Abstract
Objective:
To assess the relationship between neighborhood socioeconomic characteristics and incident stroke in a national cohort of black and white participants.
Methods:
The study comprised black (n = 10,274, 41%) and white (n = 14,601) stroke-free participants, aged 45 and older, enrolled in 2003–2007 in Reasons for Geographic and Racial Differences in Stroke (REGARDS), a national population-based cohort. A neighborhood socioeconomic score (nSES) was constructed using 6 neighborhood variables. Incident stroke was defined as first occurrence of stroke over an average 7.5 (SD 3.0) years of follow-up. Proportional hazards models were used to estimate associations between nSES score and incident stroke, adjusted for demographics (age, race, sex, region), individual socioeconomic status (SES) (education, household income), and other risk factors for stroke.
Results:
After adjustment for demographics, compared to the highest nSES quartile, stroke incidence increased with each decreasing nSES quartile. The hazard ratio (95% confidence interval) ranged from 1.28 (1.05–1.56) in quartile 3 to 1.38 (1.13–1.68) in quartile 2 to 1.56 (1.26–1.92) in quartile 1 (p < 0.0001 for linear trend). After adjustment for individual SES, the trend remained marginally significant (p = 0.085). Although there was no evidence of a differential effect by race or sex, adjustment for stroke risk factors attenuated the association between nSES and stroke in both black and white participants, with greater attenuation in black participants.
Conclusions:
Risk of incident stroke increased with decreasing nSES but the effect of nSES is attenuated through individual SES and stroke risk factors. The effect of neighborhood socioeconomic characteristics that contribute to increased stroke risk is similar in black and white participants.
Social and economic characteristics of neighborhoods have been identified as important factors in health, including cardiovascular disease, stroke, and mortality.1–8 The literature is generally consistent in showing that those living in economically disadvantaged neighborhoods have poorer health. However, many studies were cross-sectional, limiting interpretability.9 Recently, studies exploring long-term implications of neighborhood exposures on health outcomes have been reported.10–12
While much attention has focused on the impact of individual socioeconomic status (SES) on risk of stroke,11,13 there is limited research on the relationship between neighborhood socioeconomic characteristics (nSES) and stroke risk. Most nSES studies have been restricted to stroke mortality or have not accounted for individual-level SES or modifiable stroke risk factors.6,11,13–16 Further, most studies in the United States have not been national, using data from only a few communities.7,15–17
In US studies where nSES has been used as a proxy for individual SES, living in socioeconomically disadvantaged neighborhoods confers an increased risk of incident stroke in most7,15,16 but not all studies.1 In few of these was it possible to determine whether nSES contributes to stroke risk independent of individual SES. Furthermore, some studies reported differential association by sex or race groups.6,7,15,18,19 Here we examine the relationship of nSES, individual-level SES, and stroke risk factors with stroke incidence in a large, longitudinal US cohort including black and white participants across different age, sex, and SES groups living in diverse SES neighborhoods.
METHODS
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a longitudinal, population-based cohort study designed to identify factors associated with higher stroke mortality among black participants and residents of the stroke belt region, the 8 southern states of North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas.20,21 Additionally, within the stroke belt, a buckle region along the coastal plains of North Carolina, South Carolina, and Georgia has been identified with even higher stroke mortality.22 By design, black participants and residents of the stroke belt were oversampled with enrollment of 30,239 community-dwelling individuals, age 45 years and older, between 2003 and 2007. Twenty-one percent of the sample was from the stroke buckle, 35% from the rest of the stroke belt, and the remaining 44% from the other 40 contiguous states. Residents were sampled from 1,833 counties, approximately 60% of the counties in the continental United States. A map showing the distribution of participants' state of residence at time of enrollment has been previously published.23 A commercially available list was used to identify individuals who were contacted by mail, followed by telephone. Through a computer-assisted telephone interview (CATI), trained interviewers obtained demographic information, medical history, and lifestyle factors, including a selection of risk factors. Between 3 to 4 weeks after the CATI, a brief physical examination was conducted including height and weight, blood pressure measurements, blood and urine samples, and an ECG. Follow-up is by CATI every 6 months for suspected stroke (or proxy-reported in case of participants unable to respond). Additional methodologic details are provided elsewhere.21,23
Standard protocol approvals, registrations, and participant consents.
Consent was obtained initially by telephone and later in writing during the in-person evaluation. The study methods were approved by the institutional review boards of participating institutions.
Primary exposure: nSES.
Participants were linked to neighborhood of residence by home address at baseline. Addresses were geocoded using SAS/GIS batch geocoding, described in detail elsewhere.24 Only results where geocoding could match an address to a longitude/latitude with 80% or higher probability were included. A subset of results was validated against a commercially available program and found high agreement between the algorithms. The geocoded addresses were linked to the appropriate residential census block in the 2000 US census. Given their small spatial and population size, census block groups can be used as a proxy for neighborhood, and their characteristics have been shown to be robust predictors of health.25,26 A summary nSES index variable was created based on previously published methods, including 6 variables representing wealth/income, education, and occupation: (1) log of median household income, (2) log of median value of owner-occupied housing units, (3) proportion of households receiving interest, dividend, or net rental income, (4) proportion of adults aged ≥25 years with a high school diploma, (5) proportion of adults aged ≥25 years with college degree, and (6) proportion of people employed in executive, managerial, or professional occupations.3,25 The summary nSES score, constructed by summing Z scores for these variables, ranged from −11.8 to 29.0, with increasing values indicating higher nSES. Quartile 4 represents the highest nSES (most advantaged neighborhoods) and quartile 1 the lowest.
Covariates.
Individual-level SES factors, defined by annual household income and education, were used as covariates in addition to risk factors in the Framingham Stroke Risk Score (FSRS), i.e., age, sex, systolic blood pressure, use of antihypertensive medications, smoking, history of heart disease, diabetes, left ventricular hypertrophy (LVH), and atrial fibrillation. Age, race, sex, use of antihypertensive therapy, smoking (current vs not), annual household income (below $20,000/year, $20,000–$34,000/year, $35,000–$74,000/year, $75,000/year or above, refused), and education level (< high school, high school graduate, some college, ≥ college graduate) were classified according to self-report. After the participant was seated for 5 minutes, 2 blood pressure measurements were taken by a trained technician using a standard protocol and averaged. Heart disease history was defined as self-reported myocardial infarction (MI), coronary artery bypass surgery, coronary angioplasty or stenting, or ECG evidence of MI. Diabetes was defined as fasting glucose level ≥126 mL/dL (or if participant was nonfasting, ≥200 mL/dL), or self-reported medication use for glucose control. LVH was determined by centrally read ECG. Atrial fibrillation was based on self-reported history of physician diagnosis or ECG.
Stroke outcome.
Methods of determination of incident stroke have previously been reported.23 During follow-up, participants or a proxy were queried for possible stroke, transient ischemic attack (TIA), death, hospitalization, or emergency department visit. Report of death, potential stroke, TIA, brain aneurysm, brain hemorrhage, stroke symptoms, or unknown reason for hospitalization generated a request for retrieval of medical records. Initial review of records was conducted by a senior stroke nurse to exclude obvious nonstroke; then records of participants with suspected stroke were centrally adjudicated by physicians. Death certificates or proxy interviews were adjudicated for deaths with no medical records. Stroke events used the WHO definition.27 “Clinical strokes” were events not meeting this definition but with symptoms lasting >24 hours with neuroimaging consistent with acute ischemia or hemorrhage. “Probable stroke” was defined when adjudicators agreed the event was likely a stroke but insufficient information was available to meet other classifications. This analysis included WHO-defined, clinical, and probable stroke. Because risk factors such as hypertension play a dominant role in both ischemic and hemorrhagic stroke, adjustment for risk factors would be likely to play a similar role, and as such, our outcome included both subtypes. Reported events as of October 2015 were included.
Statistical analyses.
Demographics, nSES variables, nSES score, and risk factors were described as mean (SD) and n (%) as appropriate, by nSES quartile. Cox proportional hazards analysis was used to examine hazard ratios (HR) and 95% confidence intervals (95% CI) for incident stroke associated with quartiles of nSES score, with the 4th quartile used as the reference. The proportional hazards assumption was assessed and no major concerns arose. Models were adjusted for age, sex, race, and region (demographic model), further adjusted for individual-level SES (education and household income), and then for components of the FSRS. Because many census blocks (over 50%) include a small number of participants, hierarchical analysis was not feasible or appropriate. Adjusted models included an age by race interaction because of the documented higher stroke incidence for black than white participants at younger ages.23 Sensitivity analysis restricted to ischemic stroke was performed. Tests for interaction were conducted between (1) race and nSES, (2) race and sex, and (3) race, sex, and nSES. Evidence of interaction was determined by an a priori α level of 0.10. A priori, we hypothesized a differential effect in black and white participants as found in the US Cardiovascular Health Study (CHS),7 but the interaction was not significant (p = 0.94); however, for comparison purposes, race-stratified analyses were conducted. Also, because previous studies of stroke and nSES provided evidence for differential associations by sex,6,15,18,19 we conducted a 3-way interaction test among race, sex, and nSES; it was not significant (p = 0.54).
RESULTS
The analysis cohort comprised 24,875 participants (10,274, 41% black), excluding those with no follow-up (2%), self-reported stroke at baseline (6%), and missing geocoded address (9%). Those in more SES advantaged neighborhoods were older, more likely to be men, less likely to be black, less likely to live in the stroke belt/buckle, more likely to have higher individual SES, and less likely to have stroke risk factors (table 1).
Table 1.
Study population characteristics by quartile of neighborhood socioeconomic characteristics
During a mean follow-up of 7.5 years (SD 3.0), 929 first-ever strokes occurred, 49% in women, and 43% in black participants. In the unadjusted model, compared to the highest nSES quartile, stroke incidence increased with each decreasing nSES quartile (table 2). The HR (95% CI) ranged from 1.27 (1.05–1.54) in quartile 3 to 1.38 (1.14–1.68) in quartile 2 to 1.60 (1.33–1.93) in quartile 1. There was a linear trend for increasing stroke risk with decreasing nSES (p < 0.0001). The HRs changed only slightly and the association remained after adjustment for demographics and the age × race interaction. Adjustment for individual SES substantially attenuated (≈50%) the association of nSES with stroke risk, e.g., reducing the HR for the lowest nSES quartile from 1.56 (1.26–1.92) to 1.25 (0.99–1.56); however, the trend for increasing stroke risk with decreasing nSES remained marginal (p = 0.085). The HR ranged from 1.15 (0.94–1.40) in quartile 3 to 1.16 (0.95–1.44) in quartile 2 to 1.25 (0.99–1.56) in quartile 1. There was additional attenuation of the HRs after adjustment for stroke risk factors, reducing the HR for the lowest nSES quartile to 1.13 (0.89–1.42), and the test for linear trend became nonsignificant (p = 0.45). Results restricted to only ischemic stroke were similar (table e-1 at Neurology.org).
Table 2.
Association between incident stroke and neighborhood socioeconomic status (nSES)
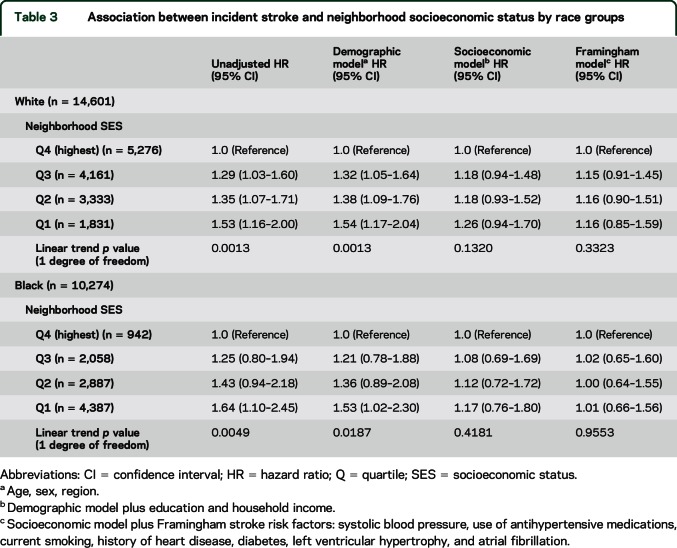
Results for black and white participants did not differ, with a monotonic increase in risk with lower nSES in both groups (table 3). However, for white participants, there was a greater risk of stroke in all 3 of the lower quartiles of nSES, while for black participants there was a higher stroke risk only in the lowest nSES quartile. This pattern remained after adjustment for demographic factors. As the combined group showed, the associations were substantially attenuated after adjustment in the individual SES model, with further attenuation in the risk factor model. The adjustment for individual SES and risk factors had a greater impact in black than white participants.
Table 3.
Association between incident stroke and neighborhood socioeconomic status by race groups
DISCUSSION
In this longitudinal study of black and white participants living across the United States, we found that residents in neighborhoods with lower socioeconomic characteristics had a higher risk of stroke compared to residents in neighborhoods with higher socioeconomic characteristics, with an increase in stroke risk with each lower quartile of nSES. This higher stroke risk remained after adjustment for age, race, sex, and an age-by-race interaction. Although the association between nSES and incident stroke was no longer significant after further adjustment for individual SES, the trend was marginally significant. The association was further weakened by adjustment for stroke risk factors. This indicates that the worse cardiovascular risk factor profile of residents in lower nSES neighborhoods may be in the pathway of the association of nSES with stroke risk.
This is consistent with evidence showing that factors associated with living in more disadvantaged neighborhoods contribute to stroke risk. However, it is difficult to separate the influence of individual SES from nSES. Individual risk factors are often intertwined with neighborhood environment. Neighborhood residence may contribute to development of stroke risk through built environment, access to care, exposure to stressors such as racial discrimination, high level of unemployment or underemployment, available choices for schooling, availability of fresh produce, and social norms influencing dietary patterns and other health behaviors and lifestyle choices. Residents living in areas of low nSES have been shown to have higher levels of unhealthy behaviors such as smoking and physical inactivity and higher prevalence of cardiovascular risk factors such as diabetes, hypertension, and obesity.28–32 Thus, these findings support ongoing calls for community development policies, urban planning, and zoning and transportation policies that address neighborhood socioeconomic contexts to improve residential environments in order to positively impact the health of the community members.10,12
Previous epidemiologic studies of incident stroke have provided evidence for an inverse association between nSES and risk of stroke in a manner similar to our results.7,14–16,33 Our study extends these findings by providing data from a national US sample with greater heterogeneity of neighborhoods, the ability to assess the independent impact of nSES after adjustment for individual SES (and traditional stroke risk factors), and with a sufficient sample size of black and white participants to assess a potential racial difference in the impact of nSES. The study most comparable to REGARDS in methodology is the CHS, a prospective cohort study of adults 65 and older (785 black and 3,834 white) living in 4 US communities.7 In CHS, for white participants, there was a trend for ischemic stroke risk to be higher for residents of lower nSES, and this trend was marginally significant after adjustment for demographic factors and individual SES (p = 0.069).7 In contrast, for black participants, there was a trend for ischemic stroke risk to be lower for residents of lower nSES, and this trend was also marginally significant after full adjustment (p = 0.08).7 While CHS did not report a p value for interaction between nSES and race, one would presume that it would have been significant since the reported race-specific associations were marginally significant and in different directions. Hence, the interpretation of the CHS findings is that nSES has a differential effect for black and white participants. Our findings stand in stark contrast to this, where, for all models considered for both black and white participants, the stroke risk was higher in lower nSES neighborhoods, and the magnitude of association was generally similar (pinteraction > 0.9). There are 2 important similarities. First, with the significant association between nSES and stroke risk attenuated to a level of marginal statistical significance, the consistent message suggests that nSES contributes to stroke risk beyond individual SES. Second, for CHS white participants, and for both white and black participants in REGARDS, further adjustment for biological risk factors substantially attenuated the association of nSES and stroke risk, supporting speculation that these worse biological risk factor profiles in lower SES neighborhoods are in the pathway to higher stroke risk. There are a number of differences between the 2 studies: REGARDS included younger ages, a larger number and more diverse neighborhoods (potentially for black participants, who could be from more homogeneous SES neighborhoods), and a larger sample size of black participants. In the United States, compared to white persons, black persons tend to live in neighborhoods of lower SES.8,34 In addition, we observed a nominally larger mediation of the association between nSES and stroke risk in black than white participants, suggesting that the correlation between nSES and stroke risk factors is larger in black than white participants.
Our findings support the consistent pattern of greater stroke incidence in persons from socioeconomically poor areas observed in other studies (table e-2)35,36; however, few studies also included measures of individual SES7,14,15,18 or prestroke risk factors.6,14,18 In some studies, there was evidence suggestive of a differential effect for men and women, although not always in the same direction.6,15,18,19,37 We found no evidence of a differential effect of nSES on stroke risk by sex.
There are many strengths to this study. First, it includes individuals of low, middle, upper-middle, and high individual SES, both white and black participants across 1,833 urban and rural counties in the United States. Other major strengths include a large sample size of both black and white participants, a wide range of neighborhoods, risk factors measured prior to stroke, and physician-adjudicated incident strokes. There are also some limitations. Those who agreed to participate in REGARDS may not be representative of the general population, potentially reducing generalizability. By design, our study only included black and white participants, so the diversity of racial/ethnic groups in the United States is not represented. Data on individual measures of SES are limited, with some recommended measures such as past socioeconomic experiences not available. A few addresses could not be geocoded. We only used baseline residence and did not include information on how long the individual lived there or whether he or she moved during the follow-up period but fewer than 10% changed address during follow-up. Current residence also may not include the time period most relevant to development of stroke risk. Because risk factors were assessed only once and could have changed over the 7-year follow-up, the possibility of residual confounding exists. Finally, individuals residing in a neighborhood may not all share the same socioeconomic characteristics.
In this national study of black and white participants across the United States, the risk of incident stroke increased with decreasing neighborhood socioeconomic characteristics. This association appeared to be explained by individual SES and risk factors for stroke. The attenuation by individual-level SES implies that it may be difficult to separate the effects of individual SES from neighborhoods where those with lower individual SES live. The further attenuation after adjustment for stroke risk factors suggests these may be in the causal pathway, i.e., lower SES (either nSES or individual) may lead to a worse stroke risk profile, which in turn leads to an increased risk of stroke. Additional research should include other race/ethnic groups and investigate potential differential effects by sex and race/ethnic groups, including risk factors for stroke that may be more prevalent in subgroups within disadvantaged neighborhoods.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their contributions. A full list of participating REGARDS investigators and institutions can be found at regardsstudy.org.
GLOSSARY
- CATI
computer-assisted telephone interview
- CHS
Cardiovascular Health Study
- CI
confidence interval
- FSRS
Framingham Stroke Risk Score
- HR
hazard ratio
- LVH
left ventricular hypertrophy
- MI
myocardial infarction
- nSES
neighborhood socioeconomic characteristics
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- SES
socioeconomic status
- TIA
transient ischemic attack
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. V.J. Howard and McClure had access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. Study concept and design: Dr. V.J. Howard. Acquisition of data: Drs. V.J. Howard, McClure, G. Howard, and Kleindorfer. Analysis and interpretation of data: Drs. V.J. Howard, McClure, G. Howard, Kleindorfer, Diez Roux, and Thrift. Drafting of the manuscript: Drs. V.J. Howard and McClure. Critical revision of the manuscript for important intellectual content: Drs. V.J. Howard, McClure, Kleindorfer, Cunningham, Thrift, Diez Roux, and G. Howard. Obtained funding: Drs. V.J. Howard, McClure, Kleindorfer, and G. Howard. Administrative, technical, and material support: Drs. V.J. Howard, McClure, and G. Howard. Study supervision and coordination: Drs. V.J. Howard and McClure.
STUDY FUNDING
The REGARDS research project is supported by cooperative agreement U01 NS041588 from the National Institute of Neurologic Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurologic Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. V.J.H., L.A.M., and S.A.C. received additional support from an investigator-initiated R01 AG039588 from the National Institute of Aging. A.G.T. is supported by a fellowship from the National Health and Medical Research Council (NHMRC 1042600).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Freedman VA, Grafova IB, Rogowski J. Neighborhoods and chronic disease onset in later life. Am J Public Health 2011;101:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley study. J Epidemiol Community Health 1998;52:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 4.Sundquist K, Winkleby M, Ahlen H, Johansson SE. Neighborhood socioeconomic environment and incidence of coronary heart disease: a follow-up study of 25,319 women and men in Sweden. Am J Epidemiol 2004;159:655–662. [DOI] [PubMed] [Google Scholar]
- 5.Steenland K, Henley J, Calle E, Thun M. Individual- and area-level socioeconomic status variables as predictors of mortality in a cohort of 179,383 persons. Am J Epidemiol 2004;159:1047–1056. [DOI] [PubMed] [Google Scholar]
- 6.Pujades-Rodriguez M, Timmis A, Stogiannis D, et al. Socioeconomic deprivation and the incidence of 12 cardiovascular diseases in 1.9 million women and men: implications for risk prediction and prevention. PLoS One 2014;9:e104671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AF, Liang LJ, Vassar SD, et al. Neighborhood disadvantage and ischemic stroke: the Cardiovascular Health Study (CHS). Stroke 2011;42:3363–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkleby MA, Cubbin C. Influence of individual and neighbourhood socioeconomic status on mortality among black, Mexican-American, and white women and men in the United States. J Epidemiol Community Health 2003;57:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen IH, Michael YL, Perdue L. Neighborhood environment in studies of health of older adults: a systematic review. Am J Prev Med 2009;37:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diez Roux AV, Mair C. Neighborhoods and health. Ann NY Acad Sci 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 11.Addo J, Ayerbe L, Mohan KM, et al. Socioeconomic status and stroke: an updated review. Stroke 2012;43:1186–1191. [DOI] [PubMed] [Google Scholar]
- 12.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 13.Cox AM, McKevitt C, Rudd AG, Wolfe CD. Socioeconomic status and stroke. Lancet Neurol 2006;5:181–188. [DOI] [PubMed] [Google Scholar]
- 14.Honjo K, Iso H, Nakaya T, et al. Impact of neighborhood socioeconomic conditions on the risk of stroke in Japan. J Epidemiol 2015;25:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisabeth LD, Diez Roux AV, Escobar JD, Smith MA, Morgenstern LB. Neighborhood environment and risk of ischemic stroke: the Brain Attack Surveillance in Corpus Christi (BASIC) Project. Am J Epidemiol 2007;165:279–287. [DOI] [PubMed] [Google Scholar]
- 16.Kleindorfer DO, Lindsell C, Broderick J, et al. Impact of socioeconomic status on stroke incidence: a population-based study. Ann Neurol 2006;60:480–484. [DOI] [PubMed] [Google Scholar]
- 17.Balamurugan A, Delongchamp R, Bates JH, Mehta JL. The neighborhood where you live is a risk factor for stroke. Circ Cardiovasc Qual Outcomes 2013;6:668–673. [DOI] [PubMed] [Google Scholar]
- 18.Hart CL, Hole DJ, Smith GD. The contribution of risk factors to stroke differentials, by socioeconomic position in adulthood: the Renfrew/Paisley Study. Am J Public Health 2000;90:1788–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimaud O, Bejot Y, Heritage Z, et al. Incidence of stroke and socioeconomic neighborhood characteristics: an ecological analysis of Dijon stroke registry. Stroke 2011;42:1201–1206. [DOI] [PubMed] [Google Scholar]
- 20.Borhani NO. Changes, and geographic distribution of mortality from cerebrovascular disease. Am J Public Health Nations Health 1965;55:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 22.Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke 1997;28:936–940. [DOI] [PubMed] [Google Scholar]
- 23.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent ST, McClure LA, Crosson WL, Arnett DK, Wadley VG, Sathiakumar N. Effect of sunlight exposure on cognitive function among depressed and non-depressed participants: a REGARDS cross-sectional study. Environ Health 2009;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diez-Roux AV, Kiefe CI, Jacobs DR Jr, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol 2001;11:395–405. [DOI] [PubMed] [Google Scholar]
- 26.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 1992;82:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroke–1989: recommendations on stroke prevention, diagnosis, and therapy: report of the WHO Task Force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 28.Cubbin C, Hadden WC, Winkleby MA. Neighborhood context and cardiovascular disease risk factors: the contribution of material deprivation. Ethn Dis 2001;11:687–700. [PubMed] [Google Scholar]
- 29.Cubbin C, Sundquist K, Ahlen H, Johansson SE, Winkleby MA, Sundquist J. Neighborhood deprivation and cardiovascular disease risk factors: protective and harmful effects. Scand J Public Health 2006;34:228–237. [DOI] [PubMed] [Google Scholar]
- 30.Diez Roux AV, Merkin SS, Hannan P, Jacobs DR, Kiefe CI. Area characteristics, individual-level socioeconomic indicators, and smoking in young adults: the Coronary Artery Disease Risk Development in Young Adults Study. Am J Epidemiol 2003;157:315–326. [DOI] [PubMed] [Google Scholar]
- 31.Yen IH, Kaplan GA. Poverty area residence and changes in physical activity level: evidence from the Alameda County Study. Am J Public Health 1998;88:1709–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keita AD, Judd SE, Howard VJ, Carson AP, Ard JD, Fernandez JR. Associations of neighborhood area level deprivation with the metabolic syndrome and inflammation among middle- and older- age adults. BMC Public Health 2014;14:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thrift AG, Dewey HM, Sturm JW, et al. Greater incidence of both fatal and nonfatal strokes in disadvantaged areas: the Northeast Melbourne Stroke Incidence Study. Stroke 2006;37:877–882. [DOI] [PubMed] [Google Scholar]
- 34.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures: the Public Health Disparities Geocoding Project. Am J Public Health 2003;93:1655–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heeley EL, Wei JW, Carter K, et al. Socioeconomic disparities in stroke rates and outcome: pooled analysis of stroke incidence studies in Australia and New Zealand. Med J Aust 2011;195:10–14. [DOI] [PubMed] [Google Scholar]
- 36.Engstrom G, Jerntorp I, Pessah-Rasmussen H, Hedblad B, Berglund G, Janzon L. Geographic distribution of stroke incidence within an urban population: relations to socioeconomic circumstances and prevalence of cardiovascular risk factors. Stroke 2001;32:1098–1103. [DOI] [PubMed] [Google Scholar]
- 37.Cesaroni G, Agabiti N, Forastiere F, Perucci CA. Socioeconomic differences in stroke incidence and prognosis under a universal healthcare system. Stroke 2009;40:2812–2819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.