Abstract
There is now general agreement that neurokinin B (NKB) acts via neurokinin-3-receptor (NK3R) to stimulate secretion of GnRH and LH in several species, including rats, mice, sheep, and humans. However, the roles of two other tachykinins, substance P (SP) and neurokinin A, which act primarily via NK1R and NK2R, respectively, are less clear. In rodents, these signaling pathways can stimulate LH release and substitute for NKB signaling; in humans, SP is colocalized with kisspeptin and NKB in the mediobasal hypothalamus. In this study, we examined the possible role of these tachykinins in control of the reproductive axis in sheep. Immunohistochemistry was used to describe the expression of SP and NK1R in the ovine diencephalon and determine whether these proteins are colocalized in kisspeptin or GnRH neurons. SP-containing cell bodies were largely confined to the arcuate nucleus, but NK1R-immunoreactivity was more widespread. However, there was very low coexpression of SP or NK1R in kisspeptin cells and none in GnRH neurons. We next determined the minimal effective dose of these three tachykinins that would stimulate LH secretion when administered into the third ventricle of ovary-intact anestrous sheep. A much lower dose of NKB (0.2 nmol) than of neurokinin A (2 nmol) or SP (10 nmol) consistently stimulated LH secretion. Moreover, the relative potency of these three neuropeptides parallels the relative selectivity of NK3R. Based on these anatomical and pharmacological data, we conclude that NKB-NK3R signaling is the primary pathway for the control of GnRH secretion by tachykinins in ewes.
Recent studies (1–3) on the reproductive effects of mutations in the TAC3 gene that encodes neurokinin B (NKB) or in the gene (TACR3) that encodes its receptor, neurokinin-3-receptor (NK3R), have focused the attention of reproductive neuroendocrinologists on the possible roles of tachykinins in control of GnRH, LH, and FSH secretion. In addition to NKB, two other tachykinins have been implicated in the control of reproduction: substance P (SP) and neurokinin A (NKA). These peptides are both encoded by the same gene (TAC1) and act via NK1R and NK2R, respectively (4, 5). At this time, there are no reports that mutations in TAC1 or the genes for NK1R or NK2R produce reproductive deficits in humans, but other approaches have implicated these two tachykinins in the control of GnRH secretion (4).
A stimulatory role for NKB in control of GnRH in humans was first proposed in 1991 based on the increase in NKB expression in postmenopausal women within the infundibular nucleus (6), which is analogous to the arcuate nucleus (ARC) in other species. These cells also contain kisspeptin and dynorphin and thus are now known as kisspeptin-NKB-dynorphin (KNDy) neurons (7, 8). More recently, work across a number of species, spurred on by the human genetic studies, has produced considerable evidence in support of this hypothesis. In most studies NKB, or more often senktide (an NK3R agonist), stimulated LH secretion via GnRH in gonadally intact animals or when LH secretion was suppressed with exogenous steroids (9–13). Conversely, antagonists to NK3R inhibited LH secretion in ovariectomized (OVX) sheep (14–16), castrated monkeys (16), and normal men and women (17). Interestingly most, but not all (18), studies indicate that these stimulatory effects are mediated by kisspeptin released from KNDy neurons. Thus, KNDy neurons contain NK3R (19–21) and administration of NKB, or senktide, increases their activity based on Fos expression, in vivo (22, 23) or electrical activity in hypothalamic slices (13, 24, 25). Moreover, these agonists did not stimulate LH secretion in the absence of the kisspeptin receptor (Kiss1r) in mice (26), in the presence of a Kiss1r antagonist in rats (27), or when Kiss1r was down-regulated in primates (28). In contrast to these stimulatory effects of senktide when endogenous GnRH secretion is low, this NK3R agonist inhibits LH secretion in OVX rats (29, 30) and mice (21), probably by stimulating dynorphin release from KNDy neurons (31).
There are fewer studies on the roles of SP and NKA, but recent work in rodents indicates that these tachykinins can have effects similar to NKB on LH and FSH secretion. Thus, NKA and SP increased the firing rate of murine KNDy neurons in vitro (25) and specific NK1R or NK2R agonists increased LH and FSH secretion in rats (32) and mice (12), with the latter effects being dependent on Kiss1r signaling (12). Similarly knockout of Tac1 delayed puberty and decreased ovulation rate and the number of pups/litter in mice (33). Finally, there may well be some redundancy among the 3 tachykinin signaling pathways in rodents because blockade of all 3 receptors is required to inhibit LH secretion in OVX rats (34). Similarly, stimulatory effects of NKB on the electrical activity of murine KNDy neurons in vitro is not blocked by each selective NKR antagonist alone, but is blocked by a cocktail of all 3 receptor antagonists (25).
The limited work on SP and NKA in other species has largely focused on the former. In humans, infusion of SP stimulated LH, but not FSH, secretion (35) and SP expression in the infundibular nucleus is higher in postmenopausal women than in premenopausal women (6) but higher than the expression observed in both young and aged men (36). Immunocytochemical studies using tissue from postmenopausal women indicated that 25% of NKB-containing and 31% of kisspeptin-containing cell bodies also expressed SP (36) and similar colocalization was observed in close contacts with GnRH axonal fibers in the median eminence (37). On the other hand, in male rhesus monkeys, SP was not found in kisspeptin-containing neurons and iv injection of SP failed to stimulate LH secretion (38). Moreover, an NK1R antagonist increased both LH and FSH concentrations during the descending phase of an estrogen-induced surge in cynomologus monkeys, suggesting a possible inhibitory effect of SP (39).
Studies in sheep have provided important information on the expression and actions of NKB (8, 9, 14–16, 19, 40), but there is no information in this species on the role of other tachykinins in control of GnRH secretion. This work addressed this gap in our knowledge by: 1) describing the expression of SP and NK1R in the ovine preoptic area (POA) and hypothalamus; 2) determining whether either was colocalized with kisspeptin- or GnRH-containing neurons and the effect of estradiol (E2) on their expression; and 3) comparing the minimal dose of NKB, SP, and NKA required to stimulate LH secretion in ovary-intact anestrous ewes. We chose to monitor LH concentrations because they provide a reliable index of episodic GnRH secretion. In contrast, patterns of FSH do not reflect endogenous GnRH pulses (41) and FSH elevations are not seen in response to exogenous GnRH injections that produce LH pulses (41, 42) in ewes.
Materials and Methods
Animals
Adult (4–8 y of age) multiparous blackface ewes of predominantly Suffolk breeding were maintained in an open barn and moved indoors 3–7 days before surgeries. Ewes were fed a pelleted alfalfa diet to maintain weight (65–85 kg) and provided free access to water and supplemental minerals. Lighting was adjusted bimonthly to mimic the duration of natural day light. All experiments used anestrous ewes and were performed between the middle of April and the end of July.
Surgical and blood collection procedures
All surgeries were performed under aseptic conditions using 2%–4% isofluorane in oxygen for anesthesia. For OVX, ovaries were exposed via midventral laporatomy, the blood supply ligated, and the ovaries removed. Any blood clots adhering to the uterus or oviducts were then removed with sterile saline, these organs returned to the abdomen, and the peritoneum and skin were sutured closed. For intracerebroventricular (icv) administration of tachykinins, an 18-gauge stainless steel cannula, was stereotaxically placed into the third ventricle, cemented in place with dental acrylic, protected with a plastic cap, and the hub plugged to prevent backflow of cerebrospinal fluid (CSF) (43). All ewes were treated with dexamethasone and penicillin from 1 day before to 5 days after surgery, and with analgesic (125 mg; Banamine, Phoenix Pharmaceutical) at the time of anesthesia induction and for 5 days after surgery. Animals were allowed to recover from surgical procedures for at least 7 days before any experimental treatments. Jugular blood samples (3–4 mL) were taken by venipuncture, placed in heparinized tubes, and plasma collected and stored at −20°C until assayed for LH. All procedures were approved by the West Virginia University Animal Care and Use Committee and conducted in accordance with National Institutes of Health (NIH) guidelines on the care and use of animals in research.
Tissue collection
Paraformaldhyde-fixed tissue was collected for immunohistochemistry and histological determination of treatment sites. Ewes were injected with 2 doses of 20 000 U of heparin (10 min apart) and then euthanized with an overdose (8–12 mL, iv) of sodium pentobarbital (Euthasol; Patterson Veterinary). The head was removed when the animal had stopped breathing and perfused via the internal carotids with 6 L of 4% paraformaldehyde in 0.1M phosphate buffer containing 0.1% NaNO3. Tissue blocks containing hypothalamus and POA were removed and stored in fixative at 4°C overnight and then in 20% sucrose. After sucrose infiltration, 45-μm-thick frozen coronal sections were cut using a freezing microtome. For immunohistochemistry, 6 parallel series of sections (270 μm apart) were stored at −20°C in cryoprotectant.
Experiments 1 and 2. Distribution, colocalization with kisspeptin or GnRH, and effect of E2 on SP and NK1R expression
All immunohistochemical studies were conducted on tissue collected from a cohort of anestrous ewes that did not undergo intracranial surgeries. To be able to determine the effects of E2, anestrous animals were OVX, as described above, and a 3-cm-long E2-containing Silastic implant was inserted sc (OVX+E2; n = 5) or sham inserted (OVX; n = 5) at the end of the surgical procedure. Animals were perfused, as described above, and tissue was collected 10 days later.
Experiment 3. LH dose response to NKB in ovary-intact anestrous ewes
Chronic cannulae were placed in the third ventricle of ovary-intact anestrous ewes (n = 4) in the middle of April. This experiment and experiment 4 were done in ovary-intact ewes to avoid multiple survival surgeries. A stock solution of NKB (Tocris Bioscience) was prepared the day before the first treatments by dissolving 0.6-mg NKB in 0.375 mL of 0.1N NaOH (1 nmol/μL) (44). Aliquots of this stock solution were stored at −20°C, and then thawed and diluted with sterile artificial CSF (aCSF) (45) on the day of treatments to produce concentrations ranging from 0.05 to 0.5 nmol/100 μL. Because the stock was diluted 1:200 to prepare the highest dose of NKB, we used 0.1N NaOH diluted 1:200 with aCSF as vehicle for the 0 nmol treatment. This study was originally designed to determine a dose of NKB that would reliably induce a physiological LH pulse for analysis of receptor turnover (46), so LH was only monitored for 2 hours after injection. Starting 9–10 days after surgery, blood samples were collected every 12 minutes from 24 minutes before to 2 hours after icv injection (100 μL) of 0 (100 μL of vehicle), 0.05 nmol NKB, 0.1 nmol NKB, 0.2 nmol NKB, or 0.5 nmol NKB. This protocol was then repeated 4 more times, with 3–5 days between treatments so that all animals received all 5 treatments in a random order. Tissue was collected after the last treatment and location of cannulae in the third ventricle confirmed. In a follow-up experiment the response to 0.2 nmol NKB was assessed in a separate set of 5 ovary-intact anestrous ewes using this protocol.
Experiment 4. What doses of NKA and SP are needed to stimulate LH secretion in ovary-intact anestrous ewes?
Chronic third ventricle cannulae were implanted in 5 ovary-intact ewes in early June. This experiment was designed to test 2 doses of NKA and SP. Based on the results of experiment 3, and the relative potency of NKB, SP, and NKA in stimulating electrical activity of KNDy neurons in mice (25), we first compared the effects of 2 nmol NKA and SP with vehicle controls. We then planned to either increase or decrease the dose of these tachykinins based on the effects at this dose, which resulted in administration of 0.5 nmol NKA and 10 nmol SP to these ewes in the second part of this experiment. Stock solutions of 0.5 nmol tachykinin/μL of aCSF were prepared before the first treatment, aliquots frozen, and diluted with aCSF to the appropriate concentration on the morning of all treatments. Starting 2 weeks after neurosurgery, ewes received icv injections of either aCSF, 2 nmol NKA, or 2 nmol SP in random order with 3–4 days between treatments. LH concentrations were measured in plasma samples collected from 36 minutes before to 4 hours after injections. One week after the last set of injections, samples were again collected for 36 minutes before to 4 hours after injection of either 0.5 nmol NKA or 10 nmol SP, and this treatment repeated with a cross-over design so that each ewe received both treatments. At the end of the experiment, tissue was collected to confirm that cannulae were in the third ventricle.
Immunohistochemistry
Free floating, double-label, immunofluorescence histochemistry was performed in order to determine the distribution of SP and NK1R and investigate their potential colocalization with kisspeptin and/or GnRH in the sheep POA and hypothalamus. Furthermore, we processed tissue sections from OVX and OVX+E2 ewes to determine whether E2 regulates protein expression of SP or NK1R. Hence, a series of every sixth section, extending from the level of the optic chiasma to the mammillary bodies, was processed for each of the following 4 combinations: 1) SP and kisspeptin; 2) NK1R and kisspeptin; 3) SP and GnRH; or 4) NK1R and GnRH using a modified protocol previously described and routinely used in our laboratory (8). Briefly, all steps were performed at room temperature and with gentle agitation, and tissue sections were washed with 0.1M PBS (pH 7.35) between each step. Antibodies were diluted in PBS+, a solution consisting of 0.1M PBS, 0.4% Triton X-100 (Sigma-Aldrich), and 4% normal goat serum (Jackson ImmunoResearch). Before the application of the first primary antibody, sections were treated with 1% hydrogen peroxide (H2O2) for 10 minutes followed by PBS+ for 1 hour to prevent nonspecific background labeling. Tissue sections were then incubated sequentially with: 1) guinea pig anti-SP (1:4000; Abcam) (Table 1) or rabbit anti-NK1R (1:10 000; Millipore) (Table 1) for 17 hours; 2) biotinylated goat antiguinea pig IgG (1:500; Vector Laboratories) or antirabbit IgG (1:500; Vector Laboratories), respectively, for 1 hour; 3) avidin and biotinylated horseradish peroxidase complex (avidin-biotin complex, 1:500; Vector Laboratories) for 1 hour; 4) biotinylated tyramine (1:250; PerkinElmer), containing 0.003% H2O2 for 10 minutes; and 5) Alexa Fluor 555 conjugated streptavidin (1:100; Invitrogen) for 30 minutes. Tissue was protected from light from this step forward. Next, sections were incubated with rabbit antikisspeptin (1:1000; Millipore) (Table 1) or rabbit anti-GnRH (1:400; Immunostar) (Table 1) for 17 hours. After overnight incubation, sections were washed and incubated with goat antirabbit DyLight green (1:100; Vector Laboratories) for 1 hour. Tissue sections were mounted onto slides, air dried, coverslipped with Gelvatol mounting medium, and stored at 4°C until analysis. Negative controls were routinely performed by omission of primary antibody, which eliminated all labeling corresponding to that antigen. In addition, specificity of the SP antibody was tested using peptide blocking controls (47). In short, preabsorption (overnight at 4°C) of the guinea pig anti-SP antibody with 15 g/mL of the corresponding SP peptide (catalog item ab38217; Abcam) abolished all staining in the ovine POA and hypothalamus. No blocking peptide was available for the NK1R antibody, but the complete mismatch with the distribution of NK3R (see Results) and reported absence of NK2R in the hypothalamus (48) support the specificity of this antibody.
Table 1.
Primary antibodies used
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| SP | CRPKPQQFFGLM | Anti-SP antibody | Abcam, ab10353; lot GR29977-16 | Guinea pig; polyclonal | 1:4000 | AB_297089 |
| NK1R | 23 aa sequence (385–407) of COOH end of rat SP receptor | Anti-SP receptor antibody | Millipore, AB5060 lot 2135068 | Rabbit; polyclonal | 1:10 000 | AB_2200636 |
| Kisspeptin | Peptide from mouse kisspeptin 10 | Antikisspeptin antibody | Millipore, AB9754 lot 2397065 | Rabbit; polyclonal | 1:1000 | AB_2296529 |
| GnRH | Synthetic GnRH coupled to keyhole limpet hemocyanin with carbodiimide linker | LHRH antibody | Immunostar, 20075 lot 1037001 | Rabbit; polyclonal | 1:400 | AB_572248 |
Image capture and analysis
The distribution of SP and NK1R was examined in sections through the POA and hypothalamus of each ewe. Colocalization of SP or NK1R with kisspeptin or GnRH as well as the comparison of total cell number between OVX and OVX+E2 ewes was evaluated in sections containing rostral, middle, or caudal levels of the ARC (4 sections each), or POA (6–8 sections each) at ×20 magnification. We used a digital camera (Microfire A/R; Optronics) attached to a microscope (DM500B; Leica Microsystems), with the appropriate excitation for DyLight 488 (green fluorescence) and Alexa Fluor 555 (red fluorescence) and Neurolucida software (MicroBrightfield Bioscience) to superimpose the 2 images and determine colocalization. For each section, the total number of single and double labeled (Kiss+SP, Kiss+NK1R, GnRH+SP, GnRH+NK1R) cells were counted. Counts were averaged per ewe, per brain area. Percentages of the total number of kisspeptin- or GnRH-immunoreactive (ir) cells containing SP or NK1R as well as the percentages of SP- or NK1R-ir cells containing kisspeptin or GnRH were calculated for each section, and averaged per ewe, per brain area.
In addition to cell counts, the effects of E2 on SP fiber density in the ARC were examined because SP cell bodies were observed infrequently. A midlevel section of the ARC was selected for imaging and quantification of the density of SP-containing fibers. Fibers were visualized and photographed with a ×20 objective lens (Microfire A/R). Data from these images were then analyzed using ImageJ software (NIH). A standardized threshold was applied to all images and the proportion of immunolabeling above threshold was quantified for each section. Therefore, the data represent the proportional area occupied by labeled fibers, not the specific number of fibers. Two images per animal were analyzed, and these data averaged to obtain values for that animal. Group data are expressed as the mean ± SEM.
LH assay
Ovine LH concentrations in plasma (200 μL) were measured in duplicate with a RIA using reagents provided by the National Hormone and Peptide Program as previously described (49). The sensitivity of the assay averaged 0.04 ng/tube (NIH S24) and the intra- and interassay coefficients of variation were 5.9% and 9.8%, respectively.
Statistical analyses
For experiment 2, the number of single-labeled kisspeptin, GnRH, or NKR1 perikarya were compared between OVX and OVX+E2 groups using a two-way ANOVA (region and hormone treatment as main factors) and Holm-Sidak post hoc test, whenever appropriate. Densities of SP fibers in the ARC were compared between OVX and OVX+E2 groups by t test. For analysis of the effects of tachykinins on LH secretion (experiments 3 and 4), an increase in LH concentrations after injection of a tachykinin was considered to be a response if it occurred within 2 samples of the injection and peak LH concentrations were 2 SD above the preinjection value, based on assay variability. For experiment 3, mean LH concentrations before and for 0–2 hours after injection were compared by two-way ANOVA with repeated measures (time and dose of NKB were the main factors), and the Holm-Sidak test was used for comparisons within treatments. LH pulse amplitudes and frequencies after injection were determined using established criteria for LH pulses (50). Amplitudes were analyzed by one-way ANOVA with repeated measures and pulse frequencies by the nonparametric Kruskal-Wallis one-way ANOVA on Ranks. For experiment 4, mean LH concentrations before, for 0–2 hours after, and for 2–4 hours after injection were analyzed for NKA and SP separately using a similar two-way ANOVA with repeated measures. Initial analysis of LH pulse frequencies and amplitudes in the 2 time bins postinjection indicated no effect of time, so these data were analyzed using a simple repeated-measures for the 4 hours after the injection period. Specifically, amplitudes were analyzed by one-way ANOVA with repeated measures and pulse frequencies by the nonparametric Friedman's repeated measures ANOVA. P < .05 was considered statistically significant.
Results
Experiment 1. Distribution of SP and NK1R and colocalization with kisspeptin or GnRH in the POA and hypothalamus
SP-ir perikarya were observed very infrequently in the mediobasal hypothalamus of OVX and OVX+E2 ewes, with more cell bodies identified in the caudal region of the ARC (range, 0–10 cell bodies) compared with middle (range, 0–4 cell bodies) and rostral (range, 0–1 cell bodies) regions (Figures 1 and 2). No SP perikarya were observed in the POA (Figures 1 and 2B). On the other hand, fibers immunopositive for SP were observed throughout the entire length of the POA and hypothalamus. In light of the paucity of SP-containing perikarya, it is not surprising that little colocalization of SP and kisspeptin was observed. Specifically, colocalization of these 2 peptides in ARC cell bodies (see Supplemental Figure 1) was only observed on 3 occasions (over 2300 kisspeptin cell bodies analyzed from 10 ewes). However, both populations of kisspeptin cells (ARC and POA) and their fibers were surrounded by a plethora of SP-ir fibers (Figure 2, A and B).
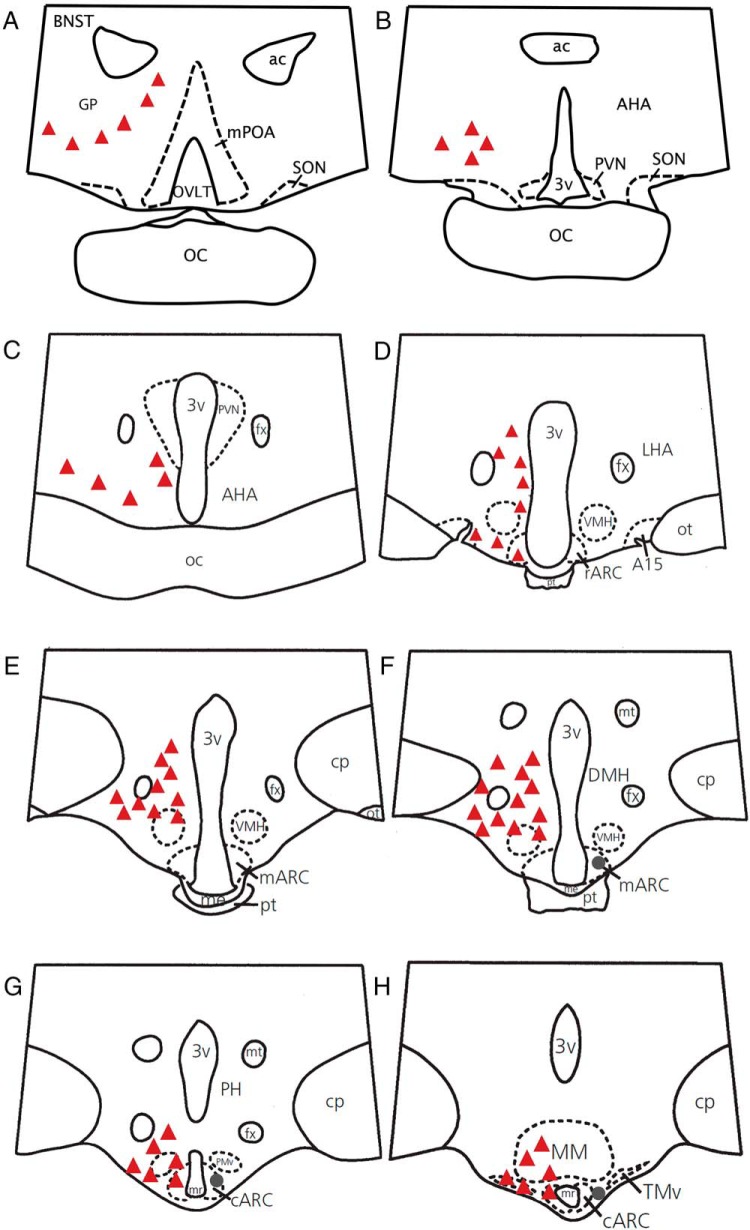
Figure 1.
A–H, Camera lucida drawings illustrating the representative distribution of SP (circles) and NK1R (triangles) cell bodies in the POA and hypothalamus of the ewe. Each marking represents approximately 10 cell bodies. Note that SP cell bodies were found only in the middle and caudal ARC (F–H). The distribution of SP and NK1R is shown unilaterally to allow visualization of labels. A15, A15 dopaminergic neurons; ac, anterior commissure; AHA, anterior hypothalamic area; r-m-c-ARC, rostral, middle, and caudal ARC; BNST, bed nucleus of the stria terminalis; CP, cerebral peduncle; DMH, dorsomedial nucleus of the hypothalamus; fx, fornix; GP, globus pallidus; LHA, lateral hypothalamic area; me, median eminence; MM, mammillary body; mPOA, medial POA; mr, mammillary recess; mt, mammillary tract; OC, optic chiasm; ot, optic tract; OVLT, organum vasculosum of lamina terminalis; PH, posterior hypothalamus; PMv, ventral premammillary nucleus; pt, pars tuberalis of the adenohypophysis; PVN, paraventricular nucleus; SON, supraoptic nucleus; TMv, ventral tuberomammillary nucleus; VMH, ventromedial nucleus of the hypothalamus; 3v, third ventricle.
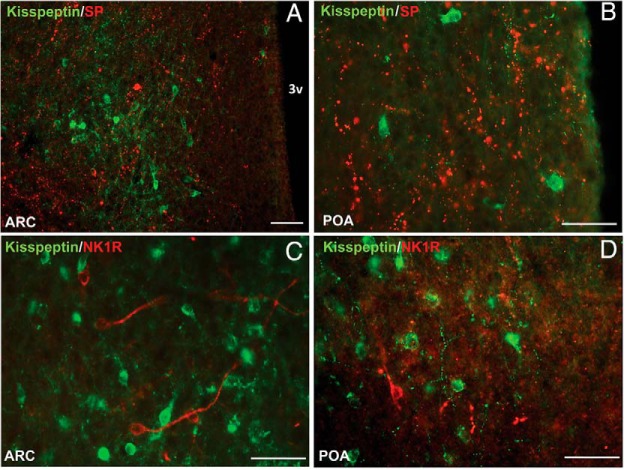
Figure 2.
Representative fluorescence photomicrographs showing lack of colocalization of kisspeptin (green) and SP (red) (A and B) and kisspeptin (green) and NK1R (red) (C and D) in the ARC (A and C) and POA (B and D). All photomicrographs are the computerized merger of 2 separate images captured sequentially with the appropriate excitation for either DyLight 488 (green, kisspeptin) or Alexa Fluor 555 (red, SP or NK1R). Scale bar, 100 μm (A) and 50 μm (B–D). 3v, third ventricle.
NK1R-containing cells were observed in the diagonal band of Broca, inner outline of the globus pallidus, anterior hypothalamic area, lateral hypothalamic area, dorsomedial hypothalamus, ventromedial nucleus, ARC, and premammillary nucleus (Figure 1). Of note, even though NK1R-ir cells were numerous throughout the hypothalamus, they were observed more sparsely in the POA (Figures 2, C and D, and 3, C and D). Within the ARC, expression of cell bodies was concentrated mainly in the rostral and caudal regions (average, 34 cell bodies for each) and less in the middle portion of this nucleus (average, 18 cell bodies). Analysis of dual-labeled kisspeptin and NK1R staining revealed infrequent colocalization of these 2 proteins in the ARC (Figure 2C). Specifically, only 6.2 ± 3.3% of kisspeptin neurons were seen to contain NK1R, and conversely, only 4.6 ± 1.7% of NK1R containing neurons detected in the ARC also expressed kisspeptin. Similarly to SP, NK1R containing fibers in the ARC where observed near kisspeptin cells (Figure 2C).
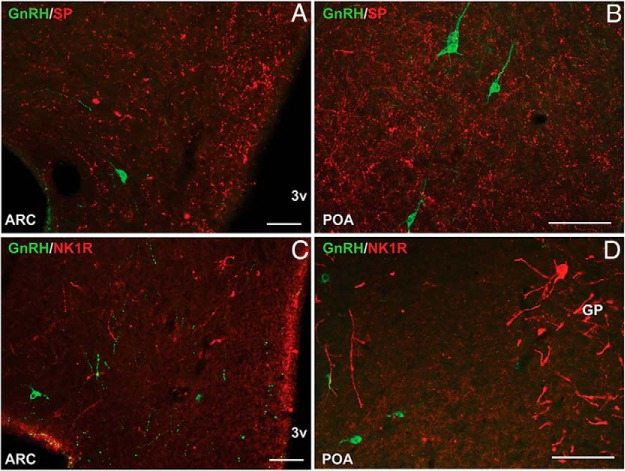
Figure 3.
Representative fluorescence photomicrographs showing lack of colocalization of GnRH (green) and SP (red) (A and B) and GnRH (green) and NK1R (red) (C and D) in the ARC (A and C) and POA (B and D). All photomicrographs are the computerized merger of 2 separate images captured sequentially with the appropriate excitation for either DyLight 488 (green) or Alexa Fluor 555 (red).Scale bar, 100 μm (A) and 50 μm (B–D). GP, globus pallidus; 3v, third ventricle.
Quantitative analysis of sections throughout the POA and mediobasal hypothalamus, revealed no colocalization of GnRH in SP or NK1R-containing neurons (over 100 GnRH cell bodies analyzed from 10 ewes, for each set of double-label staining) (Figure 3). Similarly, double-labeled fibers were not observed in the ARC or POA. However, GnRH cells and fibers were intimately surrounded by SP and NK1R-immunopositive fibers in the mediobasal hypothalamus (Figure 3 and Supplemental Figure 2).
Experiment 2. Effect of E2 on SP fiber density and NK1R expression in the ARC
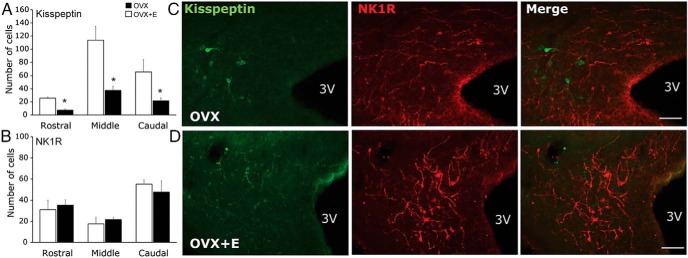
As expected, the presence of E2 down-regulated kisspeptin expression in the rostral, middle, and caudal aspects of the ARC (Figure 4) but had no effect on total GnRH cell numbers (3.9 ± 0.6 and 4.4 ± 1.3 cells in OVX and OVX+E2 ewes, respectively). Quantification of SP fiber density did not reveal differences between OVX and OVX+E2 ewes (4.23 ± 1.96% and 4.92 ± 1.26% of analyzed area above the threshold for OVX and OVX+E2 ewes, respectively). Similarly, E2 had no effect on NK1R-immunoreactivity in the rostral, middle, or caudal aspects of the ARC (Figure 4). In the POA, kisspeptin expression was up-regulated by E2 (12.3 ± 4.2 and 38.7 ± 4.2 cells for OVX and OVX+E2 ewes, respectively; P < .02), whereas there was no effect on GnRH expression (18.4 ± 2.7 and 21.4 ± 1.8 cells for OVX and OVX+E2 ewes, respectively). No cell bodies immunopositive for SP or NK1R were observed near GnRH neurons in the POA and therefore this area was not included in the analysis.
Figure 4.
Mean ± SEM total number of kisspeptin (A) and NK1R (B) cells in the rostral, middle, and caudal aspects of the ARC, in OVX (white bars) and OVX+E2 (black bars) ewes. Representative fluorescence photomicrographs from tissue stained for kisspeptin (green) and NK1R (red) in the caudal ARC of OVX (C) and OVX+E2 (D) ewes. Scale bar, 100 μm. 3v, third ventricle. *, P < .05 during comparison of OVX and OVX+E2 in each aspect of the ARC.
Experiment 3. LH dose response to NKB in ovary-intact anestrous ewes
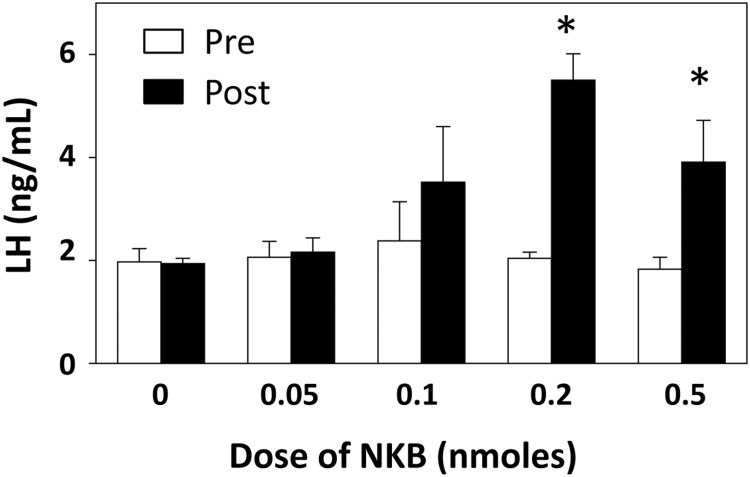
None of the ewes treated icv with 0 nmol NKB had an increase in LH concentrations that met the criteria for a response, and there was only 1 animal that responded to 0.05 nmol NKB, whereas doses of 0.1, 0.2, and 0.5 nmol NKB produced a response 100% of the time. Based on two-way ANOVA, there was a significant effect of time, dose, and dose by treatment interaction; there were no significant differences in preinjection LH concentrations, but mean LH concentrations during the 2 hours after injection of either 0.2 or 0.5 nmol NKB were significantly greater than those after control injections (Figure 5). LH pulse frequency was increased (compared with control injections) when animals were injected with 0.1, 0.2, or 0.5 nmol, but not when they received the lowest does of NKB (Table 2). There were no significant differences among groups in LH pulse amplitude, but only data from the 3 highest doses were analyzed, because there were too few pulses in ewes receiving 0 and 0.05 nmol NKB. In the follow-up study, 0.2 nmol NKB again induced a response in all 5 ewes and increased LH from 1.9 ± 0.3 ng/mL before injection to 3.1 ± 0.4 ng/mL in the 2 hours after injection (P = .015, paired t test). Pulse frequencies averaged 1.4 ± 0.2 pulses/2 hours, and amplitudes were 2.5 ± 0.5 ng/mL after the injection in these animals.
Figure 5.
Effects of different doses of NKB administered as a single injection into the third ventricle. Mean (±SEM) LH concentrations before (open bars) and 2 hours after (solid bars) injection are shown. *, P < .05 vs corresponding value for control (0 dose) injection.
Table 2.
Effect of NKB on LH Pulse Frequency and Amplitude
| Dose of NKB (nmol) | Frequency (#/2 h) | Amplitude (ng/mL) |
|---|---|---|
| 0 | 0 ± 0 | ND |
| 0.05 | 0.25 ± 0.22 | ND |
| 0.1 | 1.0 ± 0a | 2.4 ± 0.5 |
| 0.2 | 1.5 ± 0.3a | 4.7 ± 0.9 |
| 0.5 | 1.5 ± 0.3a | 3.1 ± 0.9 |
ND, not determined due to low number of pulses.
P < .05 compared with 0 nmol NKB.
Experiment 4. What doses of NKA and SP are needed to stimulate LH secretion in ovary-intact anestrous ewes?
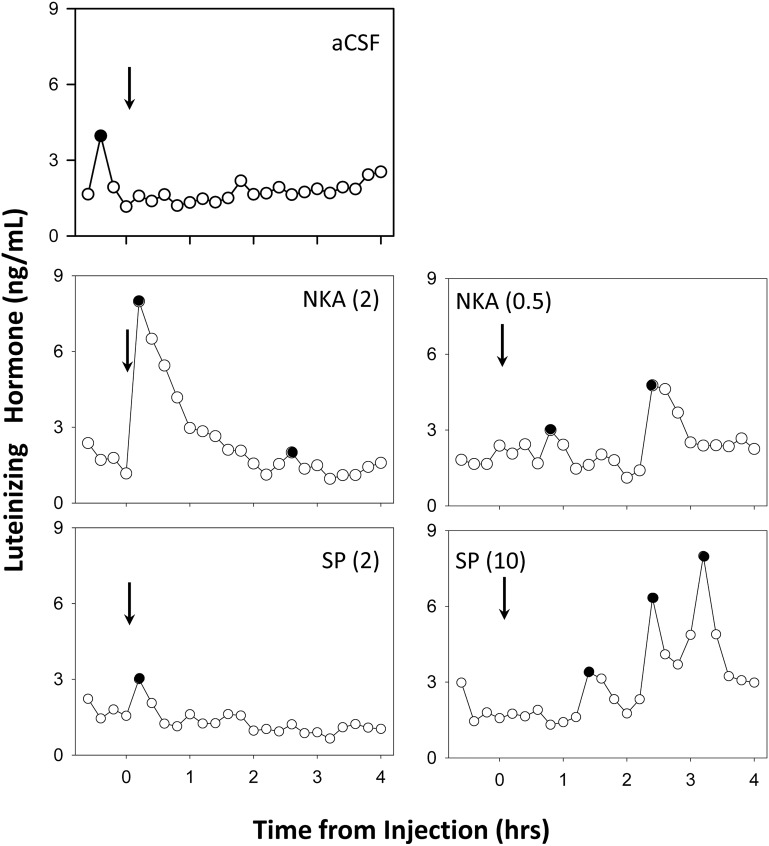
No ewes responded to icv injection of aCSF with an increase in LH concentrations, although occasional LH pulses occurred either before or 1–3 hours after injection (Figure 6). In contrast, 80% of the animals responded to icv injections of 2 nmol NKA with a robust and prolonged increment in LH, but such a response was only observed in 1 of the 5 animals given 2 nmol SP icv (Figure 6, left panels). Consequently, we next tested 0.5 nmol NKA and 10 nmol SP (Figure 6, right panels). With this lower dose of NKA, 1 ewe had a robust response immediately after injection of the tachykinin, whereas 3 ewes responded to the higher dose of SP, and the other 2 appeared to have a somewhat delayed increase in episodic LH secretion (Figure 6, right panels).
Figure 6.
Representative LH patterns before and after third ventricular injection (arrows) of aCSF, NKA, and SP. Left panels depict LH data from the same ewe for the first set of treatments, whereas data from the second set of treatments in a different ewe are depicted in the right panel. Doses (in nmol) of NKA or SP are presented in parentheses and peaks of LH pulses are indicated by solid circles.
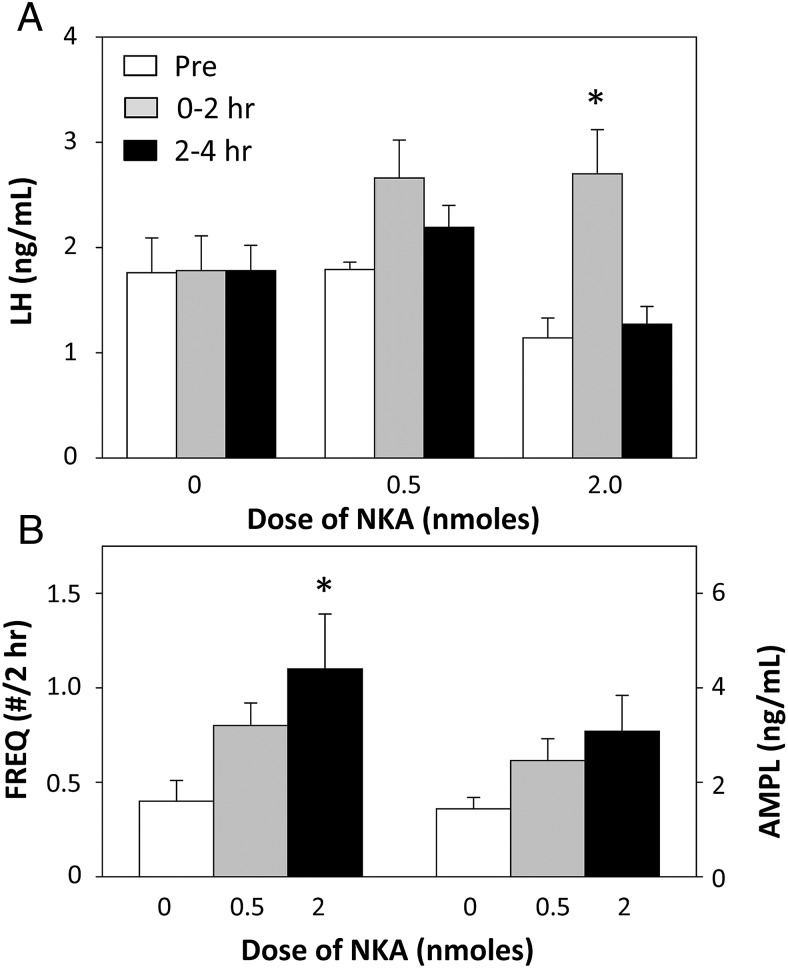
Statistical analysis of mean LH concentrations indicated there was a dose-response to NKA, with 2 nmol, but not 0.5 nmol, producing a significant increase in LH concentrations (Figure 7A). However, this response was fairly brief as LH returned to preinjection values during the 2- to 4-hour period. A similar dose-response in the effects of NKA on LH pulse frequency after injection was seen, but NKA had no significant effects on LH pulse amplitude (Figure 7B).
Figure 7.
A, Effect of 2 doses of NKA on mean LH concentrations before (open bars), 0–2 hours (gray bars), and 2–4 hours (black bars) after injection. *, P < .05 vs corresponding value for control (0 dose) treatment. B, Dose-response of NKA on LH pulse frequency (bars on left) and pulse amplitude (bars on right) during the 4 hours after injection of this tachykinin. *, P < .05 vs control (0 dose).
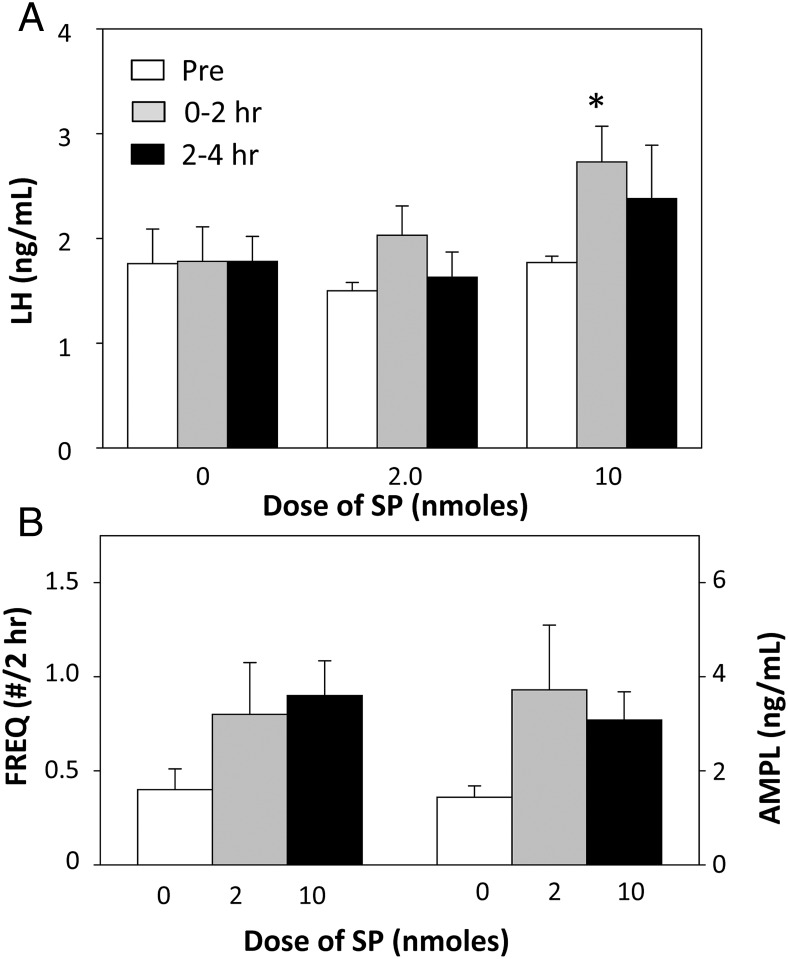
SP also produced a significant increase in mean LH concentrations in the first 2-hour period after injection, but this was only seen with the 10 nmol dose of this tachykinin (Figure 8A). Although LH values were also higher during the 2–4 hours after injection of 10 nmol SP, this was not significant because of increased variability. However, these effects of SP on mean LH concentrations were not reflected in either LH pulse frequency or pulse amplitude as neither parameter was significantly increased at either dose (Figure 8B).
Figure 8.
A, Effect of 2 doses of SP on mean LH concentrations before (open bars), 0–2 hours (gray bars), and 2–4 hours (black bars) after injection. *, P < .05 vs corresponding value for control (0 dose) treatment. B, Effect of 2 doses of SP on LH pulse frequency (bars on left) and pulse amplitude (bars on right) during the 4 hours after injection of this tachykinin. There were no statistically significant effects. Note differences in doses between NKA (Figure 7) and SP.
Discussion
This is the first detailed description of neurons containing SP and its cognate receptor, NK1R, in the ovine hypothalamus. Although SP was found in hypothalamic areas critical to the control of GnRH and LH secretion, the absence of NK1R in GnRH- or kisspeptin-containing neurons, and the inability of E2 to affect expression of SP or NK1R, argues against an important role for this tachykinin in control of reproductive function. The pharmacological data demonstrating that much higher doses of SP and NKA, than of NKB, are needed to stimulate LH secretion also supports this conclusion.
Although there has been considerable work describing the expression of SP in the ovine peripheral nervous system and one report of SP-ir cells in the pars tuberalis of sheep (51), there has been no description of SP-containing neurons in the hypothalamus of this species. We observed that these neurons were largely limited to the ARC, a distribution similar to that reported in humans (52) and monkeys (38, 53). In contrast, SP-containing neural cell bodies are found in several other hypothalamic regions in rodents, including the POA, anterior hypothalamic area, and premammillary region (PMR) (12, 54, 55). It is important to point out, however, that an immunohistochemical analysis may not detect all SP-producing neurons. For example, in situ hybridization identified cells containing mRNA for SP in the POA and PMR of humans (56), and there is a similar mismatch between mRNA and protein expression for dynorphin in some regions of the ovine hypothalamus (57). In contrast to the limited distribution of SP-ir cells, NK1R-expressing cells were observed in several different regions of the ovine diencephalon, with relatively high expression in the lateral POA, the ventromedial nucleus, the caudal ARC, and the PMR. A similar distribution of NK1R has been observed in rats (58) and guinea pigs (59). Although NK1R has been detected in the human hypothalamus (60), there is no detailed description of its expression in specific hypothalamic nuclei.
In contrast to the high level of colocalization of NKB and kisspeptin previously reported in the ovine ARC (8), we found that very few kisspeptin-containing neurons in this nucleus also expressed SP and no colocalization of SP and kisspeptin in the POA was observed. These observations conflict with the recent report that SP-immunoreactivity was found in 30% of kisspeptin neurons in the human infundibular region (36) but are consistent with the lack of Tac1 mRNA in Kiss1 neurons in either the anteroventral periventricular/periventricular nuclei or ARC of mice (12). These data raise the possibility of differences among species in expression of SP in kisspeptin neurons. In this regard, it is interesting to note that essentially no colocalization of these 2 peptides was recently observed in tissue from male monkeys (38). It is also unlikely that endocrine status can account for these differences because we examined tissue from OVX and OVX+E2 ewes and others used tissue from postmenopausal women, OVX mice, and gonadally intact and castrated monkeys.
In light of the accumulating evidence that activation of NK1R signaling stimulates LH secretion in rodents (see the introductory section), we hypothesized that NK1R would be found in either GnRH or kisspeptin neurons in the sheep. However, our data do not support this hypothesis: no GnRH neurons or POA kisspeptin neurons contained NK1R, and only 6% of KNDy neurons contained this receptor. In mice, 49% of KNDy neurons, 27% of anteroventral periventricular/periventricular nuclei Kiss1 neurons, and 23% of GnRH neurons also contain Tacr1 mRNA, based on single cell RT-PCR analysis (12); data on the expression of NK1R in these neurons are not currently available in any other species. It is possible that immunohistochemistry failed to detect NK1R in our study, but the similar anatomy of NK1R-containg neurons in rodents and sheep, and the robust expression of NK1R in ARC neurons not containing kisspeptin argue against this. Moreover, using a similar approach we have observed that most KNDy neurons contain NK3R in sheep (19). Thus, these anatomical differences in NK1R expression most likely reflect functional differences in the role of NK1R signaling between these species. Finally, although we did not examine expression of NK2R in this study, previous work found no expression of NK2R in the hypothalamus of rats (48) and no coexpression of this receptor in murine kisspeptin or GnRH neurons (12).
In the second part of this study, we determined the minimal dose of NKB, NKA, and SP needed to stimulate LH secretion when given into the third ventricle of ovary-intact anestrous ewes, animals that are endocrinologically comparable with the OVX+E2 anestrous ewes (61) used in the first 2 experiments. Ewes were much more sensitive to NKB, with 0.2 nmol producing a consistent increase in LH concentrations, whereas 2.0 nmol NKA and 10 nmol SP were needed to produce the same effect. A lower dose of NKB (0.1 nmol) consistently produced an initial increase, but mean LH concentrations during the 2 hours after injection were not significantly higher than controls because of its relatively short duration. Thus, the minimal dose of NKB needed to increase LH secretion probably falls between 0.2 and 0.1 nmol. The minimal effective dose of NKB in this study is lower than that needed to increase bursts of multiunit electrical activity in goats (44), but this may in part be due to differences in the site of administration, because NKB was injected into the lateral ventricle in that study. It is interesting to note that the relative potency of these 3 tachykinins (NKB>NKA>SP) parallels the relative selectivity of NK3R, not that of NK1R (SP>NKA>NKB) or NK2R (NKA>NKB>SP) (5). This correlation raises the possibility that each of these 3 tachykinins produces its stimulatory effects on LH secretion in the ewe via NK3R. This possibility is supported by the recent report that a selective NK3R agonist is much more potent at increasing bursts of multiunit electrical activity and LH pulses in OVX goats than selective NK1R or NK2R agonists (62) and by reports that 3 different selective NK3R antagonists each inhibits episodic LH secretion in OVX ewes (14–16).
The conclusion that NK3R signaling is the predominant pathway by which tachykinins control LH secretion in sheep and goats contrasts with recent data in rodents supporting a role for all 3 tachykinin receptors. This evidence includes reports that: 1) equivalent doses of selective agonists to NK1R, NK2R, and NK3R increase LH secretion in mice (12) and rats (32); 2) NKA and SP can stimulate electrical activity of KNDy neurons in vitro (25); and 3) antagonists to all 3 tachykinin receptors are required to completely block the stimulatory effects of NKB on the electrical activity of KNDy neurons (25) and episodic LH secretion in OVX rats (34). Thus, there appears to be species differences between rodents and ruminants in the ability of signaling via NK1R or NK2R to stimulate LH secretion.
In light of this apparent species difference in redundancy within tachykinin signaling critical for LH secretion, it is of interest to assess the situation in humans and nonhuman primates. At this time, there are no data on colocalization of any tachykinin receptor in GnRH or kisspeptin neurons in these species, but there are a few functional and more extensive genetic studies. In monkeys, the number of SP-containing neurons increases after castration (38), but iv administration of SP had no effect on LH secretion (38) and a selective antagonist to NK3R inhibited LH secretion in castrated males and ovary-intact females during the follicular phase of the menstrual cycle (16). Thus, most data are consistent with a lack of redundancy in tachykinin signaling in nonhuman primates. The situation in humans appears to be more complex. Two observations support redundancy: 1) in women, expression of mRNA for SP increases after menopause (6); and 2) in men, iv infusion of SP can stimulate LH secretion (35). On the other hand, the infertility observed in patients with mutations that disrupt NKB-NK3R signaling argues against redundancy (1–3, 63, 64), although the reversibility of this condition in some individuals (2, 65) could be due to signaling via other tachykinin receptors. Finally, the recent report that the selective NK3R antagonist, ESN364, inhibits LH secretion in men and women (17), as it does in sheep and monkeys (16), provides strong evidence that signaling through NK1R or NK2R plays a minor role in the control of LH secretion in humans. This conclusion, if correct, would provide a simple explanation for the differences in the severity of infertility produced by genetic disruption of NKB-NK3R signaling in humans (1–3, 63, 64) and mice (66, 67).
In summary, this study provides the first detailed description of the expression of SP- and NK1R-immunoreactivity within the ovine POA and hypothalamus. In contrast to data in humans and mice, but consistent with data in male monkeys, we found little colocalization of SP with kisspeptin. Moreover, the lack of expression in NK1R within GnRH- and kisspeptin-containing neurons and the relatively high doses of NKA and SP needed to stimulate LH secretion in ewes, support the hypothesis that NKB-NK3R signaling is the predominant pathway by which tachykinins control LH secretion in this species.
Acknowledgments
We thank Heather Bungard (West Virginia University Food Animal Research Facility), Gail Sager and Dr Margaret Minch for the care of the animals, and Dr Miroslav Valent for his assistance with the LH RIA. We also thank Dr Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH.
This work was supported by National Institutes of Health Grants R01-HD039916, R01-HD017864, R01-HD082135, and P20GM103434 to the West Virginia IDeA Network for Biomedical Research Excellence.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial CSF
- ARC
- arcuate nucleus
- CSF
- cerebrospinal fluid
- E2
- estradiol
- icv
- intracerebroventricular
- ir
- immunoreactive
- kiss1r
- kisspeptin-1 receptor
- KNDy
- kisspeptin-NKB-dynorphin
- NIH
- National Institutes of Health
- NKA
- neurokinin A
- NKB
- neurokinin B
- NK3R
- neurokinin-3-receptor
- OVX
- ovariectomized
- POA
- preoptic area
- SP
- substance P.
References
- 1. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287–2295. [DOI] [PubMed] [Google Scholar]
- 4. Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32(9):1972–1978. [DOI] [PubMed] [Google Scholar]
- 5. Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74(12):1445–1463. [DOI] [PubMed] [Google Scholar]
- 6. Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. [DOI] [PubMed] [Google Scholar]
- 7. Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153(11):5105–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 9. Billings HJ, Connors JM, Altman SN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Porter KL, Hileman SM, Hardy SL, Nestor CC, Lehman MN, Goodman RL. Neurokinin-3 receptor activation in the retrochiasmatic area is essential for the full pre-ovulatory luteinising hormone surge in ewes. J Neuroendocrinol. 2014;26(11):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navarro VM, Bosch MA, León S, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that neurokinin B controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinology. 2015;101(2):161–174. [DOI] [PubMed] [Google Scholar]
- 16. Fraser GL, Hoveyda HR, Clarke IJ, et al. The NK3 receptor antagonist ESN364 interrupts pulsatile LH secretion and moderates levels of ovarian hormones throughout the menstrual cycle. Endocrinology. 2015;156(11):4214–4225. [DOI] [PubMed] [Google Scholar]
- 17. Fraser GL, Ramael S, Hoveyda HR, Gheyle L, Combalbert J. The NK3 receptor antagonist ESN364 suppresses sex hormones in men and women. J Clin Endocrinol Metab. 2016;101(2):417–426. [DOI] [PubMed] [Google Scholar]
- 18. Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154(11):3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amstalden M, Coolen LM, Hemmerle AM, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 21. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakamoto K, Murata K, Wakabayashi Y, et al. Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. J Reprod Dev. 2012;58(6):700–706. [DOI] [PubMed] [Google Scholar]
- 24. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154(8):2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 26. García-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. [DOI] [PubMed] [Google Scholar]
- 27. Grachev P, Li XF, Lin YS, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One. 2012;7(9):e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026(2):307–312. [DOI] [PubMed] [Google Scholar]
- 30. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315. [DOI] [PubMed] [Google Scholar]
- 31. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894–4904. [DOI] [PubMed] [Google Scholar]
- 32. Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, et al. Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinology. 2015;156(2):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simavli S, Thompson IR, Maguire CA, et al. Substance p regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noritake K, Matsuoka T, Ohsawa T, et al. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409–415. [DOI] [PubMed] [Google Scholar]
- 35. Coiro V, Volpi R, Capretti L, et al. Luteinizing hormone response to an intravenous infusion of substance P in normal men. Metabolism. 1992;41(7):689–691. [DOI] [PubMed] [Google Scholar]
- 36. Hrabovszky E, Borsay BÁ, Racz K, et al. Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One. 2013;8(8):e72369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borsay BÁ, Skrapits K, Herczeg L, et al. Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance p immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology. 2014;100(2–3):141–152. [DOI] [PubMed] [Google Scholar]
- 38. Kalil B, Ramaswamy S, Plant TM. The distribution of substance P and kisspeptin in the mediobasal hypothalamus of the male rhesus monkey and a comparison of intravenous administration of these peptides to release GnRH as reflected by LH secretion. Neuroendocrinology. 2016;103(6):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kerdelhué B, Gordon K, Williams R, et al. Stimulatory effect of a specific substance P antagonist (RPR 100893) of the human NK1 receptor on the estradiol-induced LH and FSH surges in the ovariectomized cynomolgus monkey. J Neurosci Res. 1997;50(1):94–103. [DOI] [PubMed] [Google Scholar]
- 40. Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218–4225. [DOI] [PubMed] [Google Scholar]
- 41. Sharma TP, Nett TM, Karsch FJ, et al. Neuroendocrine control of FSH secretion: IV. Hypothalamic control of pituitary FSH-regulatory proteins and their relationship to changes in FSH synthesis and secretion. Biol Reprod. 2012;86(6):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamernik DL, Nett TM. Gonadotropin-releasing hormone increases the amount of messenger ribonucleic acid for gonadotropins in ovariectomized ewes after hypothalamic-pituitary disconnection. Endocrinology. 1988;122(3):959–966. [DOI] [PubMed] [Google Scholar]
- 43. Foradori CD, Amstalden M, Coolen LM, et al. Orphanin FQ: evidence for a role in the control of the reproductive neuroendocrine system. Endocrinology. 2007;148(10):4993–5001. [DOI] [PubMed] [Google Scholar]
- 44. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grachev P, Li XF, Hu MH, et al. Neurokinin B signaling in the female rat: a novel link between stress and reproduction. Endocrinology. 2014;155(7):2589–2601. [DOI] [PubMed] [Google Scholar]
- 46. Weems PW, Coolen LM, Hileman SM, et al. κ Opioid receptors are internalized in arcuate KNDy cells during GnRH pulse termination in the ewe. Annual Meeting of Society for Neuroscience San Diego, CA, 2016 (Abstract 60.04). [Google Scholar]
- 47. Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493(4):477–478. [DOI] [PubMed] [Google Scholar]
- 48. Saffroy M, Torrens Y, Glowinski J, Beaujouan JC. Autoradiographic distribution of tachykinin NK2 binding sites in the rat brain: comparison with NK1 and NK3 binding sites. Neuroscience. 2003;116(3):761–773. [DOI] [PubMed] [Google Scholar]
- 49. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 50. Goodman RL, Maltby MJ, Millar RP, et al. Evidence that dopamine acts via kisspeptin to hold GnRH pulse frequency in check in anestrous ewes. Endocrinology. 2012;153(12):5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Skinner DC, Lang AL, Pahl L, Wang Q. Substance P-immunoreactive cells in the ovine pars tuberalis. Neuroendocrinology. 2009;89(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dudás B, Merchenthaler I. Close juxtapositions between LHRH immunoreactive neurons and substance P immunoreactive axons in the human diencephalon. J Clin Endocrinol Metab. 2002;87(6):2946–2953. [DOI] [PubMed] [Google Scholar]
- 53. Rønnekleiv OK, Kelly MJ, Eskay RL. Distribution of immunoreactive substance P neurons in the hypothalamus and pituitary of the rhesus monkey. J Comp Neurol. 1984;224(1):51–59. [DOI] [PubMed] [Google Scholar]
- 54. Akesson TR, Micevych PE. Estrogen concentration by substance P-immunoreactive neurons in the medial basal hypothalamus of the female rat. J Neurosci Res. 1988;19:412–419, 470–471. [DOI] [PubMed] [Google Scholar]
- 55. Ljungdahl A, Hökfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat–I. Cell bodies and nerve terminals. Neuroscience. 1978;3(10):861–943. [DOI] [PubMed] [Google Scholar]
- 56. Chawla MK, Gutierrez GM, Young WS, 3rd, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1997;384(3):429–442. [DOI] [PubMed] [Google Scholar]
- 57. Foradori CD, Goodman RL, Lehman MN. Distribution of preprodynorphin mRNA and dynorphin-a immunoreactivity in the sheep preoptic area and hypothalamus. Neuroscience. 2005;130(2):409–418. [DOI] [PubMed] [Google Scholar]
- 58. Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347(2):249–274. [DOI] [PubMed] [Google Scholar]
- 59. Yip J, Chahl LA. Localization of tachykinin receptors and Fos-like immunoreactivity induced by substance P in guinea-pig brain. Clin Exp Pharmacol Physiol. 2000;27(11):943–946. [DOI] [PubMed] [Google Scholar]
- 60. Caberlotto L, Hurd YL, Murdock P, et al. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur J Neurosci. 2003;17(9):1736–1746. [DOI] [PubMed] [Google Scholar]
- 61. Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res. 1984;40:185–232. [DOI] [PubMed] [Google Scholar]
- 62. Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J Reprod Dev. 2015;61(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Francou B, Bouligand J, Voican A, et al. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PLoS One. 2011;6(10):e25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guran T, Tolhurst G, Bereket A, et al. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94(10):3633–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sidhoum VF, Chan YM, Lippincott MF, et al. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99(3):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]