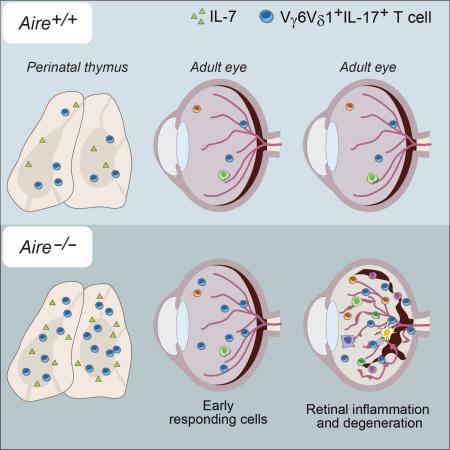
SUMMARY
Aire's primary mechanism of action is to regulate transcription of a battery of genes in medullary thymic epithelial cells (mTECs) and, consequently, negative selection of effector T cells and positive selection of regulatory T cells. We found that Aire-deficient mice had expanded thymic and peripheral populations of perinatally generated interleukin(IL)-17A+ Vγ6+Vδ1+ T cells, considered to be “early responders” to tissue stress and to drive inflammatory reactions. Aire-dependent control of Il7 expression in mTECs regulated the size of thymic IL-17A+Vγ6+Vδ1+ compartments. In mice lacking Aire and γδ T cells, certain tissues typically targeted in the “Aire-less” disease, notably the retina, were only minimally infiltrated. IL-17A+Vγ6+Vδ1+ cells were present in the retina of wild-type mice and expanded very early in Aire-deficient mice. A putatively parallel population of IL-17A+Vγ9+Vδ2+ T cells was increased in humans lacking Aire. Thus, Aire exerts multi-faceted control of autoimmunity that extends to a population of innate-like T cells.
Graphical abstract
INTRODUCTION
Humans and mice lacking the transcriptional regulator, Aire, develop a multi-organ autoimmune disease signaled by inflammatory infiltrates and autoantibodies (autoAbs) (Kisand and Peterson, 2015). Aire's primary mechanism of action is to regulate the expression of a multitude of genes in medullary thymic epithelial cells (mTECs) (Anderson et al., 2002). It accomplishes this task by intervening at a common transcriptional control-point: reversal of RNA polymerase-II pausing 50-100 nucleotides downstream of the transcriptional start-site (Oven et al., 2007; Giraud et al., 2012). This rather generic mechanism allows Aire to regulate the expression of more than one third of the mTEC transcriptome, in particular transcripts encoding antigens (Ags) characteristic of fully differentiated cells in peripheral tissues, so-called peripheral-tissue antigens (PTAs), but also messenger (m)RNAs that specify other types of molecules, e.g. chemokines, cytokines, and proteins involved in Ag processing or presentation (Sato et al., 2004; Johnnidis et al., 2005; Sansom et al., 2014; Meredith et al., 2015). By controlling the mTEC transcriptome in this manner, Aire shapes the array of self-peptides presented by major histocompatibility complex (MHC) molecules displayed at the mTEC surface and, as a consequence, both the negative selection of effector T (Teff) cells (Liston et al., 2003; Anderson et al., 2005; Taniguchi et al., 2012) and positive selection of regulatory T (Treg) cells (Lei et al., 2011; Malchow et al., 2013; Perry et al., 2014; Yang et al., 2015).
Given its influence on immunological tolerance, it was surprising to find that Aire is required only during a narrow age-window (Guerau-de-Arellano et al., 2009). Experiments on mice engineered to express Aire in a tetracycline-regulable fashion revealed its presence for the first week or two after birth to be necessary and sufficient to guard against the “Aire-less” autoimmune disease. This reliance on the perinatal window reflects age-dependent variation in the Ag-processing machinery of mTECs, thereby modifying the repertoire of peptides loaded onto MHC molecules (Yang et al., 2015). Consequently, perinatal expression of Aire promotes the positive selection of a distinct compartment of long-lived Treg cells, one that is specialized in protecting the organs targeted by autoimmunity in Aire-deficient mice (Yang et al., 2015).
Aire's pleiotropic effect on the selection of elements of the adaptive immune system – including CD4+ Teff, CD8+ Teff, and Foxp3+CD4+ Treg cells – raised the question of whether it might not extend its net of protection to effector cells of the innate immune system. Natural-killer (NK)-T cells were shown to be Aire-independent (Pitt et al., 2008), and γδ T cells of both mice and humans were reported to be indistinguishable in individuals expressing Aire or not (Tuovinen et al., 2009). Yet, several considerations argued for a re-examination of the latter case. First, our understanding of γδ T cell biology has increased on many fronts over the past several years, including new insights into their differentiation pathways in the thymus, selecting and activating ligands, effector phenotypes, and implication in disease pathogenesis (Vantourout and Hayday, 2013; Chien et al., 2014). Second, certain γδ T cell compartments are generated primarily during the Aire-relevant perinatal age-window (Chien et al., 2014). These populations often express quasi-invariant T cell receptors (TCRs) and accumulate within particular tissues, for example: the Vγ5+Vδ1+ population in the skin epidermis and the Vγ6+Vδ1+ population in the skin dermis, vagina, uterus and tongue [nomenclature according to (Heilig and Tonegawa, 1986)]. Third, certain molecules involved in thymic selection of γδ T cells have been identified and located in mTECs – such as Skint-1, which controls the maturation of epidermal Vγ5+Vγ1+ T cells (Barbee et al., 2011; Turchinovich and Hayday, 2011). In general, immature γδ T cells that encounter cognate ligand in the thymus become interferon (IFN)-γ producers, while those that do not end up making interleukin (IL)-17A (Jensen et al., 2008; Turchinovich and Hayday, 2011; Zeng et al., 2012). Lastly, there is mounting evidence that IL-17A-producing γδ (Tγδ17) cells can be critical effector cells in immune (Lockhart et al., 2006; Cho et al., 2010; Shibata et al., 2007; Meeks et al., 2009), autoimmune (Sutton et al., 2009; Roark et al., 2007; Cui et al., 2009; Cai et al., 2011), and anti-tumor (Rei et al., 2014; Wu et al., 2014) responses. In several contexts, they act as “early responders,” driving and setting the tone of the ensuing adaptive immune response (Cua and Tato, 2010).
For these reasons, we re-examined Aire's impact on γδ T cells. Mice lacking Aire showed a significant increase in the thymic production and peripheral installation of an IL-17A-producing Vγ6+Vδ1+ subset generated primarily during the perinatal age-window. We uncovered a molecular mechanism explaining the overabundance of this particular γδ T cell population, provided evidence of these cells’ role in the “Aire-less” autoimmune disorder, and translated these findings to Aire-deficient humans, i.e. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED) patients.
RESULTS
Aire-deficient mice have more IL-17A-producing γδ thymocytes and peripheral T cells
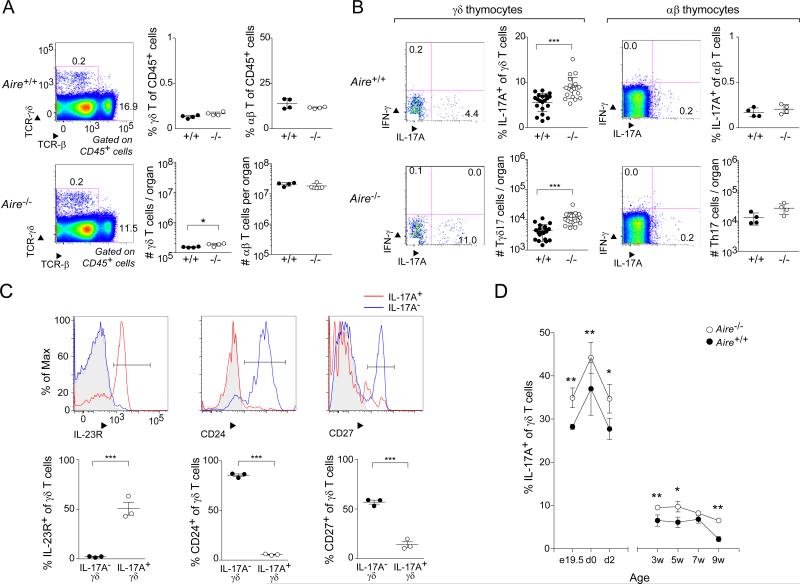
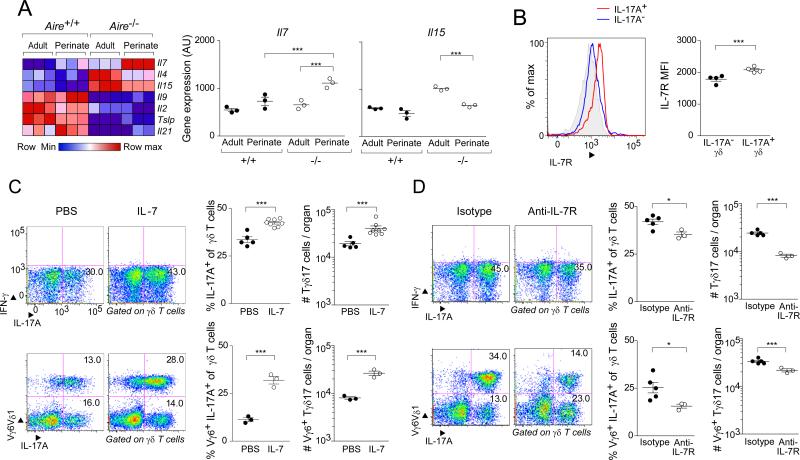
To evaluate the influence of Aire on murine γδ T cell compartments, we compared them in Aire+/+ and Aire−/− C57Bl/6 (B6) littermates. The fractional representation and number of bulk γδ thymocytes in the two types of mice were similar at 3-9 weeks of age, although there was a slightly higher number of γδ cells when Aire was absent (Fig. 1A). This difference did not represent a global divergence in the two groups – reflecting an effect on thymus size, for example – as αβ T cell frequencies and numbers were equivalent. The effect of Aire deficiency was more evident when γδ thymocytes were split into effector subsets: there were substantially more IL-17A-producing γδ T cells in Aire−/− mice, both proportionally and in numbers (Fig. 1B). Again, this difference did not hold true for αβ T cells. Few IFN-γ-producing thymocytes were detected at all. The phenotype of the enriched population in mice lacking Aire was typical of IL-17A-producing γδ T (Tγδ17) cells: high cell-surface expression of the IL-23 receptor (IL-23R) coupled with low expression of CD24 and CD27 (Fig. 1C). While the increase in IL-17A+ thymocytes was observed essentially lifelong, it was particularly prominent during the perinatal age-window (Fig. 1D), when certain populations of quasi-invariant γδ cells are typically generated (Chien et al., 2014).
Fig. 1. Mice lacking Aire had an increased frequency and number of IL-17A-producing γδ thymocytes.
Flow cytometric analysis of γδ thymocyte compartments from Aire+/+ and Aire−/− littermates. Except for panel D, mice were 3-9 weeks of age. A. Comparisons of total γδ and αβ thymocytes. Left-most panel: typical cytofluorometric profiles; top, wild-type (WT); bottom, knock-out (KO); numbers refer to the fraction of CD45+ cells in that gate. Center and right panels: summary data plots for γδ and αβ T cells, respectively (n=4); top, fractional representation; bottom, numbers per thymus. B. Comparisons of cytokine expression by γδ and αβ thymocytes. IFN-γ and IL-17A expression on gated γδ and αβ T cells. In each case, left panels: typical cytofluorometric profiles; numbers refer to the fraction of γδ or αβ cells in the indicated gate. In each case, right panels: summary data plots (n=20 or 4); top, fractional representation; bottom, numbers per thymus. C. Additional markers of IL-17A+ γδ thymocytes. Cells gated as IL-17A+ or IL-17A− as per panel B were additionally examined for expression of the indicated markers. Top, typical cytoflurometric histograms; bottom, summary data (n=3). Grey shading indicates isotype-control staining. D. IL-17A+ γδ thymocytes through ontogeny. Summary data for fractional representation (n=3-13). P=*, <0.05; **, <0.01; ***, <0.001 according to Student's t-test. Only significant differences are indicated. Dot plot scales for this figure are all the same.
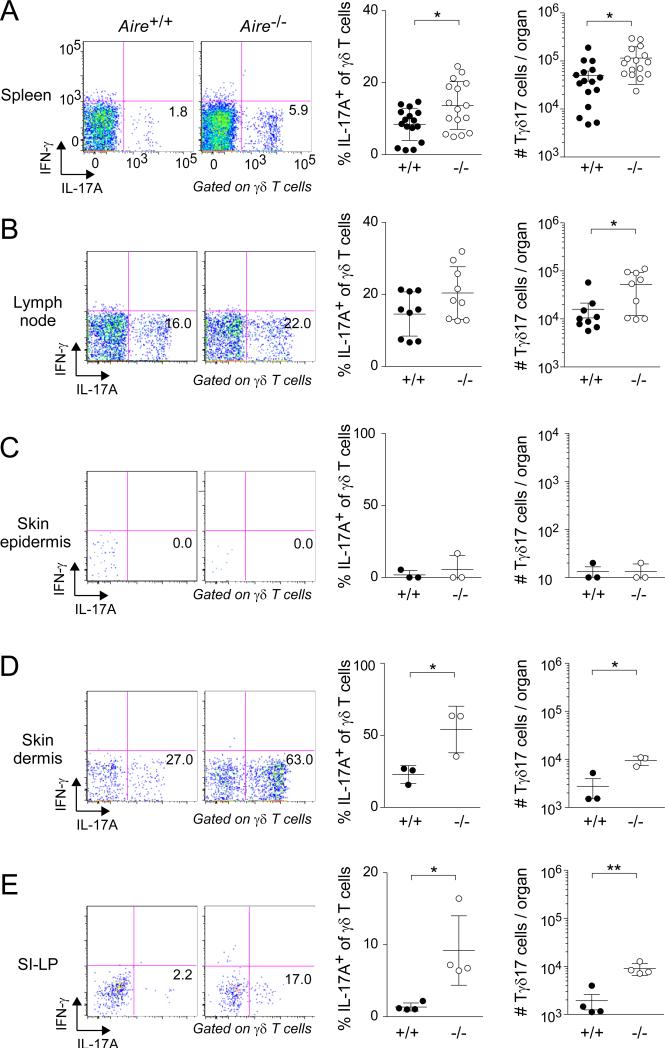
Next, we addressed whether the increase in Tγδ17 cells in Aire−/− mice persisted into the periphery. No augmentation in either the fractional representation or number of bulk γδ T cells was observed in the extra-thymic organs examined (data not shown). However, we did see more IL-17A-expressing γδ cells in the spleen, lymph nodes (LNs), skin dermis, and small-intestinal lamina propria (SI-LP) (Fig. 2). No such increase was detected in the skin epidermis. In brief, then, the absence of Aire resulted in larger thymic and peripheral Tγδ17 compartments.
Fig. 2. Increased frequency and number of Tγδ17 cells in peripheral tissues of Aire-deficient mice.
Designated organs of Aire+/+ and Aire−/− littermates were excised at 3-9 weeks of age and examined by flow cytometry, focusing on Tγδ17 cells. Left panels: typical cytofluorometric profiles. Numbers refer to fraction of total γδ T cells in that gate. SI-LP = small-intestinal lamina propria. Center panels: summary data for fractional representation (n=3-16). Right panels: corresponding summary data for number per organ. Statistics as per Fig. 1. Dot plot scales for this figure are all the same.
The expanded Tγδ17 population in Aire−/− mice consisted primarily of Vγ6+Vδ1+ cells of canonical sequence
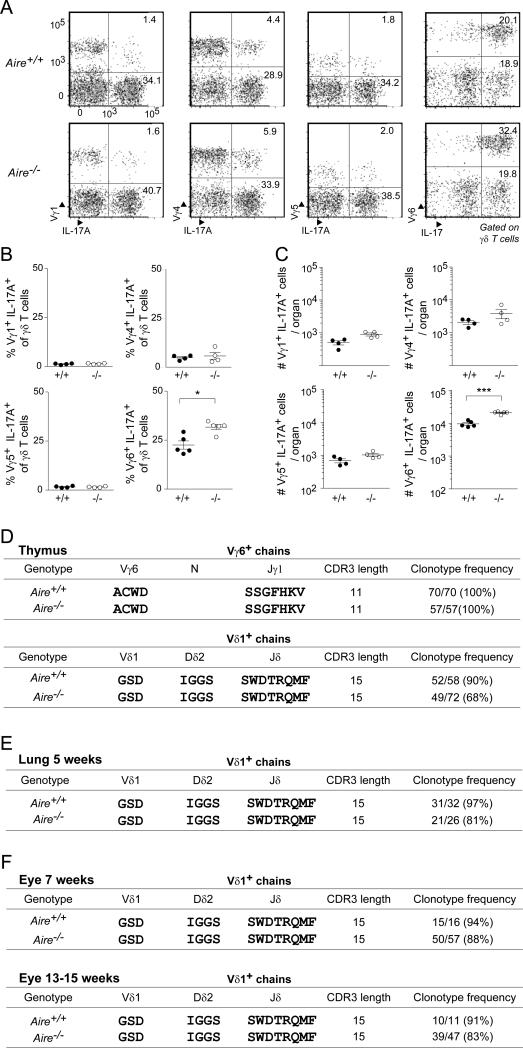
Because the IL-17A-expressing γδ thymocytes enriched in the absence of Aire were generated predominantly around birth, and because they accumulated in some peripheral tissues but not others, we wondered whether they represented a distinct γδ subset expressing a particular class of TCRs. To answer this question, we first performed a cytofluorometric analysis of perinatal thymocytes from Aire+/+ and Aire−/− littermates with available anti-Vγ reagents. There were no significant differences in the populations displaying Vγ1, Vγ4 or Vγ5 in the two types of mice; however, there was an enrichment in Vγ6+IL-17A+ thymocytes in mice lacking Aire (Fig. 3A-C).
Fig. 3. The expanded Tγδ17 population in Aire−/− mice displayed primarily Vγ6+Vδ1+ TCRs A-C.
Flow-cytometric analysis of Vγ usage. Perinatal thymocytes from Aire+/+ and Aire−/− mice were stained for IL-17A and for the indicated Vγ chains. A) Typical cytofluorometric profiles. Numbers refer to fraction of total γδ cells within the designated gate. B) Summary data on fractional representation (n=4-5). C) Corresponding summary data on numbers per thymus. D. Summary of the single-cell sequencing data detailed in Table S1. Single perinatal Vγ6+ thymocytes from Aire+/+ and Aire−/− littermates were sorted, and the CDR3 (in bold) sequences of their Vγ6+ and Vδ1+ chains determined. E. Summary of the lung single-cell sequencing data detailed in Table S2. As per panel D, except Vγ6+ cells were sorted from the lung of 5-week-old littermates. F. Summary of the eye single-cell sequencing data for 7- week and 13-15-week old mice detailed in Table S2. As per panel D, except Il-17+Vγ6+-enriched cells were identified as Vγ1,2,4,5− CD27− for sorting from the eyes of age-matched sets of Aire+/+ and Aire−/− mice (n=5-15). Statistics as per Fig. 1 (see also Tables S1 and S2). Dot plot scales for this figure are all the same.
We then employed single-cell DNA sequencing to compare the complementary-determining regions (CDR3s) of the Vγ6+ and Vδ1+ TCR chains from thymocytes of perinatal mice expressing Aire or not. In Aire-WT perinates, all of the thymocyte Vγ6+ chains had exactly the same CDR3 sequence. Similarly, all of the Vδ1+ chains (with one exception) contained Dδ2 and Jδ1 segments, with 90% of them having precisely the same CDR3 sequence (Fig. 3D and Table S1). These repeated Vγ6 and Vδ1 sequences correspond to the “canonical” sequences previously reported for a population of γδ T cells generated predominantly during the perinatal period and localized to a particular set of tissues, notably the skin dermis (O'Brien and Born, 2015). The corresponding population from Aire-KO perinates universally displayed Vγ6+ chains with the canonical CDR3; the Vδ1+ chains also frequently (~⅔ of the time) had the canonical CDR3 sequence (Fig. 3D and Table S2). However, the Vδ chain diversity observed in the absence of Aire was greater than what we saw and than what has been previously reported for wild-type mice. Similar results were obtained on lung Tγδ17-enriched cells: Vγ6+ chain CDR3s were the same for all cells examined, whether from Aire+/+ or Aire−/− mice; there was somewhat greater variability in the Vδ1+ CDR3s in the latter case (Fig. 3E and Table S2). To extend these findings to an additional tissue, and to see whether the Vδ1 repertoire matured over time, we sequenced the CDR3s of Tγδ17-enriched cells taken from the eye infiltrates of several 7- vs 13-15 week-old mice. With this tissue as well, there were more non-canonical Vδ1+ CDR3s in Aire−/− than Aire+/+ mice. The differential appeared to hold constant with age (Fig. 3F). Thus, loss of Aire had a quite precise impact on the IL-17A+ γδ compartments: an enrichment of cells displaying Vγ6+Vδ1+ TCRs, above all those with the canonical CDR3.
Increased stromal-cell expression of IL-7 by Aire-KO perinates can explain their overproduction of IL-17A+ γδ thymocytes
Two explanations for the enrichment of γδ thymocytes producing IL-17A in mice devoid of Aire seemed most likely: an increase in trophic factors and/or a decrease in selecting ligands. As concerns the first explanation, amongst the promising candidates were cytokines that signal through receptors incorporating the common gamma chain (γc), given their active roles in the differentiation and homeostasis of multiple lymphocyte subsets. In particular, it is known that IL-7 promotes the expansion specifically of IL-17A+ γδ thymocytes, while IL-15 has a parallel effect on their IFN-γ+ counterparts (Michel et al., 2012). Thus, we exploited existing microarray datasets to compare the expression of genes encoding the γc+-receptor-engaging family of cytokines in adult vs perinatal mTECs of Aire+/+ vs Aire−/− mice. Il7 transcripts were at their highest level in mTECs of Aire−/− perinates, and were also higher than in the corresponding stromal-cell populations from wild-type perinates and mutatnt or wild-type adults. In contrast, Il15 transcripts were under-represented in perinatal vis-à-vis adult Aire-KO perinates (Fig. 4A). We confirmed that IL-17A+ γδ thymocytes displayed IL-7R on their surface; actually, the mean fluorescence intensity (MFI) was significantly higher than that of their IL-17A− counterparts (Fig. 4B).
Fig. 4. IL-7 droveTγδ17 population expansion in Aire-KO mice.
A. Quantification of cytokine gene transcripts from mTECs of perinatal vs adult Aire-WT vs -KO mice. Focused on the family of cytokines that bind to receptors employing the γc chain. Microarray data on independent triplicates of mature mTECs expressing a high level of MHC-II (Yang et al., 2015). Left, heat map; right, expression values for genes deemed of particular interest. B. Flow-cytometric comparison of IL-7R expression by IL-17A+ and IL-17A− γδ thymocytes from Aire−/− perinates. Left, histogram of expression; right, mean fluorescence intensity (MFI). C. Boosting IL-7 levels in Aire+/+ mice. Recombinant IL-7 (250ng/g body weight) was ip-injected into day 0 perinates, and thymocytes were analyzed 3 days later. Left panels, typical cytofluormetric profiles; numbers represent the fraction of total γδ T cells within the indicated gate. Center and right panels, summary data on fractional representation and cell numbers, respectively (n=3-8); top, quantification of IL-17A+ γδ thymocytes; bottom, focus on IL-17A+Vγ6+ cells. D. Blocking the effect of IL-7 in Aire-KO mice. On the day of birth, 25μg of the anti-IL-7R mAb, A7R34, was injected; 3 days later, γδ thymocytes were analyzed. Data are presented as in panel C (n=3-5). Statistics as per Fig. 1. (See also Fig. S1). Dot plot scales for this figure are all the same.
To evaluate the functional relevance of IL-7 levels in this context, we performed both gain- and loss-of-function experiments. First, we administered recombinant IL-7 or just vehicle to Aire+/+ mice on the day of birth, and examined their thymocytes by flow cytometry three days later. Mice that had been injected with IL-7 showed an increase in the frequency and number of IL-17A+ γδ thymocytes (Fig. 4C, upper panels). This difference held true when we focused specifically on the IL-17A+ thymocytes that displayed Vγ6+ TCRs (Fig. 4C, lower panels). Second, we treated Aire−/− neonates with an anti-IL-7R or isotype-control monoclonal antibody (mAb), and quantified relevant thymocyte populations after three days. The mice injected with anti-IL-7R had fewer IL-17A+ γδ thymocytes, in particular those displaying Vγ6+ TCRs (Fig. 4D).
We also evaluated the possible implication of a decrease in γδ TCR ligands in the enrichment for IL-17A-expressing γδ T cells in the absence of Aire. Unfortunately, the endogenous ligand for the canonical Vγ6+Vδ1+ TCR is not known. However, Skint-1 and the non-classical MHC class 1b molecules, H2-T10 and H2-T22, have been identified as γδ TCR ligands: They are both expressed in mTECs, and their absence favors the accumulation of cognate IL-17A-producing γδ cells (Jensen et al., 2008; Barbee et al., 2011; Turchinovich and Hayday, 2011). We, therefore, checked the Aire-dependence of these ligands’ expression in perinatal mTECs, and their ability to promote selection of cognate IL-17A+ cells. Transcripts encoding both H2-T10 and H2-T22 were expressed at lower levels in mTECs of Aire−/− perinates (Fig. S1A). However, there was no corresponding increase in IL-17A+ thymocytes stained by the existing (and validated) T22 tetramer (Fig. S1B). We saw no significant difference in Skint1 transcript levels in mTECs of Aire+/+ and Aire−/− perinates – if anything, there was a slight augmentation in the absence of Aire (Fig. S1C). Correspondingly, no significant increase in IL-17A+Vγ5+ thymocytes was observed in mTECs of Aire-KO perinates (Fig. S1D).
In brief, the surfeit of Vγ6-expressing, IL-17A-producing thymocytes in perinates lacking Aire corresponded to elevated Il7 expression by thymic stromal cells; and experimental manipulation of IL-7 levels in perinates modulated the representation of Vγ6+IL-17A+ thymocytes. Our data did not provide any evidence that Aire-dependent effects on γδ TCR ligands expressed by perinatal mTECs play a role in selecting IL-17A+ γδ thymocytes. However, it remains possible that such influences will be found for other ligands, e.g. for the as-yet-unidentified endogenous ligand of Vγ6+Vδ1+ receptors.
γδ T cells played a role in the autoimmune disease characteristic of mice lacking Aire
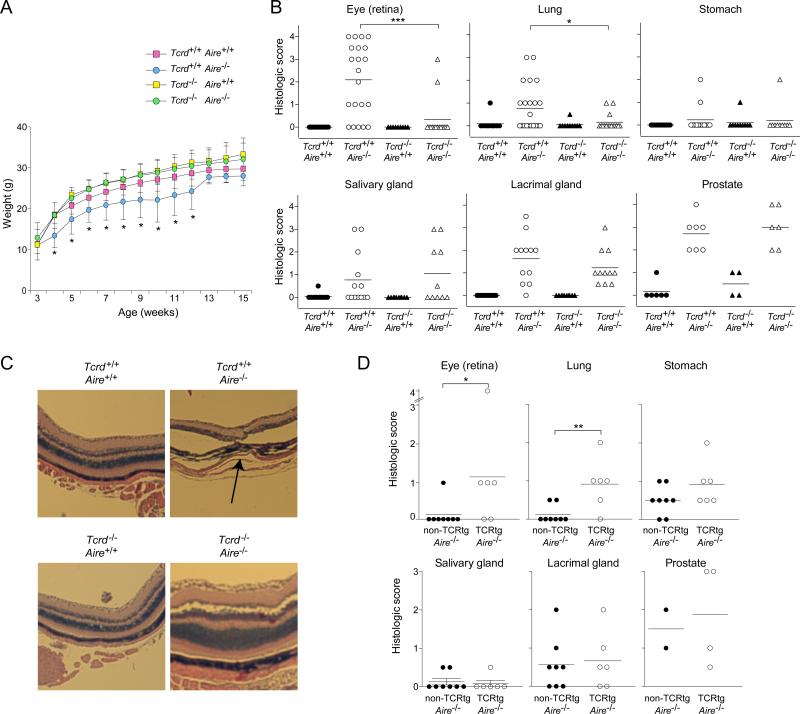
The increase in IL-17A-producing γδ T cells in Aire-KO mice, coupled with reports implicating Tγδ17 cells in the pathogenesis of diverse autoimmune reactions (Cua and Tato, 2010), prompted us to evaluate the role of γδ cells in the Aire-less autoimmune disease. To that end, we generated cohorts of B6 littermates expressing Aire or not and harboring γδ T cells or not (the latter phenotype reflecting the presence or absence of a Tcrd-null mutation).
While the group that lacked only Aire showed reduced weight gain, as usual, mice doubly deficient for Aire and TCRδ did not, their weight curve being indistinguishable from that of the singly TCRδ-deficient and doubly wild-type groups (Fig. 5A). While loss of γδ T cells in addition to Aire almost completely inhibited leukocyte attack on the retina and lung at 15 weeks of age, there was no evident decrease in prostate, lacrimal gland, or salivary gland infiltration (Fig. 5B). Infiltration into the stomach was minimal and not sufficient to draw robust conclusions. Fig. 5C illustrates the improvement in retinal pathology in the doubly-deficient compared with Aire-deficient, TCRδ-sufficient mice.
Fig 5. γδT cells played a role in the autoimmune disease characteristic of Aire-deficient mice.
Littermates carrying a homozygous null mutation of Aire, Tcrd, both or neither on the B6 genetic background were followed until 15 weeks of age, when the indicated organs were taken for histology (n=10 per group). A. Weight curves. B. Histologic scores, employing the scoring system described in Experimental Procedures. Statistics as per Fig. 1. C. Eye histology. Hematoxylin and eosin staining of retinal tissue. Arrow indicates the disrupted retinal layer. D. As per panel B, except mice harboring a homozygous null-mutation of Aire that either did or did not carry a Vγ6+Vδ1+ TCR transgene were followed until 12 weeks of age (n=2-4 for prostate, 6-8 for all other tissues). Statistics as per Fig. 1. (See also Fig. S2)
On the other hand, Aire-KO mice with an over-abundance of Tγδ17 cells showed signs of accelerated autoimmunity when compared with straight Aire−/− individuals. Mice carrying transgenes encoding the canonical Vγ6+Vδ1+ TCR generate both αβ and γδ T cells [line 134 (Sim et al., 1995a; Hayes et al., 2005) (Fig. S2A)]. However, they show a strong enrichment for γδ thymocytes, particularly around birth. We found that essentially all of the Vγ6+Vδ1+ thymocytes in perinatal mice expressed IL-17A in the presence or absence of Aire (Fig. S2B), and that there was an enrichment in γδ T cells in infiltrated organs, compared with transgene-negative counterparts (Fig. S2C). In 12-week-old mice, an age when the first signs of disease generally appear in mice lacking Aire, the Vγ6+Vδ1+ Aire−/− transgenics exhibited stronger infiltrates in certain, but not all, organs than did their transgene-negative Aire−/− littermates (Fig. 5D). The organs with significantly more infiltration (eye and lung) were precisely those whose attack was γδ T cell dependent (Fig. 5B). Therefore, γδ T cells played a role in the Aire-less autoimmune disease with an impact that was most evident in a subset of the organs generally attacked.
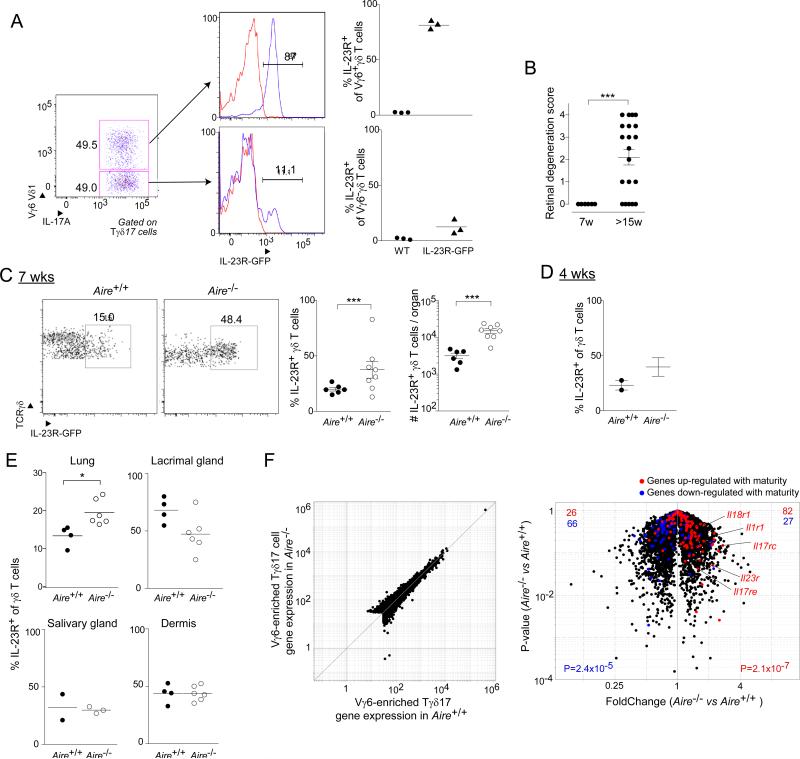
Tγδ17 cells accumulated in the retina before it undergoes autoimmune attack in Aire-deficient mice
A currently popular model for the function of Tγδ17 cells is that they exert their impact on immune and autoimmune responses by acting as “early responders,” promoting the recruitment of neutrophils and driving the polarization of myeloid and lymphocyte (notably, T helper) populations (Cua and Tato, 2010). Would this scenario be correct and generalizable, one might expect Tγδ17 cells to be already installed in tissues subject to autoimmune attack in the Aire-less disease and/or to accumulate in these tissues early during the onslaught. To evaluate this prediction, we focused mainly on the retina because it is a frequently and strongly infiltrated organ in Aire−/− mice on the B6 genetic background (Jiang et al., 2005), and because we found its infiltration to be highly dependent on γδ T cells (Fig. 5B). Robust analysis of the small number of lymphocytes in uveoretinal tissue of young mice was facilitated by use of a reporter line wherein green fluorescent protein (GFP) was expressed under the dictates of Il23r promoter and enhancer elements (Riol-Blanco et al., 2010). Most of the Vγ6+IL-17A+ cells in these mice expressed GFP, while most Vγ6−IL-17A+ and IL-17A−γδ cells did not [Fig. 6A, data not shown, and (Riol-Blanco et al., 2010)]. At 7 weeks of age, retinal degeneration was not detected in Aire−/− mice, although it was readily observed at 15 weeks or later (Fig. 6B). γδ T cells expressing GFP were found at 7 weeks in the presence of Aire, and their number increased about 6-fold in Aire's absence (Fig. 6C). Even at 4 weeks of age, there seemed to be an increase in GFP+ γδ cells in the uveoretinal tissue of Aire−/− mice (Fig. 6D). There was also a significantly increased fraction of GFP+ γδ T cells in the lung, but not in the lacrimal gland, salivary gland, or skin dermis of Aire−/− vis-à-vis -WT mice at 7 weeks of age (Fig. 6E).
Fig. 6. Tγδ17 cells accumulated in the retina prior to autoimmune attack in Aire-deficient mice.
A. IL-23 reporter validation. Flow cytometric analysis of thymic Tγδ17 cells from day-of-birth IL-23R-GFP mice. Left and center panels, typical cytofluorometric plots; right panel, summary data (n=3). B. Histologic scores, as per Experimental Procedures, of eye tissue from Aire−/− mice taken at 7 or 15 weeks of age (n=6-20). C. Cytoflurometric comparison of GFP+ Tγδ17 cells from uveoretinal tissue of 7-week-old Aire+/+ and Aire−/− mice carrying the IL-23R-GFP reporter. Left, typical profiles; right, summary data on fractional representation and number (n=6-8). D. Summary data for 4-week-old mice (n=2-3). E. As per panel C, except cells were isolated from lung, lacrimal gland, or dermis (n=2-3 for salivary gland, 4-6 for all other tissues). F. RNA-seq analysis of gene expression in Vγ1,2,4,5− CD27− (i.e. IL-17+ Vγ6+-enriched) γδ T cells isolated from the lungs of 10-week-old Aire+/+ or Aire−/− mice (n=2). Red- or blue-highlighted genes represent loci two-fold up- or down-regulated during Vγ6+ cell thymic maturation (Narayan et al., 2012), respectively. p-values reflect the significance of signature enrichment as calculated by the χ2 -test. Other statistics as per Fig. 1. (See also Fig. S3). Dot plot scales for this figure are all the same.
The observation of some IL-23R+ γδ T cells in the eye and lung of 7-week-old Aire+/+ mice, at a time point when there is an absence of autoimmune stress, suggested that their presence was not simply a secondary response to local inflammation. This notion was supported by the fact that tissues from mice lacking αβ T cells showed at least as many (and sometimes more) Vγ6-enriched, CD27-negative (i.e. IL-17A-producing) (Ribot et al., 2010; Michel et al., 2012) cells as did their wild-type littermate controls (Fig. S3).
Beyond the greater accumulation of Tγδ17 cells in certain of the tissues targeted in Aire−/− mice, it was possible that these cells also had a different phenotype. For a broad perspective on this point, we performed high-throughput mRNA sequencing (RNA-seq) analysis – on lung rather than eye tissue because the latter yielded insufficient cell numbers for robust analysis. In general, the transcriptomes of Vγ6-enriched Tγδ17 cells from Aire-WT and -KO mice were very similar (Fig. 6F, left panel). However, overlaying a signature indicative of the Vγ6+ subset's maturity (determined as per (Narayan et al., 2012)), revealed that the cells from Aire-KO mice were slightly more mature (Fig. 6F, right panel). In particular, the cells showed heightened expression of several cytokine receptor genes indicative of responsiveness to paracrine IL-17 pathway signals (e.g. Il1r1, IL23r, Il17rc, IL-17re).
In brief, then, Tγδ17 cells infiltrate parenchymal tissues early in life, independent of autoimmune stimulation. They are enriched in certain, but not all, of the tissues targeted in Aire-KO mice. Phenotypic maturation accompanies this numerical increase.
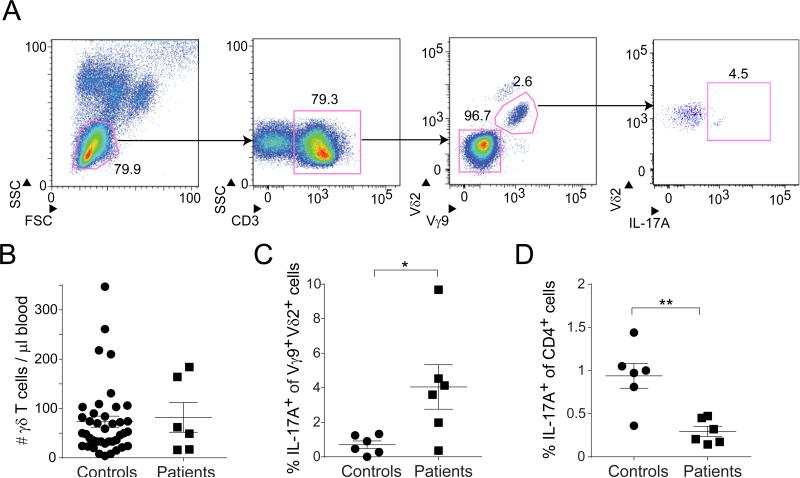
APECED patients had increased frequencies of circulating IL-17+Vγ9+Vδ2+ T cells
To explore the human relevance of these findings, we compared the IL-17+Vγ9+Vδ2+ population in APECED patients and healthy controls. We chose this class of γδ cells because it was recently reported to include most of the IL-17-producing (Caccamo et al., 2011) and perinatally generated (Dimova et al., 2015) γδ T cells in humans. The patients’ clinical features are described in the Experimental Procedures section.
Our cytofluorometric gating strategy is depicted in Fig. 7A. Frequencies of circulating γδ T cells were comparable in patients and controls (Fig. 7B). However, the APECED patients showed an average 6-7-fold enrichment in the IL-17A-producing Vγ9+Vδ2+ population (Fig. 7C). The significance of this difference was heightened by the fact that the other major IL-17A-producing T cell compartment, IL-17+CD4+ cells, was actually reduced in the patients (Fig. 7D). Thus, the absence of Aire appeared to have parallel effects in humans and mice.
Fig. 7. IL-17A+Vγ9+Vδ2+ T cells were enriched in APECED patients.
A. Gating strategy. After initial FSC/SSC gating of peripheral blood mononuclear cells, lymphocytes were gated (left panel), CD3+ T cells selected (center-left), Vγ9+Vδ2+ cells delineated (center-right), and IL-17A-producing cells quantified (right panel). B. Summary data for the absolute number of TCRγδ+CD3+ cells per μl of blood. APECED patients, n=6; healthy donors, n =40. C. Summary data on IL-17A production by PMA+ionomycin-stimulated Vγ9+Vδ2+ T cells (n=6). p, <0.05 via the unpaired t-test with Welch's correction. D. Summary data on IL-17A production by PMA+ionomycin-stimulated CD4+ T cells (n=6). p, <0.01 via the unpaired t-test.
DISCUSSION
In contrast to an earlier report (Tuovinen et al., 2009), we found that Aire had a discernable impact on γδ T cells in both mice and humans. In the absence of Aire, the quasi-invariant population of perinatally generated thymocytes expressing IL-17A and displaying Vγ6+Vδ1+ TCRs was expanded in mice, an increase reflected in the periphery of adults, notably in the skin dermis and eye. Expansion of this thymocyte compartment could be explained by a higher level of IL-7 expression by Aire-deficient mTECs. γδ T cells had clear but delimited effects on the autoimmune disease characteristic of Aire−/− mice, significantly dampening leukocyte infiltration of certain of the tissues usually targeted. In one such tissue, ocular uveoretina, IL-17A+ γδ T cells could be detected prior to and independent of autoimmune infiltration, suggesting an “early-responder” role. Our findings raise several issues that merit deeper discussion.
The first matter concerns the ontogenic timing of Aire's impact on γδ T cells. To the best of our knowledge, this report is the second one demonstrating Aire's influence on a perinatal thymocyte compartment: previously, its promotion of a distinct population of Foxp3+CD4+ Tregs (Yang et al., 2015); currently, its dampening of the IL-17A+Vγ6+Vδ1+ population. This coincidence raises the questions of whether and how the two effects might be related. One possibility is that Aire-dependent up- or down-regulation of the expression of a particular mediator by perinatal mTECs has opposing influences on Tγδ17 and Treg thymocytes or, relatedly, that the sum of Aire-dependent changes in mediator expression has opposite effects. Another possible scenario, suggested by the work of Hayday and colleagues (Pennington et al., 2006), is that Aire's impact on mTEC gene expression somehow alters the ability of the emerging thymocyte compartments in perinatal mice, e.g. CD4+CD8+ cells, to trans-regulate the differentiation of Tγδ17 and Treg cells, inhibiting the former and inducing the latter. Lastly, it is possible that Tγδ17 cells have a negative impact on the production or maintenance of thymic Tregs, via one or a combination of the mediators they secrete [e.g. IL-17A or F, GM-GSF, TNF-α (Riol-Blanco et al., 2010; Petermann et al., 2010)] or through receptor:ligand interactions reflecting co-localization of the two cell-types in the medulla (Roberts et al., 2012). Indeed, Tcrd−/− mice have expanded Treg compartments (Petermann et al., 2010). Also along these lines, IL-23-activated Tγδ17 cells have been reported to inhibit the functions of peripheral Tregs, as well as to repress conversion of conventional T into Treg cells both in vitro and in vivo (Petermann et al., 2010), of particular interest because we found Il23 to be one of the cytokine genes most highly induced in Aire-deficient mTECs (not shown).
The second issue meriting further discussion is IL-7's role in Aire-dependent expansion of the perinatal Tγδ17 compartment, argued by the up-regulation of Il7 transcripts in Aire−/− mTECs and by results from gain- and loss-of-function experiments. Such a role is consistent with previous reports that this cytokine drives the accumulation of IL-17A-expressing, at the expense of IFN-γ-expressing, γδ thymocytes (Michel et al., 2012). Somewhat paradoxically, cortical thymic epithelial cells (cTECs) are the major IL-7-producing thymic stromal cells, and they do not increase production in the absence of Aire [(Ribeiro et al., 2013) and data not shown]. But mice lacking cTECs due to a spontaneous mutation of β5t have a robust IL-17A-expressing Vγ6+ thymocyte compartment (Nitta et al., 2015). Thus, we infer that mTEC-produced IL-7 is acting locally. Co-localization of Tγδ17 cells and mTECs within the thymic medulla, as has been reported (Roberts et al., 2012), would favor such short-range IL-7-mediated crosstalk.
Third, questions about the importance of Tγδ17 cells in the Aire-less disease might be raised because of reports that both APECED patients and Aire-KO mice harbor autoAbs capable of neutralizing IL-17A, IL-17F, and IL-22 (Kisand et al., 2010; Puel et al., 2010). However, in many systems, Tγδ17 cells have been found to play an “early responder” role, accumulating early in target tissues, where they serve to recruit neutrophils, shape the complexion of the response, and perhaps inhibit Tregs (Cua and Tato, 2010; Chien et al., 2014; O'Brien and Born, 2015; Petermann et al., 2010). Thus, their role in the Aire-less disease may be an early one, prior to the generation of anti-IL-17 autoAbs, consistent with their early localization in uveoretinal tissue. In addition, it is possible that the most important IL-17A effects are local, within the target-tissue, and thereby escape autoAb neutralization. It should also be kept in mind that Tγδ17 does not equal IL-17, i.e. these cells make other cytokines (e.g. GM-CSF, TNF-α) and have other immunomodulatory activities that could escape autoAb blockade.
Fourth, while at first glance it appears that Aire's primary influence was on Tγδ17 cell representation in peripheral tissues, other possibilities remained. The transcriptomes of Tγδ17 cells in the lung of Aire+/+ and Aire−/− littermates (prior to evident autoimmune attack) were generally very similar, but the latter showed signs of greater maturation, in particular a heightened ability to respond to TCR-independent stimulants such as IL-1 and IL-23. Vγ6+Vδ1+ TCRs from Aire−/− mice had the canonical Vδ1 and CDR3 sequence less frequently than did those from -WT mice, but this differential was already evident in the thymus and did not increase with age; and thus does not appear to be a local adaptation.
Finally, what is the relevance of our findings for human APECED? γδ T cell biology in mice and humans is known to diverge (Deknuydt et al., 2009). Yet, a population of IL-17-producing Vγ9+Vδ2+ cells was recently evidenced in the pathological lesions of children with meningitis (Caccamo et al., 2011), as were Tγδ17 cells in colorectal tumors (Wu et al., 2014). Relatedly, a semi-invariant population of Vγ9+Vδ2+ cells was found to dominate the γδ compartment of the second-trimester fetus (Dimova et al., 2015). About 30% of these cells were pre-programmed for rapid IFN-γ production; their IL17A was not examined. Interestingly, human APECED patients have been reported to have elevated levels of circulating IL-7 (Laakso et al., 2011). Our finding of Vγ9+Vδ2+, but not CD4+, T cells in APECED patients is highly suggestive. But, as with all such correlations in humans, it is difficult to differentiate cause from effect.
In brief, then, we have discovered another mechanism by which Aire keeps autoimmunity in check: dampening of a population of γδ T cells capable of shaping T effector response within tissues. It is amazing how a single transcription factor can maneuver so many elements of the immune system to guard self-tolerance.
EXPERIMENTAL PROCEDURES
Mice
B6.Aire+/- mice (Anderson et al., 2002) were maintained in our colony at Jackson Laboratory, and were bred to generate Aire+/+ and Aire−/− littermates for experiments. IL-23R-GFP reporter mice (Riol-Blanco et al., 2010) came from Dr. Vijay Kuchroo, Vγ6+Vδ1+ TCRtg mice from Dr. Paul Love (Sim et al., 1995b) and Igrp-Gfp (Adig) mice (Gardner et al., 2008) from Dr. Mark Anderson. B6.Tcrd−/− mice were purchased from Jackson Laboratory, and were appropriately bred to yield Tcrd−/− and Tcrd+/+ littermates that were additionally Aire+/+ or Aire−/−. B6.Tcra−/− and age-matched controls were purchased from Jackson Laboratory. All mice were housed and bred under specific-pathogen-free conditions at the Harvard Medical School Center for Animal Resources and Comparative Medicine (Institutional Animal Care and Use Committee protocol #02954).
Tissue preparations
Single-cell suspensions of thymus, spleen and LNs were prepared according to standard procedures. Protocols for isolation of lymphocytes from skin epidermis and dermis (Jin et al., 2010) and SI-LP (Sefik et al., 2015) have been published.
To analyze eye lymphocyte populations without issues of autofluorescence, we isolated uveoretinal tissue by microscopic dissection. The two eyeballs were removed by cutting the corner of each eye; and the cornea, lens, and the vitreous body was removed under microscope with the aid of superfine scissors and forceps. Uveoretina was minced into tiny pieces in Dulbecco's Modified Eagle's Medium (DMEM) containing 2% fetal bovine serum (FBS), 10mM HEPES, 0.3% collagenase IV (Gibco), and 300 ug/ml DNase I (Roche). The minced material was incubated 1h in a 37°C waterbath with shaking. This same procedure, without microscope dissection, was used to isolate lymphocytes from the lung, lacrimal gland, and salivary gland. Minced lung and salivary gland were incubated for 1h, while lacrimal gland was incubated for 0.5h.
Staining reagents and procedures are detailed in the supplementary materials.
Single-cell TCR sequence analysis
Thymocytes were first sorted in bulk as 17D1+ TCRγδ+ TCRβ– lymphocytes from Aire+/+ or Aire−/− perinates or adults (as indicated in Fig. 3) before resorting as individual cells into 96-well plates containing the reverse-transcriptase reaction mix. This same gating and sorting was used to collect Vγ6+ T cells from the lungs of 5-week-old Aire+/+ or Aire−/− mice. cDNA was prepared as described (Burzyn et al., 2013). Resulting cDNA from each cell was split to perform multiplex nested-PCR reactions to amplify the corresponding CDR3 γ and δ transcripts using the protocol and primers published in (Pereira and Boucontet, 2004). γδ T cells from eyes of mice either 7 or 13-15 weeks of age were identified as CD45+TCRβ–TCRγδ+Vγ1,2,4,5−CD27− to enrich for the Vγ6+IL-17A+ fraction, and were sorted in bulk for resorting as individual cells.
PCR products encoding Vγ6 or Vδ1 chains were subjected to automated sequencing (Dana-Farber/Harvard Cancer Center High-Throughput Sequencing Core) using the following primers; Vγ6, GGAATTCAAAAGAAAACATTGTCT; Cδ, CGAATTCCACAATCTTCTT. Except, an updated primer scheme was used for cDNA synthesis and multiplex nested-PCR for the eye samples. (Paget et al., 2015). Raw sequencing files were filtered for sequence quality, processed in automated fashion, and parsed using IMGT/V-QUEST.
Gene-expression analyses
Microarray data for mTEChi from perinatal and adult Aire+/+ or Aire−/− mice has been published (Yang et al., 2015). For PCR analyses: RNA was isolated with Trizol as per the manufacturer's instructions, and was reverse-transcribed using SuperScript II (Invitrogen). Quantitative PCR was performed using SYBR Green PCR Master Mix and the StepOnePlus real-time PCR system (Applied Biosystems). Relative expression is displayed in arbitrary units normalized to Hprt via the ddCt method. Primer sequences were: Skint-1F, TGAAGAGCACATAACAGAGGTCA; Skint-1R, TCCATTGCATGAGAGGTCGTG; AireF, GTACAGCCGCCTGCATAGC; AireR, CCCTTTCCGGGACTGGTT.
CD45+TCRβ–TCRγδ+Vγ1,2,4,5−CD27− cells (i.e. enriched for the Vγ6+IL-17A+ subset) were double-sorted from Aire+/+ or Aire−/− lung in duplicate. Smart-Seq2 libraries were prepared by the Broad Technology Labs and sequenced by the Broad Genomics Platform (Picelli et al., 2013; Picelli et al., 2014; Trombetta et al., 2014). Transcripts were quantified by the Broad Technology Labs computational pipeline using Cuffquant version 2.2.1 (Trapnell et al., 2012; Kim et al., 2013).
Recombinant IL-7 and anti-IL-7R mAb treatments
Recombinant mouse IL-7 (250 ng/g body weight, Peprotech) or phosphate-buffered saline (PBS) was ip-injected into Aire+/+ perinates on the day of birth; 3 days later, cells were stimulated for intracellular cytokine staining as described in the supplementary materials. Anti-IL-7R (A7R34) or rat IgG isotype control mAb (both from BioLegend) was injected into Aire−/− perinates (25 ug/mouse) on the day of birth; 3 days later, cells were stimulated for intracellular cytokine staining.
Histopathology
Histopathology was assessed as previously described (Koh et al., 2010). Briefly, tissues were fixed in 10% formalin, embedded with paraffin, and stained with hematoxylin and eosin (H&E). Scores of 0, 0.5, 1, 2, 3 and 4 indicate no, trace, mild, moderate, or severe pathology, and complete destruction, respectively. For retinal degeneration: 0 = lesion present without any photoreceptor layer lost; 1 = lesion present, but less than half of the photoreceptor layer lost; 2 = more than half of the photoreceptor layer lost; 3 = entire photoreceptor layer lost without or with mild outer nuclear layer attack; and 4 = the entire photoreceptor layer and most of the outer nuclear layer destroyed. All infiltrated samples were scored blindly by two independent investigators.
Human studies
Patients with APECED and healthy donors were enrolled in protocols approved by the National Institute of Allergy and Infectious Diseases and NIH Clinical Center Institutional Review Board (IRB) committees, and provided written informed consent for participation in the study. The APECED patients’ mean age was 26 years old (range, 7-38). Five were females and one male. Among the classic triad of clinical APECED manifestations: 6 patients had chronic mucocutaneous candidiasis, 5 had adrenal insufficiency and 4 had hypoparathyroidism. Three patients had proven history of keratoconjunctivitis by ophthalmological exam.
For healthy donor enrollment, a pre-screening questionnaire along with a pre-donation assessment was performed to select individuals with no known lung, heart, kidney or bleeding disorders, and without a history of injected drug use or high-risk activities predisposing to HIV infection. The healthy donors’ mean age was 48 years old (range, 29-64). Twenty-four were females and 16 males. This study was conducted in accordance with the Helsinki Declaration.
Cytofluorometric assessments are detailed in the supplementary materials.
Statistical analyses
Data are routinely presented as mean ± SEM. Significance, generally evaluated using Student's t test, was taken to be a ≤ 0.05 level of probability.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Drs V. Kuchroo, P. Love and M. Anderson for mice; Drs R. Tigelaar and J. Lewis for the 17D1 hybridoma; K. Hattori for expert assistance with mice; Dr A. Ortiz-Lopez for histological scoring; the NIH Tetramer Core Facility for provision of tetramer reagents; Dr N. Asinovski for hybridoma culture; and Drs H. Yoshida and S. Yang for insightful discussions. This work was supported by NIH grant R01 DK060027 to D.M. and, in part, by the Division of Intramural Research (DIR), NIAID, NIH. N.F. received fellowships from the Japan Society for the Promotion of Science and the Uehara Memorial Foundation; K.B. from the American Diabetes Association (7-12-MN-51); and A.M. from the NIH (T32 AI118692).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceptualization, N.F., C.B, D.M.; Investigation, N.F., A.O.M., K.B., K.R.R., E.M.N.F., S.D.R.; Writing – Original Draft, N.F., D.M.; Writing – Review & Editing, N.F, A.O.M., K.B., M.S.L., C.B., D.M.; Supervision, M.S.L., C.B., D.M.
REFERENCES
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Barbee SD, Woodward MJ, Turchinovich G, Mention JJ, Lewis JM, Boyden LM, Lifton RP, Tigelaar R, Hayday AC. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011;108:3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, La MC, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournie JJ, Dieli F. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YH, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu. Rev. Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Cui Y, Shao H, Lan C, Nian H, O'Brien RL, Born WK, Kaplan HJ, Sun D. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. J. Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deknuydt F, Scotet E, Bonneville M. Modulation of inflammation through IL-17 production by γδ T cells: mandatory in the mouse, dispensable in humans? Immunol Lett. 2009;127:8–12. doi: 10.1016/j.imlet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, Marchant A, Vermijlen D. Effector Vγ9Vδ2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc Natl Acad Sci U S A. 2015;112:E556–E565. doi: 10.1073/pnas.1412058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, DeVoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, Mathis D, Benoist C. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci U S A. 2012;109:535–540. doi: 10.1073/pnas.1119351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp. Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Li L, Love PE. TCR signal strength influences [.alpha][.beta]/γδ lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–839. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J. Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnnidis JB, Venanzi ES, Taxman DJ, Ting JP, Benoist CO, Mathis DJ. Chromosomal clustering of genes controlled by the aire transcription factor. Proc Natl. Acad Sci U S. A. 2005;102:7233–7238. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Peterson P. Autoimmune Polyendocrinopathy Candidiasis Ectodermal Dystrophy. J. Clin. Immunol. 2015;35:463–478. doi: 10.1007/s10875-015-0176-y. [DOI] [PubMed] [Google Scholar]
- Koh AS, Kingston RE, Benoist C, Mathis D. Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci U S A. 2010;107:13016–13021. doi: 10.1073/pnas.1004436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso SM, Kekalainen E, Rossi LH, Laurinolli TT, Mannerstrom H, Heikkila N, Lehtoviita A, Perheentupa J, Jarva H, Arstila TP. IL-7 dysregulation and loss of CD8+ T cell homeostasis in the monogenic human disease autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J. Immunol. 2011;187:2023–2030. doi: 10.4049/jimmunol.1100212. [DOI] [PubMed] [Google Scholar]
- Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt RW, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J. Exp. Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J. Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- Meredith M, Zemmour D, Mathis D, Benoist C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat Immunol. 2015;16:942–949. doi: 10.1038/ni.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing γδ cells. Proc Natl Acad Sci U S A. 2012;109:17549–17554. doi: 10.1073/pnas.1204327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, Kornfeld H, Xiong N, Cohen NR, Brenner MB, et al. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nat Immunol. 2012;13:511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Muro R, Shimizu Y, Nitta S, Oda H, Ohte Y, Goto M, Yanobu-Takanashi R, Narita T, Takayanagi H, et al. The thymic cortical epithelium determines the TCR repertoire of IL-17-producing γδT cells. EMBO Rep. 2015;16:638–653. doi: 10.15252/embr.201540096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RL, Born WK. Dermal γδ T cells -What have we learned? Cell Immunol. 2015;296:62–69. doi: 10.1016/j.cellimm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–8823. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget C, Chow MT, Gherardin NA, Beavis PA, Uldrich AP, Duret H, Hassane M, Souza-Fonseca-Guimaraes F, Mogilenko DA, Staumont-Salle D, et al. CD3bright signals on gammadelta T cells identify IL-17A-producing Vgamma6Vdelta1+ T cells. Immunol Cell Biol. 2015;93:198–212. doi: 10.1038/icb.2014.94. [DOI] [PubMed] [Google Scholar]
- Pennington DJ, Silva-Santos B, Silberzahn T, Escorcio-Correia M, Woodward MJ, Roberts SJ, Smith AL, Dyson PJ, Hayday AC. Early events in the thymus affect the balance of effector and regulatory T cells. Nature. 2006;444:1073–1077. doi: 10.1038/nature06051. [DOI] [PubMed] [Google Scholar]
- Pereira P, Boucontet L. Rates of recombination and chain pair biases greatly influence the primary gammadelta TCR repertoire in the thymus of adult mice. J. Immunol. 2004;173:3261–3270. doi: 10.4049/jimmunol.173.5.3261. [DOI] [PubMed] [Google Scholar]
- Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Bjorklund AK, Faridani OR, Sagasser S, Winberg G, Sandberg R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- Pitt LA, Hubert FX, Scott HS, Godfrey DI, Berzins SP. NKT cell development in the absence of the autoimmune regulator gene (Aire). Eur. J. Immunol. 2008;38:2689–2696. doi: 10.1002/eji.200838553. [DOI] [PubMed] [Google Scholar]
- Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rei M, Goncalves-Sousa N, Lanca T, Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ, Silva-Santos B. Murine CD27(-) Vγ(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014;111:E3562–E3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AR, Rodrigues PM, Meireles C, Di Santo JP, Alves NL. Thymocyte selection regulates the homeostasis of IL-7-expressing thymic cortical epithelial cells in vivo. J. Immunol. 2013;191:1200–1209. doi: 10.4049/jimmunol.1203042. [DOI] [PubMed] [Google Scholar]
- Ribot JC, Chaves-Ferreira M, d'Orey F, Wencker M, Goncalves-Sousa N, Decalf J, Simas JP, Hayday AC, Silva-Santos B. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-gamma or IL-17-producing gammadelta T cells upon infection. J Immunol. 2010;185:6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, Kuchroo VK, Glimcher LH, Oukka M. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J. Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, et al. Rank signaling links the development of invariant γδ T cell progenitors and Aire+ medullary epithelium. Immunity. 2012;36:427–437. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom SN, Shikama-Dorn N, Zhanybekova S, Nusspaumer G, Macaulay IC, Deadman ME, Heger A, Ponting CP, Hollander GA. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014;24:1918–1931. doi: 10.1101/gr.171645.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato U, Tateishi S, Kubo K, Horikawa R, Mimura T, Yamamoto K, Kanda H. Aire downregulates multiple molecules that have contradicting immune-enhancing and immune-suppressive functions. Biochem. Biophys. Res. Commun. 2004;318:935–940. doi: 10.1016/j.bbrc.2004.04.116. [DOI] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- Sim GK, Olsson C, Augustin A. Commitment and maintenance of the alpha beta and gamma delta T cell lineages. J. Immunol. 1995a;154:5821–5831. [PubMed] [Google Scholar]
- Sim GK, Olsson C, Augustin A. Commitment and maintenance of the alpha beta and gamma delta T cell lineages. J Immunol. 1995b;154:5821–5831. [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, Anderson MS. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci U S A. 2012;109:7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta JJ, Gennert D, Lu D, Satija R, Shalek AK, Regev A. Preparation of single-cell RNA-seq libraries for next generation sequencing. Curr. Protoc. Mol Biol. 2014;107:4–17. doi: 10.1002/0471142727.mb0422s107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuovinen H, Pontynen N, Gylling M, Kekalainen E, Perheentupa J, Miettinen A, Arstila TP. γδ T cells develop independently of Aire. Cell Immunol. 2009;257:5–12. doi: 10.1016/j.cellimm.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-γ-secreting versus Interleukin-17-secreting γδ T cells. Immunity. 2011 doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. γδ T17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, Kuhns MS, Waters RW, Davis MM, Weaver CT, et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.