also consistently lower in patients compared to controls. Linear regression analysis between SOCS1 and SOCS3 message levels from SLE patients showed a positive correlation (r2=0.5696; p<0.0001) between expression levels (Figure S4 (A))., indicating that the expression levels of the two proteins were comparable in patients.
Figure-2. Reduced mRNA and Protein levels of SOCS1 and SOCS3 levels in SLE patients.
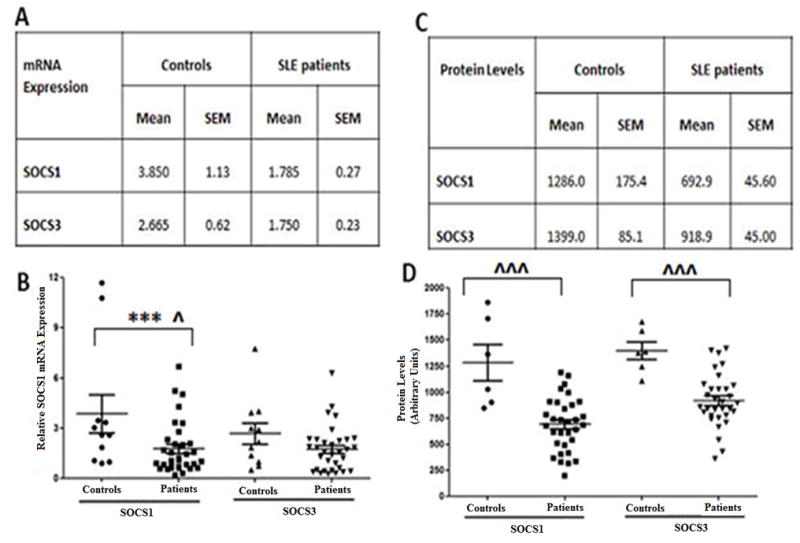
(A) table represents the average SOCS1 and SOCS3 mRNA expression (mean ± SEM) via real time RT-PCR, relative to GAPDH in SLE patients (N=33) versus controls (N=11). (B) Graph relative SOCS1 and SOCS3 message levels for SLE patients (N=33) and controls (N=11). Each dot represents an individual subject. (C) Table represents the average SOCS1 and SOCS3 protein levels (arbitrary Units mean ± SEM) for SLE patients (N=32) compared to controls (N=6). (D) Graph showing basal SOCS1 and SOCS3 protein levels present in SLE patients (N=32) and controls (N=6) as a graphical representation of densitometry values obtained through analysis of (Figure S3). Each dot represents an individual patient data points. Correlation was considered significant at 0.05 level (2 tailed).
We next assessed SOCS1 and SOCS3 protein levels within the PBMCs, of the same patients in which we evaluated message expression. Notably, reductions in the mean SOCS3 protein levels present in the PBMC of SLE patients did reach statistical significance when compared to controls (Figure 2D; p<0.001). Analysis of SOCS1 and SOCS3 protein levels in the PBMC of healthy controls, showed comparable levels of the two proteins within individual patients with relative ratio values close to one (Figure S4 (B), Table S1). Notably, in contrast to proportional levels of SOCS1 and SOCS3 protein within healthy control PBMC (grey bars present in Figure S4 (B), the ratio of SOCS1 and SOCS3 protein levels within the PBMC of individual SLE patients, showed considerable variation (Figure S4 (B). Sixty-two (15/24) percent of the SLE patients had comparable levels of SOCS1 and SOCS3 (values close to 1), 30% (7/24) had higher levels of SOCS3 compared to SOCS1 (values greater than 1) and 12.5% (3/24) had strikingly higher levels of SOCS1 (values approximately at 0.5) (Figure S4 (B). Despite variation within individual patients, together these results show that SOCS1 and SOCS3 levels are significantly lower in SLE patients compared to controls.
Reduced SOCS1 protein levels, in SLE patients, are correlated with enhanced STAT1 activation
We next assessed the activation of the inflammatory markers AKT, ERK1/2, and STAT1 by phosphorylation in SLE patients versus controls. While the average levels of pAKT and pERK1/2 protein levels were statistically indistinct (Figure 3A, 3B and 3C) as determined by average densitometry analysis of western data present in Figure S3, pSTAT1 levels were statistically higher in SLE patients compared to controls (Figure 3D, Table-S2). Higher pSTAT1 levels were present in the PBMC of SLE patients compared to controls despite overall higher AKT, ERK1/2, and STAT1 proteins levels in healthy controls (Figure 3B, 3C, 3D). On an individual level, all of the healthy controls, with the exception of HC-3, possessed pSTAT1 levels that are greater than 2 fold lower than the mean patient pSTAT1 level (Figure S3, Table S1, S2). In order to assess a relationship between the KIR containing SOCS1 and SOCS3 proteins with the inflammatory markers pAKT, pERK1/2, and pSTAT1; comparisons were performed between the individual SLE patients and the healthy controls. Figures 3E-3J are graphical representations of the ratio between SOCS1 and SOCS3 levels and that of pAKT, pERK 1/2, and pSTAT1. Healthy controls and the vast majority of SLE patients (23/26, and 20/26 respectively) possessed pAKT and pERK1/2 levels that were negatively associated with SOCS1 and SOCS3 protein levels (values less than 1). Notably, although healthy controls and the majority of SLE patients (22/26) possessed low pSTAT1/SOCS3 relative ratios (values less than 1), 15 of the 26 patients (58%) possessed pSTAT1 levels that were visibly elevated in comparison to SOCS1 levels (Figures 3E-3J). Indeed, 57% (8/14) of the SLE patients bearing SOCS1 protein levels below the SLE patient mean (692±45.6, Table S2) possessed pSTAT1 proteins levels that were elevated in relation to SOCS1 (795.4±53.6 and 500.3±63.6, Table S3). Together these data suggest that reduced levels of SOCS1, in SLE patients, is correlated to enhanced activation of STAT1.
Figure-3. Dysregulated STAT1 activation in SLE patients is correlated SOCS1 deficiency.
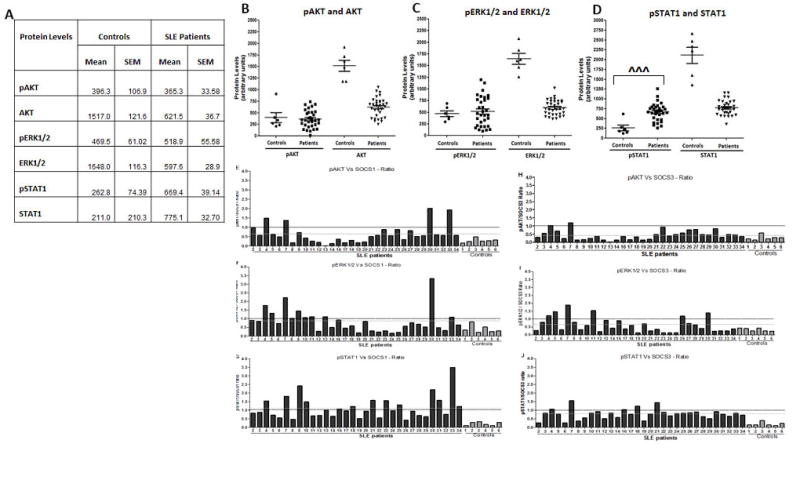
(A) Table represents Mean ± SEM of pAKT and AKT; pERK1/2 and ERK1/2; pSTAT1 and STAT1 protein levels of controls (N=6) as compared to SLE patients (N=32) expressed as arbitrary units obtained from densitometry analysis of data obtained in Figure S3. Graph represent basal protein level comparisons between (B) pAkt and AKT (C) pERK1/2 and ERK1/2 (D) pSTAT1 and STAT1 for SLE patients (N=32) and controls (N=6) where each data point represents an individual subject. Figures 3E-3G are bar graphs representing the relative ratios between (E) pAKT (F) pERK1/2, and (G) pSTAT1 compared to SOCS1 present in SLE patients (N=32) and Controls (N=6). Figures 3H-3J are bar graphs representing the relative ratios between (E) pAKT (F) pERK1/2, and (G) pSTAT1 compared to SOCS3 present in SLE patients (N=32) and Controls (N=6). The mean values of SLE patients are represented as black dotted line. ˆ represents a t-test with significant mean difference. Correlation was considered significant at 0.05 level (2 tailed).
Inverted pSTAT1/SOCS1 ratio correlates to significantly enhanced MHC class II levels amongst SLE patients
Given the visual observation that 57% of the SLE patients possessed a striking inverted pSTAT1/SOCS1 (Figure 3G, Figure S3) ratio compared to controls (Figure 3G, Figure S2), we next sought to assess the statistical significance of this observation and whether this inverted ratio could have any bearing on immunological dysregulation events associated with lupus pathology. As such, we segregated individual patients (from Figures 3G and 3J) based on bearing a high pSTAT1 to SOCS protein ratio (value greater than one) or a low pSTAT1 to SOCS protein ratio (value less than one). Comparison of the 12 patients bearing a high pSTAT1/SOCS1 ratio with 12 patients bearing a low pSTAT1/SOCS1 ratio revealed a statically significant difference in ratio values (1.776 vs. 0.602; p<0.0001), pSTAT1 levels (795.4 vs 527.8; p< 0.0001), and SOCS1 levels (500.3 vs. 863.1; p<0.0004) (Table S3). We next compared the MHC class II mRNA levels obtained from PBMCs isolated from SLE patients possessing high pSTAT1 versus SOCS protein (SOCS1 and SOCS3) ratios to those with low ratios. As can be clearly seen in Figure 4A, patients bearing a high pSTAT1/SOCS1 ratio also possessed higher levels of MHC class II message expression, which reached statistical significance with HLA-DRA. No statistically significant ratios were observed amongst comparisons between pSTAT1 and SOCS3 (Figure 4B). Given the importance of MHC associated antigen presentation to the progression of SLE, this result suggests that a high pSTAT1/SOCS1 ratio may be predictive of SLE pathology. Unfortunately, limited patient information inhibited obtaining statistical significance between the SLEDAI scores from patients with a high pSTAT1/SOCS1 ratio compared to those bearing a low pSTAT1/SOCS1 ratio or specific disease pathology (Table S4). However, it is notable that the average SLEDAI score in patients with the high pSTAT1/SOCS1 ratio was 5.8, while the average in patients with a low pSTAT1/SOCS1 ratio was 4.4 (Table S4). Together these results implicate a potential mechanism by which reduced SOCS1 signaling could be related to SLE disease progression.
Figure-4. Inverted pSTAT1/SOCS1 ratio correlates to significantly enhanced MHC class II levels amongst SLE patients.
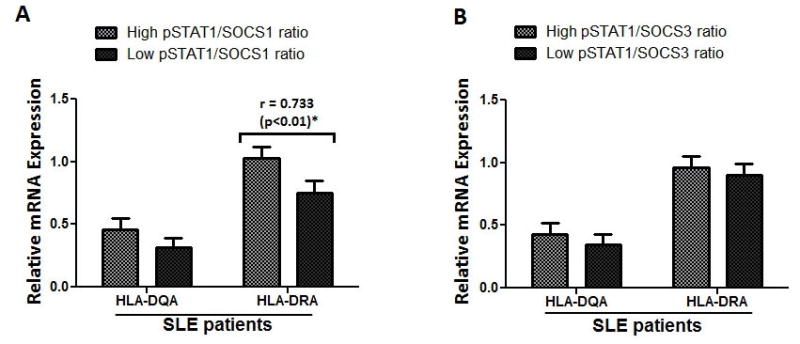
Graphs represents the comparisons of MHC II message levels (HLA-HQ-DQR and HQ-DAR) in SLE patients bearing high versus low (A) pSTAT1/SOCS1 ratio or (B) pSTAT1/SOCS3 ratio. * represents significant Pearson correlation; Correlation was considered significant at 0.05 level (2 tailed).
Prednisone treatment is correlated with enhanced SOCS1 and SOCS3 protein levels
In order to analyze the possible effects of prednisone treatment and SLE disease activity on KIR containing protein expression, subgroup analyses among SLE patients was performed. As can be seen in Figure 5 (Table-2), patients receiving prednisone consistently had higher SOCS1 protein levels than those not receiving prednisone, reaching significance among patients with an inactive SLEDAI score (n=9, p< 0.001) (Figure 5D). In contrast, prednisone treatment had no effect on SOCS3 protein levels. In terms of cellular activation, it was notable that patients with an inactive SLEDAI score and receiving prednisone had pErk1/2 activation levels that were comparable to healthy controls. In addition, prednisone treatment had no effect on either SOCS 1/3 message expression or STAT1 phosphorylation.
Figure-5. Subgroup analysis of SOCS1 and SOCS3 expression in SLE patients with and without prednisone treatment.
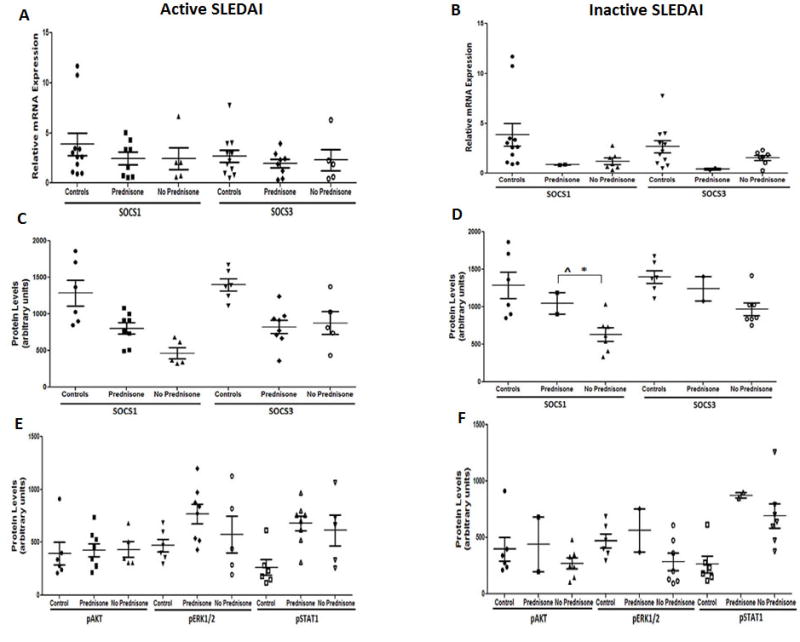
Active SLEDAI (N=13) and inactive SLEDAI (N=9) SLE patients with and without prednisone treatment were analyzed for SOCS1 and SOCS3 mRNA and protein expression relative to GAPDH in comparison to controls (N=6). mRNA expression of SOCS1 and SOCS3 is represented by graph (A) for Active SLEDAI and (B) for inactive SLEDAI. Protein expression of SOCS1 and SOCS3 is represented by graph (C) for Active SLEDAI and (D) for inactive SLEDAI. Phosphorylated protein expression for pAKT, pERK1/2, and pSTAT1 is represented by graph (E) for active SLEDAI and (F) for inactive SLEDAI. Each data point represents an individual subject. * represents significant Pearson correlation; ˆ represents a t-test with significant mean difference. Correlation was considered significant at 0.05 level (2 tailed).
Table-2.
Represents the data for figure-5 graphs; the table shows the average (mean±SEM) values and the analysis between the SLE patients (with Active (N=13) and Inactive SLEDAI (N=9) with and without prednisone in comparisons to controls (N=6), for mRNA and protein levels.
| Active SLEDAI | Inactive SLEDAI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| mRNA Expression | Controls | Prednisone | No Prednisone | Controls | Prednisone | No Prednisone | ||||||
|
| ||||||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
|
| ||||||||||||
| SOCSl | 3.850 | 1.13 | 2.42 | 0.60 | 2.42 | 1.10 | 3.850 | 1.13 | 0.86 | 0.020 | 1.20 | 0.32 |
|
| ||||||||||||
| SOCS3 | 2.665 | 0.62 | 1.93 | 0.40 | 2.28 | 1.00 | 2.665 | 0.62 | 0.42 | 0.090 | 1.54 | 0.25 |
|
| ||||||||||||
|
Protein Levels
| ||||||||||||
| SOCSl | 1286.0 | 175.4 | 801.8 | 76.3 | 463.8 | 75.5 | 1286.0 | 175.4 | 1046.0 | 143.0 | 630.3 | 89.4 |
|
| ||||||||||||
| SOCS3 | 1399.0 | 85.1 | 823.0 | 91.5 | 877.0 | 156.8 | 1399.0 | 85.1 | 1243.0 | 163.0 | 966.3 | 223.7 |
|
| ||||||||||||
|
Protein Levels
| ||||||||||||
| pAKT | 396.3 | 106.9 | 425.4 | 63.3 | 433.0 | 72.7 | 396.3 | 106.9 | 438.0 | 242.0 | 271.0 | 48.5 |
| pERKl/2 | 469.5 | 61.02 | 769.1 | 91.9 | 575.0 | 174.4 | 469.5 | 61.02 | 564.0 | 192.0 | 284.0 | 76.0 |
| pSTAT1 | 262.8 | 74.39 | 679.6 | 69.7 | 613.6 | 146.0 | 262.8 | 74.39 | 874.0 | 24.0 | 692.0 | 107.9 |
It has been previously shown that corticosteroids up-regulated SOCS1 message expression in a rodent model [35]. In order to assess the apparent distinctions between steroid induced message and protein levels in patients, and results previously published in a rodent model, we treated healthy control patients with dexamethasone and assessed SOCS1 mRNA over time. A statistically significant, dose dependent up-regulation of SOCS1 in response to steroid treatment was readily apparent at 3 hours and dissipated over time (Figure 6) Together, these data show that enhanced SOCS1 levels were present in patients receiving prednisone treatment, but with no observed differences in STAT1 phosphorylation.
Figure-6. Treatment of PBMCs with Dexamethasone, in vitro, causes SOCS1 upregulation.
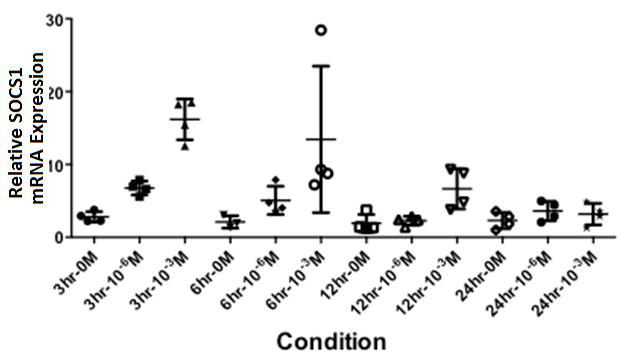
Graph showing SOCS1 mRNA expression, relative to β-actin, in healthy controls (N=4) subsequent to stimulation with dexamethasone (10-3 M or 10-6 M) for 3, 6, 12, and 24 hours
Discussion
SLE is a debilitating, multifactorial autoimmune disease possessing varied clinical manifestations and increased risk of premature mortality [1]. Although genetics play a critical role in SLE onset, notably lupus onset does not occur at birth suggesting a cumulative imbalance between SLE promoting events, and regulatory processes that promote immune homeostasis. It is therefore likely that obtaining a deeper understanding of tolerance mechanisms that inhibit lupus promoting immune responses will lead to novel intervention strategies. In this manuscript we show for the first time that SOCS1 and SOCS3, are lower in SLE patients by both message and protein expression. Although it is firmly established in rodent models that deficiencies in SOCS1 mediate lupus like pathology, to date the human studies that examine a relationship between SOCS1 and SLE bear conflicting results [17-20]. Three studies have previously shown higher levels of SOCS1 message in SLE patients, while one study did not find any difference in SOCS1 mRNA levels with respect to SLE. In two recent studies, SOCS1 message levels were indeed lower in SLE patients [36,37]. In agreement with the latter studies, our results show that message and protein levels of SOCS1 are lower in SLE patients compared to controls. Although the discrepancy in the SOCS1 levels among the patients in the various studies remains unclear, one possible area of distinction may lie in the different analysis methods used to measure the SOCS levels. Notably, the studies that observed higher levels of SOCS1 concluded their results based on only message levels, whereas our current study and Gabriela’s study [37] noted lower levels of SOCS1 through combined analysis of message and protein levels. A second possibility accounting for the discrepancy lies within the differential exclusionary criteria. In particular, the inclusion of patients receiving prednisone treatment, as our current human studies and previous rodent studies show that corticosteroids mediate the up-regulation of SOCS1 [38]. Indeed, in Chan’s study [17] , the authors note that inclusion of patients receiving prednisone may contribute to increased SOCS1 mRNA levels. In our study and Chan’s study [17] it was found that patients receiving prednisone treatment did in fact possess higher levels of SOCS1 message, although the overall levels (mRNA/Protein) in our study remained statistically lower than that of healthy controls. An additional source possibly contributing to the discrepancies within these results may lie in the well-established leukopenia/lymphopenia present in SLE patients [39]. Indeed, the PBMC of the SLE patients within our study possessed reduced amounts of CD3 mRNA (previously shown to be an accurate measure of T cell frequency) [40,41] compared to controls (Figure S1). As SOCS1 is induced by lymphoid and non-lymphoid cells, future research is required to determine whether differences within the PBMCs may be the result of distinct cell populations such as macrophages or T lymphocytes. It is also possible that genetic heterogeneity of the SLE patients with respect to inheritance, genetic susceptibility, ethnicity, disease manifestation, and disease phenotype may contribute to discrepancies in the results. As suggested by rodent studies, additional analyses of SOCS1 at the genetic and epigenetic level will need to be performed in the future [42,43]. Finally, although it is possible that limitations in sample size may have attributed to the recorded differences, we believe that our research has significant relevance as it provides a novel extension of the previous studies in showing a relationship between SOCS1 deficiency and MHC up-regulation. Therefore, in addition to showing reduced levels of SOCS1 in SLE patient PBMC in comparison to controls, our results also implicate a mechanistic role of SOCS1 deficiency in SLE disease progression as defects in antigen presentation have long been associated with the disease [44].
It is well established that the signal transducer and activator of transcription (STAT) family of intracellular proteins are essential in the propagation of cellular events mediated by the binding of cytokines to the cytokine specific extracellular receptor [45,46]. Although STAT signaling is critical in the generation of pro-inflammatory immune responses, it must also be tightly regulated to prevent the promotion of autoimmune events. Indeed, dysregulated interferon gamma (IFN) gamma and Type 1 interferon signaling, requiring STAT1, has been closely associated with the autoimmune disease SLE. Importantly, however, in addition to the transcription of pro-inflammatory factors, activated STAT1 also mediates the transcription of the immuno-modulatory protein SOCS1. Our data shows that, in contrast to healthy controls which possessed high levels of SOCS1 protein in relation to low levels of activated STAT1, a significant percentage of SLE patients possessed an inverted relationship between SOCS1 and STAT1. Mice deficient in SOCS1 die of 100% lethal, perinatal auto-inflammatory disease with excessive STAT1 activation. We have previously shown that in vivo administration of a peptide that mimicked the kinase inhibitory region of SOCS1 (SOCS1-KIR) to SOCS1 deficient mice prolonged life, limited IFN gamma production, and enhanced Foxp3+ regulatory T cells [47]. Although a positive correlation between SOCS1 and SOCS3 message levels was observed in both SLE patients and healthy controls, the overall levels were reduced in SLE patients.
Conclusions
SOCS proteins play a critical biological role in the duration of inflammation mediated by several immunological stimulations associated with SLE. The results, presented within this manuscript, are part of a growing body of evidence that suggest interventions which mimic the mechanisms of SOCS1 and SOCS3 may have efficacy in the treatment of SLE patients. Together, the results presented in this manuscript, in combination with the possible use of the therapeutics that target SOCS protein regulating mechanisms, provide necessary, relevant first steps showing the necessity of continued studies within this area. We believe that the studies presented herein, in combination with previous rodent studies utilizing immune modulating SOCS1 peptides, are critical first steps in establishing a mechanistic role of SOCS1 deficiency in the progression of SLE pathology among patients, and validating larger, more expansive translational studies.
Supplementary Material
Table S1: SOCS1 and SOCS3 protein levels in individual of Healthy controls included in the study
Table S2: Pearson correlation and t-test between SLE Patients Vs controls for SOCS1 and pSTAT1 protein levels
Table S3: Distinction in Patients bearing high pSTAT1/SOCS levels compared to low pSTAT1/SOCS1
Table S4: Disease Status information in patients possessing high pSTAT1/SOCS1 ratio compared to low pSTAT1/SOCS1 ratio
Figure S1: CD3e mRNA expression in SLE patients and Controls
Figure S2: Graphical representation of phosphorylated STAT1, AKT, ERK1/2 in comparison to SOCS1 and SOCS3.
Figure S3: Western blot analysis of SOCS1, SOCS3, pAKT, pERK1/2 and pSTAT1 in human SLE patients (N=32) and healthy controls (N=6) relative to loading control GAPDH.
Figure S4: (A) Simple linear regression correlating SOCS1 vs SOCS3 gene expression at the 95% confidence level within SLE patients. Correlation was considered significant at 0.05 level (2 tailed). (B) Graph showing SOCS3/SOCS1 ratios (protein levels) for SLE patients (N=32) and Controls (N=6), the mean values of SLE patients are represented as black dotted line. * represents significant Pearson correlation; ˆ represents a t-test with significant mean difference. Correlation was considered significant at 0.05 level (2 tailed).
Acknowledgments
We thank Dr. Howard M. Johnson for a generous gift of SOCS1 and SOCS3 antibodies. We also thank Dr. Laurence Morel for critical review of this manuscript and Dr. Tenisha Wilson for technical support. Dr. Westley Reeves and Ms. Annie Chan provided human samples through the UF Rheumatology Clinic. The study was supported by a grant from the Lupus Research Institute, a BD Biosciences Research Grant, the NIH/NCATS Clinical and Translational Science Awards to the University of Florida TL1 TR000066 and UL1TR000064, a sub-award from NIH/NIAID/ U01AI101990, and the University of Florida.
Abbreviations
- SLE
Systemic Lupus Erythematosus
- SOCS
Suppressor of cytokine signaling
- KIR
Kinase inhibitory region
- PBMC
Peripheral blood mononuclear cells
- IFN
Interferon
- SLEDAI
SLE disease activity Index
- SNP
Single nucleotide polymorphisms
- HC
Healthy Control
- JAK
Janus kinase
- STAT
Signal transducers and activators of transcription
- pSTAT
phosphorylated STAT
Footnotes
Author Contributions
BSG and JL3 wrote the first draft of manuscript. Jl3 contributed to the concept. JL3, BSG contributed to the design. All authors meet ICMJE criteria for authorship and approve this manuscript.
Competing Interests
The authors declare that there are no competing interests.
References
- 1.Yildirim-Toruner C, Diamond B. Current and novel therapeutics in the treatment of systemic lupus erythematosus. J Allergy Clin Immunol. 2011;127:303–12. doi: 10.1016/j.jaci.2010.12.1087. quiz 313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–9. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 4.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36:503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larkin J, 3rd, Ahmed CM, Wilson TD, Johnson HM. Regulation of interferon gamma signaling by suppressors of cytokine signaling and regulatory T cells. Front Immunol. 2013;4:469. doi: 10.3389/fimmu.2013.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 8.Sharabi A, Sthoeger ZM, Mahlab K, Lapter S, Zinger H, Mozes E. A tolerogenic peptide that induces suppressor of cytokine signaling (SOCS)-1 restores the aberrant control of IFN-gamma signaling in lupus-affected (NZB x NZW)F1 mice. Clin Immunol. 2009;133:61–8. doi: 10.1016/j.clim.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Hanada T, Yoshida H, Kato S, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–50. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto M, Tsutsui H, Xinshou O, et al. Inadequate induction of suppressor of cytokine signaling-1 causes systemic autoimmune diseases. Int Immunol. 2004;16:303–14. doi: 10.1093/intimm/dxh030. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp-1 in dendritic cells. J Exp Med. 2011;208:2193–9. doi: 10.1084/jem.20110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin J, 3rd, Ahmed CM, Wilson TD, Johnson HM. Regulation of interferon gamma signaling by suppressors of cytokine signaling and regulatory T cells. Front Immunol. 2013;4:469. doi: 10.3389/fimmu.2013.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–7. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 14.Seki Y, Inoue H, Nagata N, et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–54. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 15.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189:3439–48. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whyte CS, Bishop ET, Ruckerl D, et al. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol. 2011;90:845–54. doi: 10.1189/jlb.1110644. [DOI] [PubMed] [Google Scholar]
- 17.Chan HC, Ke LY, Chang LL, et al. Suppressor of cytokine signaling 1 gene expression and polymorphisms in systemic lupus erythematosus. Lupus. 2010;19:696–702. doi: 10.1177/0961203309357437. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhao S, Yi M, et al. Activation of JAK-STAT1 signal transduction pathway in lesional skin and monocytes from patients with systemic lupus erythematosus. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:109–15. doi: 10.3969/j.issn.1672-7347.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Komatsuda A, Wakui H, Iwamoto K, Sawada K. Up-regulated expression of suppressor of cytokine signalling (SOCS) proteins mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2009;27:1060. [PubMed] [Google Scholar]
- 20.Tsao JT, Kuo CC, Lin SC. The analysis of CIS, SOCS1, SOSC2 and SOCS3 transcript levels in peripheral blood mononuclear cells of systemic lupus erythematosus and rheumatoid arthritis patients. Clin Exp Med. 2008;8:179–85. doi: 10.1007/s10238-008-0006-0. [DOI] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 23.Dalpke AH, Eckerle S, Frey M, Heeg K. Triggering of Toll-like receptors modulates IFN-gamma signaling: involvement of serine 727 STAT1 phosphorylation and suppressors of cytokine signaling. Eur J Immunol. 2003;33:1776–87. doi: 10.1002/eji.200323621. [DOI] [PubMed] [Google Scholar]
- 24.Dalpke AH, Lehner MD, Hartung T, Heeg K. Differential effects of CpG-DNA in Toll-like receptor-2/-4/-9 tolerance and cross-tolerance. Immunology. 2005;116:203–12. doi: 10.1111/j.1365-2567.2005.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crespo A, Filla MB, Russell SW, Murphy WJ. Indirect induction of suppressor of cytokine signalling-1 in macrophages stimulated with bacterial lipopolysaccharide: partial role of autocrine/paracrine interferon-alpha/beta. Biochem J. 2000;349:99–104. doi: 10.1042/0264-6021:3490099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang XO, Zhang H, Kim BS, et al. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013;14:732–40. doi: 10.1038/ni.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Metze D, Nashan D, et al. Expression of SOCS-1, suppressor of cytokine signalling-1, in human melanoma. J Invest Dermatol. 2004;123:737–45. doi: 10.1111/j.0022-202X.2004.23408.x. [DOI] [PubMed] [Google Scholar]
- 28.Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008;181:3167–76. doi: 10.4049/jimmunol.181.5.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi N, Uemura H, Nagahama K, et al. Identification of miR-30d as a novel prognostic maker of prostate cancer. Oncotarget. 2012;3:1455–71. doi: 10.18632/oncotarget.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorgun G, Calabrese E, Soydan E, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–37. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sass G, Shembade ND, Tiegs G. Tumour necrosis factor alpha (TNF)-TNF receptor 1-inducible cytoprotective proteins in the mouse liver: relevance of suppressors of cytokine signalling. Biochem J. 2005;385:537–44. doi: 10.1042/BJ20040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–53. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 33.Chen XP, Losman JA, Cowan S, et al. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc Natl Acad Sci U S A. 2002;99:2175–80. doi: 10.1073/pnas.042035699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–29. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya S, Zhao Y, Kay TW, Muglia LJ. Glucocorticoids target suppressor of cytokine signaling 1 (SOCS1) and type 1 interferons to regulate Toll-like receptor-induced STAT1 activation. Proc Natl Acad Sci U S A. 2011;108:9554–9. doi: 10.1073/pnas.1017296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu LJ, Xu K, Liang Y, et al. Decreased SOCS1 mRNA expression levels in peripheral blood mononuclear cells from patients with systemic lupus erythematosus in a Chinese population. Clin Exp Med. 2014 doi: 10.1007/s10238-014-0309-2. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez-Velez G, Medina F, Ramirez-Montano L, et al. Constitutive phosphorylation of interferon receptor A-associated signaling proteins in systemic lupus erythematosus. PLoS One. 2012;7:e41414. doi: 10.1371/journal.pone.0041414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharyya S, Zhao Y, Kay TW, Muglia LJ. Glucocorticoids target suppressor of cytokine signaling 1 (SOCS1) and type 1 interferons to regulate Toll-like receptor-induced STAT1 activation. Proc Natl Acad Sci U S A. 2011;108:9554–9. doi: 10.1073/pnas.1017296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman K, Owlia MB, El-Hemaidi I, Akhtari M. Management of immune cytopenias in patients with systemic lupus erythematosus - Old and new. Autoimmun Rev. 2013;12:784–91. doi: 10.1016/j.autrev.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Ivakine EA, Gulban OM, Mortin-Toth SM, et al. Molecular genetic analysis of the Idd4 locus implicates the IFN response in type 1 diabetes susceptibility in nonobese diabetic mice. J Immunol. 2006;176:2976–90. doi: 10.4049/jimmunol.176.5.2976. [DOI] [PubMed] [Google Scholar]
- 41.Ivakine EA, Mortin-Toth SM, Gulban OM, et al. The idd4 locus displays sex-specific epistatic effects on type 1 diabetes susceptibility in nonobese diabetic mice. Diabetes. 2006;55:3611–9. doi: 10.2337/db06-0758. [DOI] [PubMed] [Google Scholar]
- 42.Dai R, Zhang Y, Khan D, et al. Identification of a common lupus disease-associated microRNA expression pattern in three different murine models of lupus. PLoS One. 2010;5:e14302. doi: 10.1371/journal.pone.0014302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Hou J, Lin L, et al. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–33. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 44.Relle M, Schwarting A. Role of MHC-linked susceptibility genes in the pathogenesis of human and murine lupus. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/584374. 584374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominguez-Gutierrez PR, Ceribelli A, Satoh M, Sobel ES, Reeves WH, Chan EK. Reduced levels of CCL2 and CXCL10 in systemic lupus erythematosus patients under treatment with prednisone, mycophenolate mofetil, or hydroxychloroquine, except in a high STAT1 subset. Arthritis Res Ther. 2014;16:R23. doi: 10.1186/ar4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karonitsch T, Feierl E, Steiner CW, et al. Activation of the interferon-gamma signaling pathway in systemic lupus erythematosus peripheral blood mononuclear cells. Arthritis Rheum. 2009;60:1463–71. doi: 10.1002/art.24449. [DOI] [PubMed] [Google Scholar]
- 47.Collins EL, Jager LD, Dabelic R, et al. Inhibition of SOCS1-/- lethal autoinflammatory disease correlated to enhanced peripheral Foxp3+ regulatory T cell homeostasis. J Immunol. 2011;187:2666–76. doi: 10.4049/jimmunol.1003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: SOCS1 and SOCS3 protein levels in individual of Healthy controls included in the study
Table S2: Pearson correlation and t-test between SLE Patients Vs controls for SOCS1 and pSTAT1 protein levels
Table S3: Distinction in Patients bearing high pSTAT1/SOCS levels compared to low pSTAT1/SOCS1
Table S4: Disease Status information in patients possessing high pSTAT1/SOCS1 ratio compared to low pSTAT1/SOCS1 ratio
Figure S1: CD3e mRNA expression in SLE patients and Controls
Figure S2: Graphical representation of phosphorylated STAT1, AKT, ERK1/2 in comparison to SOCS1 and SOCS3.
Figure S3: Western blot analysis of SOCS1, SOCS3, pAKT, pERK1/2 and pSTAT1 in human SLE patients (N=32) and healthy controls (N=6) relative to loading control GAPDH.
Figure S4: (A) Simple linear regression correlating SOCS1 vs SOCS3 gene expression at the 95% confidence level within SLE patients. Correlation was considered significant at 0.05 level (2 tailed). (B) Graph showing SOCS3/SOCS1 ratios (protein levels) for SLE patients (N=32) and Controls (N=6), the mean values of SLE patients are represented as black dotted line. * represents significant Pearson correlation; ˆ represents a t-test with significant mean difference. Correlation was considered significant at 0.05 level (2 tailed).


