Abstract
Poly(D,L-lactic acid) (PLA) has been widely used for various biomedical applications for its biodegradable, biocompatible, and nontoxic properties. Various methods, such as emulsion, salting out, and precipitation, have been used to make better PLA micro and nano-particle formulations. They are widely used as controlled drug delivery systems of therapeutic molecules, including proteins, genes, vaccines, and anti-cancer drugs. Even though PLA-based particles have challenges to overcome, such as low drug loading capacity, low encapsulation efficiency, and terminal sterilization, continuous innovations in particulate formulations will lead to development of clinically useful formulations.
Keywords: PLA, Micro-particles, Nano-particles, Fabrication methods, Drug delivery system
Graphical abstract
1. Introduction
Shortly after poly(D,L-lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic acid) (PLGA) were developed for use in surgical implants and tissue repair in the 1960s, they have been used widely for various biomedical applications, including sutures, bone plates, abdominal mesh, and controlled release drug delivery [1-5]. These polymers, which are biocompatible, biodegradable, and nontoxic, have been used for various biomedical applications for decades [5, 6]. The first PLGA-based drug delivery system approved by the Food and Drug Administration (FDA) was the Lupron Depot drug delivery system. It is made of PLGA (L:G ratio of 75:25) and leuprolide acetate for treatment of advanced prostate cancer. This system delivers the drug over a period of 4 months after a single injection [7]. The list of FDA-approved controlled release products of PLA and PLGA formulations are given in Table 1.
Table 1.
Sustained release depot formulations based on PLGA/PLA currently available for clinical use.
| Product Name | Active Ingredient | Company | Application | Formulation | Administration |
|---|---|---|---|---|---|
| Lupron®Depot (1989) | Leuprolide acetate | TAP | Prostate cancer, endometriosis |
Microparticle | I.M. |
| Zoladex® (1989) | Goserelin acetate | AstraZeneca Pharmaceuticals |
Prostate cancer, Endometriosis |
Implant | S.C. |
| Sandostatin LAR® Depot (1998) |
Octreotide acetate | Novartis | Acromegaly | Microparticle | S.C. |
| Atridox® (1998) | Doxycycline hyclate |
Zila, Inc. | Chronic adult periodontitis |
In situ forming | Local delivery |
| Nutropin®Depot (1999) | Growth hormone | Genetech | Pediatric growth hormone deficiency |
Microparticle | I.M. |
| Surodex® (1999) (Approved in China, Singapore and several other countries) |
Dexamethasone | Allergan | Post surgical inflammation after cataract surgery |
Implant | Local delivery |
| Trelstar TM Depot (2000 and 2010) |
Triptorelin pamoate | Pfizer | Prostate cancer | Microparticle | I.M. |
| Somatuline® Depot (2000) |
Lanreotide | Ipsen | Acromegaly | Microparticle | S.C. |
| Suprefact® Depot (2000) (Approved in Sweden, Netherlands and several other countries) |
Buserelin acetate | Sanofi-Aventis | Prostate cancer | Implant | S.C. |
| Arestin® (2001) | Minocycline | Orapharma | Periodontal disease |
Microparticle | Local delivery |
| Suprecur® MP (2002) (Approved in Japan) |
Buserelin acetate | Sanofi-Aventis | Prostate cancer | Microparticle | S.C. |
| Risperidal® ConstaTM
(2003) |
Risperidone | Johnson &Johnson |
Antipsychotic | Microparticle | I.M. |
| Eligard® (2004) | Leuprolide acetate injectable |
TOLMAR Pharmaceuticals Inc. |
Prostate cancer | In situ forming implant |
S.C. |
| Somatuline LA® (2004) (Approved in UK) |
Lanreotide acetate | Ipsen | Acromegaly | Microparticle | I.M. |
| Decapeptyl® (2006) (Approved in EU) |
Triptorelin pamoate | Ipsen | Prostate cancer | Microparticle | I.M. |
| Vivitrol® (2006) | Naltrexone | Alkermes | Alcohol abuse | Microparticles | I.M. |
| Decapeptyl® (2006) (Approved in EU) |
Triptorelin pamoate | Ipsen | Prostate cancer | Microparticle | I.M. |
| Ozurdex® (2009) | Dexamethasone | Allergan | Diabetic macular edema |
Implant | Local delivery |
| Lutrate Depot® (2010) | Leuprolide acetate | G P Pharm | Type 2 diabetes | Microparticle | I.M. |
| Bydureon® (2012) | Exenatide | Amylin Pharmaceuticals Inc. |
Type 2 diabetes | Microparticle | S.C. |
| PropelTM (2012) | Mometasone furoate |
Intersect ENT | Post surgical Inflammation |
Implant | Local delivery |
| Lupaneta Pack (2012) | Leuprolide acetate Norethindrone acetate |
AbbVie endocrine | Prostate cancer | Microparticle | I.M. |
| Pamorelin LA® (2012) (Approved in EU) |
Triptorelin pamoate | Galenica | Prostate cancer | Microparticle | I.M. |
(Information from Reference [7] was included in the table).
The FDA requires cGMP (current good manufacturing practice) protocols to ensure efficacy, safety, and stability for pharmaceuticals. PLGA and PLA for clinical applications are manufactured under cGMP regulation [9, 10]. The list of cGMP grade PLAs from major suppliers is shown in Table 2. Researchers can get easy access to the non-GMP grade polymers through commercial suppliers, such as Sigma-Aldrich, Vornia, Akina, and other suppliers.
Table 2.
The list of cGMP grade PLA and PLGA manufacturers.
| Company | Trade name |
| Corbion | Purasorb® |
| Lactel | Lactel® |
| Alkermes | Medisorb® |
| Evonik | Resomer® |
| PCAS | Expansorb® |
Polyesters such as PLA and PLGA have been used extensively in drug delivery because of their biodegradable and mechanical properties that can be adjustable [11, 12]. They can be designed and synthesized with different molecular weights and L:G ratios for individual applications with high reproducibility at low cost. These advantages allow researchers to make micro and nano-particles using PLA and PLGA. They have been used to make various delivery systems for low molecular weight drugs as well as peptide and protein drugs [1-4, 13, 14]. Most nano-particle formulations based on PLA and PLGA have been focused on drug delivery to target tumors [13].
One of the advantages of using PLA to make micro and nano-particles is the flexibility. Physical properties, such as size and shape, and chemical properties, including molecular weight and L:G ratio, can be easily controlled to obtain desirable pharmacokinetic and biodegradable properties. Typically, the particles include spheres, capsules, cubes, and other shapes [14, 15]. An active pharmaceutical ingredient (API) is usually dispersed homogeneously within the PLA matrix [2]. PLA-based micro and nano-particles are useful in drug delivery and biomedical applications, but there are also a variety of limitations, such as high initial burst release, as listed in Table 3.
Table 3.
Advantages and limitations of PLA micro and nano-particles [8].
| Advantages | Limitations | |
|---|---|---|
| Micro-particles | Subcutaneous injections Intramuscular injections Controlled release Reproducible processes |
Unintended toxic side effect due to high initial burst Wasteful use of expensive drugs due to initial burst release |
|
| ||
| Nano-particles | Direct injection to the blood Potentially improved vaccine responses |
Non-specific uptake by reticuloendothelial system (RES) systems Potential immunotoxicity |
Nano-particles are internalized in cells partly through fluid phase pinocytosis and also through clathrin-mediated endocytosis. They rapidly escape the endo-lysosomes and enter the cytoplasm within 10 min of incubation [12]. The micro and nano-particles can effectively incorporate the drug into their structure [8]. Micro and nano-particles with large surface-to-volume ratios provide a greater number of reaction sites than macro size particles with smaller surface areas [16]. Various methods to modify properties of PLA micro and nano-particles are available [1, 3, 5, 6, 11, 16-19]. Bulk and surface properties can be modified. The bulk modification methods include blending with different polymers, plasticization, copolymerization, and cross-linking. The surface modification methods include surface coating, entrapment, and plasma treatment. Supplemental biomaterials, such as poly(ethylene glycol) (PEG), polysaccharides, and extracellular matrix (ECM) proteins, have been used for coating PLA micro and nano-particles [2, 20]. All modifications are designed to achieve high drug encapsulation efficiency and loading, and controlled drug release rate. The following sections cover different preparation techniques for making PLA micro and nano-particles, and their applications, handling challenges, and strategies.
2. Preparation techniques of micro and nano-particles
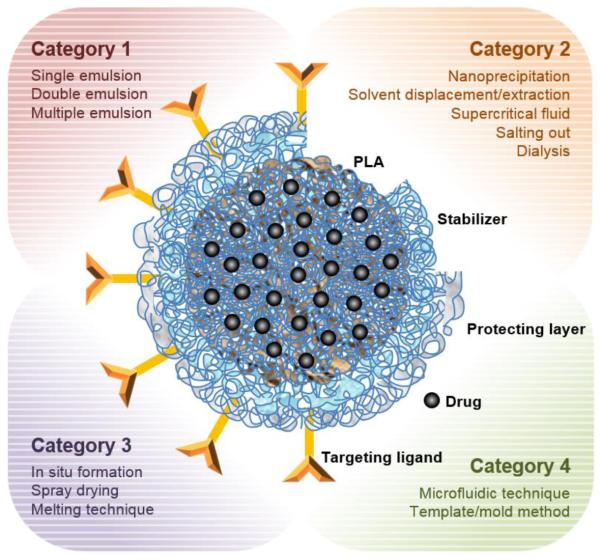
There are several techniques useful for the preparation of PLA-based micro and nano-particles [21-28]. The techniques are classified into four categories. Category 1 is traditional emulsion based methods which are single emulsion, double emulsion, and multiple emulsions. Category 2 is precipitation-based methods which include nano precipitation, rapid expansion of supercritical fluid into liquid, salting out, and dialysis. Category 3 is direct compositing methods, such as melting technique, spray drying, supercritical fluid and in situ forming micro-particles. Category 4 includes new approaches including microfluidic technique and template/mold based technique. Other criteria depend on the mode of drug encapsulation. The drug is either entrapped inside of the particles of "capsules" or dispersed in polymer matrices [6, 17, 29, 30]. Fig. 1 shows a representative micro and nano-particle structure with the four categories of preparation techniques.
Figure 1.
Schematic description of a PLA micro and nano-particle and its preparation techniques.
2.1. Single emulsion
One of the simplest methods to make micro and nano-particle is the single emulsion/solvent extraction technique. Many hydrophobic drugs are dissolved with PLA in various water-immiscible organic solvents which are then emulsified in a water phase containing a stabilizer. Emulsion, such as o/w (oil in water), o/o (oil in oil) or w/o (water in oil), can be formed to accommodate different types of dispersed phase and the dispersion medium [6, 17]. The emulsification is exposed to a high energy source, such as ultrasound, homogenizer, or milling. The oil phase is removed by evaporation under low pressure or vacuum or by solvent extraction using a large volume of water, leading to the formation of particles dispersed in the water phase. The particles are collected by centrifugation or filtration and washed with pure water or buffer solution to remove residual stabilizers and any free drug. The harvested particles is lyophilized for storage [13, 17, 31].
The emulsion/solvent extraction technique can produce various size particles ranging from nanometers to micrometers by controlling the agitation rate and other experimental parameters. The parameters for loading a water soluble drug into particles include the phase volumes of oil and water, concentration of polymer and drug, presence of oil soluble surfactant in oil, stabilizer/surfactant in oil/water, saturation solubility of drug in water, and stirring rate [1, 17, 32-34].
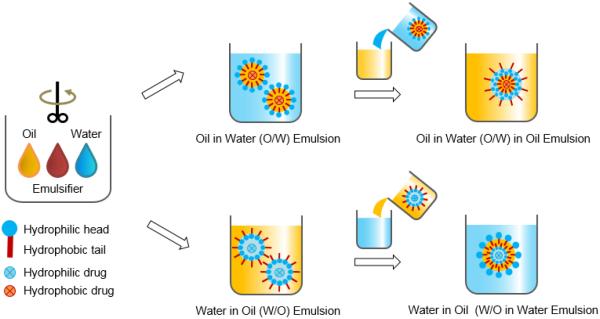
2.2. Double emulsion
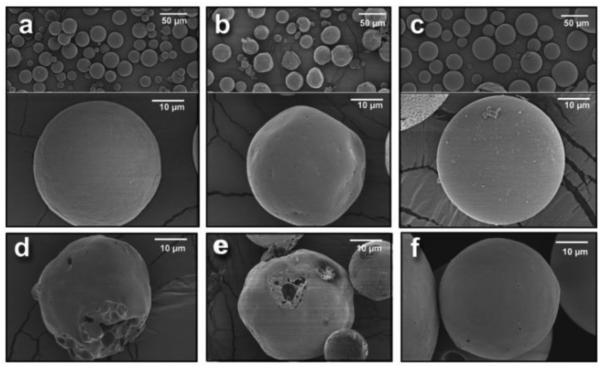
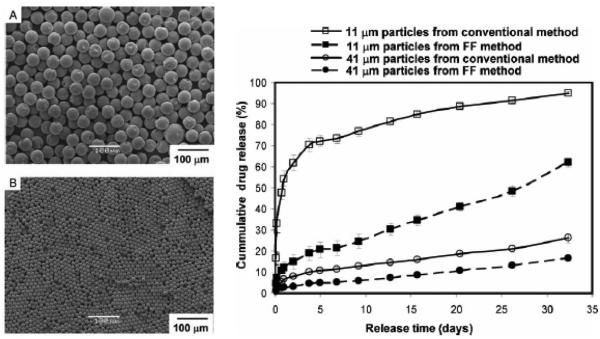
The single emulsion/extraction methods have challenges of poor encapsulation of hydrophilic drugs due to their diffusion and dispersion from the emulsified oil phase into the aqueous continuous phase. Thus, double-emulsion/extraction methods have been frequently used for improving the encapsulation efficiency of water soluble drugs, such as peptides and proteins [17, 35, 36]. In some cases, solid/oil/water (s/o/w) emulsion has been used for a high drug loading of water soluble peptides, such as insulin [17, 37, 38]. This involves addition of a water-soluble drug solution to an organic polymer solution under high energy stirring to form a w/o (or reversely o/w) emulsion. This w/o emulsion is added into a second water phase containing a stabilizer with stirring, resulting in the formation of a w/o/w (or o/w/o) emulsion. The organic solvent is removed under reduced pressure or vacuum to produce polymer particles. The harvested particles are thoroughly washed using pure water or buffer to remove residual raw materials before lyophilization [2, 17]. There are several parameters that have to be adjusted to optimize the characteristics of particles prepared by the double emulsion method. These include the amount of hydrophilic drug to be added, polymer concentration, type of solvent, stabilizer concentration, volume of the second aqueous phase, stirring rate and other variables [1, 17, 29, 32-34]. Fig. 2 shows the single and double emulsion processes with oil, emulsifier, and water. In one example, PLA three different molecular weights were used to prepare micro-particles containing prilocaine (PRL), which is an amino-amide type local anesthetic. PRL-loaded PLA micro-particles were prepared by w/o/w double emulsion methods. The particle sizes were 32 μm, 40 μm, and 68 μm. The SEM images and release profiles of three different types of PLA micro-particles are shown in Fig. 3 and Fig. 4, respectively [39].
Figure 2.
Schematic diagram of emulsion-based methods for preparation of polymer particles.
Figure 3.
SEM photos of prilocaine-loaded micro-particles with (a) Resomer R202 (PLA, average MW 10,000-18,000), (b) Resomer R203S (PLA, average MW 18,000-28,000), (c) Resomer R207 (PLA, average MW 209,000) polymers. Images of the same batches recuperated after 96 hr. of drug release studies are also reported: (d) Resomer R202, (e) Resomer R203S, (f) Resomer R207 [39].
Figure 4.
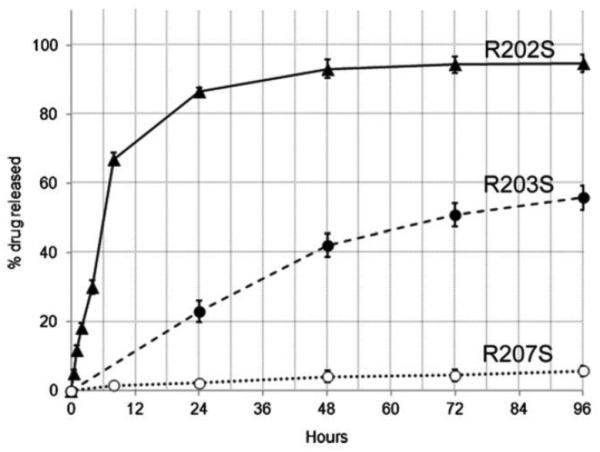
Drug release profiles of prilocaine-loaded micro-particles in pH 7.4 phosphate buffer. The total amount was calculated from the average value of encapsulation efficiency percentage obtained by the direct and the indirect method [39].
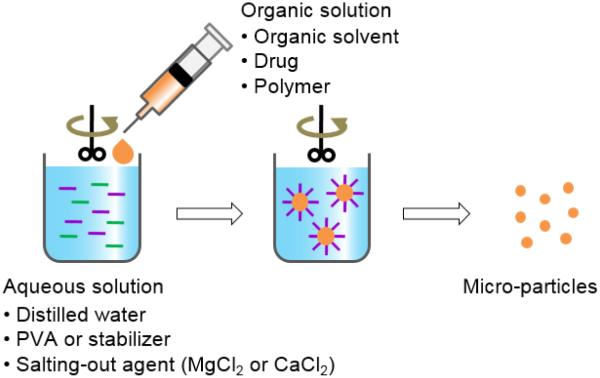
2.3. Salting out
An alternative to the widely applied emulsion-based technique is the salting-out method. This method involves the addition of polymer and drug solution in a water-miscible solvent such as acetone, acetonitrile, or tetrahydrofuran to an aqueous solution containing the salting-out agent (e.g., magnesium chloride and calcium chloride) and a colloidal stabilizer, such as polyvinylpyrrolidone, under high speed stirring (Fig. 5) [17]. When this o/w emulsion is diluted with a large amount of water, it induces the formation of particles by enhancing the diffusion of the miscible solvent into the water phase. The particles can be purified and harvested by centrifugation or cross-flow filtration [29, 40]. One of the important advantages of this method is minimizing tension to the loaded protein [17, 40]. Salting out does not need a heating process, and thus may be useful when heat sensitive drugs have to be encapsulated [41-43]. The salting out process requires optimization of the process conditions, e.g., the salt type and concentration, the type of polymer and solvent, and the ratios of these compounds in order to obtain micro-particles [17].
Figure 5.
Schematic description of the salting out method for preparation of polymer particles.
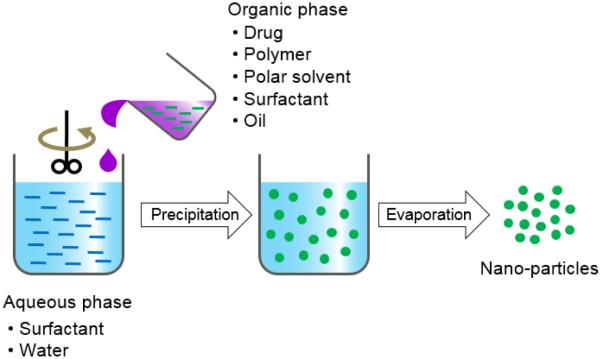
2.4. Nanoprecipitation
The nanoprecipitation method is a relatively easy and reproducible technique for the preparation of PLA based nano-particles. The nanoprecipitation is a one step process, also known as the solvent displacement method (Fig. 6) [29, 30]. The advantages of this method are: narrow size distribution; less toxic and eco-friendly solvents; and low energy source of a stirring device. The nanoprecipitation method can be applied in various ways: (i) direct pouring of an anti-solvent (e.g., water phase) into an organic solution; (ii) slow drop-wise addition of water to an organic solution; (iii) direct pouring of an organic solution into the water phase; and (iv) dilution of a polymeric dispersed phase using an anti-solvent. The solvent is then removed from the suspension under reduced pressure or vacuum [44, 45].
Figure 6.
Schematic description of nanoprecipitation for preparation of polymer particles.
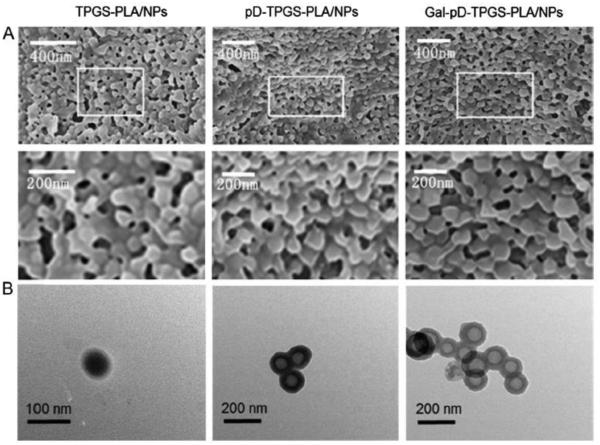
There are variable key parameters for forming nano-particles. The injection rate of the organic phase into the anti-solvent phase affects the particle size. The mixing rate affects both particle size and drug encapsulation yield. The type of the organic solvent also affects the size and encapsulation efficiency of particles. Typical solvents used for nanoprecipitation are acetone, acetonitrile, dimethylacetamide, dimethylformamide, dimethylsulfoxide (DMSO), 2-pyrrolidone, N-methyl-2-pyrrolidone (NMP), PEG, and tetrahydrofuran. Acetone is the most preferred solvent. Usually, a binary mixture of solvents is used, e.g., acetone-ethanol [28]. Other factors are the drug: polymer ratio, surfactant, and anti-solvent phase volume ratio. The difficulty faced in this method is the choice of combination of the drug/polymer/solvent/non-solvent system (usually called Ouzo region [46-48]) in which the nano-particles would be formed with a high drug encapsulation efficiency [30]. Producing the successful nano-particles, however, is restricted to a narrow condition of the Ouzo region. Beyond the Ouzo region, micro-particles rather than nano-particles are produced [29, 30]. Improved loading of procaine hydrochloride, a water soluble drug, into polymer nano-particles was achieved by increasing the aqueous phase pH and replacing procaine hydrochloride with a procaine dihydrate base [44]. Docetaxel (DTX)-loaded nano-particles were prepared by the nanoprecipitation method using polydopamine-modified tocopherol polyethylene glycol succinate (TPGS)-PLA. The distribution of the nano-particle size was around 126~209 nm. The DTX loaded PLA nano-particles reduced the tumor size most significantly on hepatoma-bearing nude mice (Fig. 7) [49].
Figure 7.
(A) Field emission SEM images of DTX-loaded TPGS-PLA/NPs, polydopamine (pD)-TPGS-PLA/NPs and galactosamine (Gal)-pD-TPGS-PLA/NPs; local images in the box are shown in the lower panel. (B) TEM images of DTX-loaded TPGS-PLA/NPs, pD-TPGS-PLA/NPs and Gal-pD-TPGS-PLA/NPs [49].
2.5. Dialysis
Dialysis is an effective, simple method for forming small size and narrowly distributed nano-particles similar to nanoprecipitation. A polymer is dissolved in an organic solvent and placed in a dialysis tube. The dialysis is performed in a non-solvent miscible with an organic solvent. The organic solvent is displaced by non-solvent resulting in a loss of polymer solubility, and subsequent formation of polymer aggregates. Nano-particles of homogeneous suspension are collected after fully displacing the organic solvent with non-solvent (Fig. 8) [6, 50-54].
Figure 8.
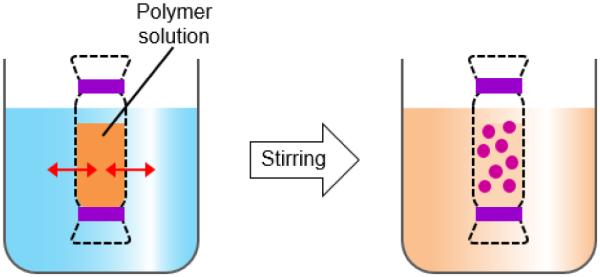
Schematic description of dialysis for preparation of polymer particles.
2.6. Spray drying
Spray drying is a useful, continuous particle production method, where the drug is dissolved or dispersed in an organic phase with a polymer that is then sprayed as ultra fine droplets in dry air flow [17, 55-58]. The organic phase is instantly evaporated. The dried particles are collected under low pressure with dry air flow (Fig. 9). This technique is easy to set up, but at the same time it is hard to control the drug distribution in the particles [4, 28]. Spray drying has been studied for protein encapsulation to improve the stability of biomacromolecules [17]. It is also useful for large scale hydrophobic drug particle production [56, 59-63]. The size distribution and morphology of the spray dried PLA micro-particles were analyzed by SEM. The largest diameter was 3 μm for making 5% of the PLA solution. There was no significant difference between 0.5% and 1% of PLA solutions (Fig. 10) [64].
Figure 9.
Schematic description of spray drying for preparation of polymer particles.
Figure 10.
PLA micro-spheres made of PEG-distearate obtained by the spray drying technique. (A) PLA 1% (w/v) and PEG-distearate 10% (w/v), (B) PLA 1% (w/v) and PEG-distearate 1% (w/v), (C) PLA 3% (w/v) and PEG-distearate 1% (w/v), (D) PLA 5% (w/v) and PEG-distearate 1% (w/v) [64].
2.7. In situ forming micro-particle
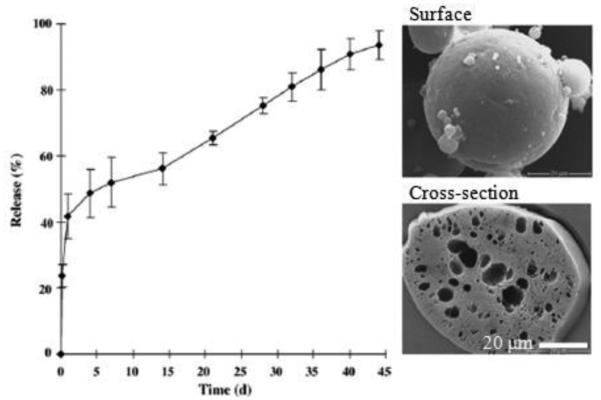
In situ forming depots have been used for site forming micro-particles or micro depots. This approach overcomes some drawbacks of conventional techniques, including manufacturing costs and complexities of other methods, e.g., drying and resuspension [17]. A drug-polymer solution is administered via injection at the target site where it is precipitated into an implant or forms micro-particles (Fig. 11), a concept that has been employed in FDA-approved long-acting release products [17]. The drug/polymer solutions are dissolved in water-miscible solvents, such as NMP and DMSO. The toxicity of solvents must be examined before selection. Some solvents show lower myotoxicities of in situ implants and in situ micro-particles [27]. Water-miscible solvents result in hardening of emulsion droplets in vivo, and the solvent removal process may be responsible for a high burst release [65, 66]. The safety issues may limit types of oils, e.g., paraffin/mineral oils, that can be used [17]. The delivery of leuprolide acetate for months was based on in situ forming micro-particle formulation. A conventional formulation is a two syringe/connector system. A solution of leuprolide and PLGA or PLA in NMP was emulsified into an external oil phase. In situ forming PLGA micro-particles showed a high initial release (~40%) because of their high porosity (Fig. 12). In situ forming PLA micro-particles exhibited a much lower initial release (~9%), which had a slow and continuous drug release (Fig. 13) [67].
Figure 11.
Schematic description of in situ forming micro-particle for preparation of polymer particles.
Figure 12.
Leuprolide release and SEM images of in situ forming micro-particles (PLGA (50:50) Resomer® (RG 503H, average MW 24,000~38,000), standard formulation) [67].
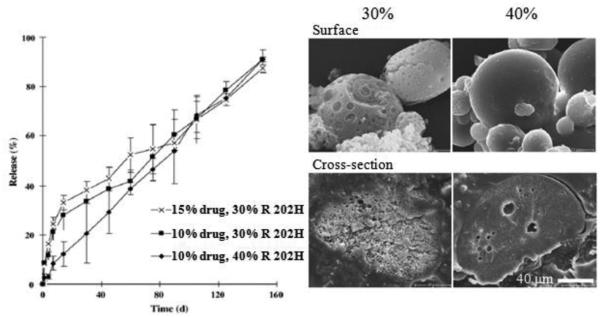
Figure 13.
Leuprolide release and SEM images of in situ forming micro-particles (PLA Resomer® (R 202H, average MW 10,000~18,000), 10% or 15%, w/w, drug loading and 30% or 40%, w/w, polymer concentration) [67].
2.8. Melting technique
The melting technique provides another option for encapsulating drugs into polymers. The melting process avoids the use of organic solvents, but the drug is dispersed in a polymer melt. The resulting drug/polymer melt is solidified by a cooling water phase or a cooling chamber with dry air flow (Fig. 14) [17, 28]. The drug/polymer melt is cooled down and then ground or milled to form particles [17]. If spherical particles and a smaller distribution are desired, the ground melt can be emulsified in a hot solution containing emulsifier or a hot gel [68]. Limitations of this approach are the thermal treatment of the drug and the multitude of steps to obtained smooth micro-particles [17].
Figure 14.
Schematic description of melting technique for preparation of polymer particles.
2.9. Supercritical fluids technique (SCF)
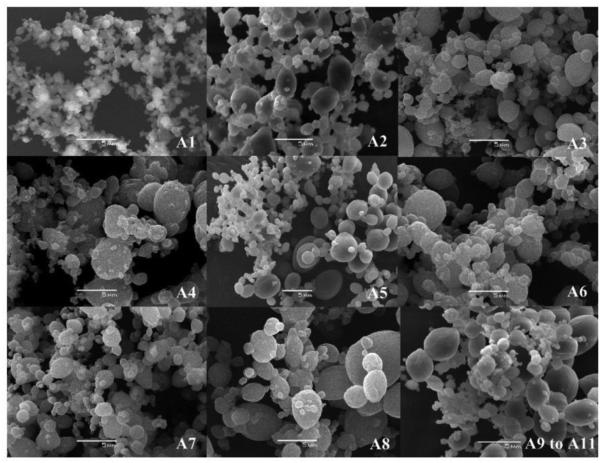
Supercritical fluid and dense gas technology offer an interesting and effective technique for particle production, avoiding most of the drawbacks of the traditional methods. The supercritical fluid method uses more environmentally friendly solvents and has the potential to produce nano-particles with high purity and no residual solvents [69-71]. Two principal processes have been developed for the production of nano-particles using supercritical fluids: rapid expansion of supercritical solution (RESS) and rapid expansion of supercritical solution into a liquid solvent (RESOLV) (Fig. 15). A limitation of the RESS is the use of low concentrations and low molecular weight PLAs [6, 17]. It was hard to control particle quality, such as size, and morphology [72]. The effects of operating conditions, such as pressure, flow rate and concentration of drug and polymer, were evaluated using different sizes and morphologies of particles. The PLA particles presented mean diameters between 5.4 ~ 20.5 μm (Table 4 and Fig. 16) [73].
Figure 15.
Schematic description of supercritical fluids technique.
Table 4.
Results of the factorial design experiment used to study the effects of pressure, concentration and flow rate of polymer solution on PLA particle mean diameters and DCM residual levels [73].
| Assay | Pressure (MPa) |
Concentration of polymer solution (%) |
Flow rate of polymer solution (mL/min) |
Mean diameter (μm) |
Residual DCM (ppm) |
|---|---|---|---|---|---|
| A1 | 8 | 0.5 | 0.5 | 6 ± 1 | 790 |
| A2 | 8 | 0.5 | 2.5 | 21 ± 1 | 1,130 |
| A3 | 16 | 0.5 | 0.5 | 5 ± 0 | 1,280 |
| A4 | 16 | 0.5 | 2.5 | 18 ± 0 | 6,490 |
| A5 | 8 | 1.5 | 0.5 | 7 ± 0 | <600 |
| A6 | 8 | 1.5 | 2.5 | 12 ± 0 | 5,980 |
| A7 | 16 | 1.5 | 0.5 | 7 ± 0 | 2,040 |
| A8 | 16 | 1.5 | 2.5 | 11 ± 0 | 2,630 |
| A9 | 12 | 1.0 | 1.5 | 10 ± 0 | 3,400 |
| A10 | 12 | 1.0 | 1.5 | 6 ± 0 | 4,160 |
| A11 | 12 | 1.0 | 1.5 | 9 ± 0 | 4,670 |
Figure 16.
Typical morphological features by scanning electron microscopy of the particles produced in the assays A1–A11 [73].
2.10. Microfluidic technique
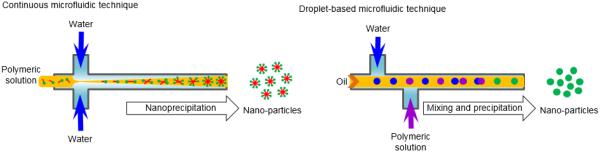
The microfluidic technique can fabricate uniform, biodegradable PLA-based particles and implants. The uniform particles may allow precisely controlled release systems, because the size of the particles is a primary determinant of drug release kinetics [1]. Droplet microfluidics deals with discrete droplets having precisely controlled volume and composition, restricted dispersion, which are ideal templates for fabricating complex particles. A number of microfluidic approaches have been developed and are widely used for fabricating single emulsion, double or multiple emulsions (Fig. 17). The polymeric micro-particles are generally fabricated by making o/w emulsions in microfluidic devices, where polymers are dissolved in an organic solvent (oil phase), by droplet solidification through solvent evaporation, diffusion or extraction. The polymer solution in the organic solvent is filled in a T- or Y-junction microfluidic device and then ejected into a large amount of stabilizing solution. The solvent is diffused from droplets into the water phase, and then the droplets are solidified to microspheres due to the relatively different solubility of the solvent in water [2, 15, 74, 75]. When the polymer solution droplets are ejected into the stabilizing solution, the size of the particles are determined by properties of the solutions (density and viscosity), the flow rate of the polymer solution, the diameter of the nozzle, and the interfacial tension between the polymer solution and the nozzle tip. There are similar limitations as other methods using physical devices such as a nozzle, a channel, a template, and a mold. The fabrication of nano-particles would apparently require a smaller nozzle or channels [18].
Figure 17.
Schematic description of microfluidic technique for preparation of polymer particles.
Applications of microfluidic based micro-particles are rapidly growing for development of controlled drug delivery systems. They have been useful for complex and multifunctional drug delivery systems as multi core-shell micro-particles [15]. With further development of microfluidic techniques and manufacturing processes, micro-particles with desired drug loading and release kinetics can be prepared. Furthermore, the low cost and high reproducibility make this technology promising for mass production of specific drug delivery systems [15, 76]. PLA particles over a wide range of size were made from w/o/w emulsion produced in a three-dimensional (3D) flow focusing glass capillary device. The droplet size is usually controlled by the fluid rate and orifice size. Also, a numerical model of drop generation in a 3D flow focusing device was developed to understand the mechanism of drop generation in the dripping regime [77]. The microfluidics technique was used to make polymeric micro-particles of two different sizes (11 μm and 41 μm) to study in vitro release profile of bupivacaine (Fig. 18). Mono dispersed particles prepared using microfluidics released drug more slowly than similar size particles prepared using a conventional method such as the emulsion method [78]. While the micro-particle manufacturing method is one parameter affecting the drug release kinetics, it is important to realize that other factors also contribute significantly to the drug release profile.
Figure 18.
SEM images of micro-particles prepared via microfluidics with 41 μm (A) and 11 μm (B). Drug-release profiles from monodispersed micro-particles prepared with microfluidic devices and polydisperse micro-particles prepared using the conventional single emulsion technique (right) [78].
2.11. Template/mold based technique
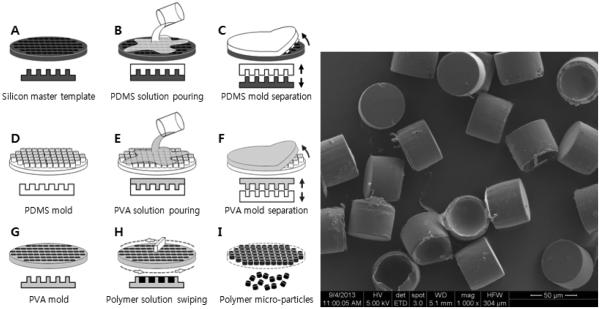
The hydrogel template method is based on the unique properties of physical gels that can undergo sol-gel phase transition upon changes in environmental conditions such as temperature. The hydrogel template is useful in producing particles in homogeneous sizes. The first step is to form a certain pattern on a hard master template. A warm aqueous hydrogel solution (e.g., gelatin solution) is poured on top of the master template, and then the template is placed under low temperature conditions for imprinting the template by a formed hydrogel mold. The solidified mold is peeled off and a polymer/drug solution, in a suitable organic solvent, is poured on the hydrogel mold and evenly spread for filling empty cavities. The filled hydrogel mold is dried to evaporate the solvent. The drug-loaded particles are collected by dissolving the hydrogel mold in water. The particles are washed in water, and collected by centrifugation or filtration [79]. The gelatin hydrogel mold, however, is easy to be damaged while spreading a drug-polymer solution on the template. Thus, an alternative polymer, poly(vinyl alcohol) (PVA), was used to make a water-soluble polymer mold. The PVA mold has many advantages over the gelatin gel mold, including stronger mechanical strength, ease of handling for making a mold, and storage in a dry chamber before use. After drying of the PVA mold having drug-containing microparticles, it is placed in distilled water and stirred at room temperature. Only the PVA mold is dissolved completely and then the drug-containing microparticles are floating freely in the water. The microparticles are washed by filtering through dual-layer meshes made of stainless steel. After collection using centrifugation, the microparticles are washed again in double distilled water to remove residual PVA, centrifuged, and the supernatant is removed. The microparticles are vacuum dried overnight. Fig. 19 shows the procedure and fabricated PLA (average MW 25,000~35,000) micro-particles produced by using this system. The shape of the microparticles is cylinderical because PVA mold having a cylinder pattern with flat bottom is used. The shape of microparaticles depends on the PVA mold pattern. Geometry of microparticles is an important parameter for the drug release rate and interaction with cells and tissues. For cylinder and spherical shapes, the surface areas may be different but the overall release mechanism, whether diffusion-controlled reservoir or matrix systems, is not expected to change due to the shape. Thus, the shape of microparticles may not affect the drug release kinetics significantly. Since the microparticle size is around 50 μm, it is not expected to make any difference in administration. The homogeneous microparticles, in fact, make it easier for administration. The PVA mold method was used to make micro-particles of three poorly water-soluble drugs: risperidone (RIS), methylprednisolone acetate (MPA), and paclitaxel (PTX). The fabricated micro-particles showed great conformity to the original template design for a wide range of formulation conditions. In addition, the micro-particles produced showed narrow size distribution, which provided advantages compared with the conventional emulsion-based method (Fig. 20) [80].
Figure 19.
Schematic of template/mold method process and fabricated PLA micro-particles.
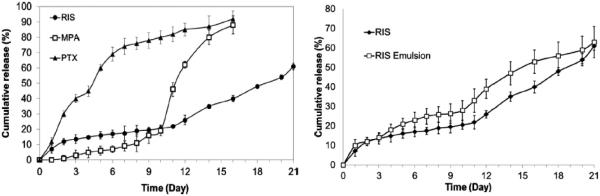
Figure 20.
Comparison of release profiles of risperidone (RIS), methylprednisolone acetate (MPA), and paclitaxel (PTX) from 85:15 PLGA (inherent viscosity 0.55~0.75 dL/g) micro-particles (left). Comparison of release profiles of RIS-loaded micro-particles prepared using hydrogel template and emulsion methods (right) [80].
The PVA mold method was extended to develop the Vacuum SpinSwiper machine to make micro-particles more efficiently in large quantities [81]. There are several advantages of the template based techniques. The preferred advantages are mono-dispersed and predetermined micro-particles dimension, easy scale-up, and a reproducible process. The drug loading by the PVA mold approach can be high for water-soluble drugs, which is not easy to produce by conventional methods. There are also limitations, however. The size of the particle is currently in the micron scale, i.e., larger than 1 μm because of the use of UV lithographic technique in fabricating silicon water master templates. Fabrication of nano-particles requires preparation of the master templates with nano size patterns.
3. Applications of PLA micro and nano-particles
PLA micro and nano-particles have been proposed for improving oral bioavailability of poorly water soluble drugs. Nano-particles are thought to be absorbed from the gastrointestinal tract after oral administration [2, 82]. Poorly water soluble drugs are difficult to make into suitable dosage forms with adequate oral bioavailability [16]. Particles loaded with a poorly soluble drug can significantly increase the drug dissolution rate. The intestine has a special mechanism to absorb particles of certain sizes. The 100 nm particles showed a significantly higher uptake than larger particles [83, 84]. Although gene therapy has been extensively studied for treating genetic diseases and acquired diseases [85], the safety and efficiency of gene delivery have not been examined in depth. PLA-based micro and nano-particles have shown particular promise in improving protection from plasma enzymes, alternative routes of administration (e.g., nasal, oral, pulmonary, and mucosal), and prolonged gene delivery efficacy [86-89]. Cationic PEG-PLA nano-particles are one of the major delivery systems for the small interference RNA (siRNA) system. Systemic delivery of small interfering polo-like kinase 1 (siPlk1) by PEG-PLA nano-particles significantly suppressed tumor growth in an MDA-MB-435s (cancer cells) murine xenograft model [90]. siRNA encapsulated in PEG-PLA nano-particles were shown to have successfully entered the cells and resulted in remarkable gene-specific knockdown in the adult zebrafish heart [91]. PLA based nano-particles containing polyethyleneimine (PEI) on their surfaces were used for incorporating genes. PEO-PLA-PEI was also used for co-delivery of supercoiled minicircle (mc) DNA vectors and Dox. These nano carrier systems have the advantage of non-fouling oxazolines to confer biological stability, of PLA to provide hydrophobicity for Dox encapsulation and of bioreductive PEI to provide gene complexation. The dual delivery of mcDNA-Dox to B16F10 (Musmusculus skinmelanoma cell line; ATCC® CRL-6475™)-Luciferase tumor bearing mice resulted in significantly reduced tumor size and cancer cells' viability [92]. Recently, dual or multi drug delivery systems have shown great potential in the drug delivery field for cancer and gene therapy.
Vaccinations have been highly successful for preventing many infectious diseases using micro-particles [93, 94]. New vaccines are focused on AIDS, hepatitis B, anthrax, SARS, and MERS. Many research groups are focused on developing micro-particle based single shot vaccines using PLA-based materials [95-98]. The T cell activation in response to antigen-encapsulated micro-particles has increased up to 100~1,000 fold more than antigens alone [99]. HIV Gag antigens (p24)-coated PLA nano-particles captured by monocyte-derived dendritic cells (MDDCs) from HIV-1 individuals stimulated MDDC maturation and increased HIV-specific CD8+ T-cell proliferation as compared with p24 alone [100]. PEG-PLA-PEG block copolymer nano-particles were evaluated for encapsulating the hepatitis B surface antigen (HBsAg) as an oral vaccine delivery system. HBsAg encapsulated copolymer and PLA nano-particles were used for adjuvanticity in generating immune stimulations after oral administration. PEG-PLA-PEG copolymer nano-particles exhibited effective levels of humoral immunity along with the mucosal (sIgA) and cellular immune response (TH1) [101]. In the study of the relationship between PLA-PEG particle size and efficacy of transport across the nasal mucosal, tetanus toxoid was encapsulated into PLA-PEG particles of different sizes (200 nm, 1.5 μm, 5 μm, and 10 μm) prepared by the w/o/w double emulsion solvent evaporation technique. The nasal bioavailability of tetanus toxoid encapsulated into 200 nm nano-particles was higher than into larger particles. PLA-PEG nano-particles and aluminum phosphate have been used as a potential adjuvant system using tetanus toxoid. The encapsulation efficiency was increased to nearly 90% in PLA-PEG nano-particles as compared to 55% in a conventional vaccine. PLA-PEG-aluminum (Al) and PLA-Al showed 80% and 50% survival rates, respectively, even at 180 days as compared to a 30% survival rate in the conventional tetanus vaccine [102]. Cyclosporine A (CyA) entrapped in PLA micro and nano-particles showed enhanced bioavailability and sustained release kinetics for extended periods of time [103]. The effects of the concentration of PLA, surfactant, and aqueous phase volume on the PLA microsphere size and nimesulide (a non-steroidal anti-inflammatory drug) encapsulation efficiency were studied using particles made by emulsion methods. A specific aqueous phase volume was selected for small size particles, because an increased volume resulted in microdroplet's coalescence [104]. The in vitro release of albumin from PLA micro-particles was sustained for one month after the particles were blended with PEG [105]. Insulin loaded PEG-PLA nano-particles provided a sustained release for more than two months. The burst release amount increased as PEG molecular weight or PEG content increased [106, 107]. Insulin-loaded PLA based particles had more specific and selective release at high pH conditions, which might increase the effect of the insulin in the blood stream (pH 7.4) [108]. One of the extensive drug delivery fields is cancer chemotherapy. Paclitaxel loaded particles have significantly enhanced anti-tumoral efficacy as compared with free drugs. Paclitaxel loaded PLA-PEG-PLA micro-particles showed 49.6% sustained release of paclitaxel within 1 month [109]. In vitro cytotoxicity testing in cancer cell lines revealed that the PLA-PEG nanoparticles compared with free paclitaxel exhibited similar cytotoxicity [110]. Gemcitabine hydrochloride (GEM) loaded PEG-PLA nanoparticles had zero-order release profiles. The particles increased antitumor effect compared with the free drug on different cancer cell lines and showed a significant improvement of cell interaction. Two xenograft murine models of human solid tumors were used for in vivo anticancer activity of the particles. GEM-PEG-PLA nano-particles significantly inhibited the tumor growth and the mice survival rate increased compared with the free drug [111]. Docetaxel and tamoxifen are potent drugs against breast cancer. There is an antagonistic problem when both drugs are used in combination because they have different metabolisms. Docetaxel and tamoxifen loaded TPGS-PLA showed a significant reduction of the drug antagonism in the MCF7 cell line [112].
Some representative published results on PLA/PLGA based particles as drug delivery systems are summarized in Table 5 [5, 16]. There are variable drugs and suitable methods applied for their encapsulation. The suitability of the method was evaluated by loading efficiency and pharmacokinetic release results.
Table 5.
Investigations on PLA/PLGA particles as drug delivery systems.
| Material | Drug | Method | Result | Ref. |
|---|---|---|---|---|
| PLA | Irinotecan hydrochloride |
Single emulsion | Smooth surface and less initial burst release |
[113] |
| PLA | Nimesulide | Single emulsion | Initial burst followed by sustained release |
[114] |
| PLA-PEG | Tetanus toxoid | Single emulsion | Enhanced transport across the rat nasal mucosa |
[115] |
| PLA | Vanillin | Single emulsion | Slow and sustained release, stable particles over 3 months Inferior free radical scavenging activity than free vanillin |
[116] |
| PLA | BSA | Double emulsion | Encapsulation efficiency up to 71.6% | [23] |
| PLA-PEG | Hemoglobin | Double emulsion | Less macrophage uptake | [117] |
| PLA | Protein-C | Double emulsion | Release of protein C seems to increase with the hydrophilic character of PLA |
[118] |
| PLA- TPGS |
BSA | Double emulsion | longer blood circulation time than free drug |
[119] |
| PLA | Neurotoxin-1 | Double emulsion | Brain delivery of NT-1 enhanced | [120] |
| PLA | Triclosan | Double emulsion | High encapsulation efficiency | [121] |
| PLA | Oridonin | Modified spontaneous emulsion solvent diffusion |
Slow drug release up to 72hrs. | [22] |
| PLA-PEG | Lactoferrin | Modified double emulsion/solvent evaporation |
Increased uptake by bEnd.3 cells | [122] |
| PLA-PEG | Zidovudine | Solvent evaporation | Less phagocytosis | [21] |
| PLA- TPGS |
Paclitaxel | Modified solvent extraction/evaporation |
Initial burst followed by sustained release |
[24] |
| PLA- mPEG |
Salting out | Less interaction with leukocytes | [123] | |
| PLA- PEG-PLA |
Savoxepine | Salting out | Controlled drug release up to 1 week | [124] |
| PLA | BSA | Salting out/coacervation | High encapsulation efficiency and acceptable burst release |
[125] |
| PLA | Cloricromene | Nanoprecipitation | Faster dissolution than free drug | [26] |
| PLA | Tamoxifen | Nanoprecipitation | Significant therapeutic efficacy with reduced side effects |
[126] |
| PLA- Pluronic |
Stevioside | Nanoprecipitation | High potential safe and effective | [127] |
| Chitosan- PLA |
Anthraquinone | Nanoprecipitation | Continuous and sustained release, pH dependent release profiles |
[128] |
| PLA- pluronic |
Insulin | Dialysis/ nanoprecipitation |
Good control over blood glucose concentration |
[129] |
| PEG | Gene delivery | Dialysis | Improved transfection activity | [25] |
| PLA | HIV p24 protein | Dialysis | Induced mucosal antibody production | [130] |
| PLA | Progesterone Theophylline Vitamin D3 |
Spray drying | Alternative method | [56, 59] |
| PLA | Piroxicam | Spray drying | Small initial burst release | [60] |
| PLA | Ketotifen | Spray drying | Released in plasma between 336 and 384 hr, and the mean residence time increased between 30 and 70 times compared to solution treatment |
[131] |
| PLA/ PLGA |
Implant | In situ forming micro- particles |
Lower myotoxicity | [27, 132] |
| PLA | Ivermectin | In situ forming gel | Slow in vitro release and 80% cumulative release in 80 days 110-120 days maintained effective gel in vivo pharmacokinetic results |
[133] |
| PLA/ PLGA |
Diltiazem hydrochloride, Buserelin acetate |
In situ forming micro- particles |
Significantly reduced burst effect | [134] |
| PLA | Bupivacaine hydrochloride |
In situ forming micro- particles |
Significantly slower release compared to conventional particle formulation |
[135] |
| HA-PLA | Methylprednisolone | In situ forming micro gel | Entrap a hydrophobic drug and prolong release profile |
[136] |
| PLA | Steroid norethisterone |
Melting | Zero order release | [137, 138] |
| PLA | Naltrexone | Melting | Long effective blocking action to morphine |
[139] |
| PLA | Prednisolone | Melting | Sustained release over 30 days | [140] |
| PLA/F68 | Dexamethasone | Hot melt extrusion | No negative influence in the body | [141] |
| PLA | Hyoscine Butylbromide Indomethacin Piroxicam Thymopentin |
Supercritical fluids technique |
High encapsulation efficiency | [142] |
| PLA | Enzyme Insulin Calcitonin |
Supercritical fluids technique |
Low loss of enzyme activity, retention of protein activity |
[143] |
| PLA | Rifampin Gentamycin Naltrexone |
Supercritical fluids technique |
Lower initial burst | [144] |
| PLA | Paclitaxel | Microfluidic technique | Monodisperse paclitaxel particles | [145] |
| PLA/ PLGA |
Risperidone Paclitaxel |
Template method | Low initial burst and controlled drug release for 1 month |
[80] |
4. Challenges and strategies
Current micro and nano-particle production methods have been constrained by limitations of processes. The conventional techniques have several disadvantages, including the relatively high cost of particle production, the potential toxicity of solvents and reagents like stabilizers, emulsifiers, and other additives for forming particles, the use of a high energy mechanical mixer and homogenizer, which may be damaging to biological drugs such as proteins, peptides and macromolecules, the difficulty of reproducing biologically stable particles, and the low drug encapsulation efficiency [2, 7, 8, 17, 29, 146]. The high energy mechanical mixer and homogenizer generate high shearing forces. These high energy shearing forces can cause disorder which changes the natural structure of the macromolecules. Biopharmaceuticals, or protein drugs, may be denatured by exposure to the water/solvent interface or organic solvents. The reported processes are small laboratory scale or small test production scale under 1 g. Some of the large production scales have resulted in different particle size distributions increasing the process volume because the solvent evaporation rate may be different [30]. The most significant challenge is to understand the particle forming mechanism and encapsulation process [2, 8, 147].
Physico-chemical characterization of nano- and micro-particles have not been complete [2, 8, 148]. The nano-particles have several unique physico-chemical properties that can present difficulty in characterization. The nano-particles need higher cost characterization methods related to size, shape, surface charge, surface area and other physico-chemical properties [7, 149]. Incomplete characterization of the nano-particles may lead to an incomplete understanding of the correlation between nano-particle properties and various biological effects. The properties of the nano-particles may be easily changed by the surrounding environments, such as the blood stream, cell types, and physico-chemical environments (temperature, pH, pressure, volume, etc.). PLA-based nano-particles need to be analyzed both in dry or lyophilized form and in the test media, such as with or without serum based culture media for complete characterization [2, 7, 8, 17, 149].
One important consideration for making drug delivery systems is sterilization of PLA-based particles [7, 149]. Most useful sterilization methods cannot be applied to PLA based particles. Steam sterilization cannot be used with PLA based particles because high temperature and pressure can affect the particle that softens, melts, deforms, and undergoes hydrolysis. Heat sterilization exposes the particle to high temperature for long periods of time, which can destroy the PLA matrix structure and drug. Ethylene oxide (EO) is known as a polymer softener and plasticizer. The residual EO gas causes mutagenic, carcinogenic, and allergenic effects. Gamma radiation can breakdown polymer chains, resulting in decreased molecular weight and increased biodegradation rates, significantly altering drug release profiles. Therefore, GMP grade production of particles has to be done by aseptic processing. It is very effective for preventing contamination of particles but an expensive technique for manufacturing PLA based particles. It requires clean room control and the use of GMP protocols.
Various particle production techniques have been investigated for improving the encapsulation efficiency of drugs, optimizing the scale up process for mass production, and enhancing the reproducibility of the methods [2, 147, 149]. Table 6 lists a summary of challenges associated with developing PLA based particles as drug delivery applications [7-9, 150].
Table 6.
Various challenges for making PLA particles for drug delivery [7].
|
One of the most useful strategies to overcoming certain challenges is surface modification of PLA based micro and nano-particles for improving the stability of the particles. Surface modification is important for escaping the immune system when administrating particles to the bloodstream [29, 151]. Similarly, other strategies have been used to make a hydrophilic cloud around the particles to reduce their uptake by RES systems. These strategies comprise surface modifications of particles with Tween 80, PEG or PEO, poloxamers and poloxamines, polysorbate 80, TPGS, functional amino acids and polysaccharides [29, 124]. The most preferred surface modification is the adsorption or grafting of PEG (known as PEGylation) to the surface of particles. Grafting of PEG and PEG-containing copolymers onto the surface of particles augmented the blood circulation half-life. Increasing the molecular weight of the PEG chains has been shown to reduce opsonization of particles and improve retention in the circulation [29, 110, 151-153]. In addition, PEG may have good interactions with blood components. The other option is a copolymerization with PGA/PCL, PEG, and other polymers. PLA-copolymers have worked well as biocompatible polymer particles for drug delivery systems [2, 9, 29].
There is a huge amount of knowledge scattered around the world. The data on each PLA-based micro and nano-particles are unique in that the fabrication method, the drug used, and the efficacy testing methods are all different. This makes it difficult to compare properties of PLA particles, and PLGA particles for that matter, to find the right formulation for specific applications. It is time to assemble a data bank that presents detailed information correlating PLA particle properties and their in vivo functions. Such a data bank will propel more systematic development of future PLA micro and nano-particles that can be developed for specific in vivo applications.
Acknowledgments
This work was supported by the Showalter Research Trust Fund and the National Institute of Health through CA129287 and GM095879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. International Journal of Pharmaceutics. 2004;282:1–18. doi: 10.1016/j.ijpharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- [2].Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chemical Society reviews. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stloukal P, Kucharczyk P, Sedlarik V, Bazant P, Koutny M. Low molecular weight poly(lactic acid) microparticles for controlled release of the herbicide metazachlor: preparation, morphology, and release kinetics. Journal of agricultural and food chemistry. 2012;60:4111–4119. doi: 10.1021/jf300521j. [DOI] [PubMed] [Google Scholar]
- [4].Thomasin C, Merkle HP, Gander B. Drug microencapsulation by PLA/PLGA coacervation in the light of thermodynamics. 2. Parameters determining microsphere formation. J Pharm Sci. 1998;87:269–275. doi: 10.1021/js970048j. [DOI] [PubMed] [Google Scholar]
- [5].Lassalle V, Ferreira ML. PLA nano- and microparticles for drug delivery: an overview of the methods of preparation. Macromol Biosci. 2007;7:767–783. doi: 10.1002/mabi.200700022. [DOI] [PubMed] [Google Scholar]
- [6].Allouche J. Synthesis of Organic and Bioorganic Nanoparticles: An Overview of the Preparation Methods. In: Brayner R, Fiévet F, Coradin T, editors. Nanomaterials: A Danger or a Promise? Springer; London: 2013. pp. 27–74. [Google Scholar]
- [7].Kumar G, Shafiq N, Malhotra S. Drug-loaded PLGA nanoparticles for oral administration: fundamental issues and challenges ahead. Critical reviews in therapeutic drug carrier systems. 2012;29:149–182. doi: 10.1615/critrevtherdrugcarriersyst.v29.i2.20. [DOI] [PubMed] [Google Scholar]
- [8].Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Madhavan Nampoothiri K, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresource Technology. 2010;101:8493–8501. doi: 10.1016/j.biortech.2010.05.092. [DOI] [PubMed] [Google Scholar]
- [10].Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Critical reviews in therapeutic drug carrier systems. 2004;21:387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- [11].Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- [12].Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews. 2012;64(Supplement):72–82. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- [13].Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- [14].Pagels RF, Prud'homme RK. Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. Journal of Controlled Release. 2015;219:519–535. doi: 10.1016/j.jconrel.2015.09.001. [DOI] [PubMed] [Google Scholar]
- [15].Zhao C-X. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Advanced Drug Delivery Reviews. 2013;65:1420–1446. doi: 10.1016/j.addr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- [16].Coelho JF, Ferreira PC, Alves P, Cordeiro R, Fonseca AC, Góis JR, Gil MH. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. The EPMA Journal. 2010;1:164–209. doi: 10.1007/s13167-010-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- [18].Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. Journal of Controlled Release. 2005;102:313–332. doi: 10.1016/j.jconrel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- [19].Rao JP, Geckeler KE. Polymer nanoparticles: Preparation techniques and size-control parameters. Progress in Polymer Science. 2011;36:887–913. [Google Scholar]
- [20].Lin YM, Zhang A, Rippon HJ, Bismarck A, Bishop AE. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue engineering. Part A. 2010;16:1515–1526. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
- [21].Mainardes RM, Gremiao MP, Brunetti IL, da Fonseca LM, Khalil NM. Zidovudine-loaded PLA and PLA-PEG blend nanoparticles: influence of polymer type on phagocytic uptake by polymorphonuclear cells. J Pharm Sci. 2009;98:257–267. doi: 10.1002/jps.21406. [DOI] [PubMed] [Google Scholar]
- [22].Xing J, Zhang D, Tan T. Studies on the oridonin-loaded poly(d,l-lactic acid) nanoparticles in vitro and in vivo. International Journal of Biological Macromolecules. 2007;40:153–158. doi: 10.1016/j.ijbiomac.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [23].Gao H, Wang YN, Fan YG, Ma JB. Synthesis of a biodegradable tadpole-shaped polymer via the coupling reaction of polylactide onto mono(6-(2-aminoethyl)amino-6-deoxy)-beta-cyclodextrin and its properties as the new carrier of protein delivery system. J Control Release. 2005;107:158–173. doi: 10.1016/j.jconrel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- [24].Zhang Z, Feng S-S. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: Synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27:262–270. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- [25].Ataman-Onal Y, Munier S, Ganee A, Terrat C, Durand PY, Battail N, Martinon F, Le Grand R, Charles MH, Delair T, Verrier B. Surfactant-free anionic PLA nanoparticles coated with HIV-1 p24 protein induced enhanced cellular and humoral immune responses in various animal models. J Control Release. 2006;112:175–185. doi: 10.1016/j.jconrel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- [26].Leo E, Brina B, Forni F, Vandelli MA. In vitro evaluation of PLA nanoparticles containing a lipophilic drug in water-soluble or insoluble form. International Journal of Pharmaceutics. 2004;278:133–141. doi: 10.1016/j.ijpharm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- [27].Kranz H, Brazeau GA, Napaporn J, Martin RL, Millard W, Bodmeier R. Myotoxicity studies of injectable biodegradable in-situ forming drug delivery systems. International Journal of Pharmaceutics. 2001;212:11–18. doi: 10.1016/s0378-5173(00)00568-8. [DOI] [PubMed] [Google Scholar]
- [28].Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S, Hanifehpour Y, Joo SW. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pacific journal of cancer prevention : APJCP. 2014;15:517–535. doi: 10.7314/apjcp.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- [30].Sah E, Sah H. Recent Trends in Preparation of Poly(lactide-co-glycolide) Nanoparticles by Mixing Polymeric Organic Solution with Antisolvent. Journal of Nanomaterials. 2015;2015:22. [Google Scholar]
- [31].Quintanar-Guerrero D, Allemann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug development and industrial pharmacy. 1998;24:1113–1128. doi: 10.3109/03639049809108571. [DOI] [PubMed] [Google Scholar]
- [32].Martín-Sabroso C, Fraguas-Sánchez AI, Aparicio-Blanco J, Cano-Abad MF, Torres-Suárez AI. Critical attributes of formulation and of elaboration process of PLGA-protein microparticles. International Journal of Pharmaceutics. 2015;480:27–36. doi: 10.1016/j.ijpharm.2015.01.008. [DOI] [PubMed] [Google Scholar]
- [33].Li M, Rouaud O, Poncelet D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. International Journal of Pharmaceutics. 2008;363:26–39. doi: 10.1016/j.ijpharm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- [34].Tan MXL, Danquah MK. Drug and Protein Encapsulation by Emulsification: Technology Enhancement Using Foam Formulations. Chemical Engineering & Technology. 2012;35:618–626. [Google Scholar]
- [35].Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- [36].Lamprecht A, Ubrich N, Hombreiro Pérez M, Lehr CM, Hoffman M, Maincent P. Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. International Journal of Pharmaceutics. 1999;184:97–105. doi: 10.1016/s0378-5173(99)00107-6. [DOI] [PubMed] [Google Scholar]
- [37].Zambaux MF, Bonneaux F, Gref R, Maincent P, Dellacherie E, Alonso MJ, Labrude P, Vigneron C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. Journal of Controlled Release. 1998;50:31–40. doi: 10.1016/s0168-3659(97)00106-5. [DOI] [PubMed] [Google Scholar]
- [38].Lu JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert review of molecular diagnostics. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bragagni M, Beneitez C, Martín C, de la Ossa DHP, Mura PA, Gil-Alegre ME. Selection of PLA polymers for the development of injectable prilocaine controlled release microparticles: Usefulness of thermal analysis. International Journal of Pharmaceutics. 2013;441:468–475. doi: 10.1016/j.ijpharm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- [40].Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JX, Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? European Journal of Pharmaceutics and Biopharmaceutics. 2000;50:147–160. doi: 10.1016/s0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- [41].Lambert G, Fattal E, Couvreur P. Nanoparticulate systems for the delivery of antisense oligonucleotides. Advanced Drug Delivery Reviews. 2001;47:99–112. doi: 10.1016/s0169-409x(00)00116-2. [DOI] [PubMed] [Google Scholar]
- [42].Ghaderi R, Artursson P, Carlfors J. A new method for preparing biodegradable microparticles and entrapment of hydrocortisone in dl-PLG microparticles using supercritical fluids. European Journal of Pharmaceutical Sciences. 2000;10:1–9. doi: 10.1016/s0928-0987(99)00079-2. [DOI] [PubMed] [Google Scholar]
- [43].Allémann E, Gurny R, Doelker E. Preparation of aqueous polymeric nanodispersions by a reversible salting-out process: influence of process parameters on particle size. International Journal of Pharmaceutics. 1992;87:247–253. [Google Scholar]
- [44].Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. Journal of Controlled Release. 1999;57:171–185. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- [45].Barichello JM, Morishita M, Takayama K, Nagai T. Absorption of insulin from Pluronic F-127 gels following subcutaneous administration in rats. International Journal of Pharmaceutics. 1999;184:189–198. doi: 10.1016/s0378-5173(99)00119-2. [DOI] [PubMed] [Google Scholar]
- [46].Francois G, Katz JL. Nanoparticles and nanocapsules created using the Ouzo effect: spontaneous emulisification as an alternative to ultrasonic and high-shear devices. Chemphyschem : a European journal of chemical physics and physical chemistry. 2005;6:209–216. doi: 10.1002/cphc.200400527. [DOI] [PubMed] [Google Scholar]
- [47].Vitale SA, Katz JL. Liquid Droplet Dispersions Formed by Homogeneous Liquid−Liquid Nucleation: “The Ouzo Effect”. Langmuir. 2003;19:4105–4110. [Google Scholar]
- [48].Anton N, Benoit JP, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates-a review. J Control Release. 2008;128:185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- [49].Zhu D, Tao W, Zhang H, Liu G, Wang T, Zhang L, Zeng X, Mei L. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomaterialia. 2016;30:144–154. doi: 10.1016/j.actbio.2015.11.031. [DOI] [PubMed] [Google Scholar]
- [50].Kostag M, Köhler S, Liebert T, Heinze T. Pure Cellulose Nanoparticles from Trimethylsilyl Cellulose. Macromolecular Symposia. 2010;294:96–106. [Google Scholar]
- [51].Chronopoulou L, Fratoddi I, Palocci C, Venditti I, Russo MV. Osmosis based method drives the self-assembly of polymeric chains into micro- and nanostructures. Langmuir. 2009;25:11940–11946. doi: 10.1021/la9016382. [DOI] [PubMed] [Google Scholar]
- [52].Na K, Lee KH, Lee DH, Bae YH. Biodegradable thermo-sensitive nanoparticles from poly(l-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. European Journal of Pharmaceutical Sciences. 2006;27:115–122. doi: 10.1016/j.ejps.2005.08.012. [DOI] [PubMed] [Google Scholar]
- [53].Hornig S, Heinze T. Efficient approach to design stable water-dispersible nanoparticles of hydrophobic cellulose esters. Biomacromolecules. 2008;9:1487–1492. doi: 10.1021/bm8000155. [DOI] [PubMed] [Google Scholar]
- [54].Jeong Y-I, Cho C-S, Kim S-H, Ko K-S, Kim S-I, Shim Y-H, Nah J-W. Preparation of poly(DL-lactide-co-glycolide) nanoparticles without surfactant. Journal of Applied Polymer Science. 2001;80:2228–2236. [Google Scholar]
- [55].Liu W, Chen XD, Selomulya C. On the spray drying of uniform functional microparticles. Particuology. 2015;22:1–12. [Google Scholar]
- [56].Bodmeier R, Chen HG. Preparation of biodegradable poly(+/−)lactide microparticles using a spray-drying technique. J Pharm Pharmacol. 1988;40:754–757. doi: 10.1111/j.2042-7158.1988.tb05166.x. [DOI] [PubMed] [Google Scholar]
- [57].Sosnik A, Seremeta KP. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Advances in Colloid and Interface Science. 2015;223:40–54. doi: 10.1016/j.cis.2015.05.003. [DOI] [PubMed] [Google Scholar]
- [58].Chavalitkul J, Likittanaprasong N, Seansala P, Suttiruengwong S, Seadan M. Application of Ultrasonic Atomization for Biopolymer Particles and Tube Fabrication. Energy Procedia. 2014;56:458–465. [Google Scholar]
- [59].Pavanetto F, Genta I, Giunchedi P, Conti B. Evaluation of spray drying as a method for polylactide and polylactide-co-glycolide microsphere preparation. J Microencapsul. 1993;10:487–497. doi: 10.3109/02652049309015325. [DOI] [PubMed] [Google Scholar]
- [60].Wagenaar BW, Müller BW. Piroxicam release from spray-dried biodegradable microspheres. Biomaterials. 1994;15:49–54. doi: 10.1016/0142-9612(94)90196-1. [DOI] [PubMed] [Google Scholar]
- [61].Benelli P, Conti B, Genta I, Costantini M, Montanari L. Clonazepam microencapsulation in poly-D,L-lactide-co-glycolide microspheres. J Microencapsul. 1998;15:431–443. doi: 10.3109/02652049809006870. [DOI] [PubMed] [Google Scholar]
- [62].Mu L, Feng SS. Fabrication, characterization and in vitro release of paclitaxel (Taxol) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release. 2001;76:239–254. doi: 10.1016/s0168-3659(01)00440-0. [DOI] [PubMed] [Google Scholar]
- [63].Mu L, Teo M-M, Ning H-Z, Tan C-S, Feng S-S. Novel powder formulations for controlled delivery of poorly soluble anticancer drug: Application and investigation of TPGS and PEG in spray-dried particulate system. Journal of Controlled Release. 2005;103:565–575. doi: 10.1016/j.jconrel.2004.12.023. [DOI] [PubMed] [Google Scholar]
- [64].Lacasse FX, Hildgen P, McMullen JN. Surface and morphology of spray-dried pegylated PLA microspheres. International Journal of Pharmaceutics. 1998;174:101–109. [Google Scholar]
- [65].Royals MA, Fujita SM, Yewey GL, Rodriguez J, Schultheiss PC, Dunn RL. Biocompatibility of a biodegradable in situ forming implant system in rhesus monkeys. Journal of biomedical materials research. 1999;45:231–239. doi: 10.1002/(sici)1097-4636(19990605)45:3<231::aid-jbm11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [66].Jain RA, Rhodes CT, Railkar AM, Malick AW, Shah NH. Comparison of various injectable protein-loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices: in-situ-formed implant versus in-situ-formed microspheres versus isolated microspheres. Pharmaceutical development and technology. 2000;5:201–207. doi: 10.1081/pdt-100100535. [DOI] [PubMed] [Google Scholar]
- [67].Luan X, Bodmeier R. In situ forming microparticle system for controlled delivery of leuprolide acetate: Influence of the formulation and processing parameters. European Journal of Pharmaceutical Sciences. 2006;27:143–149. doi: 10.1016/j.ejps.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [68].Wichert B, Rohdewald P. A new method for the preparation of drug containing polylactic acid microparticles without using organic solvents. Journal of Controlled Release. 1990;14:269–283. [Google Scholar]
- [69].Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles, Nanomedicine: Nanotechnology. Biology and Medicine. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- [70].Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Advanced Drug Delivery Reviews. 2008;60:373–387. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- [71].Kim J-H, Paxton TE, Tomasko DL. Microencapsulation of Naproxen Using Rapid Expansion of Supercritical Solutions. Biotechnology Progress. 1996;12:650–661. [Google Scholar]
- [72].Yeo Y, Baek N, Park K. Microencapsulation methods for delivery of protein drugs. Biotechnology and Bioprocess Engineering. 6:213–230. [Google Scholar]
- [73].Sacchetin PSC, Morales AR, Moraes ÂM, Rosa P.d.T.V.e. Formation of PLA particles incorporating 17α-methyltestosterone by supercritical fluid technology. The Journal of Supercritical Fluids. 2013;77:52–62. [Google Scholar]
- [74].Watanabe T, Ono T, Kimura Y. Continuous fabrication of monodisperse polylactide microspheres by droplet-to-particle technology using microfluidic emulsification and emulsion-solvent diffusion. Soft Matter. 2011;7:9894–9897. [Google Scholar]
- [75].Liao CY, Su YC. Formation of biodegradable microcapsules utilizing 3D, selectively surface-modified PDMS microfluidic devices. Biomedical microdevices. 2010;12:125–133. doi: 10.1007/s10544-009-9367-8. [DOI] [PubMed] [Google Scholar]
- [76].Jyothi NV, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, Srawan GY. Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul. 2010;27:187–197. doi: 10.3109/02652040903131301. [DOI] [PubMed] [Google Scholar]
- [77].Vladisavljević GT, Shahmohamadi H, Das DB, Ekanem EE, Tauanov Z, Sharma L. Glass capillary microfluidics for production of monodispersed poly (dl-lactic acid) and polycaprolactone microparticles: Experiments and numerical simulations. Journal of Colloid and Interface Science. 2014;418:163–170. doi: 10.1016/j.jcis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- [78].Xu Q, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, Langer R, Anderson DG. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery, Small (Weinheim an der Bergstrasse. Germany) 2009;5:1575–1581. doi: 10.1002/smll.200801855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Acharya G, Shin CS, McDermott M, Mishra H, Park H, Kwon IC, Park K. The hydrogel template method for fabrication of homogeneous nano/microparticles. Journal of Controlled Release. 2010;141:314–319. doi: 10.1016/j.jconrel.2009.09.032. [DOI] [PubMed] [Google Scholar]
- [80].Lu Y, Sturek M, Park K. Microparticles produced by the hydrogel template method for sustained drug delivery. Int J Pharm. 2014;461:258–269. doi: 10.1016/j.ijpharm.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Park K, Skidmore SM, Fultz L, Yun Y, Lee B.k. Device for large-scale microparticle production and method of using the same. patent pending.
- [82].Takeuchi H, Yamamoto H, Kawashima Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Advanced Drug Delivery Reviews. 2001;47:39–54. doi: 10.1016/s0169-409x(00)00120-4. [DOI] [PubMed] [Google Scholar]
- [83].Hsu CH, Cui Z, Mumper RJ, Jay M. Preparation and characterization of novel coenzyme Q10 nanoparticles engineered from microemulsion precursors. AAPS PharmSciTech. 2003;4:E32. doi: 10.1208/pt040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Murakami H, Kobayashi M, Takeuchi H, Kawashima Y. Utilization of poly(dl-lactide-co-glycolide) nanoparticles for preparation of mini-depot tablets by direct compression. Journal of Controlled Release. 2000;67:29–36. doi: 10.1016/s0168-3659(99)00288-6. [DOI] [PubMed] [Google Scholar]
- [85].Dwarki VJ, Belloni P, Nijjar T, Smith J, Couto L, Rabier M, Clift S, Berns A, Cohen LK. Gene therapy for hemophilia A: production of therapeutic levels of human factor VIII in vivo in mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1023–1027. doi: 10.1073/pnas.92.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chen J, Tian B, Yin X, Zhang Y, Hu D, Hu Z, Liu M, Pan Y, Zhao J, Li H, Hou C, Wang J, Zhang Y. Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems. Journal of Biotechnology. 2007;130:107–113. doi: 10.1016/j.jbiotec.2007.02.007. [DOI] [PubMed] [Google Scholar]
- [87].Munier S, Messai I, Delair T, Verrier B, Ataman-Önal Y. Cationic PLA nanoparticles for DNA delivery: Comparison of three surface polycations for DNA binding, protection and transfection properties. Colloids and Surfaces B: Biointerfaces. 2005;43:163–173. doi: 10.1016/j.colsurfb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [88].Chen CK, Jones CH, Mistriotis P, Yu Y, Ma X, Ravikrishnan A, Jiang M, Andreadis ST, Pfeifer BA, Cheng C. Poly(ethylene glycol)-block-cationic polylactide nanocomplexes of differing charge density for gene delivery. Biomaterials. 2013;34:9688–9699. doi: 10.1016/j.biomaterials.2013.08.063. [DOI] [PubMed] [Google Scholar]
- [89].Basarkar A, Devineni D, Palaniappan R, Singh J. Preparation, characterization, cytotoxicity and transfection efficiency of poly(dl-lactide-co-glycolide) and poly(dl-lactic acid) cationic nanoparticles for controlled delivery of plasmid DNA. International Journal of Pharmaceutics. 2007;343:247–254. doi: 10.1016/j.ijpharm.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yang X-Z, Dou S, Sun T-M, Mao C-Q, Wang H-X, Wang J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. Journal of Controlled Release. 2011;156:203–211. doi: 10.1016/j.jconrel.2011.07.035. [DOI] [PubMed] [Google Scholar]
- [91].Gu G, Hu Q, Feng X, Gao X, Menglin J, Kang T, Jiang D, Song Q, Chen H, Chen J. PEG-PLA nanoparticles modified with APTEDB peptide for enhanced anti-angiogenic and anti-glioma therapy. Biomaterials. 2014;35:8215–8226. doi: 10.1016/j.biomaterials.2014.06.022. [DOI] [PubMed] [Google Scholar]
- [92].Gaspar VM, Baril P, Costa EC, de Melo-Diogo D, Foucher F, Queiroz JA, Sousa F, Pichon C, Correia IJ. Bioreducible poly(2-ethyl-2-oxazoline)-PLA-PEI-SS triblock copolymer micelles for co-delivery of DNA minicircles and Doxorubicin. J Control Release. 2015;213:175–191. doi: 10.1016/j.jconrel.2015.07.011. [DOI] [PubMed] [Google Scholar]
- [93].Yu XJ, Luo C, Lin JC, Hao P, He YY, Guo ZM, Qin L, Su J, Liu BS, Huang Y, Nan P, Li CS, Xiong B, Luo XM, Zhao GP, Pei G, Chen KX, Shen X, Shen JH, Zou JP, He WZ, Shi TL, Zhong Y, Jiang HL, Li YX. Putative hAPN receptor binding sites in SARS_CoV spike protein. Acta pharmacologica Sinica. 2003;24:481–488. [PubMed] [Google Scholar]
- [94].Thermet A, Rollier C, Zoulim F, Trepo C, Cova L. Progress in DNA vaccine for prophylaxis and therapy of hepatitis B. Vaccine. 2003;21:659–662. doi: 10.1016/s0264-410x(02)00575-3. [DOI] [PubMed] [Google Scholar]
- [95].Liu Y, Chen X, Wang L, Yang T, Yuan Q, Ma G. Surface charge of PLA microparticles in regulation of antigen loading, macrophage phagocytosis and activation, and immune effects in vitro. Particuology. 2014;17:74–80. [Google Scholar]
- [96].Gupta RK, Goswami DG, Singh RR, Surolia A, Panda AK. Soybean agglutinin coated PLA particles entrapping candidate vaccines induces enhanced primary and sustained secondary antibody response from single point immunization. European Journal of Pharmaceutical Sciences. 2012;45:282–295. doi: 10.1016/j.ejps.2011.11.022. [DOI] [PubMed] [Google Scholar]
- [97].Johansen P, Moon L, Tamber H, Merkle HP, Gander B, Sesardic D. Immunogenicity of single-dose diphtheria vaccines based on PLA/PLGA microspheres in guinea pigs. Vaccine. 1999;18:209–215. doi: 10.1016/s0264-410x(99)00191-7. [DOI] [PubMed] [Google Scholar]
- [98].Guillon C, Mayol K, Terrat C, Compagnon C, Primard C, Charles M-H, Delair T, Munier S, Verrier B. Formulation of HIV-1 Tat and p24 antigens by PLA nanoparticles or MF59 impacts the breadth, but not the magnitude, of serum and faecal antibody responses in rabbits. Vaccine. 2007;25:7491–7501. doi: 10.1016/j.vaccine.2007.08.060. [DOI] [PubMed] [Google Scholar]
- [99].Walsh MC, Banas JA, Mudzinski SP, Preissler MT, Graziano RF, Gosselin EJ. A two-component modular approach for enhancing T-cell activation utilizing a unique anti-FcgammaRI-streptavidin construct and microspheres coated with biotinylated-antigen. Biomolecular engineering. 2003;20:21–33. doi: 10.1016/s1389-0344(02)00089-8. [DOI] [PubMed] [Google Scholar]
- [100].Climent N, Munier S, Piqué N, García F, Pavot V, Primard C, Casanova V, Gatell JM, Verrier B, Gallart T. Loading dendritic cells with PLA-p24 nanoparticles or MVA expressing HIV genes induces HIV-1-specific T cell responses. Vaccine. 2014;32:6266–6276. doi: 10.1016/j.vaccine.2014.09.010. [DOI] [PubMed] [Google Scholar]
- [101].Jain AK, Goyal AK, Mishra N, Vaidya B, Mangal S, Vyas SP. PEG–PLA–PEG block copolymeric nanoparticles for oral immunization against hepatitis B. International Journal of Pharmaceutics. 2010;387:253–262. doi: 10.1016/j.ijpharm.2009.12.013. [DOI] [PubMed] [Google Scholar]
- [102].Bansal V, Kumar M, Dalela M, Brahmne HG, Singh H. Evaluation of synergistic effect of biodegradable polymeric nanoparticles and aluminum based adjuvant for improving vaccine efficacy. International Journal of Pharmaceutics. 2014;471:377–384. doi: 10.1016/j.ijpharm.2014.05.061. [DOI] [PubMed] [Google Scholar]
- [103].Lee W.-k., Park J.-y., Yang EH, Suh H, Kim SH, Chung DS, Choi K, Yang CW, Park J.-s. Investigation of the factors influencing the release rates of cyclosporin A-loaded micro- and nanoparticles prepared by high-pressure homogenizer. Journal of Controlled Release. 2002;84:115–123. doi: 10.1016/s0168-3659(02)00239-0. [DOI] [PubMed] [Google Scholar]
- [104].Tan Q, Jiang R, Xu M, Liu G, Li S, Zhang J. Nanosized sustained-release pyridostigmine bromide microcapsules: process optimization and evaluation of characteristics. International journal of nanomedicine. 2013;8:737–745. doi: 10.2147/IJN.S40860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jiang W, Schwendeman SP. Stabilization and controlled release of bovine serum albumin encapsulated in poly(D, L-lactide) and poly(ethylene glycol) microsphere blends. Pharmaceutical research. 2001;18:878–885. doi: 10.1023/a:1011009117586. [DOI] [PubMed] [Google Scholar]
- [106].Elvassore N, Bertucco A, Caliceti P. Production of insulin-loaded poly(ethylene glycol)/poly(l-lactide) (PEG/PLA) nanoparticles by gas antisolvent techniques. J Pharm Sci. 2001;90:1628–1636. doi: 10.1002/jps.1113. [DOI] [PubMed] [Google Scholar]
- [107].Caliceti P, Salmaso S, Elvassore N, Bertucco A. Effective protein release from PEG/PLA nano-particles produced by compressed gas anti-solvent precipitation techniques. Journal of Controlled Release. 2004;94:195–205. doi: 10.1016/j.jconrel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- [108].Mimi N, Belkacemi H, Sadoun T, Sapin A, Maincent P. How the composition and manufacturing parameters affect insulin release from polymeric nanoparticles. Journal of Drug Delivery Science and Technology. 2015;30:458–466. Part B. [Google Scholar]
- [109].Ruan G, Feng S-S. Preparation and characterization of poly(lactic acid)–poly(ethylene glycol)–poly(lactic acid) (PLA–PEG–PLA) microspheres for controlled release of paclitaxel. Biomaterials. 2003;24:5037–5044. doi: 10.1016/s0142-9612(03)00419-8. [DOI] [PubMed] [Google Scholar]
- [110].Adesina SK, Holly A, Kramer-Marek G, Capala J, Akala EO. Polylactide-based paclitaxel-loaded nanoparticles fabricated by dispersion polymerization: characterization, evaluation in cancer cell lines, and preliminary biodistribution studies. J Pharm Sci. 2014;103:2546–2555. doi: 10.1002/jps.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cosco D, Paolino D, De Angelis F, Cilurzo F, Celia C, Di Marzio L, Russo D, Tsapis N, Fattal E, Fresta M. Aqueous-core PEG-coated PLA nanocapsules for an efficient entrapment of water soluble anticancer drugs and a smart therapeutic response. European Journal of Pharmaceutics and Biopharmaceutics. 2015;89:30–39. doi: 10.1016/j.ejpb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- [112].Tan G-R, Feng S-S, Leong DT. The reduction of anti-cancer drug antagonism by the spatial protection of drugs with PLA–TPGS nanoparticles. Biomaterials. 2014;35:3044–3051. doi: 10.1016/j.biomaterials.2013.12.033. [DOI] [PubMed] [Google Scholar]
- [113].Yoshizawa H, Nishino S, Shiomori K, Natsugoe S, Aiko T, Kitamura Y. Surface morphology control of polylactide microspheres enclosing irinotecan hydrochloride. Int J Pharm. 2005;296:112–116. doi: 10.1016/j.ijpharm.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [114].Freitas MN, Marchetti JM. Nimesulide PLA microspheres as a potential sustained release system for the treatment of inflammatory diseases. International Journal of Pharmaceutics. 2005;295:201–211. doi: 10.1016/j.ijpharm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [115].Vila A, Sánchez A, Évora C, Soriano I, McCallion O, Alonso MJ. PLA-PEG particles as nasal protein carriers: the influence of the particle size. International Journal of Pharmaceutics. 2005;292:43–52. doi: 10.1016/j.ijpharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [116].Dalmolin LF, Khalil NM, Mainardes RM. Delivery of vanillin by poly(lactic-acid) nanoparticles: Development, characterization and in vitro evaluation of antioxidant activity. Materials Science and Engineering: C. 2016;62:1–8. doi: 10.1016/j.msec.2016.01.031. [DOI] [PubMed] [Google Scholar]
- [117].Sheng Y, Yuan Y, Liu C, Tao X, Shan X, Xu F. In vitro macrophage uptake and in vivo biodistribution of PLA-PEG nanoparticles loaded with hemoglobin as blood substitutes: effect of PEG content. Journal of materials science. Materials in medicine. 2009;20:1881–1891. doi: 10.1007/s10856-009-3746-9. [DOI] [PubMed] [Google Scholar]
- [118].Zambaux MF, Bonneaux F, Gref R, Dellacherie E, Vigneron C. Preparation and characterization of protein C-loaded PLA nanoparticles. Journal of Controlled Release. 1999;60:179–188. doi: 10.1016/s0168-3659(99)00073-5. [DOI] [PubMed] [Google Scholar]
- [119].Lee SH, Zhang Z, Feng S-S. Nanoparticles of poly(lactide)—tocopheryl polyethylene glycol succinate (PLA-TPGS) copolymers for protein drug delivery. Biomaterials. 2007;28:2041–2050. doi: 10.1016/j.biomaterials.2007.01.003. [DOI] [PubMed] [Google Scholar]
- [120].Cheng Q, Feng J, Chen J, Zhu X, Li F. Brain transport of neurotoxin-I with PLA nanoparticles through intranasal administration in rats: a microdialysis study. Biopharm Drug Dispos. 2008;29:431–439. doi: 10.1002/bdd.621. [DOI] [PubMed] [Google Scholar]
- [121].Pinon-Segundo E, Ganem-Quintanar A, Alonso-Perez V, Quintanar-Guerrero D. Preparation and characterization of triclosan nanoparticles for periodontal treatment. Int J Pharm. 2005;294:217–232. doi: 10.1016/j.ijpharm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- [122].Hu K, Li J, Shen Y, Lu W, Gao X, Zhang Q, Jiang X. Lactoferrin-conjugated PEG– PLA nanoparticles with improved brain delivery: In vitro and in vivo evaluations. Journal of Controlled Release. 2009;134:55–61. doi: 10.1016/j.jconrel.2008.10.016. [DOI] [PubMed] [Google Scholar]
- [123].Nguyen CA, Allémann E, Schwach G, Doelker E, Gurny R. Cell interaction studies of PLA-MePEG nanoparticles. International Journal of Pharmaceutics. 2003;254:69–72. doi: 10.1016/s0378-5173(02)00685-3. [DOI] [PubMed] [Google Scholar]
- [124].Leroux J-C, Allémann E, De Jaeghere F, Doelker E, Gurny R. Biodegradable nanoparticles — From sustained release formulations to improved site specific drug delivery. Journal of Controlled Release. 1996;39:339–350. [Google Scholar]
- [125].Thomasin C, Merkle HP, Gander BA. Physico-chemical parameters governing protein microencapsulation into biodegradable polyesters by coacervation. International Journal of Pharmaceutics. 1997;147:173–186. [Google Scholar]
- [126].Pandey SK, Ghosh S, Maiti P, Haldar C. Therapeutic efficacy and toxicity of tamoxifen loaded PLA nanoparticles for breast cancer. International Journal of Biological Macromolecules. 2015;72:309–319. doi: 10.1016/j.ijbiomac.2014.08.012. [DOI] [PubMed] [Google Scholar]
- [127].Barwal I, Sood A, Sharma M, Singh B, Yadav SC. Development of stevioside Pluronic-F-68 copolymer based PLA-nanoparticles as an antidiabetic nanomedicine. Colloids and Surfaces B: Biointerfaces. 2013;101:510–516. doi: 10.1016/j.colsurfb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- [128].Jeevitha D, Amarnath K. Chitosan/PLA nanoparticles as a novel carrier for the delivery of anthraquinone: Synthesis, characterization and in vitro cytotoxicity evaluation. Colloids and Surfaces B: Biointerfaces. 2013;101:126–134. doi: 10.1016/j.colsurfb.2012.06.019. [DOI] [PubMed] [Google Scholar]
- [129].Xiong XY, Li YP, Li ZL, Zhou CL, Tam KC, Liu ZY, Xie GX. Vesicles from Pluronic/poly(lactic acid) block copolymers as new carriers for oral insulin delivery. Journal of Controlled Release. 2007;120:11–17. doi: 10.1016/j.jconrel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [130].Aline F, Brand D, Pierre J, Roingeard P, Séverine M, Verrier B, Dimier-Poisson I. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27:5284–5291. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- [131].Guerrero S, Muniz E, Teijon C, Olmo R, Teijon JM, Blanco MD. Ketotifen-loaded microspheres prepared by spray-drying poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) polymers: characterization and in vivo evaluation. J Pharm Sci. 2008;97:3153–3169. doi: 10.1002/jps.21241. [DOI] [PubMed] [Google Scholar]
- [132].Rungseevijitprapa W, Brazeau GA, Simkins JW, Bodmeier R. Myotoxicity studies of O/W-in situ forming microparticle systems. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69:126–133. doi: 10.1016/j.ejpb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- [133].Geng Z, Luo X, Zhang Z, Li H, Tian J, Yu Z. Study of an injectable in situ forming gel for sustained-release of Ivermectin in vitro and in vivo. Int J Biol Macromol. 2016;85:271–276. doi: 10.1016/j.ijbiomac.2015.12.028. [DOI] [PubMed] [Google Scholar]
- [134].Kranz H, Bodmeier R. A novel in situ forming drug delivery system for controlled parenteral drug delivery. International Journal of Pharmaceutics. 2007;332:107–114. doi: 10.1016/j.ijpharm.2006.09.033. [DOI] [PubMed] [Google Scholar]
- [135].Kranz H, Bodmeier R. Structure formation and characterization of injectable drug loaded biodegradable devices: In situ implants versus in situ microparticles. European Journal of Pharmaceutical Sciences. 2008;34:164–172. doi: 10.1016/j.ejps.2008.03.004. [DOI] [PubMed] [Google Scholar]
- [136].Pitarresi G, Palumbo FS, Fiorica C, Calascibetta F, Di Stefano M, Giammona G. Injectable in situ forming microgels of hyaluronic acid-g-polylactic acid for methylprednisolone release. European Polymer Journal. 2013;49:718–725. [Google Scholar]
- [137].Anderson LC, Wise DL, Howes JF. An injectable sustained release fertility control system. Contraception. 1976;13:375–384. doi: 10.1016/s0010-7824(76)80047-9. [DOI] [PubMed] [Google Scholar]
- [138].Nykamp G, Carstensen U, Müller BW. Jet milling—a new technique for microparticle preparation. International Journal of Pharmaceutics. 2002;242:79–86. doi: 10.1016/s0378-5173(02)00150-3. [DOI] [PubMed] [Google Scholar]
- [139].Yolles S, Leafe TD, Woodland JH, Meyer FJ. Long acting delivery systems for narcotic antagonists II: release rates of naltrexone from poly(lactic acid) composites. J Pharm Sci. 1975;64:348–349. doi: 10.1002/jps.2600640239. [DOI] [PubMed] [Google Scholar]
- [140].Smith A, Hunneyball l.M. Evaluation of poly(lactic acid) as a biodegradable drug delivery system for parenteral administration. International Journal of Pharmaceutics. 1986;30:215–220. [Google Scholar]
- [141].Li D, Guo G, Fan R, Liang J, Deng X, Luo F, Qian Z. PLA/F68/dexamethasone implants prepared by hot-melt extrusion for controlled release of anti-inflammatory drug to implantable medical devices: I. Preparation, characterization and hydrolytic degradation study. Int J Pharm. 2013;441:365–372. doi: 10.1016/j.ijpharm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- [142].Bleich J, Muller BW. Production of drug loaded microparticles by the use of supercritical gases with the aerosol solvent extraction system (ASES) process. J Microencapsul. 1996;13:131–139. doi: 10.3109/02652049609052902. [DOI] [PubMed] [Google Scholar]
- [143].Whitaker MJ, Hao J, Davies OR, Serhatkulu G, Stolnik-Trenkic S, Howdle SM, Shakesheff KM. The production of protein-loaded microparticles by supercritical fluid enhanced mixing and spraying. Journal of Controlled Release. 2005;101:85–92. doi: 10.1016/j.jconrel.2004.07.017. [DOI] [PubMed] [Google Scholar]
- [144].Falk R, Randolph TW, Meyer JD, Kelly RM, Manning MC. Controlled release of ionic compounds from poly (l-lactide) microspheres produced by precipitation with a compressed antisolvent. Journal of Controlled Release. 1997;44:77–85. [Google Scholar]
- [145].He T, Liang Q, Zhang K, Mu X, Luo T, Wang Y, Luo G. A modified microfluidic chip for fabrication of paclitaxel-loaded poly(l-lactic acid) microspheres. Microfluidics and Nanofluidics. 2011;10:1289–1298. [Google Scholar]
- [146].van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharmaceutical research. 2000;17:1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- [147].Katou H, Wandrey AJ, Gander B. Kinetics of solvent extraction/evaporation process for PLGA microparticle fabrication. International Journal of Pharmaceutics. 2008;364:45–53. doi: 10.1016/j.ijpharm.2008.08.015. [DOI] [PubMed] [Google Scholar]
- [148].Thomasin C, Ho NT, Merkle HP, Gander B. Drug microencapsulation by PLA/PLGA coacervation in the light of thermodynamics. 1. Overview and theoretical considerations. J Pharm Sci. 1998;87:259–268. doi: 10.1021/js970047r. [DOI] [PubMed] [Google Scholar]
- [149].Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. Journal of biomaterials science. Polymer edition. 2006;17:247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- [150].Yun YH, Lee BK, Park K. Controlled Drug Delivery: Historical perspective for the next generation. J Control Release. 2015;219:2–7. doi: 10.1016/j.jconrel.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sah H, Thoma LA, Desu HR, Sah E, Wood GC. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. International journal of nanomedicine. 2013;8:747–765. doi: 10.2147/IJN.S40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. J Control Release. 2014;193:324–340. doi: 10.1016/j.jconrel.2014.09.003. [DOI] [PubMed] [Google Scholar]
- [153].Palacio J, Agudelo NA, Lopez BL. PEGylation of PLA nanoparticles to improve mucus-penetration and colloidal stability for oral delivery systems. Current Opinion in Chemical Engineering. 2016;11:14–19. [Google Scholar]