Abstract
Mammals use endogenously produced heat to maintain a high and relatively constant core body temperature (Tb). How they regulate their Tb during reproduction might inform us as to what thermal conditions are necessary for optimal development of offspring. However, few studies have measured Tb in free-ranging animals for sufficient periods of time to encounter reproductive events. We measured Tb continuously in six free-ranging adult female African lions (Panthera leo) for approximately 1 year. Lions reduced the 24 h amplitude of Tb by about 25% during gestation and decreased mean 24 h Tb by 1.3 ± 0.1°C over the course of the gestation, reducing incidences of hyperthermia (Tb > 39.5°C). The observation of improved homeothermy during reproduction may support the parental care model (PCM) for the evolution of endothermy, which postulates that endothermy arose in birds and mammals as a consequence of more general selection for parental care. According to the PCM, endothermy arose because it enabled parents to better control incubation temperature, leading to rapid growth and development of offspring and thus to fitness benefits for the parents. Whether the precision of Tb regulation in pregnant lions, and consequently their reproductive success, will be influenced by changing environmental conditions, particularly hotter and drier periods associated with climate change, remains to be determined.
Keywords: endothermy, evolution, Panthera leo, parental care
1. Introduction
Reproduction is regarded as a particularly costly period of life, with gestation and lactation often requiring the most resources and potentially leading to energetic trade-offs [1]. Measuring how adult females allocate resources during these phases might help us understand how and why different homeostatic processes are prioritized, particularly under free-ranging conditions which can vary unpredictably and in which resources are limited. We measured the Tb and monitored the life histories of six free-ranging lionesses over a sufficient period to encounter pregnancies—approximately 1 year. During this time, three lionesses experienced pregnancies and gave birth to litters of cubs, and three did not, allowing us to compare Tb patterns in lionesses before, during and after pregnancies, and between lionesses that did, and did not, experience pregnancy.
2. Material and methods
We measured the Tb of six adult free-ranging lionesses, by surgically implanting miniature temperature bio-loggers (DST centi loggers, Star Oddi, Gardabaer, Iceland) between the parietal peritoneum and the transversus abdominis muscle. Bio-loggers were removed approximately 1 year later. During our study, three lionesses gave birth to litters of cubs (three cubs each), and three did not. All study animals were monitored frequently by direct observation throughout the study.
We used the statistics program R [2] and package ‘nlme’ [3] to perform a linear mixed effects analysis of the relationship between 24 h amplitude of Tb and reproductive status. The 24 h amplitude of Tb was defined as the difference between the maximum and minimum 24 h Tb. We recognized three reproductive states: pregnant, non-pregnant and lactating. Non-pregnant is taken to mean non-pregnant and non-lactating. We included 24 h dry-bulb temperature range and reproductive status (without an interaction term) as fixed effects in the model. As random effects, we had intercepts for individual. The 24 h amplitude of Tb was likely to be temporally auto-correlated so we included a correlation structure with an autoregressive process of order 1. P-values were obtained by likelihood ratio tests of the full model that contained the effect in question (reproductive status), against a null model that did not include the effect in question. We used multcomp [4] to perform a post hoc multiple variable comparison to determine in which reproductive states 24 h amplitude of Tb differed. Linear mixed effects analysis was also used to determine the relationship between mean 24 h Tb and ‘pregnancy day’ (the number of days into a gestation), and only data recorded during gestation were included in the analysis. We included mean 24 h dry-bulb temperature and pregnancy day as fixed effects, and included individuals as random intercepts, with an autoregressive correlation structure of order 1. Once again, p-values were obtained by likelihood ratio tests of the full model with the effect in question (pregnancy day), against a null model that did not include the pregnancy day. Raw data are accessible online [5].
3. Results
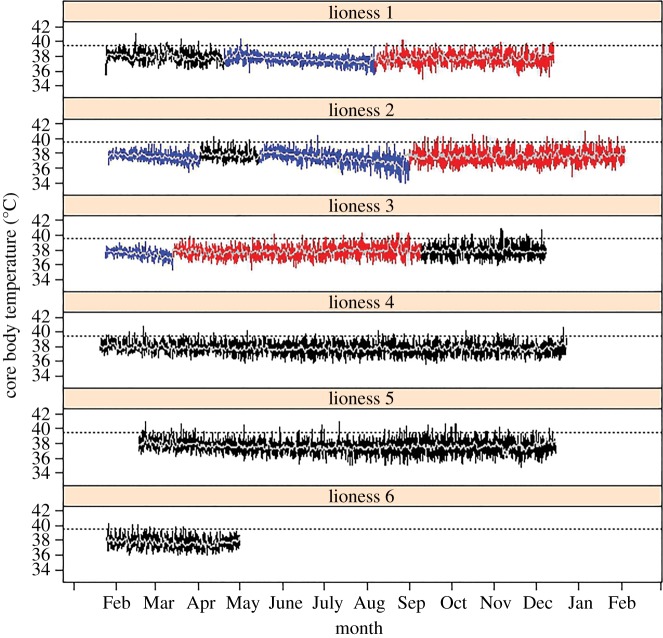
Mean female 24 h Tb was 37.7 ± 0.1°C (1 s.d. calculated between individual means) outside of pregnancy. Pregnancy day had an effect on mean 24 h Tb (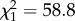 , p < 0.0001). In a 108-day gestation, Tb decreased by 1.3 ± 0.1°C (approximately 0.01°C per day, s.d. estimated by model, figure 1). Reproductive status affected 24 h amplitude of Tb (
, p < 0.0001). In a 108-day gestation, Tb decreased by 1.3 ± 0.1°C (approximately 0.01°C per day, s.d. estimated by model, figure 1). Reproductive status affected 24 h amplitude of Tb (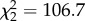 , p < 0.0001), which was approximately 25% lower during pregnancy (mean = 1.7 ± 0.3°C) than when females were lactating (mean = 2.4 ± 0.3°C; z = 10.8, p < 0.0001) or not pregnant (mean = 2.2 ± 0.3°C; z = 7.9, p < 0.0001). Mean 24 h amplitude of Tb was not different between non-pregnant and lactating (z = 1.7, p = 0.2) reproductive states. Reduced 24 h amplitude of Tb during pregnancy indicates that lionesses improved homeothermy during pregnancy compared with non-pregnant and non-lactating periods. Incidences of hyperthermia (Tb > 39.5°C) occurred less frequently in females during pregnancy than when they were lactating or non-pregnant (figure 1).
, p < 0.0001), which was approximately 25% lower during pregnancy (mean = 1.7 ± 0.3°C) than when females were lactating (mean = 2.4 ± 0.3°C; z = 10.8, p < 0.0001) or not pregnant (mean = 2.2 ± 0.3°C; z = 7.9, p < 0.0001). Mean 24 h amplitude of Tb was not different between non-pregnant and lactating (z = 1.7, p = 0.2) reproductive states. Reduced 24 h amplitude of Tb during pregnancy indicates that lionesses improved homeothermy during pregnancy compared with non-pregnant and non-lactating periods. Incidences of hyperthermia (Tb > 39.5°C) occurred less frequently in females during pregnancy than when they were lactating or non-pregnant (figure 1).
Figure 1.
Reproductive state and Tb of lionesses. Raw Tb data recorded every 5 min for each lioness included in the study coloured by reproductive state (blue, pregnant; red, lactating; black, non-pregnant and non-lactating). The light grey line indicates the trend in Tb. The temperature logger in lioness 6 did not produce reliable data after the beginning of May.
4. Discussion
Improved homeothermy, and a continuous decline in mean Tb, which we refer to as gestational hypothermia [6], appear to be thermal characteristics of gestation in lions. As far as we are aware, our study is the first to demonstrate gestational hypothermia in free-ranging mammals, with mean 24 h Tb 1.3°C lower at the end of pregnancy. Over the same period, there was no change in Tb of non-pregnant lions. The clear trend of decreasing mean Tb in lionesses began in the first trimester (figure 1), earlier than in reports of gestational hypothermia in laboratory animals [7–9]. In addition to the decline in mean Tb, pregnant lions also regulated Tb with greater precision over 24 h, with the mean amplitude of Tb 0.6°C lower than that when non-pregnant or lactating.
Gestational hypothermia has been observed in a variety of species under laboratory conditions [7–11], and appears to represent a regulated reduction in Tb rather than an inability to regulate a high Tb. The threshold temperature for initiating cooling mechanisms was reduced in pregnant compared with non-pregnant rats, indicating that pregnant rats defended a lower Tb [12]. Pregnant rats, like non-pregnant rats, also did not select a warmer microclimate when it was made available to them [7]. In rats, a central angiotensin AT1 receptor mediated mechanism appears to play a role in generating the gestational hypothermia [11].
The improved homeothermy during pregnancy is likely the result of increased thermoregulatory effort, by a combination of physiological and behavioural mechanisms. Maintaining a narrow 24 h range of Tb during pregnancy requires both food energy and water [13]. The requirement of extra resources makes improving homeothermy a costly process and a Darwinian argument posits that there should be some corresponding gain in fitness, or selection would act against this waste of resources. One advantage of improved homeothermy during gestation might be accelerated offspring development, resulting from rapid cell division occurring in conditions that better approximate the optimum temperature for cell division. For large endothermic mammals that regulate Tb at a high temperature (usually between approx. 36°C and 39°C [13]), further increases in Tb to accelerate offspring development are not feasible. Rather, to accelerate offspring development, large mammals may focus on spending more time at the optimum temperature for development by increasing thermoregulatory effort during gestation, as we see here in lions.
Lions have a particularly strong incentive to accelerate offspring development. Male lions dominate a pride of females for only a short period (about 2 years [14]), during which they must sire and raise cubs to maturity. If cubs have not reached independence by the time incumbent males are forced out, the cubs face a high likelihood of being killed by incoming males [15,16]. The imminent threat of infanticide gives females a strong incentive to achieve rapid fetal development as this will reduce the time between the date on which cub(s) are conceived and the date at which they reach independence. As cubs that reach independence faster are less likely to be exposed to and killed by infanticidal males, this may explain why lionesses invest additional resources to increase thermoregulatory control during gestation.
If rapid fetal development is the primary adaptive advantage of improved homeothermy during gestation, as we suggest, then the presence of improved homeothermy during gestation in lions would support the parental care model (PCM) for the evolution of endothermy. The PCM, as proposed by Farmer [17], seeks to explain the convergent evolution of endothermy as a by-product of more general selection for parental care. According to the PCM, endothermy would have enabled parents to better control incubation temperature, leading to rapid growth and development of offspring and thus to fitness benefits for the parents.
Support for the PCM has been found in tenrecs, primitive eutherian mammals, which were more homeothermic during pregnancy than at other times [18]. Tighter regulation of Tb during near-term gestation has also been demonstrated in echidnas (Tachyglossus aculeatus), bats (Eptesicus fuscus) and dunnarts (Sminthopsis macroura) [19], and more recently support for the PCM has been found in the remarkable discovery of reproductive endothermy in a small (approx. 2 kg) reptile (tegu lizard; Salvator merianae) [20,21]. Although data on free-ranging large mammals during reproduction are scarce, if the PCM does explain increased homeothermy during gestation in lions, we might expect to observe it also in other species. However, evidence in the literature is mixed. Bears practise delayed implantation (embryonic diapause) and pregnancy occurs during hibernation [22], making their thermal biology complex. However, free-ranging bears (Ursus arctos) also displayed improved homeothermy during the gestation period [22]. In springbok (Antidorcas marsupialis), neither homeothermy nor gestational hypothermia was evident in pregnant females [23]. However, the mean 24 h amplitude of Tb in springbok was only 1.2°C, and further decreases in amplitude of Tb may only be possible at a prohibitive cost.
Gestational hypothermia is also likely to facilitate fetal development. Fetal Tb is necessarily higher than maternal Tb, because the metabolically active and rapidly dividing fetal cells generate more heat than those of the mother, and fetal Tb has been found to be approximately 0.5°C higher than maternal Tb for all species measured so far [9]. Cell division has been found to cease at temperatures higher than 40°C [24], and developing fetuses are vulnerable to damage by episodes of hyperthermia [25]. Gestational hypothermia therefore might be necessary to protect the fetus from damage caused by episodes of hyperthermia. A lower maternal body temperature may also protect the fetus from hypoxia by reducing metabolism, and therefore oxygen demand in the mother, or by causing a leftward shift in the oxyhaemoglobin dissociation curve—increasing oxygen affinity and saturation as a result [6].
Thermoregulatory responses of free-ranging animals exposed to complex stressors differ from those of captive animals [26], and insights from free-living animals are critical if we are to understand how large mammals might respond to future changing climates [27]. This study highlights the importance of thermoregulatory control and protection from hyperthermia in lions (and potentially other large carnivores) during gestation, and has implications for conservation initiatives. For example, large carnivores living in more extreme future environments may be able to cope with normal daily thermoregulatory demands but might fail to reproduce successfully.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the shareholders of Bubye Valley Conservancy (BVC) for allowing us to conduct the research, and the BVC management team, particularly Blondie Leathem, for practical field advice and assistance. We also acknowledge Dallas Safari club for their support of BVC. Paul Trethowan also thanks Dr Megan Masters for consultation concerning the manuscript.
Ethics
The study received approval from the University of Oxford Animal Welfare and Ethical Review Board and the University Veterinary Services Department, and from the Animal Ethics Screening Committee of the University of the Witwatersrand (clearance certificate 2013/54/04).
Data accessibility
Miniature bio-logger temperature data: Dryad, http://dx.doi.org/10.5061/dryad.32nm0 [5].
Authors' contributions
P.D.T. participated in study design, carried out the fieldwork, did the data analysis and drafted the manuscript. A.F. participated in study design, data interpretation and critically revised the manuscript. A.H. participated in fieldwork and data acquisition and critiqued the manuscript. T.H. contributed towards data analysis and interpretation and revision of the manuscript. A.J.L. and D.W.M. contributed to study conception, design and critically revised the manuscript. All authors gave final approval for publication and agreed to be held accountable for the content of this manuscript.
Competing interests
We have no competing interests.
Funding
This research was funded by: the Wildlife Conservation Research Unit (WildCRU) of the Zoology Department, University of Oxford, with the core support of the Recanti-Kaplan Foundation and Panthera, University of Witwatersrand and the National Research Foundation, South Africa.
References
- 1.Archie EA, Altmann J, Alberts SC. 2014. Costs of reproduction in a long-lived female primate: injury risk and wound healing. AAPG Bull. 68, 1183–1193. ( 10.1007/s00265-014-1729-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org (accessed 10 April 2016). [Google Scholar]
- 3.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team. 2015. nlme: linear and nonlinear mixed effects models. R package version 3.1-120 Vienna, Austria: R Foundation for Statistical Computing; See http://CRAN.R-project.org/package=nlme (accessed 20 February 2015). [Google Scholar]
- 4.Hothorn T, Bretz F, Westfall P. 2016. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 5.Trethowan P, Hart T, Loveridge AJ, Haw A, Fuller A, Macdonald DW. 2016. Data from: Improved homeothermy and hypothermia in African lions during gestation. Dryad Digital Repository. ( 10.5061/dryad.32nm0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozak W. 1997. Regulated decreases in body temperature. In Fever: basic mechanisms and management, 2nd edn (ed. PA Mackowiak), pp. 467–478. Philadelphia, PA: Lippincott-Raven. [Google Scholar]
- 7.Eliason HL, Fewell JE. 1997. Thermoregulatory control during pregnancy and lactation in rats. J. Appl. Physiol. 83, 837–844. [DOI] [PubMed] [Google Scholar]
- 8.Fewell JE. 1995. Body temperature regulation in rats near term of pregnancy. Can. J. Physiol. Pharmacol. 73, 364–368. ( 10.1139/y95-046) [DOI] [PubMed] [Google Scholar]
- 9.Laburn HP, Mitchell D, Goelst K. 1992. Fetal and maternal body temperatures measured by radiotelemetry in near-term sheep during thermal stress. J. Appl. Physiol. 72, 894–900. [DOI] [PubMed] [Google Scholar]
- 10.Concannon PW, Hansel W. 1977. Prostaglandin F 2α induced luteolysis, hypothermia, and abortions in beagle bitches. Prostaglandins 13, 533–542. ( 10.1016/0090-6980(77)90030-2) [DOI] [PubMed] [Google Scholar]
- 11.Cairns MJ, Burns P, Di Nicolantonio R, McKinley MJ, Mathai ML. 2005. Influence of brain angiotensin on thermoregulation and hydromineral balance during pregnancy in rats. J. Appl. Physiol. 98, 1813–1819. ( 10.1152/japplphysiol.00842.2004) [DOI] [PubMed] [Google Scholar]
- 12.Wilson NE, Stricker EM. 1979. Thermal homeostasis in pregnant rats during heat stress. J. Comp. Physiol. Psychol. 93, 585 ( 10.1037/h0077578) [DOI] [PubMed] [Google Scholar]
- 13.Hetem RS, Maloney SK, Fuller A, Mitchell D. 2016. Heterothermy in large mammals: inevitable or implemented? Biol. Rev. 91, 187–205. ( 10.1111/brv.12166) [DOI] [PubMed] [Google Scholar]
- 14.Pusey AE, Packer C. 1987. The evolution of sex-biased dispersal in lions. Behaviour 101, 275–310. ( 10.1163/156853987X00026) [DOI] [Google Scholar]
- 15.Packer C, Pusey AE. 1983. Adaptations of female lions to infanticide by incoming males. Am. Nat. 121, 716–728. ( 10.1086/284097) [DOI] [Google Scholar]
- 16.Macdonald DW, Loveridge AJ. 2010. Biology and conservation of wild felids. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Farmer C. 2000. Parental care: the key to understanding endothermy and other convergent features in birds and mammals. Am. Nat. 155, 326–334. ( 10.1086/303323) [DOI] [PubMed] [Google Scholar]
- 18.Levesque DL, Lovegrove BG. 2014. Increased homeothermy during reproduction in a basal placental mammal. J. Exp. Biol. 217, 1535–1542. ( 10.1242/jeb.098848) [DOI] [PubMed] [Google Scholar]
- 19.Nicol S, Andersen NA. 2006. Body temperature as an indicator of egg-laying in the echidna, Tachyglossus aculeatus. J. Therm. Biol 31, 483–490. ( 10.1016/j.jtherbio.2006.05.001) [DOI] [Google Scholar]
- 20.Farmer CG. 2016. Evolution: a lizard that generates heat. Nature 529, 470–472. ( 10.1038/529470a) [DOI] [PubMed] [Google Scholar]
- 21.Tattersall GJ, Leite CA, Sanders CE, Cadena V, Andrade DV, Abe AS, Milsom WK. 2016. Seasonal reproductive endothermy in tegu lizards. Sci. Adv. 2, e1500951 ( 10.1126/sciadv.1500951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friebe A, Evans AL, Arnemo JM, Blanc S, Brunberg S, Fleissner G, Swenson JE. 2014. Factors affecting date of implantation, parturition, and den entry estimated from activity and body temperature in free-ranging brown bears. PLoS ONE 9, e101410 ( 10.1371/journal.pone.0101410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller A, Kamerman PR, Maloney SK, Matthee A, Mitchell G, Mitchell D. 2005. A year in the thermal life of a free-ranging herd of springbok Antidorcas marsupialis. J. Exp. Biol. 208, 2855–2864. ( 10.1242/jeb.01714) [DOI] [PubMed] [Google Scholar]
- 24.López-Sáez J, Gimenez-Martin G, González-Fernández A. 1966. Duration of the cell division cycle and its dependence on temperature. Z. Zellforschung Mikroskopische Anatomie 75, 591–600. ( 10.1007/BF00341516) [DOI] [PubMed] [Google Scholar]
- 25.Laburn HP. 1996. How does the fetus cope with thermal challenges? Physiology 11, 96–100. [Google Scholar]
- 26.Mitchell D, Maloney SK, Jessen C, Laburn HP, Kamerman PR, Mitchell G, Fuller A. 2002. Adaptive heterothermy and selective brain cooling in arid-zone mammals. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 131, 571–585. ( 10.1016/S1096-4959(02)00012-X) [DOI] [PubMed] [Google Scholar]
- 27.Lovegrove BG. 2012. The evolution of endothermy in Cenozoic mammals: a plesiomorphic-apomorphic continuum. Biol. Rev. 87, 128–162. ( 10.1111/j.1469-185X.2011.00188.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Miniature bio-logger temperature data: Dryad, http://dx.doi.org/10.5061/dryad.32nm0 [5].