Abstract
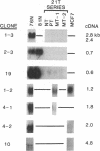
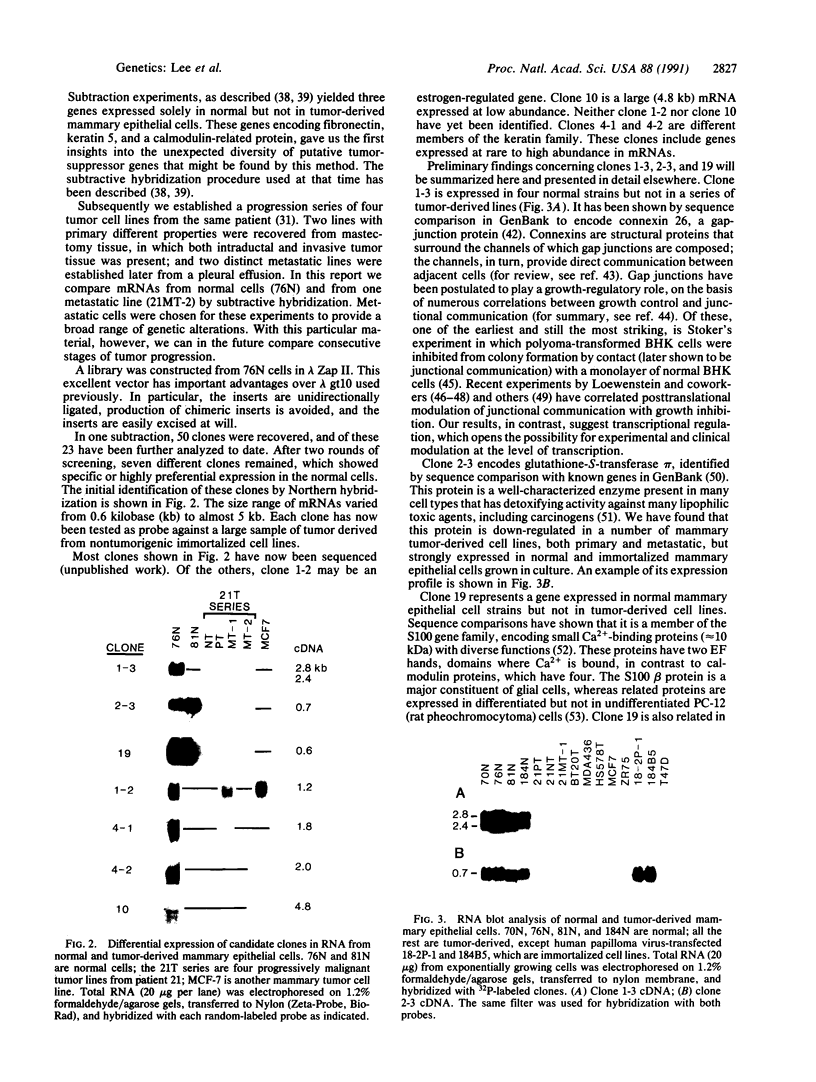
A positive selection system designed to identify and recover candidate tumor-suppressor genes is described. The system compares mRNA expression of genes from normal and tumor-derived human mammary epithelial cells grown in a special medium that supports similar growth rates of the two cell types. mRNAs uniquely expressed in normal cells are recovered as cDNAs after subtraction with mRNA from tumor cells. Seven different clones, from 0.6 to 4.8 kilobases in transcript size and including both rare and abundunt transcripts, were recovered in the first 23 clones analyzed. Among the isolated clones were genes encoding the gap-junction protein connexin 26, two different keratins, and glutathione-S-transferase pi, as well as an unknown gene in the S100 family of small calcium-binding proteins. In principle, tumor-suppressor genes include two classes: class I, in which loss of function results from mutation or deletion of DNA and class II, in which loss of function is from a regulatory block to expression. A class II suppressor gene is assumed to be regulated by a different suppressor gene that lost its function by mutation or deletion. Both classes of tumor-suppressor genes may provide valuable proteins with clinical applications in cancer diagnosis or therapy. Class II suppressors may be especially useful because the normal genes are present and their reexpression may be inducible by drugs or other treatments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azarnia R., Mitcho M., Shalloway D., Loewenstein W. R. Junctional intercellular communication is cooperatively inhibited by oncogenes in transformation. Oncogene. 1989 Oct;4(10):1161–1168. [PubMed] [Google Scholar]
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Band V., Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Stenman G., Morton C. C., Kulesa V., Connolly J., Sager R. A newly established metastatic breast tumor cell line with integrated amplified copies of ERBB2 and double minute chromosomes. Genes Chromosomes Cancer. 1989 Sep;1(1):48–58. doi: 10.1002/gcc.2870010109. [DOI] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Swisshelm K., Trask D., Kulesa V., Cohen C., Connolly J., Sager R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990 Nov 15;50(22):7351–7357. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Blessing M., Jorcano J. L., Franke W. W. Enhancer elements directing cell-type-specific expression of cytokeratin genes and changes of the epithelial cytoskeleton by transfections of hybrid cytokeratin genes. EMBO J. 1989 Jan;8(1):117–126. doi: 10.1002/j.1460-2075.1989.tb03355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein R., Shew J. Y., Chen P. L., Scully P., Lee W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990 Feb 9;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Battini R., Kaczmarek L., de Riel J. K., Baserga R. Molecular cloning of the cDNA for a growth factor-inducible gene with strong homology to S-100, a calcium-binding protein. J Biol Chem. 1986 Sep 25;261(27):12628–12632. [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow D. S., Beyer E. C., Paul D. L., Kobe S. S., Lau A. F. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990 Apr;10(4):1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Cohen D. I., Nielsen E. A., Steinmetz M., Paul W. E., Hood L. Cell-type-specific cDNA probes and the murine I region: the localization and orientation of Ad alpha. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2194–2198. doi: 10.1073/pnas.81.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear T. N., Ramshaw I. A., Kefford R. F. Differential expression of a novel gene, WDNM1, in nonmetastatic rat mammary adenocarcinoma cells. Cancer Res. 1988 Sep 15;48(18):5203–5209. [PubMed] [Google Scholar]
- Fargnoli J., Holbrook N. J., Fornace A. J., Jr Low-ratio hybridization subtraction. Anal Biochem. 1990 Jun;187(2):364–373. doi: 10.1016/0003-2697(90)90471-k. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J. 1985 Nov;4(11):2917–2920. doi: 10.1002/j.1460-2075.1985.tb04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Guo X. J., Chambers A. F., Parfett C. L., Waterhouse P., Murphy L. C., Reid R. E., Craig A. M., Edwards D. R., Denhardt D. T. Identification of a serum-inducible messenger RNA (5B10) as the mouse homologue of calcyclin: tissue distribution and expression in metastatic, ras-transformed NIH 3T3 cells. Cell Growth Differ. 1990 Jul;1(7):333–338. [PubMed] [Google Scholar]
- Harris H., Miller O. J., Klein G., Worst P., Tachibana T. Suppression of malignancy by cell fusion. Nature. 1969 Jul 26;223(5204):363–368. doi: 10.1038/223363a0. [DOI] [PubMed] [Google Scholar]
- Haskill S., Peace A., Morris J., Sporn S. A., Anisowicz A., Lee S. W., Smith T., Martin G., Ralph P., Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kligman D., Hilt D. C. The S100 protein family. Trends Biochem Sci. 1988 Nov;13(11):437–443. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Lee G. W., Ziff E. B., Vilcek J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol Cell Biol. 1990 May;10(5):1982–1988. doi: 10.1128/mcb.10.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. The cell-to-cell channel of gap junctions. Cell. 1987 Mar 13;48(5):725–726. doi: 10.1016/0092-8674(87)90067-5. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Shooter E. M. Nerve growth factor induces the genes for two proteins related to a family of calcium-binding proteins in PC12 cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1277–1281. doi: 10.1073/pnas.85.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. The actions of retinoids on cellular growth correlate with their actions on gap junctional communication. J Cell Biol. 1989 Mar;108(3):1053–1065. doi: 10.1083/jcb.108.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- Nikaido T., Bradley D. W., Pardee A. B. Molecular cloning of transcripts that accumulate during the late G1 phase in cultured mouse cells. Exp Cell Res. 1991 Jan;192(1):102–109. doi: 10.1016/0014-4827(91)90163-o. [DOI] [PubMed] [Google Scholar]
- Noda M., Kitayama H., Matsuzaki T., Sugimoto Y., Okayama H., Bassin R. H., Ikawa Y. Detection of genes with a potential for suppressing the transformed phenotype associated with activated ras genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):162–166. doi: 10.1073/pnas.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odink K., Cerletti N., Brüggen J., Clerc R. G., Tarcsay L., Zwadlo G., Gerhards G., Schlegel R., Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987 Nov 5;330(6143):80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Rasmussen U. B., Basset P., Daniel J. Y. Direct amplification of cDNA inserts from lambda libraries using the cloning-adapter as primer for PCR. Nucleic Acids Res. 1989 Apr 25;17(8):3308–3308. doi: 10.1093/nar/17.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation. Adv Cancer Res. 1985;44:43–68. doi: 10.1016/s0065-230x(08)60025-1. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation: a new frontier in cancer research. Cancer Res. 1986 Apr;46(4 Pt 1):1573–1580. [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Stoker M. G. Transfer of growth inhibition between normal and virus-transformed cells: autoradiographic studies using marked cells. J Cell Sci. 1967 Sep;2(3):293–304. doi: 10.1242/jcs.2.3.293. [DOI] [PubMed] [Google Scholar]
- Timblin C., Battey J., Kuehl W. M. Application for PCR technology to subtractive cDNA cloning: identification of genes expressed specifically in murine plasmacytoma cells. Nucleic Acids Res. 1990 Mar 25;18(6):1587–1593. doi: 10.1093/nar/18.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask D. K., Band V., Zajchowski D. A., Yaswen P., Suh T., Sager R. Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2319–2323. doi: 10.1073/pnas.87.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland I., Bolger G., Asouline G., Wigler M. A method for difference cloning: gene amplification following subtractive hybridization. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2720–2724. doi: 10.1073/pnas.87.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Oltz E. M., Rathbun G., Berman J. E., Smith R. K., Lansford R. D., Rothman P., Okada A., Lee G., Morrow M. Isolation of coordinately regulated genes that are expressed in discrete stages of B-cell development. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5759–5763. doi: 10.1073/pnas.87.15.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen P., Smoll A., Peehl D. M., Trask D. K., Sager R., Stampfer M. R. Down-regulation of a calmodulin-related gene during transformation of human mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7360–7364. doi: 10.1073/pnas.87.19.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]