Abstract
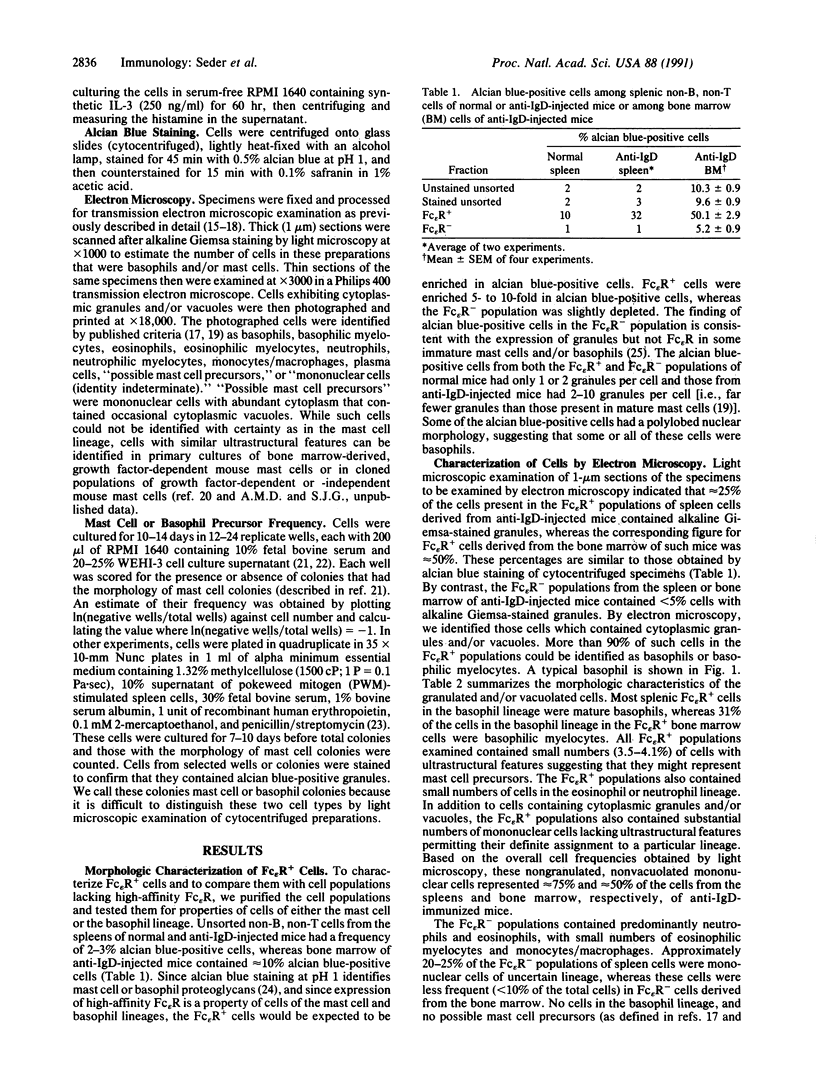
Splenic and bone marrow cells from normal mice, and from mice that have been polyclonally activated by injection of anti-IgD antibody, contain cells that produce interleukin 4 (IL-4) in response to crosslinkage of Fc epsilon receptors (Fc epsilon R) or Fc gamma R or to ionomycin. Isolated Fc epsilon R+ cells have recently been shown to contain all of the IL-4-producing capacity of the nonlymphoid compartment of spleen and bone marrow. Here, purified Fc epsilon R+ cells are shown to be enriched in cells that contain histamine and express alcian blue-positive cytoplasmic granules. By electron microscopy, the vast majority of cytoplasmic granule-containing cells are basophils; they constitute approximately 25% and approximately 50%, respectively, of Fc epsilon R+ spleen and bone marrow cells from anti-IgD-injected mice. The Fc epsilon R- populations contain cells that form colonies typical of mast cells. The Fc epsilon R+ populations also contain cells that, upon culture with IL-3, form colonies of alcian blue-positive cells, but (in contrast to colonies derived from Fc epsilon R- populations) the colonies are small, and all the cells die within 2-3 weeks. The Fc epsilon R+ cells synthesize histamine during a 60-hr culture with IL-3, while the Fc epsilon R- cells do not. These results indicate that IL-4-producing Fc epsilon R+ cells are highly enriched in basophils.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W. Role of basophils, mast cells, and vasoamines in hypersensitivity reactions with a delayed time course. Prog Allergy. 1977;23:199–320. [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Le Gros G., Conrad D. H., Finkelman F. D., Paul W. E. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1421–1425. doi: 10.1073/pnas.87.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher R. R., Verburg K. M., Henry D. P. Rat histamine N-methyltransferase. Quantification, tissue distribution, purification, and immunologic properties. J Biol Chem. 1983 Oct 25;258(20):12215–12220. [PubMed] [Google Scholar]
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Brown M. J., Ind P. W., Causon R., Lee T. H. A novel double-isotope technique for the enzymatic assay of plasma histamine: application to estimation of mast cell activation assessed by antigen challenge in asthmatics. J Allergy Clin Immunol. 1982 Jan;69(1 Pt 1):20–24. doi: 10.1016/0091-6749(82)90082-3. [DOI] [PubMed] [Google Scholar]
- Brown S. J., Galli S. J., Gleich G. J., Askenase P. W. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol. 1982 Aug;129(2):790–796. [PubMed] [Google Scholar]
- Burd P. R., Rogers H. W., Gordon J. R., Martin C. A., Jayaraman S., Wilson S. D., Dvorak A. M., Galli S. J., Dorf M. E. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989 Jul 1;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth E. N., Hood A. F., Soter N. A., Kagey-Sobotka A., Norman P. S., Lichtenstein L. M. Cutaneous late-phase response to allergen. Mediator release and inflammatory cell infiltration. J Clin Invest. 1989 May;83(5):1519–1526. doi: 10.1172/JCI114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Hood L. E., Kent S. B. Role of disulfide bridges in determining the biological activity of interleukin 3. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7897–7901. doi: 10.1073/pnas.85.21.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad D. H., Ben-Sasson S. Z., Le Gros G., Finkelman F. D., Paul W. E. Infection with Nippostrongylus brasiliensis or injection of anti-IgD antibodies markedly enhances Fc-receptor-mediated interleukin 4 production by non-B, non-T cells. J Exp Med. 1990 May 1;171(5):1497–1508. doi: 10.1084/jem.171.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapper R. M., Schrader J. W. Frequency of mast cell precursors in normal tissues determined by an in vitro assay: antigen induces parallel increases in the frequency of P cell precursors and mast cells. J Immunol. 1983 Aug;131(2):923–928. [PubMed] [Google Scholar]
- Dayton E. T., Pharr P., Ogawa M., Serafin W. E., Austen K. F., Levi-Schaffer F., Stevens R. L. 3T3 fibroblasts induce cloned interleukin 3-dependent mouse mast cells to resemble connective tissue mast cells in granular constituency. Proc Natl Acad Sci U S A. 1988 Jan;85(2):569–572. doi: 10.1073/pnas.85.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Dvorak H. F., Galli S. J. Ultrastructural criteria for identification of mast cells and basophils in humans, guinea pigs, and mice. Am Rev Respir Dis. 1983 Aug;128(2 Pt 2):S49–S52. doi: 10.1164/arrd.1983.128.2P2.S49. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Galli S. J., Morgan E., Galli A. S., Hammond M. E., Dvorak H. F. Anaphylactic degranulation of guinea pig basophilic leukocytes. I. Fusion of granule membranes and cytoplasmic vesicles formation and resolution of degranulation sacs. Lab Invest. 1981 Feb;44(2):174–191. [PubMed] [Google Scholar]
- Dvorak A. M., Monahan-Earley R. A., Estrella P., Kissell S., Donahue R. E. Ultrastructure of monkey peripheral blood basophils stimulated to develop in vivo by recombinant human interleukin 3. Lab Invest. 1989 Dec;61(6):677–690. [PubMed] [Google Scholar]
- Dvorak A. M., Nabel G., Pyne K., Cantor H., Dvorak H. F., Galli S. J. Ultrastructural identification of the mouse basophil. Blood. 1982 Jun;59(6):1279–1285. [PubMed] [Google Scholar]
- Dvorak A. M., Newball H. H., Dvorak H. F., Lichtenstein L. M. Antigen-induced IgE-mediated degranulation of human basophils. Lab Invest. 1980 Aug;43(2):126–139. [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr Basophilic leukocytes in allergic contact dermatitis. J Exp Med. 1972 Feb 1;135(2):235–254. doi: 10.1084/jem.135.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy M., Lebel B., Kamoun P., Hamburger J. Histamine production during the anti-allograft response. Demonstration of a new lymphokine enhancing histamine synthesis. J Exp Med. 1981 Feb 1;153(2):293–309. doi: 10.1084/jem.153.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982 Nov;95(2 Pt 1):435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990 Jul 19;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Dy M., Luffau G., Vassalli P. Gut mucosal mast cells. Origin, traffic, and differentiation. J Exp Med. 1984 Jul 1;160(1):12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado I., Urbina C. Presence of basophils in PHA-reacted skin sites of mice. An electron microscopic study. Int Arch Allergy Appl Immunol. 1983;72(2):116–118. doi: 10.1159/000234852. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Senn J. S., Till J. E., McCulloch E. A. Colony formation by normal and leukemic human marrow cells in culture: effect of conditioned medium from human leukocytes. Blood. 1971 Jan;37(1):1–5. [PubMed] [Google Scholar]
- Ishizaka T., Okudaira H., Mauser L. E., Ishizaka K. Development of rat mast cells in vitro. I. Differentiation of mast cells from thymus cells. J Immunol. 1976 Mar;116(3):747–754. [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Conrad D. H., Clark-Lewis I., Finkelman F. D., Plaut M., Paul W. E. IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J Immunol. 1990 Oct 15;145(8):2500–2506. [PubMed] [Google Scholar]
- Nabarra B., Dy M. Ultrastructural study on long-term cultures of bone marrow cells with histamine-producing stimulating factor (HCSF). Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46(3):175–185. doi: 10.1007/BF02890307. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Proud D., Togias A. G., Adkinson N. F., Jr, Meyers D. A., Kagey-Sobotka A., Plaut M., Norman P. S., Lichtenstein L. M. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med. 1985 Jul 11;313(2):65–70. doi: 10.1056/NEJM198507113130201. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Askenase P. W., Rose M. E. Basophils and eosinophils in three strains of rats and in athymic (nude) rats following infection with the nematodes Nippostrongylus brasiliensis or Trichinella spiralis. Immunology. 1980 Mar;39(3):385–389. [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Schneider E., Pollard H., Lepault F., Guy-Grand D., Minkowski M., Dy M. Histamine-producing cell-stimulating activity. Interleukin 3 and granulocyte-macrophage colony-stimulating factor induce de novo synthesis of histidine decarboxylase in hemopoietic progenitor cells. J Immunol. 1987 Dec 1;139(11):3710–3717. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves E. B., Allen J. R. Basophils in skin reactions of mast cell-deficient mice infested with Dermacentor variabilis. Int J Parasitol. 1990 Aug;20(5):655–667. doi: 10.1016/0020-7519(90)90124-6. [DOI] [PubMed] [Google Scholar]
- Thompson H. L., Metcalfe D. D., Kinet J. P. Early expression of high-affinity receptor for immunoglobulin E (Fc epsilon RI) during differentiation of mouse mast cells and human basophils. J Clin Invest. 1990 Apr;85(4):1227–1233. doi: 10.1172/JCI114557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina C., Ortiz C., Hurtado I. A new look at basophils in mice. Int Arch Allergy Appl Immunol. 1981;66(2):158–160. doi: 10.1159/000232814. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Yamatodani A., Maeyama K., Wada H. Pharmacology of alpha-fluoromethylhistidine, a specific inhibitor of histidine decarboxylase. Trends Pharmacol Sci. 1990 Sep;11(9):363–367. doi: 10.1016/0165-6147(90)90181-7. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]



