Abstract
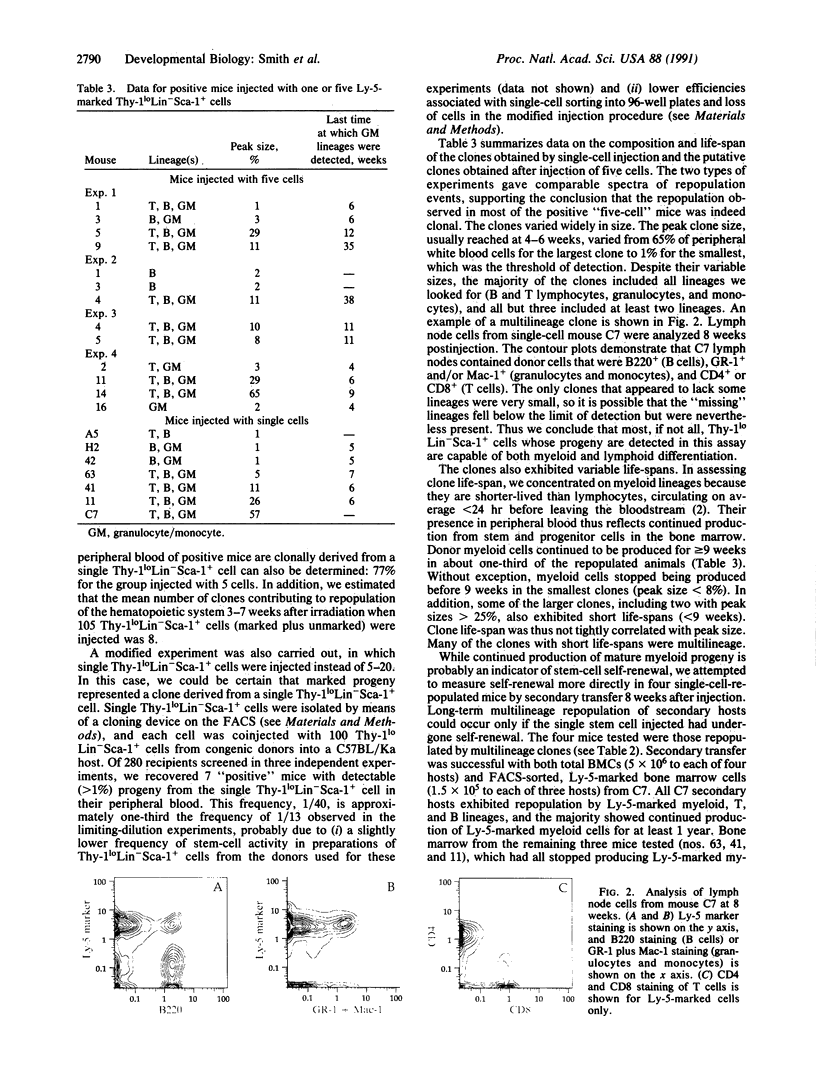
Previous work has shown that the 0.02-0.05% of adult mouse bone marrow cells that bear the cell surface phenotype Thy-1loLin-Sca-1+ are enriched 1000- to 2000-fold for hematopoietic stem-cell activity in a variety of assays. When 50-100 cells of this phenotype are injected into an irradiated animal, they can permanently repopulate the entire hematopoietic system. In the present study, limiting-dilution and single-cell experiments were used to address the question of how individual Thy-1loLin-Sca-1+ stem cells contribute to repopulation of the hematopoietic system following irradiation. We calculated that 1 of 13 Thy-1loLin-Sca-1+ cells formed a clone comprising greater than 1% of peripheral white blood cells 3-7 weeks after injection. The majority of these clones included both lymphoid and myeloid lineages. Approximately one-third of the clones continued to produce new blood cells for 9 weeks or more, but the remainder disappeared earlier, including many that were multilineage. Thus, while the majority of Thy-1loLin-Sca-1+ bone marrow cells whose progeny are detected in the in vivo repopulation assay are pluripotential, only a subset undergo long-term self-renewal in vivo. Repopulation appears to be oligoclonal when limiting numbers of Thy-1loLin-Sca-1+ cells are injected. However, the number of clones contributing to hematopoiesis increases in proportion to the number of Thy-1loLin-Sca-1+ cells injected, bringing into question the notion that steady-state hematopoiesis in normal individuals is oligoclonal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. R., Boggs S. S., Saxe D. F., Gress L. A., Canfield D. R. Hematopoietic stem cells with high proliferative potential. Assay of their concentration in marrow by the frequency and duration of cure of W/Wv mice. J Clin Invest. 1982 Aug;70(2):242–253. doi: 10.1172/JCI110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B., Hawley R. G., Mintz B. Long- and short-lived murine hematopoietic stem cell clones individually identified with retroviral integration markers. Blood. 1990 Jun 15;75(12):2267–2270. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., Lerner C. Number and continuous proliferative pattern of transplanted primitive immunohematopoietic stem cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Jordan C. T., McKearn J. P., Lemischka I. R. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990 Jun 15;61(6):953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990 May 1;171(5):1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lord B. I., Spooncer E. Isolation of haemopoietic spleen colony forming cells. Lymphokine Res. 1986 Winter;5(1):59–72. [PubMed] [Google Scholar]
- Micklem H. S., Ford C. E., Evans E. P., Ogden D. A. Compartments and cell flows within the mouse haemopoietic system. I. Restricted interchange between haemopoietic sites. Cell Tissue Kinet. 1975 May;8(3):219–232. doi: 10.1111/j.1365-2184.1975.tb01221.x. [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Lennon J. E., Ansell J. D., Gray R. A. Numbers and dispersion of repopulating hematopoietic cell clones in radiation chimeras as functions of injected cell dose. Exp Hematol. 1987 Mar;15(3):251–257. [PubMed] [Google Scholar]
- Mintz B., Anthony K., Litwin S. Monoclonal derivation of mouse myeloid and lymphoid lineages from totipotent hematopoietic stem cells experimentally engrafted in fetal hosts. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7835–7839. doi: 10.1073/pnas.81.24.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder A. H., Visser J. W., van den Engh G. J. Thymus regeneration by bone marrow cell suspensions differing in the potential to form early and late spleen colonies. Exp Hematol. 1985 Sep;13(8):768–775. [PubMed] [Google Scholar]
- Muller-Sieburg C. E., Whitlock C. A., Weissman I. L. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986 Feb 28;44(4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Müller-Sieburg C. E., Townsend K., Weissman I. L., Rennick D. Proliferation and differentiation of highly enriched mouse hematopoietic stem cells and progenitor cells in response to defined growth factors. J Exp Med. 1988 Jun 1;167(6):1825–1840. doi: 10.1084/jem.167.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Isolation of hemopoietic stem cell subsets from murine bone marrow: I. Radioprotective ability of purified cell suspensions differing in the proportion of day-7 and day-12 CFU-S. Exp Hematol. 1988 Jan;16(1):21–26. [PubMed] [Google Scholar]
- Spangrude G. J. Enrichment of murine haemopoietic stem cells: diverging roads. Immunol Today. 1989 Oct;10(10):344–350. doi: 10.1016/0167-5699(89)90192-8. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Weissman I. L. Mature T cells generated from single thymic clones are phenotypically and functionally heterogeneous. J Immunol. 1988 Sep 15;141(6):1877–1890. [PubMed] [Google Scholar]
- Szilvassy S. J., Fraser C. C., Eaves C. J., Lansdorp P. M., Eaves A. C., Humphries R. K. Retrovirus-mediated gene transfer to purified hemopoietic stem cells with long-term lympho-myelopoietic repopulating ability. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8798–8802. doi: 10.1073/pnas.86.22.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn M., Heimfeld S., Spangrude G. J., Weissman I. L. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]