Screening with synthesized two-dimensional imaging combined with digital breast tomosynthesis (DBT) maintains overall cancer detection rates and reduces recall rates while reducing radiation dose compared with screening with digital mammography combined with DBT.
Abstract
Purpose
To evaluate the early implementation of synthesized two-dimensional (s2D) mammography in a population screened entirely with s2D and digital breast tomosynthesis (DBT) (referred to as s2D/DBT) and compare recall rates and cancer detection rates to historic outcomes of digital mammography combined with DBT (referred to as digital mammography/DBT) screening.
Materials and Methods
This was an institutional review board–approved and HIPAA-compliant retrospective interpretation of prospectively acquired data with waiver of informed consent. Compared were recall rates, biopsy rates, cancer detection rates, and radiation dose for 15 571 women screened with digital mammography/DBT from October 1, 2011, to February 28, 2013, and 5366 women screened with s2D/DBT from January 7, 2015, to June 30, 2015. Two-sample z tests of equal proportions were used to determine statistical significance.
Results
Recall rate for s2D/DBT versus digital mammography/DBT was 7.1% versus 8.8%, respectively (P < .001). Biopsy rate for s2D/DBT versus digital mammography/DBT decreased (1.3% vs 2.0%, respectively; P = .001). There was no significant difference in cancer detection rate for s2D/DBT versus digital mammography/DBT (5.03 of 1000 vs 5.45 of 1000, respectively; P = .72). The average glandular dose was 39% lower in s2D/DBT versus digital mammography/DBT (4.88 mGy vs 7.97 mGy, respectively; P < .001).
Conclusion
Screening with s2D/DBT in a large urban practice resulted in similar outcomes compared with digital mammography/DBT imaging. Screening with s2D/DBT allowed for the benefits of DBT with a decrease in radiation dose compared with digital mammography/DBT.
© RSNA, 2016
An earlier incorrect version of this article appeared online. This article was corrected on August 11, 2016.
Introduction
Digital breast tomosynthesis (DBT) combined with full-field digital mammography decreases recall rates and improves invasive breast carcinoma detection rates compared with screening with digital mammography alone in both reader studies and prospective clinical trials (1–5). Although it is beneficial to outcomes, the addition of DBT to digital mammography screening increases radiation dose to patients (6–8) even though phantom doses still fall within the Mammography Quality Standards Act regulation limit of 3.0 mGy (9). Ideally, mammography screening should be performed at the lowest possible dose that maintains optimal benefits (10).
Synthesized two-dimensional (s2D) mammography, generated via slab reconstruction from the tomosynthesis acquisition, was approved by the U.S. Food and Drug Administration in May 2013 as an alternative to digital mammography in DBT screening. When s2D mammography is used in the place of digital mammography, the dose received by patients is only from the DBT study; this was estimated to be approximately 45% lower than that of a combination digital mammography and DBT (referred to as digital mammography/DBT) study (11). There have been a few reader studies and limited prospective studies that compared the performance of s2D mammography versus digital mammography/DBT in a clinical setting (11–15). Results were mixed: two prospective studies and one reader study showed similar performance between both combination modalities (11,13–15), whereas another reader study showed lower sensitivity and comparable specificity in s2D mammography and DBT (referred to as s2D/DBT) versus digital mammography/DBT. In one prospective study, symptomatic patients were included in the s2D mammography and digital mammography/DBT screening group so this cohort was not a true screening population (11). In another analysis, digital mammography was used to select cancer cases for reader interpretation, which may have led to a selection bias (14,15).
The purpose of our study was to evaluate the early implementation of s2D mammography in a population screened entirely with s2D/DBT and compare recall rates and cancer detection rates to historic outcomes of digital mammography/DBT screening. We hypothesized that there would be no difference in recall rates or cancer detection rates between groups.
Materials and Methods
Patient Data
Informed consent was waived for this Health Insurance Portability and Accountability Act–compliant and institutional review board–approved retrospective analysis of prospectively acquired data. We screened 15 571 women (mean age, 56.7 years) by using digital mammography/DBT from October 1, 2011, to February 28, 2013 (Dimension; Hologic, Bedford, Mass). From January 7, 2015, to June 30, 2015, 5366 women (mean age, 56.7 years) were screened with s2D/DBT. All patients who were referred for breast cancer screening at our institution during each time-point were included in our study populations.
The screening results were accessed through the electronic medical record (RIS, GE Centricity, Milwaukee, Wis; and Epic Systems Corporation, Madison, Wis). Studies were interpreted on dedicated workstations by one of six radiologists who specialized in breast imaging (including E.F.C., S.P.W., and E.S.M.) with experience that ranged from 8 to 26 years (median, 17 years; mean, 16.5 years). DBT imaging was added to the practice in September 2011. Our institution previously published results that compared the digital mammography/DBT group with 10 728 patients from a digital mammography–only screening population (2,3,16–19).
Image Acquisition
Technologic specifications for both the digital mammography and s2D mammographic examinations were similar and are as follows: The pixel size for digital mammography images is 0.070 × 0.070 mm. The pixel size for s2D varies depending on breast size and plane of reconstruction, but is approximately 0.100 × 0.100 mm. The larger pixel size arises because s2D is produced from reconstructed DBT images. As a result, the spatial resolution of s2D is slightly reduced compared with the digital mammography images (approximately 5.8 line pairs per millimeter vs 7.6 line pairs per millimeter measured at the midplane of a 5-cm breast).
Data Acquisition
Bilateral craniocaudal and mediolateral oblique images were obtained for each screening study. Patients were given a Breast Imaging Reporting and Data System (known as BI-RADS) score of 1 (negative), 2 (benign), or 0 (additional imaging needed) for each screening study. Patients who were given a BI-RADS score of 0 were reviewed in the digital mammography/DBT and s2D/DBT groups. Patient age, race, breast density, history of prior mammogram, lesion subtype that prompted recall (calcification, mass, asymmetry, architectural distortion, or technical recall), final BI-RADS assessment category, and biopsy outcome, if applicable, were recorded for each study. Breast density was recorded as the following: almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, or extremely dense (20). If more than one finding type was mentioned at the time of recall, both were recorded. Percentage recall rate, cancer detection rate per 1000 patients screened, and positive predictive values were calculated.
Statistical Analysis
Differences in demographics, breast density, and the availability of prior mammograms between women recalled after undergoing digital mammography/DBT versus s2D/DBT were assessed by using χ2 tests for categorical variables and two-sided t tests for continuous variables. Two-sample z tests of equal proportions were used to compare the rates of specific findings that prompted recall between the two cohorts and rates of screening outcomes. All statistical analyses in this study were performed in Stata 13.1 (Stata Statistical Software Release 13; StataCorp, College Station, Tex), and all statistical tests were two sided at the standard α significance level of .05. P values less than .05 were considered to indicate statistical significance. Post-hoc power analysis was conducted by using the PASS 14 statistical software package (PASS 14 Power Analysis and Sample Size Software; NCSS, Kaysville, Utah; www.ncss.com/software/pass).
Dose Calculations
A custom dose-tracking system was used to extract the exposure factors from the Digital Communications in Medicine (known as DICOM) header of the digital mammography and DBT images and store them in a relational database. The system consists of a custom DICOM service class provider and an intermediate data-cleaning component that maps the meta-data into a structured query language database by using an object relational mapper. Various DICOM meta-data were recorded, including the compressed breast thickness, and the radiographic factors (kilovolt, milliampere-second, filter material, and exposure). The radiographic factors with physical measurements of air kerma from each system were used to calculate the average glandular dose per image. The average glandular dose per study was then calculated from the per-image doses summed between views and averaged between breasts (6). Differences in average glandular dose and breast thickness were assessed with two-sided t tests for continuous variables.
Results
Patient characteristics of both screening groups are compared in Table 1. There were no clinically relevant differences in the two cohorts relative to age (P = .99) or race (P = .01). There was a significant difference in breast density between the groups with lower subjective assessment of density on s2D/DBT images compared with the digital mammography/DBT images (P < .001). There was also a significant difference in breast thickness between the groups with greater breast thickness in s2D/DBT (P < .001). The s2D/DBT group was also more likely to undergo baseline screenings or have no previous studies available for comparison (P = .002).
Table 1.
Patient Characteristics
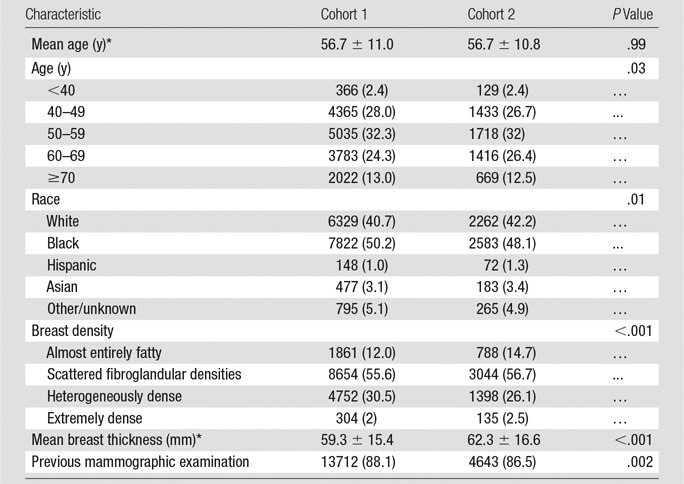
Note.—Unless otherwise indicated, data are numbers of patients and data in parentheses are percentages. Cohort 1 is digital mammography/DBT (n = 15 571). Cohort 2 is s2D/DBT (n = 5366).
*Data are ± standard deviation.
Recalls from the s2D/DBT and digital mammography/DBT cohorts were analyzed. Recall rate was significantly lower for s2D/DBT than with digital mammography/DBT (7.1% vs 8.8%, respectively; P < .001). There was no change in recalls for the finding types of masses (s2D/DBT vs digital mammography/DBT, 2.4% vs 2.7%, respectively; P = .31) or architectural distortion (s2D/DBT vs digital mammography/DBT, 1.1% vs 1.0%, respectively; P = .70), while significant decreases were observed for asymmetries (s2D/DBT vs digital mammography/DBT, 3.2% vs 4.5%, respectively; P ≤ .001) and calcifications (s2D/DBT vs digital mammography/DBT, 1.1% vs 1.6%, respectively; P = .02). There was also a significant decrease in technical recalls or recalls because of poor positioning, motion, or other technical factors (s2D/DBT vs digital mammography/DBT, 0.1% vs 0.2%, respectively; P = .03) (Table 2).
Table 2.
Comparison of Recall Types
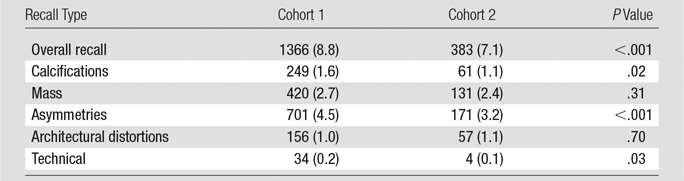
Note.—Unless otherwise indicated, data are numbers of patients and data in parentheses are percentages. Cohort 1 is digital mammography/DBT (n = 15 571). Cohort 2 is s2D/DBT (n = 5366).
Screening outcomes were compared between s2D/DBT and digital mammography/DBT. Biopsy rates were significantly decreased for s2D/DBT compared with digital mammography/DBT (s2D/DBT vs digital mammography/DBT, 1.3% vs 2.0%, respectively; P = .001). The number of cancers detected per 1000 screened was not statistically different (s2D/DBT vs digital mammography/DBT, 5.03 vs 5.45, respectively; P = .723). There was also no statistical difference between the proportion of invasive cancers versus ductal carcinoma in situ detected at s2D/DBT compared with digital mammography/DBT (invasive cancer: 4.10 per 1000 screened s2D/DBT vs 3.85 per 1000 screened [P = .840]; ductal carcinoma in situ: 0.9 per 1000 screened s2D/DBT vs 1.48 per 1000 screened [P = .301]). There was no difference in positive predictive value 1 (s2D/DBT vs digital mammography/DBT, 7.1% vs 6.2%, respectively; P = .548). Positive predictive value 2 trended toward an increase (s2D/DBT vs digital mammography/DBT, 35.5% vs 24.7%, respectively; P = .054). Positive predictive value 3 trended toward an increase (s2D/DBT vs digital mammography/DBT, 38.6% vs 27.0%, respectively; P = .053) (Table 3).
Table 3.
Screening Outcomes
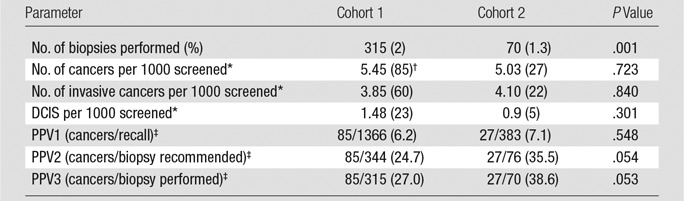
Note.—Unless otherwise indicated, data in parentheses are percentages. Cohort 1 is digital mammography/DBT (n = 15 571). Cohort 2 is s2D/DBT (n = 5366). DCIS = ductal carcinoma in situ, PPV = positive predictive value.
*Data in parentheses are total number of cancers.
†Includes one lymphoma and one lung metastasis in DM/DBT cohort, not counted as invasive or ductal carcinoma in situ.
‡Data are number of cancers/number of biopsies performed.
A post hoc secondary power analysis was performed to evaluate the power of our sample size to test for noninferiority with an α level of .025. We had 80% power to detect noninferiority from our historic recall rate of 8.8% with a margin of 1.257%, approximately the difference between our historical rate and the benchmark national average for recall rate of 10%, which provided a noninferiority threshold of 10.057%. Our findings suggest s2D/DBT, with a recall rate of 7.1% (95% confidence interval: 6.4%, 7.8%), could be considered noninferior to digital mammography/DBT because the upper limit of the 95% confidence interval for the difference between s2D/DBT and digital mammography/DBT recall rates (95% confidence interval: –2.5%, –0.88%) is less than the stated noninferiority margin of 1.257%.
Regarding biopsy rate, our sample size provided 80% power to detect noninferiority by using a margin of 0.63% from our baseline historical rate (2%), which provided a noninferiority threshold of 2.63% that is also below the national recommended limit of biopsy of 3%. Given this noninferiority threshold, we found that biopsy rate of s2D/DBT (observed biopsy rate of 1.3% [95% confidence interval: 1.0%, 1.6%]) may also be considered a noninferior alternative to digital mammography/DBT because the upper limit of the 95% confidence interval for the difference between s2D/DBT and digital mammography/DBT biopsy rates (95% confidence interval: –1.1%, –0.32%) falls below the noninferiority margin of 0.63%. However, the above findings need to be prospectively validated because the present study is underpowered to detect noninferiority in cancer detection rate and, subsequently, positive predictive value because the event is rare and proportions are low.
The average glandular dose per study was compared between s2D/DBT and digital mammography/DBT. The total dose was 39% lower in s2D/DBT compared with digital mammography/DBT (4.88 mGy vs 7.97 mGy, respectively; P < .001). For digital mammography/DBT, the average digital mammography and DBT doses were 3.77 mGy and 4.20 mGy, respectively. The s2D/DBT dose is attributable to the DBT images only (Table 4).
Table 4.
Average Glandular Dose

Note.—Data in parentheses are percentages. Cohort 1 is digital mammography/DBT (n = 15 571). Cohort 2 is s2D/DBT (n = 5366). DM = digital mammography.
Discussion
Digital mammography/DBT screening decreases recall rates and increases cancer detection rates compared with digital mammography alone. Early studies that evaluated s2D mammography found an initial drop in cancer detection when digital mammography was not available, though decreased recall remained (11–13,15). Our study indicates that s2D/DBT screening maintains overall cancer detection rates while reducing recall rates and significantly reducing radiation dose compared with digital mammography/DBT screening.
Our cohorts were well matched for age and race. There was a significant difference between our cohorts in breast density, with a trend toward decreased density in the s2D/DBT group. Breast density is a subjective assessment; decreased density may be a perceptive difference on the basis of the assessment of s2D mammography. Further research will evaluate whether the observed density differences persist after several years of s2D/DBT screening. Breast thickness was statistically different between both cohorts, but the actual difference was only 3 mm and thus is likely not clinically relevant; the statistical difference observed is secondary to our large sample sizes. Our s2D cohort was also more likely to not have undergone baseline screening or have previous studies available, despite attempts to retrieve earlier studies. While the reason for this is unknown, this should increase recall rates in the s2D population because women at baseline screening or without previous mammograms have higher recall rates (16).
Our analysis demonstrated decreased recall rates with s2D/DBT compared with digital mammography/DBT. When s2D mammography was approved by the U.S. Food and Drug Administration, there were concerns that it would lead to increased recalls for calcification-only lesions (12). Ligaments are enhanced in the s2D reconstruction algorithm and may appear as radio-opaque, calcific-like lesions (17). However, our results showed a decrease in recalls secondary to calcifications. This may be secondary to the ability of our readers to better evaluate calcifications while reviewing DBT after potential calcifications are observed with s2D. Long-term follow-up is needed to ensure that clinically significant calcific lesions are not passed as normal structures.
There was also a decrease of recalls secondary to asymmetries. This may be secondary to our increased experience with DBT and may not be related to s2D or digital mammography because there was a 2-year difference between both cohorts. Additionally, there was a decrease of recalls secondary to technical factors. This may be secondary to increased technologist familiarity with the DBT equipment over time. In addition, s2D/DBT may be less sensitive to patient motion because both the synthetic image and the DBT images were reconstructed, which made linear and feature sharpness more difficult to detect.
The recall rates for architectural distortions and masses were similar. This is likely because both masses and distortions are best resolved on DBT images, rather than on digital mammograms or s2D mammographic images. Overall, there was no increase in any recall finding type and overall recall rate decreased, which suggests a possible learning curve over time.
Our preliminary data showed a decrease in the number of biopsies performed, but a similar rate of both cancer detection and cancers detected per number of patients recalled. We found no statistical difference between the proportions of invasive cancer versus ductal carcinoma in situ detected in each group. Thus, the benefit of DBT for increased cancer detection remains with s2D/DBT, which contradicts an earlier report (15).
Screening with s2D/DBT reduced dose compared with digital mammography/DBT. The increase in DBT dose in the s2D cohort relative to the DBT dose in the digital mammography/DBT cohort is attributable to the practice of performing extra DBT views for large breasts in the s2D/DBT mode. Previously in the digital mammography/DBT mode, tiling of additional areas of tissue in larger breasts was performed with digital mammography, not with DBT. The difference is also in part because of the increase in breast thickness in the s2D cohort.
Our study had limitations. We only evaluated the use of s2D mammography at a single institution and with a single vendor, and we have not yet performed subanalyses on the basis of breast densities and thickness. Further research is necessary to see if this modality is suitable for women of all breast densities and thicknesses because the appearance of s2D mammography may vary greatly according to these factors (17). Additionally, we do not have adequate follow-up data to calculate the rate of false-negative findings at s2D/DBT screenings to ensure that this new technology is not missing cancers, as was suggested (15). Finally, our cohorts are from different periods, and s2D mammography was implemented after several years of experience interpreting DBT examinations. This may make our findings less generalizable to groups that are just implementing DBT. However, we recently published (18) data from 3 consecutive years of DBT screening from 2011 to 2014; our s2D/DBT recall rate is still significantly reduced compared with each year of DM/DBT screening (P < .001). Also, our overall cancer detection rate per 1000 women screened with s2D/DBT is not significantly different in each of the 3 consecutive years of screening (P = .68, .52, and .38, respectively). With these limitations, our study presents data from the prospective evaluation of an entire population screened with only s2D/DBT. Our data suggest that replacing DM with s2D mammography in DBT breast cancer screening yields screening outcomes similar to combination DM/DBT with a significantly lower radiation dose. The use of DBT in clinical practice is rapidly increasing, with some estimates now as high as 50% of practices that use DBT in the United States (21). Thus, this study has potential for clinical effect by providing preliminary evidence for a new technology that can reduce radiation exposure while maintaining improved breast cancer screening outcomes.
Advances in Knowledge
■ The cancer detection rate for synthesized two-dimensional (s2D) mammography with digital breast tomosynthesis (DBT)(referred to as s2D/DBT) was not significantly different compared with digital mammography and DBT (referred to as digital mammography/DBT) screening (5.03 of 1000 women screened vs 5.45 of 1000 screened, respectively; P = .72), which maintained one of the most important benefits of DBT screening.
■ Cancers per biopsy performed were higher in s2D/DBT compared with digital mammography/DBT screening (38.6% vs 27.0%, respectively; P = .053).
■ The recall rate for s2D/DBT significantly decreased compared with digital mammography/DBT screening (7.1% vs 8.8%, respectively; P < .001).
■ The recall rate for calcified lesions and asymmetries was lower with s2D/DBT compared with digital mammography/DBT screening (1.1% vs 1.6% [P = .02] and 3.2% vs 4.5% [P < .001], respectively).
■ The average glandular dose was 39% lower in s2D/DBT compared with digital mammography/DBT screening (4.88 mGy vs 7.97 mGy; P < .001).
Implications for Patient Care
■ Because of the reduction in radiation dose, the replacement of digital mammography with s2D mammography is beneficial to women who undergo breast cancer screening.
■ The results from this early implementation study demonstrate that s2D mammography may be an acceptable replacement to digital mammography/DBT in screening because reduced recall rates and cancer detection rates are maintained.
Received February 14, 2016; revision requested March 29; revision received April 24; accepted May 20; final version accepted May 24.
Study supported by National Cancer Institute (U54CA163313).
Disclosures of Conflicts of Interest: S.P.Z. disclosed no relevant relationships. E.F.C. Activities related to the present article: author disclosed consulting fees for Hologic and Siemens Healthcare. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. B.M.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author disclosed employment by Hologic that began after this study was completed. Other relationships: disclosed no relevant relationships. A.D.A.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author disclosed money paid by Gamma Medica and Real-time Tomography for board membership; grants from the Komen Foundation and NIH; payment for lectures including speakers bureaus from University of Washington and University of North Carolina; patents pending for a system and method for assessing cancer risk, radiographic contrast agents for temporal subtraction and dual-energy x-ray imaging, Super Resolution Tomosynthesis Systems and Methods, and dynamic four-dimensional contrast enhanced tomosynthesis; and stock or stock options in Real-time Tomography. Other relationships: disclosed no relevant relationships. B.B. disclosed no relevant relationships. S.P.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author disclosed personal fees from Siemens for consultancy. Other relationships: disclosed no relevant relationships. M.S. disclosed no relevant relationships. E.S.M. disclosed no relevant relationships.
Abbreviations:
- DBT
- digital breast tomosynthesis
- s2D
- synthesized two-dimensional
References
- 1.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013;14(7):583–589. [DOI] [PubMed] [Google Scholar]
- 2.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014;311(24):2499–2507. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy AM, Kontos D, Synnestvedt M, et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. J Natl Cancer Inst 2014;106(11):dju316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafferty EA, Park JM, Philpotts LE, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology 2013;266(1):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rafferty EA, Park JM, Philpotts LE, et al. Diagnostic accuracy and recall rates for digital mammography and digital mammography combined with one-view and two-view tomosynthesis: results of an enriched reader study. AJR Am J Roentgenol 2014;202(2):273–281. [DOI] [PubMed] [Google Scholar]
- 6.Barufaldi B, Zuckerman SP, Synnestvedt M, et al. Impact of 2D reconstructed mammograms on patient dose in the clinical practice of tomosynthesis [abstr]. In: Radiological Society of North America Scientific Assembly and Annual Meeting Program. Oak Brook, Ill: Radiological Society of North America, 2015; 223. [Google Scholar]
- 7.Skaane P, Bandos AI, Gullien R, et al. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening programme using independent double reading with arbitration. Eur Radiol 2013;23(8):2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015;24(2):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mammography Quality Standards Act Regulations, Part 900.12(e)(5)(vi). Effective April 28, 1999. Amended February 6, 2002.
- 10.Miglioretti DL, Lange J, van den Broek JJ, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med 2016;164(4):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaane P, Bandos AI, Eben EB, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology 2014;271(3):655–663. [DOI] [PubMed] [Google Scholar]
- 12.Gur D, Zuley ML, Anello MI, et al. Dose reduction in digital breast tomosynthesis (DBT) screening using synthetically reconstructed projection images: an observer performance study. Acad Radiol 2012;19(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuley ML, Guo B, Catullo VJ, et al. Comparison of two-dimensional synthesized mammograms versus original digital mammograms alone and in combination with tomosynthesis images. Radiology 2014;271(3):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert FJ, Tucker L, Gillan MG, et al. Accuracy of digital breast tomosynthesis for depicting breast cancer subgroups in a UK retrospective reading study (TOMMY Trial). Radiology 2015;277(3):697–706. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert FJ, Tucker L, Gillan MG, et al. The TOMMY trial: a comparison of TOMosynthesis with digital MammographY in the UK NHS Breast Screening Programme–a multicentre retrospective reading study comparing the diagnostic performance of digital breast tomosynthesis and digital mammography with digital mammography alone. Health Technol Assess 2015;19(4):i–xxv, 1–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald ES, McCarthy AM, Akhtar AL, Synnestvedt MB, Schnall M, Conant EF. Baseline screening mammography: performance of full-field digital mammography versus digital breast tomosynthesis. AJR Am J Roentgenol 2015;205(5):1143–1148. [DOI] [PubMed] [Google Scholar]
- 17.Zuckerman SP, Conant EF, Weinstein S, Korhonen K, Synnestvedt M, McDonald E. Early implementation of synthesized 2D in screening with digital breast tomosynthesis: a pictorial essay of early outcomes [abstr]. In: Radiological Society of North America Scientific Assembly and Annual Meeting Program. Oak Brook, Ill: Radiological Society of North America, 2015; 220. [Google Scholar]
- 18.McDonald ES, Oustimov A, Weinstein SP, Synnestvedt M, Schnall M, Conant EC. Effectiveness of tomosynthesis in combination with digital mammography: outcomes analysis from three years of breast cancer screening. JAMA Oncol 2016;2(6):737–743. [DOI] [PubMed] [Google Scholar]
- 19.Conant EF, Beaber EF, Sprague BL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat 2016;156(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: ACR BI-RADS Atlas , Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 21.Houssami N, Miglioretti DL. Digital breast tomosynthesis: a brave new world of mammography screening. JAMA Oncol 2016;2(6):725–727. [DOI] [PubMed] [Google Scholar]


