Abstract
Mangrove crabs influence ecosystem processes through bioturbation and/or litter feeding. In Brazilian mangroves, the abundant and commercially important crab Ucides cordatus is the main faunal modifier of microtopography establishing up to 2 m deep burrows. They process more than 70% of the leaf litter and propagule production, thus promoting microbial degradation of detritus and benefiting microbe-feeding fiddler crabs. The accelerated nutrient turn-over and increased sediment oxygenation mediated by U. cordatus may enhance mangrove tree growth. Such positive feed-back loop was tested in North Brazil through a one year crab removal experiment simulating increased harvesting rates in a mature Rhizophora mangle forest. Investigated response parameters were sediment salinity, organic matter content, CO2 efflux rates of the surface sediment, and reduction potential. We also determined stipule fall of the mangrove tree R. mangle as a proxy for tree growth. Three treatments were applied to twelve experimental plots (13 m × 13 m each): crab removal, disturbance control and control. Within one year, the number of U. cordatus burrows inside the four removal plots decreased on average to 52% of the initial number. Despite this distinct reduction in burrow density of this large bioturbator, none of the measured parameters differed between treatments. Instead, most parameters were clearly influenced by seasonal changes in precipitation. Hence, in the studied R. mangle forest, abiotic factors seem to be more important drivers of ecosystem processes than factors mediated by U. cordatus, at least within the studied timespan of one year.
Introduction
Burrowing crabs are ecosystem engineers and their importance for sediment processes has been discussed for many years [1–5], along with effects of their feeding activities on forest structure and nutrient cycling [2,6–11]. To determine the ecological roles and ecosystemic importance of burrowing crabs, addition or exclusion and removal experiments have been performed. In such experiments the size of the experimental plots varies depending on the size and density of the crabs and the underlying research questions. For example, for determining the effects of exclusion of small fiddler crabs (Uca spp.) on the growth of mangrove seedlings, small scale experiments with plot sizes of 1 m × 1 m proved to be sufficient [12]. Assessing the effects of larger crabs in mature forests requires larger plot sizes and longer experimental time frames, and thus considerably more effort. Only one such study, excluding crabs for 12 months from three 225 m2 plots, has been performed to date in Australia [5].
Exclusion of burrowing fiddler crabs from salt marshes led to an increase in meiofaunal density, probably due to reduced competition for food (bacteria, microphytobenthos) on the sediment surface [13,14]. In other studies, however, a reduction of crab burrows decreased the density of associated meio- and macrobentic infauna [15,16]. Reduced bioturbation can result in more saline and reduced sediment conditions and also affect the growth of mangrove seedlings [12,17]. Removal of bioturbating fiddler crabs also decreased microbial activity, fungal decomposition and leaching rates of organic matter in the upper sediment layer of salt marshes [18,19]. Similarly, crab removal in an Australian mangrove forest, and the only experiment to date with larger plot sizes, resulted in increased concentrations of sulfide and ammonium in the sediment and decreased leaf production of trees, as indicated by a significantly reduced stipule fall rate [5].
Most past research efforts to investigate the ecosystemic role of crabs have focussed on smaller burrowing species and small-scale experiments, and on crabs from the Indo-West-Pacific (IWP). Results from the IWP region are not necessarily representative for the Atlantic-east-Pacific (AEP) system, since flora and invertebrate fauna of the latter biogeographical area is much less diverse [20].
On the Atlantic side of the AEP, mangrove microtopography is dominated by the large burrowing crab Ucides cordatus (Ucididae). The species is an obligate mangrove dweller, living in up to 2 m deep burrows [9]. In Northern Brazil, U. cordatus occurs at average densities of 1.7 individuals m-2 [21]. Due to its large size (carapace width up to 10 cm [22]), it provides 63% of the total faunal biomass compared to sympatric fiddler crabs, which contribute 12% with approximately 19 individuals m-2 [7]. U. cordatus sustains its high biomass by processing more than two thirds of the annual mangrove litter and propagule production in the high intertidal N-Brazilian forest, most of which would otherwise be exported by the tides [8,9]. However, since U. cordatus assimilates only parts of the energy inherent in the food, a large percentage of the matter remains as leaf fragments, due to sloppy feeding, or as faeces, becoming available for decomposing bacteria.
Koch and Wolff [7] hypothesized for this system that fiddler crabs feed on bacteria, which in turn feed on the leaf remains from U. cordatus. They further postulated a positive feedback effect on the primary production, since nutrients in the leaf litter are first retained by the feeding activity of U. cordatus and then re-mineralized by bacteria, thereby becoming available to the trees. The authors additionally hypothesized that a significant reduction or loss of one of the model's components would have a negative impact on the remaining components, consequently affecting primary production If, for example, crab numbers decreased significantly in an otherwise healthy mangrove forest, nutrients locked in leaf litter would no longer be retained in the system under this scenario. Mangrove trees would suffer from decreased bioturbation, since e.g. the crabs’ burrows facilitate sediment oxygenation, preventing the formation of phytotoxins such as H2S [7]. Burrowing crabs may also impact sediment desalination, the reduction state, the organic matter decomposition and thereby CO2 efflux rates of the sediment, which in turn may drive changes in stipule production.
No manipulative experiment has been conducted yet to assess the above predictions. It is however important to understand the functional role of U. cordatus in the mangrove ecosystem, given re-occurring crab population declines in North-eastern Brazil caused by a spreading fungal disease (“Lethargic crab disease” [23,24]) and increasing fishing pressure across the country due to the application of a new illegal capture technique [25]. U. cordatus is economically important, providing the livelihood for thousands of artisanal fishermen in Brazil. In Northern Brazil, approximately 7 tons per km2 are captured per year [26]. Due to its slow growth [27,28] the species is listed in Brazilian legislation under the category “species at risk of overexploitation or overfished” [29].
We have conducted a one year U. cordatus removal experiment in the mangrove forest of the Northern Brazilian Caeté estuary to investigate possible ecosystemic effects of significantly decreased crab numbers, such as would result from a significantly increased fishing pressure. The following sediment parameters were monitored: salinity, organic matter content, CO2 efflux rate of the surface sediment (as a proxy for microbial carbon degradation) and reduction potential. To assess impacts on biota, tree leaf production using stipule fall as proxy [27] were assessed.
We hypothesize that a reduction of the number of U. cordatus leads to 1) increasing sediment salinity, 2) decreasing organic matter content of the sediment, 3) reduced CO2 efflux rates of the surface sediment due to a decrease in organic matter and 4) more reduced conditions in the sediment. Further, we predict that the reduction of U. cordatus will 5) decrease stipule production.
Material and Methods
Study area
The study was performed in a mangrove forest in the Caeté estuary, Pará state, North Brazil. Field work permission was granted by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO) and the Stakeholder Council of the Extrativist Reserve Caeté-Taperaçu, SISBIO Authorization number: 30007–1. The removal experiment was implemented near the tidal channel Furo Grande on the Ajuruteua peninsula (46°38’W 0°50’S). At the study site, the dominant mangrove tree species is Rhizophora mangle L. (Rhizophoraceae). Other mangrove tree species are Avicennia germinans (L.) L. (Acanthaceae) and Laguncularia racemosa (L.) C. F. Gaertn. (Combretaceae) [30].
The region has semidiurnal tides with amplitudes of 2 to 5 m [31,32]. Mean annual temperature for the study years 2011 and 2012 was 26.1°C (Tracuateua weather station, 50 km from the study site). Precipitation was 2621 mm in 2011 and decreased to 1552 mm in 2012 [33]. The wet season occurs typically from January to August and the dry season (monthly precipitation < 100 mm) from September to December [32].
Experimental design
Twelve experimental plots (13 m × 13 m) were established in the high intertidal zone containing exclusively R. mangle trees (6–18 trees per plot, up to 15 m height), with a minimum distance of 15 m in-between them. To reduce variability, plots with similar inundation frequencies were chosen, as indicated by height range of algal growth on the trees' stems, and visually similar sediment characteristics. Each plot was placed around a central mature R. mangle tree. The diameter of central trees (measured in the cylindrical portion of the stem 50 cm above the highest stilt root) ranged between 18 and 26.5 cm (mean ± standard error: 21.7 ± 0.8), central tree heights ranged between 10–14 m. All above ground stilt roots of the central tree, which roughly mirror the extension of below ground roots [32], were within the borders of the plot at the onset of the experiment, and none had grown to the outside by the end of the experiment. We therefore assume that the central tree's root system was exclusively under influence of the sediment conditions induced by the experimental treatments of the respective plots (see below).
Plots were randomly assigned to three treatments (crab removal, disturbance control and control; with four replicates each). Possible side effects of the crab removal procedure (see details below) were assessed by the disturbance control plots, where crab removal was only simulated, following the approach of Smith et al. [5]. We added an additional replication per treatment (n = 4), compared to the experimental design of Smith et al. [5]. In each plot, sediment samples were taken to measure a number of abiotic sediment parameters (sediment salinity, sediment organic matter content, reduction potential, CO2 efflux rates from the sediment) and one biotic parameter (leaf production estimated by the stipule fall rate); a detailed description of the respective procedures is given below. Three replicate sampling points were randomly chosen inside each plot during each sampling campaign, but in a distance of at least 15 cm away from U. cordatus burrow entrances and from spots where superficial R. mangle roots touched the sediment surface. This allowed the investigation of potential larger scale ecosystemic changes beyond the immediate neighbourhood of burrows or roots. The selected parameters were measured at all three points (reduction potential, CO2 efflux rate) or at a subset of two (salinity, organic matter).
The experiment ran from 19/11/2011 until 04/11/2012. Sediment salinity, organic matter, CO2 efflux rate and reduction potential were measured during eight sampling campaigns. From November 2011 until January 2012 the sampling of the above parameters was conducted monthly, thereafter in intervals of six weeks until April 2012 and after that every two months until November 2012. Stipule fall was assessed by biweekly collection of material in litter traps (24 samplings in total).
Tidal inundation
Water pressure data loggers (HOBO U20, onset) were employed from 05/03/2012 to 15/03/2012 to determine water levels and calculate inundation frequencies. To extrapolate the data to the entire study period, the pressure data obtained for the 10 days were matched with the tide table for the nearest site from the Brazilian National Oceanographic Database (Banco Nacional de Dados Oceanográficos, BNDO, Fundeadouro de Salinópolis, http://www.mar.mil.br, 2012, 80 km northwest from the study site). Salinity and temperature readings of tidal surface water were taken in the middle of the tidal channel Furo Grande, approximately 300 m away from the experimental plots. Readings were taken in the morning and late afternoon of each sampling day.
Crab removal
The term “crab removal” rather than of “crab exclusion” is used since no fences or other artificial borders were applied around the experimental plots. This way likely side effects of fencing in this dynamic macrotidal environment, such as changes in sediment deposition and leaf export, were avoided [12,13,19]. U. cordatus specimens were caught from removal plots by deploying approximately 400 nylon nets (20 cm × 30 cm) per sampling day. The capture technique was modified after a technique called “redinha” (tangle-netting), used illegally by crab fishermen in many other parts of Brazil [34,35]. Each net was fixed to the ground with one cutting of R. mangle aerial roots (25 cm, long; obtained outside the experimental plots) inserted into the sediment in front a crab burrow. Cuttings were rinsed and dried before applying them to minimize leaching into the sediment. The nets were then slightly pushed into the burrow entrances. The following day, crabs entangled in the nets were counted and the carapace width (cm) of all or every second individual (if there were more than 10) recorded. Due to the activity of crab eating raccoons (Procyon cancrivorus) and other predators, captured crabs had frequently been consumed before the tangle-nets could be controlled, indicated by crab remains. These remains were also counted and carapace widths measured, if possible. All survivors were released sufficiently far away from the experimental plots to prevent re-immigration. During the application of nets, care was taken to minimize sediment disturbance, e.g. by using firm stilt roots as walkways. Crab removal started with the first sampling campaign in November 2011 and was conducted biweekly for 3–6 days (3 days sampling during crab catching campaigns and 6 days during campaigns for sampling environmental parameters only) for each plot during neap tides over one year. Crab removal was conducted during neap tides, because crabs are more active then, and close their burrows less frequently than during spring tides [36]. Capture success was calculated for each removal plot by dividing the number of captured living crabs and carapace remains by the number of installed nets and the number of capture days (crabs d-1 net-1). Additional manual removal of crabs moving around freely inside the plots was necessary during mass mate searching events during spring tides [37]. These events occurred after new moon in January 2012 and after full moon in February and March 2012. Crab burrow density (burrows m-2) was monitored every 4–5 weeks over the entire study period by counting closed and open burrows in always the same two 1 m × 13 m subplots per removal plot. For the disturbance control plots the applied capture technique was simulated by pushing the mangrove cuttings without nets into the ground, followed by removal of the cutting, and stressing the tree roots by walking over them to a similar extent as in the removal plots.
Organic matter content and salinity of the sediment
Two sediment cores were taken per plot and sampling campaign with a peat sampler (Eijkelkamp) of 50 cm length and 6 cm diameter. Subsamples of the extracted sediment were taken at core depths of 1, 5, 10, 20, 30, 40, and 50 cm, filled into plastic vials and stored at temperatures ≤ 0°C until further processing. Samples were homogenized and divided into two portions. One portion was used for the gravimetrical determination of the water content of the sediment through weight loss by drying at 104°C. The organic matter content of the dry sample was obtained subsequently through weight loss by combustion at 450°C. The second portion was used to analyse sediment salinity. Two grams of the sediment were mixed with 10 ml of distilled water and shaken for 24 h on a mechanical shaker (MA136, Marconi). Afterwards, the salinity of the sediment extract was measured with a WTW TetraCon 325 conductivity meter connected to a WTW multi-parameter instrument (340i). Sediment salinity was calculated based on the previously measured original water content of the respective subsample [38].
CO2 efflux rate of the surface sediment
Six CO2 efflux rate measurements of the surface sediment were performed in each of the twelve plots at each sampling date. At each of the three sampling points (see above), two PVC collars of 20 cm diameter were inserted several centimetres into the sediment void of visible roots, U. cordatus burrows and mostly also void of burrows of other crab species. These two collars were handled as replicates for one sampling point. A distance of 40–50 cm was maintained between the two collars at each sampling point, large enough to ensure that the disturbance created by the insertion of one collar into the sediment would not affect the sediment of the other collar and small enough to represent the sampling point. To avoid any influence of CO2 release due to the insertion of the collars, measurements were started 1 h after the installation. An opaque respiration chamber was connected to a CO2/H2O infrared gas analyser (LI-8100A, LI-COR, Biosciences) and fitted on top of the PVC collar. The CO2 concentration inside the chamber was recorded for 2 min. The measurement was repeated four times per collar. Between replicates, the chamber was opened for 25 s to release the accumulated CO2. Sediment temperature was measured outside the collar at 2 cm sediment depth by thermocouple (OMEGA Engineering). The CO2 efflux rates were calculated [38], assuming a linear increase in CO2 concentration over time. A correction for changing sediment temperature was applied.
Reduction potential
Three sediment cores were taken in each plot per sampling date at the three sampling points. Redox potential (± 1.0 mV), pH (± 0.1) and temperature (± 0.1°C) were measured within the sediment cores immediately after their extraction at 1, 5, 10, 20, 30, 40, and 50 cm depth with a Sartorius ORP (redox) combination electrode and a WTW Sentix 41 pH-electrode connected to a WTW portable meter (Multi 340i), respectively.
As indicator of the reduction force of a reduction system, the rH value was calculated including the redox potential, temperature and pH value of each measurement [39]. rH values range between 0 (strongly reducing conditions) and 42 (strongly oxidizing conditions).
Stipule production
Stipule fall of R. mangle trees is related to the unfolding of new pairs of leaves. It can therefore be used as an indicator for the leaf production of these trees [40–42]. Stipule fall in the genus Rhizophora is known to be influenced by sediment characteristics [40–42] and was shown to respond to changing sediment conditions within one year [5].
Litter of the central tree in each plot was sampled with two litter traps (0.25 m2 each) fixed to the stem at 50 cm horizontal distance in 5–7 m height to ensure autochthonous litter. Traps were emptied biweekly. Stipules were separated from other litter components and dried at 104°C to constant weight (g). Stipule dry matter from both collectors was pooled and stipule fall rates (g m-2 d-1) calculated.
Statistical analyses
The statistical analyses were carried out following the protocols for data exploration and analysis of Zuur et al. [43,44] using the statistical programming environment R [45] with the packages “nlme” [46], “mgcv” [47], “lattice” [48] and “ggplot2” [49]. Presented values are shown as mean ± standard error (se). Before further analyses, data were checked for outliers (Cook's distance), which were removed, if necessary. All data for the analysis are available in the supporting information (S1 File).
Carapace widths of the captured crabs at each plot were analysed for differences over time with a one-way ANOVA. Linear mixed-effects models (LME) and generalized additive mixed-effects models (GAMM) [47,50,44,51] were used to model individual response variables (sediment salinity, organic matter content, CO2 efflux rate and reduction potential/rH) in relation to different treatments, time and their interaction effect. In some models (including sediment salinity, organic matter content and rH), sediment depth was considered as an additional covariate. Plot and sampling points within plots (when appropriate) were used as random terms to account for the nested structure of the experimental design. When trends over sediment depth or time were not linear, these covariates were set as categorical covariates. To find the optimal fixed terms, stepwise backward model selection was used based on the maximum likelihood ratio test (ML) and/or the Akaike Information Criterion (AIC). When an interaction was part of the final model, all participating main factors were automatically retained. The validity of the models was checked by examining diagnostic plots of residual versus fitted values and residuals versus covariates. Independence was examined by plotting residuals versus time. Final models were presented with the restricted maximum likelihood estimation method (REML).
Stipule data were analysed with a GAMM model for differences among treatments over time. GAMM's are non-parametric regression models and allow, in the case of the stipule data, for nonlinear trends over time with a smoothing function for the predictor variable time.
Results
Inundation levels
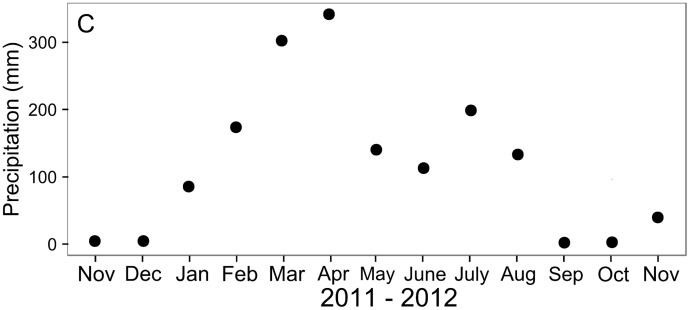
All twelve experimental plots had similar inundation levels and were flooded during high tide on 131 days out of the 355 days of the study period. This corresponded to a flooding frequency of 14–19 days per month. Surface water salinity at the Furo Grande varied from 22.8 to 36.9 during the twelve months. Lowest salinities were recorded during periods of high rainfall (Fig 1). Surface water temperatures ranged between 27.1°C and 30.5°C.
Fig 1. Precipitation data of the study area.
Total monthly precipitation (mm) recorded at the weather station in Tracuateua, 50 km southwest from the study site (INMET, 2013). Data sets are from November 2011 until November 2012.
Crab removal
It proved logistically feasible to set up to approximately 400 nylon nets per sampling day and per removal plot, which initially covered around 50% of all U. cordatus burrows and, at six months of the experiment, around 90–100% of all U. cordatus burrows. In total, 4866 crabs were caught during the one year study, including live crabs (2872), and remains of dead crabs (1994). Additional crab parts scattered by predators within plots of further 1563 captured crabs were counted, but not included in the capture success calculation for a more conservative estimate. During mass mate searching events 844 crabs were additionally caught by hand inside the removal plots. Capture success varied over the year from 0 to 0.2 crabs d-1 net-1 with a higher success during the mass mate searching events between January and March 2012 (S1 Fig). Crab burrow density in the removal plots slowly decreased until stabilizing more or less towards the end of the experiment. Burrow density inside the removal plots decreased on average to 52% of the initial number (Table 1, data from other plots and samplings are listed in in the supporting material in S1 Table). The carapace width of the captured U. cordatus specimens decreased over time in removal plot 1 (F-value = 50.4, df = 1, p-value < 0.001) and 3 (F-value = 38.9, df = 1, p-value < 0.001), showing that the larger animals (the more efficient bioturbators) were constantly removed. However, it did not differ over the year in removal plot 2 (F-value = 2.6, df = 1, p-value = 0.1) and 4 (F-value = 0.3, df = 1, p-value = 0.6) (Table 2).
Table 1. Crab burrow density.
U. cordatus burrow density (burrows m-2) inside the four removal plots for the first and last sampling. Decrease in burrow density from the first until the last sampling is given in %. Data from the other plots and sampling occasions are listed in the supporting material (S1 Table).
| Removal plot | 1. sampling | 8. sampling | Decrease in % |
|---|---|---|---|
| 1 | 4.7 | 2.1 | 55.3 |
| 2 | 6.7 | 3.9 | 41.8 |
| 3 | 3.1 | 0.5 | 83.9 |
| 4 | 4.4a | 3.2 | 27.3 |
a Crab burrow density of plot 4 was estimated one month after the first sampling; decrease in % may thus be underestimated.
Table 2. Mean carapace width of U. cordatus.
Mean carapace width ± standard deviation of captured crabs and carapace remains of the first and last sampling. Sample size is given in the brackets. The change of the carapace width from the first to the last sampling is given in % and its statistical significance is given in the brackets (p).
| Removal plot | 1. sampling | 8. sampling | Change in % (p-value) |
|---|---|---|---|
| 1 | 6.5 ± 0.8 (35) | 5.2 ± 1.0 (25) | - 20 (p < 0.001) |
| 2 | 5.1 ± 0.7 (35) | 5.4 ± 0.9 (30) | + 6 (p = 0.1) |
| 3 | 6.1 ± 0.9 (18) | 5.7 ± 1.0 (25) | - 7 (p < 0.001) |
| 4 | 5.0 ± 0.7 (50) | 5.1 ± 1.0 (43) | + 2 (p = 0.6) |
Sediment parameters
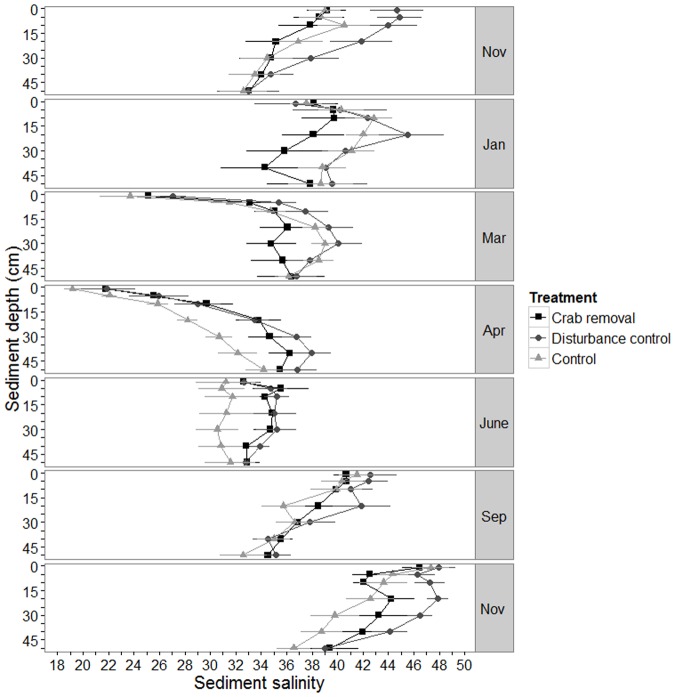
No consistent difference between treatments in respect to sediment salinity across depth and time were detected. In months with significant rainfall, salinity was lowest near the sediment surface and increased gradually with depth (Fig 2, March and April). In the dryer months salinities tended to be high over the whole sediment depth range. This relationship was reflected by the final LME model including a three way interaction (L. Ratio = 26.2, df = 12, p-value = 0.01, S2 File).
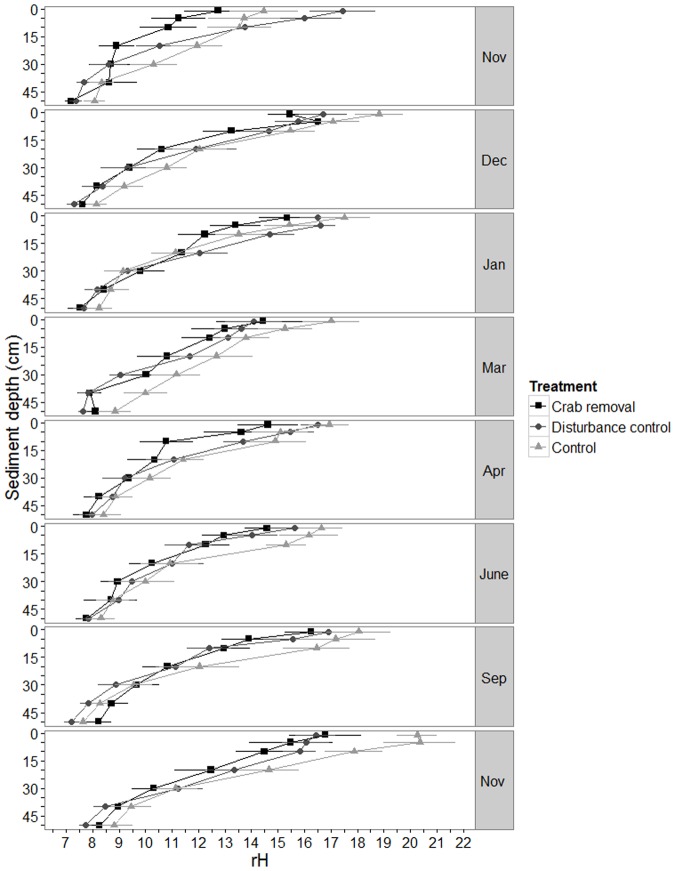
Fig 2. Measured sediment salinity.
Mean sediment salinity ± standard error (se) over sediment depth (cm) in crab removal plots, disturbance control plots and control plots (see legend for symbols). The data of seven sampling campaigns between November 2011 and November 2012 are plotted. Values for the second sampling in December are missing because of technical problems.
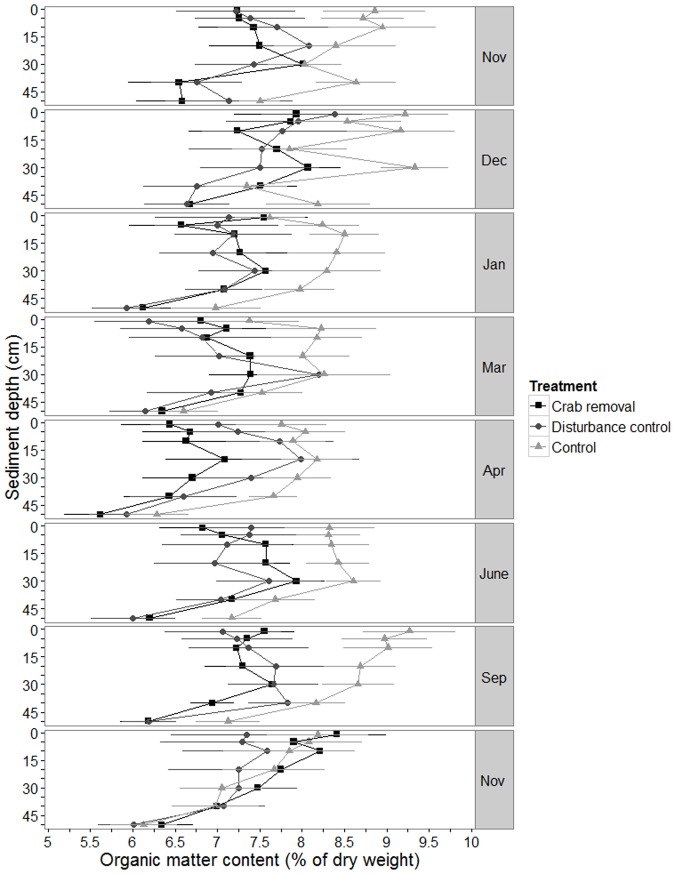
Treatments for the organic matter content did not differ among each other over time (Fig 3) (interaction term treatment × time was not significant, L. Ratio = 2.0, df = 2, p-value = 0.4, S3 File). However, organic matter content was generally lowest at the greatest depth. The form of the organic matter-depth curves differed slightly between treatments at each sampling campaign (interaction term sediment depth × treatment: L. Ratio = 8.7, df = 2, p-value = 0.01, S2 Table); control values were higher than those of the other two treatments for most sampling date/depth combinations (Fig 3). Changes also occurred among sampling campaigns as reflected by the significant interaction term sediment depth × time included in the final model (L. Ratio = 6.2, df = 1, p-value = 0.01, S3 File).
Fig 3. Measured organic matter content.
Mean organic matter content ± standard error (se) (% of dry mass) over sediment depth (cm) in crab removal plots, disturbance control plots and control plots (see legend for symbols). The data of eight sampling campaigns between November 2011 and November 2012 are plotted.
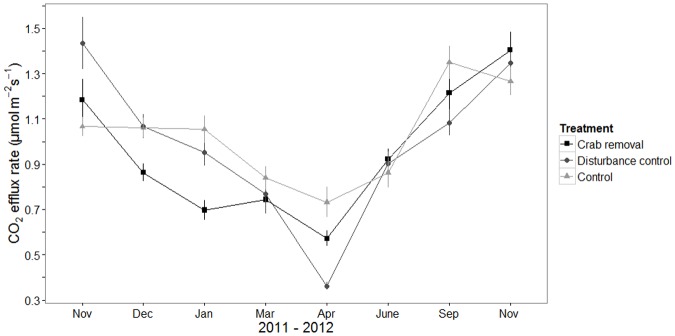
The CO2 efflux rate of the surface sediment showed the same seasonal trend in all treatments, with lowest CO2 efflux rates in the peak wet season (Fig 4). Only the variable time was significant (L. Ratio = 100.2, df = 7, p-value < 0.001, S4 File).
Fig 4. Measured CO2 efflux rate.
Mean CO2 efflux rate (μmol m-2 s-1) ± standard error (se) in crab removal plots, disturbance control plots and control plots (see legend for symbols). The data of eight sampling campaigns between November 2011 and November 2012 are plotted.
rH values in all treatments decreased with depth during all sampling campaigns. However, no distinct difference among treatments evolved over time (Fig 5). The final model retained a three-way interaction (L. Ratio = 29.04, df = 12, p-value = 0.004, S5 File), indicating that the specific form of the rH-depth curves was not consistent over all treatment and sampling dates.
Fig 5. Measured rH values.
Mean rH ± standard error (se) over sediment depth (cm) in crab removal plots, disturbance control plots and control plots (see legend for symbols). The data of eight sampling campaigns between November 2011 and November 2012 are plotted.
Stipule production
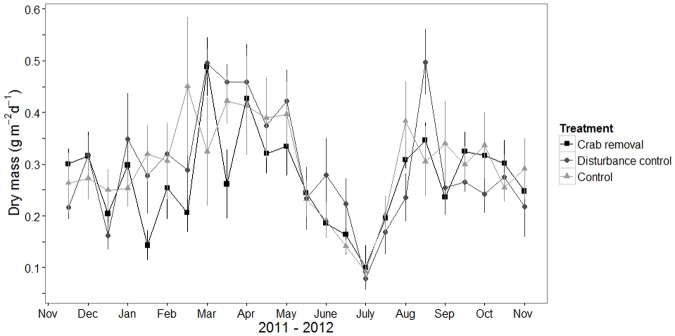
Stipule fall rate did not differ significantly between treatments (F-value = 0.3, df = 2, p-value = 0.7, S6 File). However, it showed a distinct bimodal temporal pattern, therefore the inclusion of a smoothing function for the variable time improved the model (F-value = 16.9, df = 7.9, p-value < 0.001, S6 File). Peaks appeared in March-April and in August 2012 (Fig 6).
Fig 6. Measured stipule fall rate.
Mean stipule fall rate in dry mass ± standard error (se) (g m-2 d-1) for R. mangle trees in crab removal plots, disturbance control plots and control plots (see legend for symbols). The data of 24 biweekly sampling campaigns between November 2011 and November 2012 are plotted.
Discussion
None of the studied response parameters was affected by the decrease in U. cordatus burrow numbers in the one year removal experiment, hence all initially stated hypotheses have to be rejected. As in the crab removal study of Smith et al. [5] in Australia, crabs were not completely removed from the exclusion plots in our experiment (we simulated a distinctly decreased fishing pressure rather than catastrophic mass mortality), but their numbers significantly decreased by 52% of the initial overall burrow density of 3.7–6.7 burrows-1 m-2. We increased the number of replication by a factor one compared to the Australian study (a further increase of plot numbers was logistically unachievable) and due to the inclusion of a disturbance control treatment in our experimental design, we can exclude that the findings are procedural artefacts. In fact, here we show for the first time that the mangrove ecosystem of the North Brazilian Amazon may be resilient to reduced U. cordatus numbers within a time span of one year, at least regarding the measured parameters.
Salinity
The rational of our initial hypothesis that large U. cordatus burrows would significantly desalinise the sediment was based on findings of studies of other species [12,52,53]. For example, Smith et al. [12] found an increase in sediment salinity after experimental reduction of the number of Uca spp. burrows. However, in contrast to their study which was performed in relatively open plots with small mangrove seedlings, our study was performed in a mature, closed-canopy R. mangle forest (compare Table 3). The lack of salinity reduction through U. cordatus burrows suggests that the amounts of salt removed by flushing of U. cordatus burrows are insignificant compared to the amount of salt accumulated by the extensive root systems of the central mangrove trees during water uptake [53–55]. Furthermore, burrows with one opening, typical for U. cordatus [56] as well as for Uca spp., may not be as efficient as desalinators as the burrows with multiple openings of many sesarmid crabs in the IWP which allow a flow through of tidal water between openings [57–60].
Table 3. Comparison of exclusion/removal experiments with burrowing crabs from the literature.
| Study | This study | Smith et al. 1991 [5] | Smith et al. 2009 [12] | Dye & Lasiak 1986 [13] | Thomas & Blum 2010 [19] |
|---|---|---|---|---|---|
| Habitat | Mangroves | Mangroves | Restored coastal marsh | Salt marsh | Salt marsh |
| Tide | semidiurnal | na | semidiurnal | semidiurnal | na |
| Tidal amplitude | 3–5 m | na | 1 m | 1.5 m | 0.25 m |
| Daily flooding | Only during spring tide | na | Not daily | na | 29 times in one year |
| Study site | High intertidal | Low intertidal | na | Mid tide level | na |
| Tree density in plots | 6–18 | 52–81 | 1 | 0 | na |
| Tree species in plots | Rhizophora mangle | Rhizophora apiculata, R. stylosa, R. lamarckii | Languncularia racemosa | - | Spartina alterniflora |
| Number of plots | 12 | 9 | 15 | 5 | 12 |
| Size of plots | 13 m × 13 m | 15 m × 15 m | 1 m × 1 m | Exclosure: 10–20 cm diameter PVC pipes, control: 0.25 m2 | Exclosure 1.5 m2, others 1 m2 |
| Tree height | 10–14 m | na | ca. 34–65 cm | - | - |
| Crab species | Ucides cordatus | Sesarma spp. | Uca spp. | Uca vocans, U. polita | Uca pugnax |
| Crab catching | Nylon nets | Pitfall traps | By hand, enclosure | Exclosure | Exclosure |
| Removed crabs | 4866 | over 1500 | na | na | na |
| Time of catching | Biweekly for 3–6 days | constant | Before experiment started | na | Before experiment started |
| Removal efficiency | In average 52% | 70–80% | na | na | na |
| Sampling time | 1 year | 1 year | 11 months | 14 days | 18 months |
| Treatments | Removal, disturbance control, control | Removal, disturbance control, control | Exclusion, control | Exclusion, control | Exclusion, adding artificial burrows, crabs naturally (not) present |
| Effects due to crab removal or exclusion | none | Soil sulphide and ammonium concentration increased | Height, trunk diameter and leaf production decreased | Abundance of meiobenthos increased 2 to 5-fold | Decrease in soil redox potential |
| Decrease of forest growth (by stipule fall) | Increase of interstitial water salinity | Decrease in sediment decomposition | |||
| Less reproductive output (by mature propagule fall) | Decreased the oxidation-reduction potential of the lower organic sediments | Accumulation of carbon in the sediment |
Organic matter content and CO2 efflux rate
Our hypothesis of decreased sediment organic matter storage due to removal of U. cordatus was based on the fact that these crabs are the dominant litter feeder in Brazilian mangrove forests; as such, the animals retain litter material in the mangrove forest that would otherwise be flushed away by the tides [8,9]. In addition to their (sloppy) feeding at the sediment surface, the crabs carry litter into their burrows where it is often only partially consumed [36,61]. A removal of crabs should therefore lead to an increase in sediment organic matter content. However, U. cordatus does not only accumulate organic matter, but at the same time also facilitates organic matter processing by other organisms, leading to a decrease in organic matter stock. Enhanced organic matter decomposition can be increased under drier conditions (e.g. low tide, dry season), when burrow walls are more oxidized due to contact with atmospheric oxygen. Consequently, carbon oxidation is facilitated by the presence of burrows, resulting in diminishing sediment organic matter [1,62]. A decreasing number of crabs would therefore affect both (antagonistic) processes and could potentially result in a zero net change. In addition, competition for leaf litter among crabs is strong [8]. Lower crab densities (i.e. inside the removal plots) may therefore allow the remaining crabs to increase their per capita food uptake, allowing them to process the same amount of leaf litter as in a situation with higher crab numbers.
Regarding sediment CO2 efflux rates we assumed that crab removal would lead to a decrease in this parameter. Sediment CO2 efflux rates reflect the activity of microbes, and a reduced crab feeding activity would lead to a decrease in substrate availability for these organisms. However, since no changes in the organic matter content of the sediment occurred, it is not surprising that sediment CO2 efflux rates did also not change.
rH
Crab burrows may influence the reduction state of the sediment. Pülmanns et al. [63] showed such an effect at least for the immediate neighborhood of U. cordatus burrow walls. In a North-Eastern Brazilian area with burrow densities much higher than at our study site (12 ± 3 burrows m−2 versus 6.7 burrows m−2 [64]), U. cordatus bioturbation led to more oxidizing conditions. However, our rH results do not support a general, i.e. far-reaching effect of the burrows on sediment reduction state, probably due to the limited reach of aeration effects at individual burrows [63] in combination with the relatively low burrow densities (also in our control plots). Under these conditions, overlap between oxidized zones around burrows is minimal.
Several other studies focusing on fiddler crabs, with manifold higher burrow densities (60 to more than 200 burrows m-2), also recorded substantial changes in the reduction potential for the upper sediment layer in bioturbated areas [15,65–69]. However, most authors do not report the distance between sampling points and the nearest burrows, making it difficult to compare their data with ours.
Stipule production
Since none of the measured sediment parameters changed with crab removal, it is not surprising that stipule fall rate did not change in the removal plots.
Experimental removal of mostly sesarmid mangrove crabs (which are generally much smaller than U. cordatus) resulted in a distinct decrease in stipule fall rate during a one year study period in Australia [5]. In contrast to our one year experiment, sediment conditions in the Australian crab removal study changed inside the exclusion plots (increased concentration of sulfide and ammonium) as well as stipule fall [5]. It remains unclear why stipule fall in the Australian mangrove ecosystem was affected by the reduction of burrow density, while in North Brazilian it was not. This is even more intriguing since the total number of caught crabs in our study was more than threefold higher than that of the Australian study (Brazil: in total 4866 U. cordatus caught with nets, Australia: approximately 1500 crabs—mostly Sesarma messa and Sesarma semperi longicristatum—caught with pitfall traps), and our design included four replicate plots, compared to only three in the Australian study. One reason for this outcome could be that our plots contained 6–18 relatively large trees each, whereas the Australian plots contained 52–81 smaller trees. Younger trees with smaller root system extension may react faster to changes in sediment characteristics than more mature trees with high root biomass. Furthermore, the location of the study site along the tidal gradient differed between the two sites. Our study was conducted in the high intertidal, whereas Smith III et al. [5] worked in the lower intertidal which was probably more frequently inundated. Thus, in the Australian system, regular tidal flushing of crab burrows is an important factor amplifying the role of burrows in contrast to the situation in our study, where the effects of (rare) flushing of the burrows may be too insignificant to influence sediment characteristics within one year.
Seasonal effects
In contrast to the lack of consistent differences in any of the measured parameters between crab removal and both control treatments, most parameters exhibited distinct seasonal changes. Precipitation is an important abiotic factor influencing sediment salinity conditions in mangrove forests [70,71]. Changes in sediment salinity can influence growth and phenology of R. mangle trees, and tree growth and flower bud production is enhanced during the wet season [72–74]. This agrees with the observed highest stipule fall rates in our plots from March to April when sediment salinities were lowest. Precipitation does not only affect salinity, but can, independently from the tidal cycle, saturate the sediment with water, creating less oxidized conditions over extended time intervals. This may lead to sulfate reduction in the upper sediment layer [70]. In our study, slightly lower rH values were recorded at the sediment surface during the wet season (Fig 2). Consequently, waterlogged and more anoxic sediment conditions in the upper sediment layers may have led to reduced carbon oxidation rates, resulting in lower CO2 release (Fig 1) as observed elsewhere [1,70,75]. Overall, our results suggest that seasonal changes in precipitation are more important drivers for the measured parameters than the U. cordatus burrows at the given low natural crab density at our macrotidal study site.
Conclusion
At our Amazonian mangrove study site, all measured parameters remained unaffected by the artificial removal of more than 4866 U. cordatus over one year from four 13 m × 13 m plots. We substantially reduced the initial burrow density by more than 50% and thus simulated a clear substantial increase in fishery or pathogen pressure. However, during our one year study the pronounced seasonal changes in precipitation had a much stronger influence on the measured parameters than the crabs’ bioturbation and leaf litter feeding. An experimental duration of several years could yield different results, due to potential accumulation of (subtle) effects of reduced crab numbers. A different experimental outcome than ours could also be thinkable for areas with higher initial crab densities and/or less pronounced rainfall during the rainy season than in Amazonian. Comparative removal studies involving the same crab species in different environmental contexts would further improve our understanding of the relative importance (and plasticity) of abiotic versus biotic factors as drivers of mangrove ecosystem functioning.
Supporting Information
(PDF)
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank the Instituto do Estudos Costeiros (IECOS) of the Federal University of Pará (UFPa). Further we are grateful to all crab catchers, Cassiano Sousa Silva, Leiliane Oliveira dos Santos, Gleiciane de Olivera Silva and Amanda Reis da Silva for their help during field and laboratory work. We thank the technicians from the ZMT for their help.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
NP and IN were funded by the Leibniz Center for Tropical Marine Ecology (project 6201, http://zmt-bremen.de). KD received funding from the MASTS pooling initiative (The Marine Alliance for Science and Technology for Scotland, http://www.masts.ac.uk) and its support is gratefully acknowledged. MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions. UM was funded by the Instituto do Estudos Costeiros (IECOS) of the Federal University of Pará (UFPa). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Andreetta A, Fusi M, Cameldi I, Cimò F, Carnicelli S, Cannicci S. Mangrove carbon sink. Do burrowing crabs contribute to sediment carbon storage? Evidence from a Kenyan mangrove system. J Sea Res. Elsevier B.V.; 2014. September;85:524–33. [Google Scholar]
- 2.Kristensen E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J Sea Res. 2008. February;59(1–2):30–43. [Google Scholar]
- 3.Penha-Lopes G, Bartolini F, Limbu S, Cannicci S, Kristensen E, Paula J. Are fiddler crabs potentially useful ecosystem engineers in mangrove wastewater wetlands? Mar Pollut Bull. Elsevier Ltd; 2009. November;58(11):1694–703. [DOI] [PubMed] [Google Scholar]
- 4.Robertson AI. Leaf-burying crabs: Their influence on energy flow and export from mixed mangrove forests (Rhizophora spp.) in northeastern Australia. J Exp Mar Bio Ecol. 1986;102(340):237–48. [Google Scholar]
- 5.Smith TJ III, Boto KG, Frusher SD, Giddins RL. Keystone species and mangrove forest dynamics: the influence of burrowing by crabs on soil nutrient status and forest productivity. Estuar Coast Shelf Sci. 1991;33:419–32. [Google Scholar]
- 6.Emmerson WD, McGwynne LE. Feeding and assimilation of mangrove leaves by the crab Sesarma meinerti de Man in relation to leaf-litter production in Mgazana, a warm-temperate southern African mangrove swamp. J Exp Mar Bio Ecol. 1992. May;157(1):41–53. [Google Scholar]
- 7.Koch V, Wolff M. Energy budget and ecological role of mangrove epibenthos in the Caeté estuary, North Brazil. Mar Ecol Prog Ser. 2002;228:119–30. [Google Scholar]
- 8.Nordhaus I, Wolff M, Diele K. Litter processing and population food intake of the mangrove crab Ucides cordatus in a high intertidal forest in northern Brazil. Estuar Coast Shelf Sci. 2006;67:239–50. [Google Scholar]
- 9.Schories D, Barletta-Bergan A, Barletta M, Krumme U, Mehlig U, Rademaker V. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetl Ecol Manag. 2003;11:243–55. [Google Scholar]
- 10.Smith TJ III. Seed predation in relation to tree dominance and distribution in mangrove forests. Ecology. Ecological Society of America; 1987. April 1;68(2):266–73. [Google Scholar]
- 11.Twilley RR, Pozo M, Garcia VH, Rivera-Monroy VH, Zambrano R, Bodero A. Litter dynamics in riverine mangrove forests in the Guayas River estuary, Ecuador. Oecologia. 1997. June 6;111(1):109–22. [DOI] [PubMed] [Google Scholar]
- 12.Smith NF, Wilcox C, Lessmann JM. Fiddler crab burrowing affects growth and production of the white mangrove (Laguncularia racemosa) in a restored Florida coastal marsh. Mar Biol. 2009. July 14;156(11):2255–66. [Google Scholar]
- 13.Dye AH, Lasiak TA. Microbenthos, meiobenthos and fiddler crabs: trophic interactions in a tropical mangrove sediment. Mar Ecol Prog Ser. 1986;32:259–64. [Google Scholar]
- 14.Hoffman JA, Katz J, Bertness MD. Fiddler crab deposit-feeding and meiofaunal abundance in salt marsh habitats. J Exp Mar Bio Ecol. 1984. November;82(2–3):161–74. [Google Scholar]
- 15.Botto F, Iribarne O. Effect of the burrowing crab Chasmagnathus granulata (Dana) on the benthic community of a SW Atlantic coastal lagoon. J Exp Mar Bio Ecol. 1999. August;241(2):263–84. [Google Scholar]
- 16.Dittmann S. Effects of macrobenthic burrows on infaunal communities in tropical tidal flats. Mar Ecol Prog Ser. 1996;134:119–30. [Google Scholar]
- 17.Kristensen E, Alongi DM. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron, and sulfur biogeochemistry in mangrove sediment. Limnol Oceanogr. 2006;51(4):1557–71. [Google Scholar]
- 18.Fanjul E, Grela MA, Iribarne O. Effects of the dominant SW Atlantic intertidal burrowing crab Chasmagnathus granulatus on sediment chemistry and nutrient distribution. Mar Ecol Prog Ser. 2007;341:177–90. [Google Scholar]
- 19.Thomas CR, Blum LK. Importance of the fiddler crab Uca pugnax to salt marsh soil organic matter accumulation. Mar Ecol Prog Ser. 2010. September 13;414:167–77. [Google Scholar]
- 20.Ellison AM. Managing mangroves with benthic biodiversity in mind: Moving beyond roving banditry. J Sea Res. 2008. February;59(1–2):2–15. [Google Scholar]
- 21.Diele K, Koch V, Saint-Paul U. Population structure, catch composition and CPUE of the artisanally harvested mangrove crab Ucides cordatus (Ocypodidae) in the Caeté estuary, North Brazil: Indications for overfishing? Aquat Living Resour. 2005;18:169–78. [Google Scholar]
- 22.Tavares M. True Crabs In: Carpenter KE, editor. The living marine resources of the Western Central Atlantic. Rome, FAO; 2003. pp. 327–352. [Google Scholar]
- 23.Boeger WA, Pie MR, Ostrensky A, Patella L. Lethargic crab disease: multidisciplinary evidence supports a mycotic etiology. Mem Inst Oswaldo Cruz. 2005;100(2):161–7. [DOI] [PubMed] [Google Scholar]
- 24.Guerra R, do Nascimento M, Miesch S, Najafzadeh M, Ribeiro R, Ostrensky A, et al. Black Yeast Biota in the Mangrove, in Search of the Origin of the Lethargic Crab Disease (LCD). Mycopathologia. Springer Netherlands; 2013;175(5–6):421–30. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento DM, Alves AGC, Alves RRN, Barboza RRC, Diele K, Mourao JS. An examination of the techniques used to capture mangrove crabs, Ucides cordatus, in the Mamanguape River estuary, northeastern Brazil, with implications for management. Ocean Coast Manage 2016; 130: 50–57. [Google Scholar]
- 26.Diele K, Araújo ARR, Glaser M, Salzmann U. Artisanal fishery of the mangrove crab Ucides cordatus (Ucididae) and first steps toward a successful co-management in Bragança, North Brazil In: Saint-Paul U, Schneider H, editors. Mangrove Dynamics and Management in North Brazil SE—19. Springer; Berlin Heidelberg; 2010. p. 287–97. [Google Scholar]
- 27.Diele K, Koch V. Comparative population dynamics and life histories of North Brazilian mangrove crabs, genera Uca and Ucides (Ocypodoidea) In: Saint-Paul U, Schneider H, editors. Mangrove Dynamics and Management in North Brazil. Springer; Berlin Heidelberg; 2010. p. 275–85. [Google Scholar]
- 28.Pinheiro MAA, Hattori GY. Relative growth of the mangrove crab Ucides cordatus (Linnaeus, 173) (Crustacea, Brachyura, Ocypodidae) at Iguape São Paulo, Brazil. Brazilian Arch Biol Technol. 2006;49:813–23. [Google Scholar]
- 29.Anexo II, Instrução Normativa, MMA 05/2004. http://www.prpe.mpf.mp.br/internet/content/download/2830/22487/file/in052004mma.pdf
- 30.Mehlig U, Menezes MPM, Reise A, Schories D, Medina E. Mangrove vegetation of the Caeté estuary In: Saint-Paul U, Schneider H, editors. Mangrove Dynamics and Management in North Brazil. Springer; Berlin Heidelberg; 2010. p. 71–107. [Google Scholar]
- 31.Krause G. The geography of the Bragança coastal region In: Saint-Paul U, Schneider H, editors. Mangrove Dynamics and Management in North Brazil. Springer; Berlin Heidelberg; 2010. p. 19–34. [Google Scholar]
- 32.Krause G, Schories D, Glaser M, Diele K. Spatial patterns of mangrove ecosystems: the bragantinian mangroves of northern Brazil (Bragança, Pará). Ecotropica. 2001;7:93–107. Available: http://www.gtoe.de/public_html/publications/pdf/7-1-2/KrauseGetal.2001,Ecotropica7_93-107.pdf [Google Scholar]
- 33.INMET. http://www.inmet.gov.br. Brasilia: Instituto Nacional de Meterologia (INMET); 2013.
- 34.Gomes de Santa Fé UMG, da Rocha Araujo AR. Seletividade e eficiênca das artes de pesca utilizadas na captura de Ucides cordatus (Linnaeus, 1973), Sergipe, Brasil. ACTAPESCA—Acta Fish Aquac. 2013;1:29–44. Available: http://seer.ufs.br/index.php/actapesca/article/view/1669 [Google Scholar]
- 35.de Magalhães HF, Costa Neto EM, Schiavetti A. Saberes pesqueiros relacionados à coleta de siris e caranguejos (Decapoda: Brachyura) no município de Conde, Estado da Bahia. Biota Neotrop. scielo; 2011;11:45–54. [Google Scholar]
- 36.Nordhaus I, Diele K, Wolff M. Activity patterns, feeding and burrowing behaviour of the crab Ucides cordatus (Ucididae) in a high intertidal mangrove forest in North Brazil. J Exp Mar Bio Ecol. Elsevier B.V.; 2009. June;374(2):104–12. [Google Scholar]
- 37.Schmidt AJ, Bemvenuti CE, Diele K. Effects of geophysical cycles on the rhythm of mass mate searching of a harvested mangrove crab. Anim Behav. 2012. August;84(2):333–40. [Google Scholar]
- 38.Steubing L, Fangmeier A. Pflanzenökologisches Praktikum: Gelände- und Laborpraktikum der terrestrischen Pflanzenökologie. Stuttgart: Ulmer; 1992. 205 p. [Google Scholar]
- 39.Pöpel F. Lehrbuch für Abwassertechnik und Gewässerschutz. Müller Jur.Vlg.C.F.; 2000. [Google Scholar]
- 40.Boto KG, Wellington JT. Phosphorus and nitrogen nutritional status of a northern Australian mangrove forest. Mar Ecol Prog Ser. 1983;11:63–9. [Google Scholar]
- 41.Boto KG, Wellington JT. Soil characteristics and nutrient status in a northern Australian mangrove forest. Estuaries. 1984;7(1):61–9. Available: http://link.springer.com/article/10.2307/1351957 [Google Scholar]
- 42.Duke NC, Bunt JS, Williams WT. Observations on the floral and vegetative phenologies of North-eastern Australian mangroves. Aust J Bot. 1984;32:87–99. [Google Scholar]
- 43.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York: Springer; 2009. 574 p. [Google Scholar]
- 44.Zuur AF, Ieno EN, Elphick CS. A protocol for a data exploration to avoid common statistical problems. Methods Ecol Evol. 2010; 1: 3–14. [Google Scholar]
- 45.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria; 2012. http://www.r-project.org
- 46.Pinheiro JC, Bates DM, DebRoy S, Sarkar D, R Core Team. nlme: Linear and nonlinear mixed effects models. 2012. p. 1–102.
- 47.Wood S. Generalized additive models: An introduction with R. London: Chapman & Hall; 2006. [Google Scholar]
- 48.Sarkar D. Lattice: Multivariate data visualitzation with R. New York: Springer; 2008. [Google Scholar]
- 49.Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- 50.Pinheiro JC, Bates DM. Mixed effects models in S and S-Plus. New York: Springer; 2000. 530 p. [Google Scholar]
- 51.Zuur AF, Ieno EN, Smith GM. Analysing Ecological Data. New York: Springer; 2007. [Google Scholar]
- 52.Stieglitz TC, Ridd P V, Müller P. Passive irrigation and functional morphology of crustacean burrows in a tropical mangrove swamp. Hydrobiologia. 2000;421:69–76. [Google Scholar]
- 53.Xin P, Jin G, Li L, Barry DA. Effects of crab burrows on pore water flows in salt marshes. Adv Water Resour. Elsevier Ltd; 2009. March;32(3):439–49. [Google Scholar]
- 54.Passioura JB, Ball MC, Knight JH. Mangroves may salinize the soil and in so doing limit their transpiration rate. Funct Ecol. 1992;6(4):476–81. [Google Scholar]
- 55.Reise A. Estimates of biomass and productivity in fringe mangroves of North-Brazil. University Bremen, Germany; 2003. http://books.google.de/books/about/Estimates_of_Biomass_and_Productivity_in.html?id=BcuCtgAACAAJ&pgis=1 [Google Scholar]
- 56.Araújo M de SLC de, Calado TC dos S. Burrows architecture of the crab Ucides cordatus (LINNAEUS, 1763) (Crustacea, Decapoda, Ucididae) in a mangrove swamp of Brazil. Trop Oceanogr. 2011;39(2):155–65. [Google Scholar]
- 57.Heron SF, Ridd P V. The tidal flushing of multiple-loop animal burrows. Estuar Coast Shelf Sci. 2008. June;78(1):135–44. [Google Scholar]
- 58.Heron SF, Ridd P V. The effect of water density variations on the tidal flushing of animal burrows. Estuar Coast Shelf Sci. 2003. September;58(1):137–45. [Google Scholar]
- 59.Heron SF, Ridd P V. The use of computational fluid dynamics in predicting the tidal flushing of animal burrows. Estuar Coast Shelf Sci. 2001. April;52(4):411–21. [Google Scholar]
- 60.Ridd P V. Flow through animal burrows in mangrove creeks. Estuar Coast Shelf Sci. 1996. November;43(5):617–25. [Google Scholar]
- 61.Nordhaus I, Wolff M. Feeding ecology of the mangrove crab Ucides cordatus (Ocypodidae): food choice, food quality and assimilation efficiency. J Mar Biol. 2007; 151(5):1665–1681. [Google Scholar]
- 62.Alongi DM, Wattayakorn G, Pfitzner J, Tirendi F, Zagorskis I, Brunskill G, et al. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Mar Geol. 2001. September;179(1–2):85–103. [Google Scholar]
- 63.Pülmanns N, Diele K, Mehlig U. Burrows of the semiterrestrial crab Ucides cordatus enhance CO2 release in a North Brazilian mangrove forest. Plos ONE. 2014. October 14 9(10):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Araújo JMC Jr., Otero XL, Marques AGB, Nóbrega GN, Silva JRF, Ferreira TO. Selective geochemistry of iron in mangrove soils in a semiarid tropical climate: effects of the burrowing activity of the crabs Ucides cordatus and Uca maracoani. Geo-Marine Lett. 2012. December 14;32(4):289–300. [Google Scholar]
- 65.Bortolus A, Iribarne O. Effects of the SW Atlantic burrowing crab Chasmagnathus granulata on a Spartina salt marsh. Mar Ecol Prog Ser. 1999;178:79–88. Available: http://www.intres.com/articles/meps/178/m178p079.pdf [Google Scholar]
- 66.Ferreira TO, Otero XL, Vidal-Torrado P, Macías F. Effects of bioturbation by root and crab activity on iron and sulfur biogeochemistry in mangrove substrate. Geoderma. 2007. November;142(1–2):36–46. [Google Scholar]
- 67.Kostka JE, Gribsholt B, Petrie E, Dalton D, Skelton H, Kristensen E. The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments. Limnol Oceanogr. 2002;47(1):230–40. [Google Scholar]
- 68.Morrisey DJ, DeWitt TH, Roper DS, Williamson RB. Variation in the depth and morphology of burrows of the mud crab Helice crassa among different types of intertidal sediment in New Zealand. Mar Ecol Prog Ser Prog. 1999;182:231–42. [Google Scholar]
- 69.Nielsen OI, Kristensen E, Macintosh DJ. Impact of fiddler crabs (Uca spp.) on rates and pathways of benthic mineralization in deposited mangrove shrimp pond waste. J Exp Mar Bio Ecol. 2003. April;289(1):59–81. [Google Scholar]
- 70.Marchand C, Baltzer F, Lallier-Vergès E, Albéric P. Pore-water chemistry in mangrove sediments: relationship with species composition and developmental stages (French Guiana). Mar Geol. 2004;208(2–4):361–81. [Google Scholar]
- 71.Wolanski E, Gardiner R. Flushing of salt from mangrove swamps. Mar Freshw Res. 1981;32(4):681–3. [Google Scholar]
- 72.Lara RJ, Cohen MCL. Sediment porewater salinity, inundation frequency and mangrove vegetation height in Bragança, North Brazil: an ecohydrology-based empirical model. Wetl Ecol Manag. 2006. August;14(4):349–58. [Google Scholar]
- 73.Mehlig U. Phenology of the red mangrove, Rhizophora mangle L., in the Caeté Estuary, Pará, equatorial Brazil. Aquat Bot. 2006. February;84(2):158–64. [Google Scholar]
- 74.Menezes M, Berger U, Worbes M. Annual growth rings and long-term growth patterns of mangrove trees from the Bragança peninsula, North Brazil. Wetl Ecol Manag. 2003;11:233–42. [Google Scholar]
- 75.Leopold A, Marchand C, Deborde J, Allenbach M. Temporal variability of CO2 fluxes at the sediment-air interface in mangroves (New Caledonia). Sci Total Environ. 2015. January 1;502(0):617–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.