Abstract
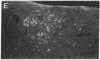
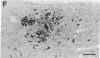
NADPH diaphorase histochemistry selectively labels a number of discrete populations of neurons throughout the nervous system. This simple and robust technique has been used in a great many experimental and neuropathological studies; however, the function of this enzyme has remained a matter of speculation. We, therefore, undertook to characterize this enzyme biochemically. With biochemical and immunochemical assays, NADPH diaphorase was purified to apparent homogeneity from rat brain by affinity chromatography and anion-exchange HPLC. Western (immunoblot) transfer and immunostaining with an antibody specific for NADPH diaphorase labeled a single protein of 150 kDa. Nitric oxide synthase was recently shown to be a 150-kDa, NADPH-dependent enzyme in brain. It is responsible for the calcium/calmodulin-dependent synthesis of the guanylyl cyclase activator nitric oxide from L-arginine. We have found that nitric oxide synthase activity and NADPH diaphorase copurify to homogeneity and that both activities could be immunoprecipitated with an antibody recognizing neuronal NADPH diaphorase. Furthermore, nitric oxide synthase was competitively inhibited by the NADPH diaphorase substrate, nitro blue tetrazolium. Thus, neuronal NADPH diaphorase is a nitric oxide synthase, and NADPH diaphorase histochemistry, therefore, provides a specific histochemical marker for neurons producing nitric oxide.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. J., Martin J. B. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986 May 8;321(6066):168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- East S. J., Garthwaite J. Nanomolar N(G)-nitroarginine inhibits NMDA-induced cyclic GMP formation in rat cerebellum. Eur J Pharmacol. 1990 Aug 10;184(2-3):311–313. doi: 10.1016/0014-2999(90)90623-e. [DOI] [PubMed] [Google Scholar]
- Ferrante R. J., Kowall N. W., Beal M. F., Richardson E. P., Jr, Bird E. D., Martin J. B. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985 Nov 1;230(4725):561–563. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- Ferriero D. M., Arcavi L. J., Sagar S. M., McIntosh T. K., Simon R. P. Selective sparing of NADPH-diaphorase neurons in neonatal hypoxia-ischemia. Ann Neurol. 1988 Nov;24(5):670–676. doi: 10.1002/ana.410240512. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G. Cellular origins of cyclic GMP responses to excitatory amino acid receptor agonists in rat cerebellum in vitro. J Neurochem. 1987 Jan;48(1):29–39. doi: 10.1111/j.1471-4159.1987.tb13123.x. [DOI] [PubMed] [Google Scholar]
- Hirsch E. C., Graybiel A. M., Duyckaerts C., Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5976–5980. doi: 10.1073/pnas.84.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B. T., Vincent S. R. Histochemical characterization of neuronal NADPH-diaphorase. J Histochem Cytochem. 1989 May;37(5):653–661. doi: 10.1177/37.5.2703701. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. C., Kuonen D. R., Sutton A., Roberts P. J. Rat brain NADPH-dependent diaphorase. A possible relationship to cytochrome P450 reductase. Biochem Pharmacol. 1988 Aug 15;37(16):3063–3070. doi: 10.1016/0006-2952(88)90302-4. [DOI] [PubMed] [Google Scholar]
- Knigge K. M., Piekut D. T., Abood L. G., Joseph S. A., Michael G. J., Xin L., Berlove D. J. Immunocytochemistry of receptors using anti-idiotypic antibodies. Methods Enzymol. 1989;178:212–221. doi: 10.1016/0076-6879(89)78017-4. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Vulnerability of cultured cortical neurons to damage by excitotoxins: differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci. 1988 Jun;8(6):2153–2163. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Peters S., Choi D. W. Neurons containing NADPH-diaphorase are selectively resistant to quinolinate toxicity. Science. 1986 Oct 3;234(4772):73–76. doi: 10.1126/science.2875522. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F. Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: normal anatomy and alterations in Alzheimer's disease. Ann Neurol. 1988 Feb;23(2):105–114. doi: 10.1002/ana.410230202. [DOI] [PubMed] [Google Scholar]
- Kuonen D. R., Kemp M. C., Roberts P. J. Demonstration and biochemical characterisation of rat brain NADPH-dependent diaphorase. J Neurochem. 1988 Apr;50(4):1017–1025. doi: 10.1111/j.1471-4159.1988.tb10567.x. [DOI] [PubMed] [Google Scholar]
- Miki N., Kawabe Y., Kuriyama K. Activation of cerebral guanylate cyclase by nitric oxide. Biochem Biophys Res Commun. 1977 Apr 25;75(4):851–856. doi: 10.1016/0006-291x(77)91460-7. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Mash D. C., Hersh L. B. Neurofibrillary tangles in cholinergic pedunculopontine neurons in Alzheimer's disease. Ann Neurol. 1988 Nov;24(5):623–629. doi: 10.1002/ana.410240506. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Bredt D., Snyder S. H. Messenger molecules in the cerebellum. Trends Neurosci. 1990 Jun;13(6):216–222. doi: 10.1016/0166-2236(90)90163-5. [DOI] [PubMed] [Google Scholar]
- Scherer-Singler U., Vincent S. R., Kimura H., McGeer E. G. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods. 1983 Nov;9(3):229–234. doi: 10.1016/0165-0270(83)90085-7. [DOI] [PubMed] [Google Scholar]
- Uemura Y., Kowall N. W., Beal M. F. Selective sparing of NADPH-diaphorase-somatostatin-neuropeptide Y neurons in ischemic gerbil striatum. Ann Neurol. 1990 Jun;27(6):620–625. doi: 10.1002/ana.410270606. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Johansson O., Hökfelt T., Skirboll L., Elde R. P., Terenius L., Kimmel J., Goldstein M. NADPH-diaphorase: a selective histochemical marker for striatal neurons containing both somatostatin- and avian pancreatic polypeptide (APP)-like immunoreactivities. J Comp Neurol. 1983 Jul 1;217(3):252–263. doi: 10.1002/cne.902170303. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Satoh K., Armstrong D. M., Fibiger H. C. NADPH-diaphorase: a selective histochemical marker for the cholinergic neurons of the pontine reticular formation. Neurosci Lett. 1983 Dec 23;43(1):31–36. doi: 10.1016/0304-3940(83)90124-6. [DOI] [PubMed] [Google Scholar]