Abstract
Objective
Validating objective, brain‐based indices of consciousness in behaviorally unresponsive patients represents a challenge due to the impossibility of obtaining independent evidence through subjective reports. Here we address this problem by first validating a promising metric of consciousness—the Perturbational Complexity Index (PCI)—in a benchmark population who could confirm the presence or absence of consciousness through subjective reports, and then applying the same index to patients with disorders of consciousness (DOCs).
Methods
The benchmark population encompassed 150 healthy controls and communicative brain‐injured subjects in various states of conscious wakefulness, disconnected consciousness, and unconsciousness. Receiver operating characteristic curve analysis was performed to define an optimal cutoff for discriminating between the conscious and unconscious conditions. This cutoff was then applied to a cohort of noncommunicative DOC patients (38 in a minimally conscious state [MCS] and 43 in a vegetative state [VS]).
Results
We found an empirical cutoff that discriminated with 100% sensitivity and specificity between the conscious and the unconscious conditions in the benchmark population. This cutoff resulted in a sensitivity of 94.7% in detecting MCS and allowed the identification of a number of unresponsive VS patients (9 of 43) with high values of PCI, overlapping with the distribution of the benchmark conscious condition.
Interpretation
Given its high sensitivity and specificity in the benchmark and MCS population, PCI offers a reliable, independently validated stratification of unresponsive patients that has important physiopathological and therapeutic implications. In particular, the high‐PCI subgroup of VS patients may retain a capacity for consciousness that is not expressed in behavior. Ann Neurol 2016;80:718–729
The clinical evaluation of disorders of consciousness (DOCs) in severely brain‐injured patients relies on their ability to connect to the surrounding environment and demonstrate their subjective experience through motor behavior.1 However, some patients may become unable to respond to stimuli despite still having conscious experiences.2, 3 This may happen because of motor or executive function impairments4, 5 and/or because of sensory disconnection from the environment.6 States of disconnected consciousness can occur in healthy subjects during dreaming7 and some forms of anesthesia,8 and may result from severe brain injury.9 In the latter case, covertly conscious patients may be misdiagnosed as being in a vegetative state (VS).10
This discrepancy advocates the development of ancillary brain‐based measures of consciousness that are independent of sensory processing, motor outputs, and subject participation. With this in mind, a novel metric—the Perturbational Complexity Index (PCI)11—has recently been developed based on a quantification of the electroencephalographic (EEG) responses to transcranial magnetic stimulation (TMS). Inspired by theoretical considerations,12, 13 PCI directly gauges the ability of many functionally specialized modules of the thalamocortical system (differentiation) to interact rapidly and effectively (integration), thus producing complex patterns of activity. In a reduced sample, PCI has been shown to distinguish between conscious and unconscious subjects,11 prompting further validation and testing especially in VS patients, in whom unequivocal signs of consciousness are lacking by definition. This task, however, is not straightforward; because behavior‐based clinical diagnosis may fail to recognize brain‐injured patients who are conscious but disconnected and unresponsive, the true state of affairs necessary to define the accuracy and the optimal cutoff for a given brain‐based measure of consciousness remains unknown.14, 15
Here, we apply PCI to a cohort of 38 minimally conscious state (MCS) and 43 VS patients while attempting to overcome this problem. Hence, we first validate this index on a large benchmark population of 150 subjects who could confirm the presence or absence of conscious experience through immediate or delayed reports.9, 16 This population included: (1) healthy subjects of different age (range = 18–80 years) and conscious brain‐injured patients who were awake and able to communicate; (2) unresponsive subjects who reported no conscious experience upon awakening from non–rapid eye movement (NREM) sleep or midazolam, xenon, or propofol anesthesia; and (3) subjects who were disconnected and unresponsive during rapid eye movement (REM) sleep and ketamine anesthesia but retrospectively reported having had vivid conscious experiences upon awakening. Considering subjects' reports as the provisional gold standard for assessing consciousness (no report = unconscious condition; immediate or delayed report = conscious condition), we perform receiver operating characteristic (ROC) curve analysis. Next, we identify an empirical PCI cutoff that discriminates with 100% accuracy between conscious and unconscious conditions, irrespectively of connectedness, responsiveness, and presence of brain lesions. Finally, we apply this independently validated cutoff to a large population of DOC patients (1) to assess the sensitivity of PCI in detecting MCS patients and (2) to objectively stratify VS patients based on the complexity of their brain responses to TMS.
Materials and Methods
Participants
Benchmark Population
The benchmark population consisted of 102 healthy subjects and 48 conscious brain‐injured patients. Healthy volunteers with history or presence of major medical/neurological disorders and of drug/alcohol abuse were excluded. All participants underwent neurological screening to exclude those at risk of potential adverse effects of TMS. TMS/EEG data were recorded from healthy subjects (female, n = 63; age range = 18–80 years) in the following conditions: (1) while they were unresponsive and did not provide any subjective report upon awakening (NREM sleep, n = 18; midazolam sedation at anesthetic concentrations, n = 6; anesthesia with xenon, n = 6; anesthesia with propofol, n = 6); (2) while they were unresponsive but able to provide a delayed subjective report upon awakening (dreaming during REM sleep, n = 8; and during ketamine anesthesia, n = 6); and (3) while they were awake and able to provide an immediate subjective report (n = 102, including 48 subjects also recorded in the previously described unresponsive conditions). The experimental protocols applied during sleep and anesthesia have been detailed elsewhere.11, 17, 18 Brain‐injured conscious patients were always recorded during wakefulness and encompassed (1) individuals affected by locked‐in syndrome (LIS; n = 5); (2) conscious individuals affected by ischemic or hemorrhagic stroke involving subcortical (n = 16) or cortical regions (n = 18); and (3) individuals who recovered functional communication after a previous DOC (emergence from minimally conscious state [EMCS], n = 9). Overall, this large benchmark population included subjects (47 of 150) reported in previous works.11, 17
Test Population
The test population consisted of 81 brain‐injured patients (12 previously reported)11 with severe DOC (Supplementary Table). Besides screening for potential adverse effects of TMS, exclusion criteria were medical instability, refractory generalized seizures, and history of neurodegenerative or psychiatric disease. All included patients had anoxic, traumatic, or vascular etiology. Each DOC patient was repeatedly evaluated with the Coma Recovery Scale‐Revised (CRS‐R)1 for a period of 1 week (4 times, every other day). Patients showing only reflexive behavior across all evaluations were considered as being in a VS, whereas patients showing signs of nonreflexive behaviors in at least 1 evaluation were considered as minimally conscious (MCS+/MCS−). The best CRS‐R score ensured the detection of minimal signs of consciousness even when behavioral responsiveness was fluctuating. One TMS/EEG recording session was scheduled in the same evaluation week, at least 20 days after DOC onset and 3 days after withdrawal of sedation.
Experimental Protocol and Data Collection
The experimental protocols were approved by the local ethical committees of the following Institutions: Istituto di Ricovero e Cura a Carattere Scientifico Fondazione Don Gnocchi Onlus, Azienda Socio‐Sanitaria Territoriale, Grande Ospedale Metropolitano Niguarda Cà Granda, and Fondazione Europea per la Ricerca Biomedica in Milan, Italy; Medical School of the University of Liège in Liège, Belgium. Written informed consent was obtained from healthy subjects, from communicative patients, and from legal surrogates of DOC patients. EEG responses to TMS were recorded from all participants to compute PCI. In addition, EEG data at rest were collected from DOC patients to characterize their background activity. DOC patients were recorded without sedation, and in case of behavioral signs of drowsiness (e.g., eye closure) recordings were momentarily interrupted to apply the CRS‐R arousal facilitation protocols.1 All experiments were conducted between 9.30 am and 4.30 pm so as to maximize the chance of having a stable vigilance level. During the recordings, vigilance was assessed according to the arousal subscale of the CRS‐R and continuously monitored so as to ascertain that the patients always had their eyes open either spontaneously or with stimulation.
Resting EEG and artifact‐free EEG responses to TMS were recorded with a 60‐channel TMS‐compatible amplifier. EEG recordings were referenced to an additional electrode on the forehead, band‐pass filtered between 0.1 and 350Hz, and sampled at 1,450Hz. Two extra sensors were used to record the electrooculogram. During TMS stimulation, participants wore inserted earplugs continuously playing a masking noise that abolishes the auditory potentials elicited by TMS‐associated clicks. Structural magnetic resonance (MR) images were always recorded within 1 week prior to the TMS/EEG assessment, to provide reliable anatomical information to the navigated‐TMS equipment. Using this navigation system, single TMS pulses were delivered with a focal biphasic stimulator. TMS targets were selected bilaterally within the middle‐caudal portion of the superior frontal gyrus (BA6 and BA8) and within the superior parietal lobule (BA7), about 1cm lateral to the midline. These targets were chosen because they are part of a cortical network that has been suggested to be relevant for consciousness19, 20, 21 and because they are far from the insertion of head muscles that may induce TMS‐related artifacts.22 In brain‐injured patients, the stimulation of targets affected by cortical lesions identified on individual MR images was deliberately avoided because, in these cases, TMS is ineffective and does not evoke measureable responses.23 Moreover, in 2 MCS patients, TMS pulses could not be delivered in 3 cortical targets that were close to skull breaches and internal drain placement for safety reasons. Each eligible cortical target was stimulated with an estimated electric field, orthogonal to the gyral crown, of about 120V/m. This intensity, applied to the selected cortical targets, has been shown to produce robust and reproducible EEG responses.24, 25 If no large muscular or magnetic artifacts were visible on single‐pulse responses, a few tens of trials were averaged and displayed in average reference to check online the presence of an early (0–50 milliseconds) evoked potential with a minimum peak‐to‐peak amplitude of 10 µV. In this case, at least 200 trials were recorded and subsequently analyzed for computing PCI offline. Otherwise, stimulation intensity was increased up to 160V/m and at least 200 trials were recorded, provided that muscular or magnetic artifacts were still absent. When these artifacts were unavoidable even after rotating and translating the coil within the same area, the stimulation session at that site was aborted. The same procedure was also applied in case of diffuse postanoxic damage, which was associated with diffuse cortical atrophy.
Data Analysis
PCI Computation and ROC Analysis
EEG responses to TMS were visually inspected to reject single trials and channels with bad signal quality. Recording sessions either with <80 good trials or with >10 bad channels were excluded from further analysis. Independent component analysis was applied to reduce ocular and muscular artifacts. The subsequent analysis was fully automatic and accurately complied with the procedure described in Casali et al,11 including 0.1 to 45Hz band‐pass filtering, downsampling at 362.5Hz, estimation of cortical current density, and related statistical analysis to extract the deterministic pattern of TMS‐evoked responses at the source level. PCI was obtained as the Lempel–Ziv complexity of the matrix of significant cortical source activity, normalized by source entropy, resulting in a positive real number between 0 (minimally complex patterns) and 1 (maximally complex patterns).11 If the percentage of spatiotemporal activations surviving statistical analysis was <1% (corresponding to the maximum rate of false positives), PCI was set to 0, indicating that cortical neurons failed to engage in any significant activation pattern in response to TMS perturbation. The absolute PCI values necessarily depend on the specific data acquisition/analysis protocol (e.g., sampling rate, type of source modeling, statistical analysis) applied in the present as well as in previous11, 17 studies.
For each individual, the maximum value of PCI (PCImax) was considered for ROC analysis. This choice aims at detecting the presence of cortical islands with high complexity, reflects the need for a univocal brain‐based classification parameter as an input to ROC analysis, and parallels the diagnostic use of the best behavioral (CRS‐R) score. PCImax values computed in the benchmark population were subdivided into 2 clusters, respectively representing “unconscious condition” (unresponsive individuals who did not provide any subjective report) and “conscious condition” (responsive healthy and brain‐injured individuals combined with unresponsive subjects able to provide a delayed subjective report). Based on this dichotomy, ROC curve analysis was applied to PCImax values to identify an optimal empirical cutoff (PCI*) that discriminates between the unconscious and the conscious conditions. Then, PCI* was first used to assess the sensitivity of PCImax in objectively detecting MCS patients. Finally, the same independently validated cutoff was employed to slice through and stratify the VS population.
Qualitative EEG Assessment
Continuous resting EEG recordings, re‐referenced to the standard double banana montage after 1 to 70Hz band‐pass filtering and downsampling to 725Hz, were evaluated according to the clinical neurophysiological descriptors proposed by Forgacs et al,26 namely predominant background EEG frequency, organization of the anteroposterior gradient, and presence of any diffuse/focal slowing. Accordingly, 4 EEG categories with increasing degree of abnormality were defined (i.e., normal, mildly abnormal, moderately abnormal, severely abnormal) and used to classify the resting EEG recording of each patient. This classification was then compared to behavioral diagnosis and PCImax results.
Results
Validation of PCImax in a Large Benchmark Population
Overall, 540 sets of TMS‐evoked potentials were analyzed in the benchmark population during different conditions (Table 1) and the corresponding PCI values are displayed in Figure 1A. At the group level, in awake healthy subjects PCImax was not significantly different (Wilcoxon test, p = 0.38) between male (n = 57) and female (n = 45) and between younger (<50 years, n = 80) and older (≥50 years, n = 22) subjects (Wilcoxon test, p = 0.25). To evaluate a possible effect of the stimulation site, we selected a subgroup of healthy awake subjects (n = 50) who were stimulated at least once in both frontal and parietal sites. In this subset, we found that the likelihood of finding maximum complexity values (PCImax) did not differ between frontal and parietal sites (z test for proportions, p = 0.30). Instead, we found a significant reduction of complexity (PCImax) at the group level (Wilcoxon test, p < 0.016) in conscious brain‐injured patients (LIS, stroke, and EMCS; median = 0.48, range = 0.34–0.61) as compared to healthy awake controls (median = 0.53, range = 0.39–0.70).
Table 1.
PCI values in the Benchmark Population
| Responsiveness | Report | Condition | PCI | PCImax | Subjects | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Median | Min | Max | No. | Median | Min | Max | |||||
| Behavioral unresponsiveness | No report | NREM sleep | 31 | 0.23 | 0.12 | 0.31 | 18 | 0.25 | 0.15 | 0.31 | Healthy subjects | |
| Behavioral unresponsiveness | No report | Midazolam | 6 | 0.30 | 0.23 | 0.31 | 6 | 0.30 | 0.23 | 0.31 | Healthy subjects | |
| Behavioral unresponsiveness | No report | Xenon | 6 | 0.23 | 0.11 | 0.31 | 6 | 0.23 | 0.11 | 0.31 | Healthy subjects | |
| Behavioral unresponsiveness | No report | Propofol | 20 | 0.23 | 0.13 | 0.31 | 6 | 0.26 | 0.23 | 0.31 | Healthy subjects | |
| Behavioral unresponsiveness | Delayed report | REM sleep | 10 | 0.48 | 0.35 | 0.56 | 8 | 0.48 | 0.36 | 0.56 | Healthy subjects | |
| Behavioral unresponsiveness | Delayed report | Ketamine | 6 | 0.43 | 0.36 | 0.52 | 6 | 0.43 | 0.36 | 0.52 | Healthy subjects | |
| Behavioral responsiveness | Immediate report | Wakefulness | 314 | 0.51 | 0.24 | 0.70 | 102 | 0.53 | 0.39 | 0.70 | Healthy subjects | |
| Behavioral responsiveness | Immediate report | LIS | 14 | 0.44 | 0.32 | 0.60 | 5 | 0.47 | 0.44 | 0.60 | Brain‐injured patients | |
| Behavioral responsiveness | Immediate report |
Subcortical stroke |
52 | 0.46 | 0.31 | 0.61 | 16 | 0.52 | 0.36 | 0.61 | Brain‐injured patients | |
| Behavioral responsiveness | Immediate report |
Cortical stroke |
50 | 0.40 | 0.26 | 0.57 | 18 | 0.46 | 0.34 | 0.57 | Brain‐injured patients | |
| Behavioral responsiveness | Immediate report | EMCS | 31 | 0.43 | 0.30 | 0.61 | 9 | 0.52 | 0.39 | 0.61 | Brain‐injured patients | |
EMCS = emergence from minimally conscious state; LIS = locked‐in syndrome; NREM = non‐rapid‐eye‐movement; PCI = Perturbational Complexity Index; PCImax = individual maximum value of Perturbational Complexity Index; REM = rapid‐eye‐movement.
Figure 1.
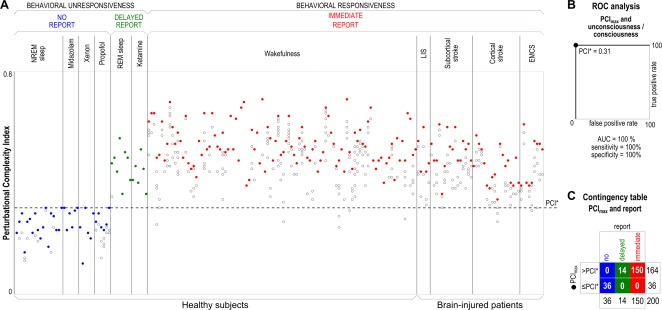
(A) Each circle represents the Perturbational Complexity Index (PCI) value computed from the cortical responses to transcranial magnetic stimulation (TMS) of one stimulation site. Several PCI values computed in each individual are aligned along vertical columns. PCI values are computed from TMS‐evoked potentials recorded in healthy subjects and conscious brain‐injured patients during different conditions. Individuals are grouped by condition, and within each condition are sorted by increasing age. For each individual, the maximum PCI value (PCImax) is represented by a solid circle, whereas lower PCI values are represented by open circles. During non–rapid eye movement (NREM) sleep and anesthesia with midazolam, xenon, and propofol, subjects were behaviorally unresponsive and did not provide any report upon awakening. During dreaming and ketamine anesthesia, subjects were behaviorally unresponsive but provided delayed subjective reports upon awakening. During wakefulness, both healthy subjects and conscious brain‐injured patients could immediately report their subjective experience. (B) Receiver operating characteristic (ROC) curve analysis applied to PCImax values for computing the optimal cutoff (PCI* = 0.31) that discriminates between unconsciousness (as assessed through the absence of any subjective report) and consciousness (as assessed through the presence of either an immediate or a delayed subjective report). Area under the curve (AUC) is 100%; using PCI* as a cutoff, sensitivity and specificity both result in 100%. (C) Contingency table obtained by slicing through the PCImax values with PCI*, also highlighted by a dashed horizontal line in panel A. EMCS = emergence from minimally conscious state; LIS = locked‐in syndrome; REM = rapid eye movement. [Color figure can be viewed at wileyonlinelibrary.com]
At the individual level, PCImax was invariably higher in the conscious (as assessed through immediate or delayed reports) as compared to the unconscious (no report) conditions (see Fig 1A). Although a few PCI values computed during conscious wakefulness (13 of 461 measurements) fell within the distribution obtained during the unconscious condition, the corresponding distributions of PCImax did not overlap. Thus, ROC curve analysis applied to PCImax resulted in 100% area under the curve and yielded an empirical cutoff PCI* of 0.31, which discriminated between the unconscious and the conscious conditions with 100% sensitivity and 100% specificity (see Fig 1B, C). Crucially, this accuracy was obtained irrespectively of behavioral responsiveness, age, gender, stimulation site, and presence of brain lesions.
Clinical Assessment of DOC Patients
The DOC population consisted of 38 MCS and 43 VS patients diagnosed according to the best CRS‐R score. The best total score was significantly lower in the VS as compared to the MCS cohort (Wilcoxon test, p < 0.0001) and in the MCS− (n = 21) as compared to the MCS+ subgroup (n = 17; Wilcoxon test, p < 0.001). To rule out a possible unbalance of vigilance between MCS and VS patients, we analyzed the arousal subscale of the CRS‐R administered during the recordings. All patients were scored either 1 (i.e., eye opening with stimulation) or 2 (i.e., eye opening without stimulation); although the proportion of VS patients with vigilance score = 1 was slightly higher than that of MCS patients, the difference between these cohorts was not significant (z test for proportions, p = 0.07).
Etiology distribution was not significantly different in the VS and MCS cohorts as assessed by z test for proportions (postanoxic damage: 18 VS and 9 MCS patients, p = 0.07 with a slight prevalence in the VS cohort; traumatic insult: 14 VS and 12 MCS patients, p = 0.93; vascular lesion: 11 VS and 17 MCS, p = 0.084 with a slight prevalence in the MCS cohort). Splitting patients into subacute and chronic subgroups based on the time lag (shorter or longer than 3 months, respectively) between DOC onset and TMS/EEG recording, we did not observe a significantly different prevalence between VS (15 subacute and 28 chronic patients) and MCS (10 subacute and 28 chronic patients) cohorts (z test for proportions, p = 0.41).
Sensitivity of PCImax in Detecting MCS Patients
Overall, 254 sets of TMS‐evoked potentials were analyzed in the DOC population and the corresponding PCI values are displayed in Figure 2. In all patients, 4 TMS targets were selected from the superior frontal gyrus and superior parietal lobule bilaterally. Based on previous studies,23 TMS was not targeted on cortical lesions because it would have failed to elicit any significant brain response. As a result, at least 2 PCI values were actually computed in each patient (except for Patient 33; see Supplementary Table); on average, the number of PCI measurements was 3.30 and 2.95 in the VS and MCS cohorts, respectively. At the group level, PCImax was lower in MCS patients (median = 0.40, range = 0.27–0.55) as compared to conscious brain‐injured patients (Wilcoxon test, p < 0.0001). However, PCImax was not significantly different between MCS+ and MCS− patients (Wilcoxon test, p = 0.92).
Figure 2.
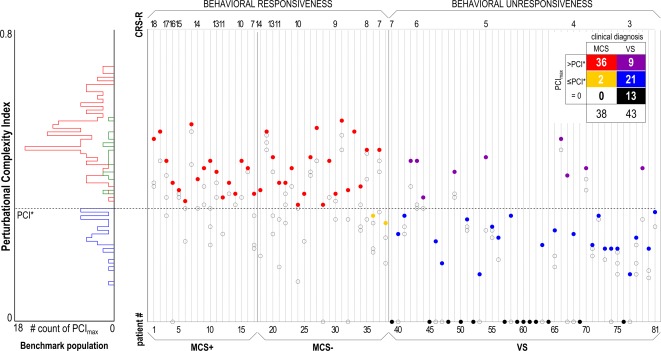
The histogram (left) summarizes the distribution of maximum Perturbational Complexity Index values (PCImax) in the benchmark population, specifically obtained in the absence of subjective report (blue) and in the presence of subjective report (delayed, green; immediate, red) conditions. The dashed horizontal line highlights the optimal cutoff (PCI*) computed from receiver operating characteristic curve analysis on the benchmark population. The scatter plot (right) shows all the PCI values obtained in minimally conscious state (MCS+/MCS−) and vegetative state (VS) patients. The PCI values computed in each patient (2–4 values) are aligned along vertical columns. Within each diagnostic group, patients are sorted by the Coma Recovery Scale‐Revised (CRS‐R) total score in decreasing order. For each patient, the PCImax is represented by a color‐filled circle, whereas lower PCI values are represented by empty circles. The contingency table (right upper corner) is obtained by slicing through the PCImax values with PCI* and shows that 36 MCS patients resulted in PCImax > PCI* (red), whereas in 2 MCS− patients PCImax was lower than PCI* (yellow). In addition, VS patients could be divided into 3 subgroups according to PCImax: 9 patients with PCImax > PCI* (purple), 21 patients with PCImax ≤ PCI* (blue), and 13 patients with PCImax = 0 (black).
To assess the sensitivity of PCImax in detecting patients showing minimal but unequivocal behavioral signs of consciousness, we sliced through the PCImax distribution of the MCS cohort with the empirical cutoff derived from the benchmark population. Considering all measurements, we found that the stimulation of some cortical sites could result in PCI values lower than PCI* (37 of 112 measurements). Crucially, however, PCImax was higher than PCI* in 36 of 38 patients, indicating that PCImax had a sensitivity of 94.7% in detecting minimal signs of consciousness. In 2 MCS− patients, TMS delivered at different cortical sites could only trigger a simple response, resulting in PCImax lower than PCI*.
According to conventional EEG assessment (Fig 3A), MCS patients were distributed across 3 categories: 18.4% in the severely abnormal, 44.7% in the moderately abnormal, and 36.9% in the mildly abnormal background. Of note, the 2 MCS− patients with PCImax lower than PCI* showed a severely abnormal background. No patient showed a normal EEG pattern. Grouping MCS patients according to background EEG did not result in significantly different PCImax values (Kruskal–Wallis test, p = 0.41; see Fig 3B). Thus, in MCS patients TMS typically triggered complex spatial–temporal dynamics, despite a heterogeneous EEG background, including 5 patients with a severely abnormal pattern. This finding is exemplified in Figure 3C, where the TMS‐evoked cortical responses in 3 representative MCS patients are shown together with the corresponding background EEG traces.
Figure 3.
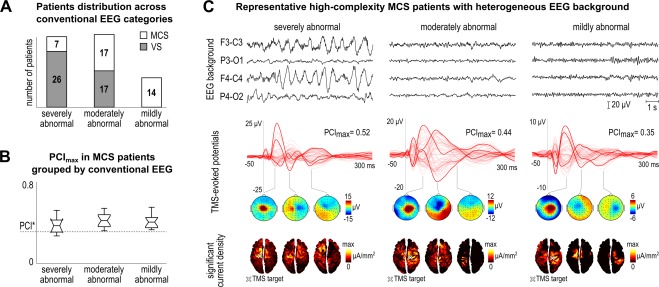
(A) Distribution of vegetative state (VS) and minimally conscious state (MCS) patients across conventional electroencephalographic (EEG) categories (i.e., severely abnormal, moderately abnormal, and mildly abnormal). The number of patients in each EEG category is explicitly indicated within the bars for VS and MCS patients. (B) Boxplot of the maximum individual Perturbational Complexity Index values (PCImax) computed in MCS patients as a function of conventional EEG category. The dashed horizontal line highlights the optimal cutoff (PCI*) obtained from the benchmark population. (C) The first row shows 10‐second continuous EEG recordings from 4 bipolar channels (F3‐C3, P3‐O1, F4‐C4, P4‐O2) in 3 representative MCS patients with PCImax higher than PCI* (from left to right: Patients 19, 10, and 25), and respectively with a severely abnormal (left), a moderately abnormal (center), and a mildly abnormal (right) background. The second row shows the corresponding average transcranial magnetic stimulation (TMS)‐evoked potentials (all channels superimposed, with 3 illustrative channels highlighted in bold) together with the PCImax values. Three voltage scalp topographies (third row) and significant current density cortical maps (fourth row) are shown at selected time points for each patient. A white cross on the cortical map indicates the stimulation target. [Color figure can be viewed at wileyonlinelibrary.com]
Stratification of VS Patients
After ascertaining the sensitivity of PCImax in detecting MCS patients who showed unequivocal behavioral signs of consciousness, we employed PCImax to stratify unresponsive patients. We found that the VS population could be stratified in 3 different subgroups: a “no‐response” subgroup (PCImax = 0) of 13 patients (30%), a “low‐complexity” subgroup (PCImax ≤ PCI*) of 21 patients (49%), and finally a smaller “high‐complexity” subgroup (PCImax > PCI*) of 9 patients (21%). The TMS‐evoked responses together with selected structural images of 3 representative VS patients belonging to these subgroups are displayed in Figure 4A. In the no‐response subgroup TMS targeted over different cortical areas failed to engage any significant cortical response, whereas in the low‐complexity subgroup TMS triggered a local and stereotypical positive–negative response, similar to the one observed in healthy controls during unconscious NREM sleep and anesthesia. Notably, in the high‐complexity subgroup, TMS engaged a rapidly changing and spatially differentiated cortical response, similar to the one observed in MCS patients and in responsive (wakefulness) or unresponsive (REM sleep and ketamine anesthesia) conscious controls.
Figure 4.
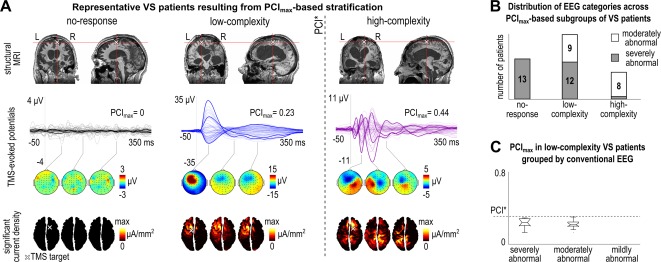
(A) The first row shows structural images of 3 representative vegetative state (VS) patients (from left to right: Patients 57, 78, and 42), with individual maximum value of Perturbational Complexity Index (PCImax) respectively = 0 (left), PCImax lower than the optimal empirical cutoff obtained from the benchmark population (PCI*; center), and PCImax higher than PCI* (right). One coronal and 1 sagittal view for each patient are displayed in correspondence with the stimulation site (white cross). The second row shows the corresponding average transcranial magnetic stimulation (TMS)‐evoked potentials (all channels superimposed, with 3 illustrative channels highlighted in bold), together with the PCImax values. Three voltage scalp topographies (third row) and significant current density cortical maps (fourth row) are shown at selected time points for each patient. A white cross on the cortical map indicates the stimulation target. The dashed vertical line highlights the PCI* obtained from the benchmark population. (B) Distribution of the severely abnormal and moderately abnormal background patterns across the 3 subgroups of VS patients defined by PCImax‐based stratification, namely no‐response, low‐complexity, and high‐complexity. (C) Boxplot of the PCImax values computed in VS patients with PCImax lower than PCI* as a function of conventional electroencephalographic (EEG) category. The dashed horizontal line highlights the PCI* obtained from the benchmark population. L = left; MRI = magnetic resonance imaging; R = right. [Color figure can be viewed at wileyonlinelibrary.com]
Comparing the 3 subgroups of VS patients, we did not find a significant effect of the best CRS‐R total score (Kruskal–Wallis test, p = 0.28) or of the vigilance level (z test for proportions: no‐response vs low‐complexity, p = 0.45; no‐response vs high‐complexity, p = 0.94; low‐complexity vs high‐complexity, p = 0.53). Considering etiology and EEG category, the low‐complexity subgroup was composed of patients with heterogeneous etiology (5 postanoxic, 6 traumatic, 10 vascular) showing 2 different EEG patterns (12 severely and 9 moderately abnormal background; see Fig 4B); patients with vascular or traumatic etiology equally showed either a moderately or a severely abnormal pattern, whereas 4 of 5 postanoxic patients showed a severely abnormal pattern. Accordingly, within the low‐complexity subgroup, PCImax did not significantly differ between patients with different EEG background (see Fig 4C). However, the 2 extreme subgroups identified by the PCImax‐based stratification were associated with a characteristic prevalence of etiology and EEG background organization (see Fig 4B); all except 1 no‐response VS patient had suffered severe postanoxic damage and were all characterized by a severely abnormal background, whereas the high‐complexity subgroup had a prevalent traumatic etiology (7 of 9) and almost all showed a moderately abnormal EEG (8 of 9). Concerning the outcome at 6 months, we found that 6 of 9 (1 unknown) high‐complexity VS patients transitioned to a behavioral MCS, whereas such transition was observed in 5 of 21 (2 unknown) low‐complexity patients. None of the no‐response subgroup showed any improvement.
Discussion
Towards a Calibration of PCI
Validating and calibrating an objective index of the brain's capacity for consciousness when behavioral signs of consciousness are unreliable or inconsistent represents a formidable challenge.14, 15 Here we consider PCI, a measure that gauges the ability of thalamocortical circuits to integrate information irrespectively of the integrity of sensory processing, motor behavior, and subject participation. To validate PCI, we chose benchmark conditions15 in which subjects could provide a report about the absence or presence of conscious experience, including delayed reports upon awakening from sleep27 and anesthesia.9 Retrospective reports, either immediate or delayed, are the current gold standard for assessing the presence and content of subjective experience16 and therefore for validating objective measures of consciousness.9 Based on these premises, if a brain‐based test of consciousness is positive in subjects who are fully unresponsive at the time of measurement but provide a delayed report of a vivid dream upon awakening, the result should be considered a true positive. The contingency table based on PCI* cutoff (see Fig 1C) shows that in the delayed report conditions PCImax always provides positive results. This finding is relevant, especially concerning ketamine anesthesia and REM sleep, conditions in which consciousness is present but is disconnected from the external environment.27, 28 Importantly, the benchmark population included not only conscious subjects disconnected from the environment but also a large group of conscious brain‐injured patients (LIS, subcortical and cortical stroke, EMCS) as a necessary requirement for the calibration of a test aimed at a target population of MCS and VS patients with severe neurological damage.
Overall, referring to subjective reports as ground truth and to PCImax as a test, we find a PCI* that optimally discriminates between the conscious and the unconscious conditions in the benchmark population, irrespectively of behavioral unresponsiveness and brain lesions (see Fig 1C). Although subjective reports are currently the gold standard for assessing the presence of consciousness, they are not necessarily always reliable unless the context is carefully considered. Thus, in certain situations people may be conscious but subsequently forget their experiences, or be unconscious and confabulate upon awakening. Accordingly, PCI* should not be interpreted as an absolute boundary between consciousness and unconsciousness, but rather should be used as an operational threshold to be applied to conditions in which no reliable behavioral reference is available.
PCI‐Based Detection of MCS Patients
Although MCS patients are unable to provide a verbal report about their subjective experience, they show behavioral signs of consciousness.1, 29 The presence of such signs, albeit fluctuating, can be considered as a minimal standard against which the sensitivity of any candidate index of consciousness should be tested. This check is important because the reliable detection of MCS patients represents a general challenge for bedside, brain‐based indices of consciousness. For example, the P3b potential elicited by global violations of auditory regularities, a candidate signature of the presence of consciousness, can be found in only up to 31% of MCS patients,30 measures of resting EEG connectivity such as weighted symbolic mutual information result in a 71% sensitivity, and the best combination of 92 quantitative measures derived from both resting and evoked EEG achieves a sensitivity of 78%.31 Besides quantitative analysis, the clinical qualitative assessment of the EEG background offers a practical tool at the bedside that, if properly interpreted, may outperform some of the above indices.26 In the present data set, a cutoff between severely and moderately abnormal EEG patterns reached a remarkable sensitivity of 81.6% (see Fig 3A). Importantly, however, the highest sensitivity (94.7%) was obtained by quantifying with PCImax the complexity of the EEG responses to a direct cortical perturbation (see Fig 2). Notably, TMS perturbations revealed complex cortical responses (PCImax > PCI*) also in 5 MCS patients, who according to the clinical classification of the resting EEG were characterized by a severely abnormal pattern (see Fig 3B, C). This suggests that assessing the joint presence of integration and differentiation through direct cortical perturbations17, 32 reveals additional information that is not immediately captured by conventional EEG descriptors and may direct further refinement of the classification of EEG resting activity.
PCI‐Based Assessment of VS Patients
In VS patients, the absence of behavioral signs of consciousness per se cannot be considered a proof of the absence of consciousness.2, 33 For this reason, the alternative term unresponsive wakefulness syndrome, which is more descriptive and more neutral regarding the patient's capacity for consciousness, has recently been recommended.34 This widely acknowledged notion also implies that in principle, these patients should not be employed for calibrating measures of consciousness; rather, they ought to represent the endpoint for the application of brain‐based measures that have been validated on an independent gold standard.14, 15 Here, by applying an externally validated cutoff (PCI*), we find that 9 of 43 unresponsive patients had PCImax > PCI* (see Fig 2). Are these false or true positives? Across 200 measurements in the benchmark population, PCImax was always higher than PCI* when consciousness was present and never when consciousness was absent, as assessed through subjective report. Notably, the PCImax values found in the high‐complexity VS patients (0.34–0.50; see Supplementary Table) were well within the range (0.32–0.70) found in conscious controls (see Table 1), and did not depend on the level of arousal as assessed by the CRS‐R vigilance subscale (see Supplementary Table). Hence, it is parsimonious to assume that these high‐complexity unresponsive patients may retain a capacity for consciousness that is not expressed in behavior. A similar dissociation between consciousness and behavior is observed in ketamine‐anesthetized subjects who lie eyes‐open, completely unresponsive at the time of measurement, but provide a delayed report upon awakening8, 17, 28; unlike these high‐complexity anesthetized subjects, however, high‐complexity VS patients might remain unable to report due to pathological disconnection/unresponsiveness.
From this perspective, we suggest that PCI should not be considered as a prognostic marker—although the outcome at 6 months was more favorable in the high‐complexity subgroup—but rather as an index of the current capacity for consciousness that is independent of the patient's ability to process external inputs or to engage in motor behavior. Practically, by approximating an optimal tradeoff between sensitivity and specificity, the PCI test may represent an important step within a hierarchical diagnostic flow of DOCs. For example, one may apply PCI after a first screening with tests characterized by high sensitivity and low specificity, such as positron emission tomography assessment of cortical metabolic rates35; then, patients with PCImax > PCI* could be selected to confirm the presence of covert consciousness through more demanding tests characterized by maximal specificity, such as functional MR imaging active paradigms.33
Physiopathological Implications
We observed a progressive group‐level reduction of PCImax from healthy controls to conscious patients with focal brain injury to the MCS cohort, suggesting that overall lesion load may eventually affect the upper bound of complexity. In parallel, and confirming the impact of lesions, we found a larger number of TMS/EEG sessions resulting in PCI measurements lower than PCI* (empty circles in Fig 2) in MCS patients, suggesting that some cortical areas may fail to engage in complex activation patterns when stimulated. Despite this, the TMS mapping yielded PCImax values higher than PCI* in 84 of 86 (97.7%) brain‐injured conscious patients, including LIS, stroke, EMCS, and MCS. Thus, instances of high perturbational complexity linked to behavioral signs of consciousness could be detected even in brains affected by multifocal, widespread lesions. Clearly, the cohort of brain‐injured patients enrolled in the present study did not allow inferences regarding the functional role of specific brain networks in consciousness, because of their different etiology and because their anatomical lesions were characterized by heterogeneous extent and spatial location. Nonetheless, this finding highlights the sensitivity of PCI and raises the basic question of what are the minimal anatomical and functional requirements to sustain such complex interactions, a question that is even more relevant for those unresponsive patients in whom severe brain damage spares the function of large brain islands.36
We describe 3 possible TMS/EEG patterns in clinically vegetative patients (see Fig 4A); when directly perturbed at multiple locations, the patients' cerebral cortex may (1) fail to engage in any significant response, (2) engage in a low‐complexity response similar to the one observed in NREM sleep and anesthesia unconsciousness, or (3) engage in complex spatiotemporal dynamics similar to that observed in conscious awake or dreaming subjects. The no‐response subgroup was mostly (12 of 13) composed of postanoxic patients who showed diffuse cortical necrosis (see Fig 4A) and a severely abnormal, voltage‐suppressed EEG. The low‐ and the high‐complexity patterns, conversely, could be found in the presence of similar lesion load as roughly inspected by structural imaging (see Fig 4A). Several factors, in addition to the extent of structural lesions, may affect the ability of the residual brain to engage in complex interactions. One possibility to be investigated is whether the integrity of some specific structures, such as the precuneus,20, 37 the thalamus,5, 38 and the claustrum,39, 40 or a critical level of overall anatomical connectivity41 may be key in sustaining such interactions. Another non–mutually exclusive possibility is that the complexity of residual thalamocortical networks may be reduced by functional imbalances leading to an excessive degree of neuronal bistability.42 This may happen, for example, following changes in the neuromodulatory milieu, when potassium currents are abnormally increased and when the balance between excitation and inhibition is disrupted.43, 44 In this regard, it is worth recalling that low complexity responses to cortical stimulation are the rule during states such as anesthesia18 and NREM sleep,45 when bistability is present but can be readily reversed.46
The proposed physiopathological stratification has practical implications for patient management. For example, whereas no‐response patients may be further investigated in search of preserved cortical and subcortical metabolic activations that may have escaped the TMS probing, the patients in whom TMS triggered a significant response with PCImax < PCI* should be directed toward neuromodulation with medications or brain stimulation techniques47 aimed at restoring complex patterns of activity. For example, although anatomical lesions and disconnections cannot be easily reversed, it may still be possible to reduce sleep‐like bistability by deep brain stimulation48 or by acting pharmacologically on intrinsic neuronal properties.49 In this process, longitudinal TMS/EEG measurements also offer the fundamental readout to direct and titrate intervention toward the desired endpoint of PCImax > PCI*. Finally, unresponsive patients in whom TMS/EEG already documents a core of high complexity should be selected for intensive interventions aimed at restoring responsiveness to the external environment, such as by increasing behavioral output through thalamic stimulation50 or by establishing communication through active paradigms or brain–machine interface.51, 52
Author Contributions
Conception and design of the study: G.D., S.L., G.T., M.Mas. Acquisition and analysis of data: S.C., A.C., M.R., S.S., M.F., M.N., A.P., A.G.C., P.D.T., M.B., O.G., O.B., F.C., C.L., M.Mar. Drafting a significant portion of the manuscript or figures: S.C., A.C., M.Mas.
Potential Conflicts of Interest
Nothing to report.
Supporting information
Additional supporting information can be found in the online version of this article
Supporting Information
Acknowledgment
This work was supported by Prin 2010 “Connage” (Italian Government), European Union (EU) grant FP7‐ICT‐2011‐9 n. 600806 “Corticonic,” James S. McDonnell Foundation Scholar Award 2013, EU grant H2020‐FETOPEN‐2014‐2015‐RIA n. 686764 “Luminous,” and EU grant H2020 grant agreement 720270‐Human Brain Project SGA1 (M.Mas.); by the Belgian National Fund for Scientific Research (O.G.); and by the Templeton World Charity Foundation, McDonnell Foundation, and Distinguished Chair in Consciousness Science at the University of Wisconsin (G.T.).
We thank P. Cecconi and A. Bo for helping with the data acquisition.
References
- 1. Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale‐Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–2029. [DOI] [PubMed] [Google Scholar]
- 2. Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 2012;61:478–491. [DOI] [PubMed] [Google Scholar]
- 3. Fernández‐Espejo D, Owen AM. Detecting awareness after severe brain injury. Nat Rev Neurosci 2013;14:801–809. [DOI] [PubMed] [Google Scholar]
- 4. Fernández‐Espejo D, Rossit S, Owen AM. A thalamocortical mechanism for the absence of overt motor behavior in covertly aware patients. JAMA Neurol 2015;72:1442–1450. [DOI] [PubMed] [Google Scholar]
- 5. Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci 2010;33:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanders RD, Tononi G, Laureys S, Sleigh J. Unresponsiveness ≠ unconsciousness. Anesthesiology 2012;116:946–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stickgold R, Malia A, Fosse R, et al. Brain‐mind states: I. Longitudinal field study of sleep/wake factors influencing mentation report length. Sleep 2001;24:171–179. [DOI] [PubMed] [Google Scholar]
- 8. Domino EF. Taming the ketamine tiger. Anesthesiology 2010;113:678–684. [DOI] [PubMed] [Google Scholar]
- 9. Sanders RD, Raz A, Banks MI, et al. Is consciousness fragile? Br J Anaesth 2016;116:1–3. [DOI] [PubMed] [Google Scholar]
- 10. Majerus S, Gill‐Thwaites H, Andrews K, Laureys S. Behavioral evaluation of consciousness in severe brain damage. Prog Brain Res 2005;150:397–413. [DOI] [PubMed] [Google Scholar]
- 11. Casali AG, Gosseries O, Rosanova M, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013;5:198ra105. [DOI] [PubMed] [Google Scholar]
- 12. Tononi G. An information integration theory of consciousness. BMC Neurosci 2004;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tononi G, Boly M, Massimini M, Koch C. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci 2016;17:450–461. [DOI] [PubMed] [Google Scholar]
- 14. Harrison AH, Connolly JF. Finding a way in: A review and practical evaluation of fMRI and EEG for detection and assessment in disorders of consciousness. Neurosci Biobehav Rev 2013;37:1403–1419. [DOI] [PubMed] [Google Scholar]
- 15. Peterson A, Cruse D, Naci L, et al. Risk, diagnostic error, and the clinical science of consciousness. Neuroimage Clin 2015;7:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noreika V, Jylhänkangas L, Móró L, et al. Consciousness lost and found: subjective experiences in an unresponsive state. Brain Cogn 2011;77:327–334. [DOI] [PubMed] [Google Scholar]
- 17. Sarasso S, Boly M, Napolitani M, et al. Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr Biol 2015;25:3099–3105. [DOI] [PubMed] [Google Scholar]
- 18. Ferrarelli F, Massimini M, Sarasso S, et al. Breakdown in cortical effective connectivity during midazolam‐induced loss of consciousness. Proc Natl Acad Sci U S A 2010;107:2681–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laureys S, Goldman S, Phillips C, et al. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. Neuroimage 1999;9:377–382. [DOI] [PubMed] [Google Scholar]
- 20. Fridman EA, Beattie BJ, Broft A, et al. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci U S A 2014;111:6473–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Perri C, Stender J, Laureys S, Gosseries O. Functional neuroanatomy of disorders of consciousness. Epilepsy Behav 2014;30:28–32. [DOI] [PubMed] [Google Scholar]
- 22. Mutanen T, Mäki H, Ilmoniemi RJ. The effect of stimulus parameters on TMS–EEG muscle artifacts. Brain Stimul 2013;6:371–376. [DOI] [PubMed] [Google Scholar]
- 23. Gosseries O, Sarasso S, Casarotto S, et al. On the cerebral origin of EEG responses to TMS: insights from severe cortical lesions. Brain Stimul 2015;8:142–149. [DOI] [PubMed] [Google Scholar]
- 24. Casali AG, Casarotto S, Rosanova M, et al. General indices to characterize the electrical response of the cerebral cortex to TMS. Neuroimage 2010;49:1459–1468. [DOI] [PubMed] [Google Scholar]
- 25. Casarotto S, Romero Lauro LJ, Bellina V, et al. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One 2010;5:e10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forgacs PB, Conte MM, Fridman EA, et al. Preservation of electroencephalographic organization in patients with impaired consciousness and imaging‐based evidence of command‐following. Ann Neurol 2014;76:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siclari F, LaRocque JJ, Postle BR, Tononi G. Assessing sleep consciousness within subjects using a serial awakening paradigm. Front Psychol 2013;4:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collier BB. Ketamine and the conscious mind. Anaesthesia 1972;27:120–134. [DOI] [PubMed] [Google Scholar]
- 29. Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state definition and diagnostic criteria. Neurology 2002;58:349–353. [DOI] [PubMed] [Google Scholar]
- 30. King JR, Faugeras F, Gramfort A, et al. Single‐trial decoding of auditory novelty responses facilitates the detection of residual consciousness. Neuroimage 2013;83:726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sitt JD, King J‐R, El Karoui I, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014;137:2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 2016;17:307–321. [DOI] [PubMed] [Google Scholar]
- 33. Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010;362:579–589. [DOI] [PubMed] [Google Scholar]
- 34. Laureys S, Celesia GG, Cohadon F, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Medicine 2010;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stender J, Gosseries O, Bruno M‐A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 2014;384:514–522. [DOI] [PubMed] [Google Scholar]
- 36. Gosseries O, Di H, Laureys S, Boly M. Measuring consciousness in severely damaged brains. Annu Rev Neurosci 2014;37:457–478. [DOI] [PubMed] [Google Scholar]
- 37. Lant ND, Gonzalez‐Lara LE, Owen AM, Fernández‐Espejo D. Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage Clin 2016;10:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lutkenhoff ES, Chiang J, Tshibanda L, et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann Neurol 2015;78:68–76. [DOI] [PubMed] [Google Scholar]
- 39. Yin B, Terhune DB, Smythies J, Meck WH. Claustrum, consciousness, and time perception. Curr Opin Behav Sci 2016;8:258–267. [Google Scholar]
- 40. Crick FC, Koch C. What is the function of the claustrum? Phil Trans R Soc B 2005;360:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernández‐Espejo D, Soddu A, Cruse D, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol 2012;72:335–343. [DOI] [PubMed] [Google Scholar]
- 42. Massimini M, Ferrarelli F, Sarasso S, Tononi G. Cortical mechanisms of loss of consciousness: insight from TMS/EEG studies. Arch Ital Biol 2012;150:44–55. [DOI] [PubMed] [Google Scholar]
- 43. Sanchez‐Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 2000;3:1027–1034. [DOI] [PubMed] [Google Scholar]
- 44. Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep‐wake cycle: an intracellular study. Proc Natl Acad Sci U S A 2001;98:1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Massimini M, Ferrarelli F, Esser SK, et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U S A 2007;104:8496–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pigorini A, Sarasso S, Proserpio P, et al. Bistability breaks‐off deterministic responses to intracortical stimulation during non‐REM sleep. Neuroimage 2015;112:105–113. [DOI] [PubMed] [Google Scholar]
- 47. Fridman EA, Schiff ND. Neuromodulation of the conscious state following severe brain injuries. Curr Opin Neurobiol 2014;29:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea‐pig cerebral cortex in vitro. J Physiol 1986;375:169–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 2007;448:600–603. [DOI] [PubMed] [Google Scholar]
- 51. Naci L, Monti MM, Cruse D, et al. Brain‐computer interfaces for communication with nonresponsive patients. Ann Neurol 2012;72:312–323. [DOI] [PubMed] [Google Scholar]
- 52. Chatelle C, Chennu S, Noirhomme Q, et al. Brain–computer interfacing in disorders of consciousness. Brain Inj 2012;26:1510–1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information can be found in the online version of this article
Supporting Information


