Abstract
Objective:
To determine whether race/ethnicity and socioeconomic status are associated with amyotrophic lateral sclerosis (ALS) mortality in the United States.
Methods:
The National Longitudinal Mortality Study (NLMS), a United States–representative, multistage sample, collected race/ethnicity and socioeconomic data prospectively. Mortality information was obtained by matching NLMS records to the National Death Index (1979–2011). More than 2 million persons (n = 1,145,368 women, n = 1,011,172 men) were included, with 33,024,881 person-years of follow-up (1,299 ALS deaths , response rate 96%). Race/ethnicity was by self-report in 4 categories. Hazard ratios (HRs) for ALS mortality were calculated for race/ethnicity and socioeconomic status separately and in mutually adjusted models.
Results:
Minority vs white race/ethnicity predicted lower ALS mortality in models adjusted for socioeconomic status, type of health insurance, and birthplace (non-Hispanic black, HR 0.61, 95% confidence interval [CI] 0.48–0.78; Hispanic, HR 0.64, 95% CI 0.46–0.88; other races, non-Hispanic, HR 0.52, 95% CI 0.31–0.86). Higher educational attainment compared with < high school was in general associated with higher rate of ALS (high school, HR 1.23, 95% CI 1.07–1.42; some college, HR 1.24, 95% CI 1.04–1.48; college, HR 1.10, 95% CI 0.90–1.36; postgraduate, HR 1.31, 95% CI 1.06–1.62). Income, household poverty, and home ownership were not associated with ALS after adjustment for race/ethnicity. Rates did not differ by sex.
Conclusion:
Higher rate of ALS among whites vs non-Hispanic blacks, Hispanics, and non-Hispanic other races was not accounted for by multiple measures of socioeconomic status, birthplace, or type of health insurance. Higher rate of ALS among whites likely reflects actual higher risk of ALS rather than ascertainment bias or effects of socioeconomic status on ALS risk.
Amyotrophic lateral sclerosis (ALS) is a degenerative neurologic disorder with few identified risk factors. Even fundamental demographic associations with ALS, including race/ethnicity and socioeconomic status (SES), are not well-understood. In the United States, few studies have tested race/ethnic differences,1 and studies of SES have focused almost exclusively on occupation.2–4 Moreover, for the most part, studies of race/ethnicity and ALS have not accounted for SES, and studies of SES have not accounted for race/ethnicity.
Apparent differences in ALS risk by race/ethnicity and SES may be driven in part by underascertainment of ALS due to poorer access to health care among minorities or persons of low SES, or because immigrants who develop ALS may return to their birth country when symptoms emerge. If ALS risk differs by SES, this would suggest that ALS risk is affected by factors commonly associated with SES, including health behaviors,5 environmental stressors,6 and workplace exposures.7 Thus, it is critical to jointly examine race/ethnicity, SES, access to health care, and immigrant status, which no studies to date have done.8–13
In the present study, we examined the association of ALS mortality with race/ethnicity and SES in a very large United States–representative study14 with multiple measures of SES and data on health insurance and birthplace collected prospectively.
METHODS
Study population.
The National Longitudinal Mortality Study (NLMS) comprises US Census Bureau Annual Social and Economic Supplements (March 1973 to March 2011), Current Population Surveys (CPS, February 1978, April 1980, August 1980, December 1980, and September 1985), and one 1980 Census cohort.14–16 Surveys gather data about the noninstitutionalized US population, are conducted in-person and via telephone interview, and emphasize the topics of employment, education, and income. The response rate is approximately 96%. So that NLMS participants' reported SES more accurately reflected their adulthood circumstances, we restricted the sample to the 1,011,172 men and 1,145,368 women who were 25 years or older at the time of their survey.
Standard protocol approvals, registrations, and patient consents.
The Institutional Review Board of the Harvard T. H. Chan School of Public Health approved this work.
Case ascertainment.
Mortality information for 1979 through 2011 was obtained by matching NLMS records to the National Death Index (NDI).17 ALS, like many noninfectious diseases, is not a reportable disease in the United States. Therefore, mortality is often used as a surrogate for ALS incidence. The NDI contains death certificate data and is managed by the National Center for Health Statistics.18 Cause of death was obtained from the NDI and coded using the ICD-9 (deaths in or before 1998) or ICD-10 (deaths after 1998). Deaths with either the underlying or a contributing cause listed as ICD-9 code 335.2 or ICD-10 code G12.2 were considered ALS cases. In studies in the United States and other industrialized nations, death certificates have had good validity for ALS mortality.19
Measures.
The head or responsible adult for each household responded for all household members. Participants self-reported their ethnicity as Hispanic or non-Hispanic and self-reported their race as white, black, or other races (including Asian, Pacific Islander, Aleut, Eskimo, and Native American). Education was in 5 levels: less than high school, high school graduate, some college, college graduate, postgraduate, or missing. Household income was coded as less than $20,000, $20,000–$35,000, $35,000–$50,000, $50,000–$75,000, and more than $75,000 dollars per year, or missing. Current home ownership was coded yes/no/missing. Place of birth was coded as in the United States, Puerto Rico, or within an outlying possession of the United States; not one of these places; or missing. Type of health insurance was Medicare, Medicaid, private, employer, government health, no insurance, or missing. Presence of a Social Security number on Census records in the matches to the NDI was coded as yes or no.
Statistical analyses.
Race/ethnicity and ALS mortality.
To examine the association of race/ethnicity with risk of ALS mortality, we estimated hazard ratios (HR) using proportional hazards models adjusted for sex, with age as the time scale in order to closely control for age. To ascertain whether associations of race/ethnicity with ALS differed in women and men, we compared models with and without sex-by-race/ethnicity interaction terms using the likelihood ratio test.
To calculate the extent to which SES might account for racial/ethnic differences in risk of ALS, we further adjusted for indicators of SES, including educational attainment, household income, household income in relation to the poverty line, and home ownership. Presence and type of health insurance coverage may have affected ascertainment of ALS, and emigration of participants to their country of birth following onset of ALS symptoms may have resulted in underascertainment of ALS mortality among immigrants. We therefore further adjusted for presence and type of health insurance coverage and 2 indicators of immigrant status: birthplace (United States or non–United States) and presence or absence of a Social Security number on Census records in the matches to the NDI. We additionally examined rates of ALS death by race/ethnicity restricted to US-born persons in which a Social Security number was used in the matches to the NDI. As an association of military service with rate of ALS has previously been found in this cohort,20 we conducted sensitivity analyses that further adjusted for military service, coded as any or none.
We also conducted sensitivity analyses to examine the association of race/ethnicity and SES with ALS under restrictions that may have improved the ascertainment of ALS and SES. As ALS cases are less reliably diagnosed in death certificates for persons at older ages,21 we restricted follow-up to age 75. We next implemented exclusions that may have improved the ascertainment of SES. First, as ALS symptoms may have led to unemployment or lower income, we excluded the first 5 years of follow-up and the first 8 years of follow-up. A detailed natural history study of ALS found that by 5 years after first symptom, 80% of patients had died and by 8 years after first symptom, 96% of patients had died.22 Second, as occupation is less stable in younger vs older persons and may therefore less accurately reflect adulthood income, we restricted analyses to persons ages 35–75 at enrollment. To ensure that missing data on SES were not reducing its explanatory power to account for race/ethnic differences in ALS, we restricted to participants with complete data on SES and estimated models of race/ethnicity as a predictor of ALS mortality unadjusted and adjusted for all SES measures. In addition, to check on the possibility of differences in competing risks by race/ethnicity accounting for differences in ALS mortality, we also conducted analyses restricting follow-up to age 67 (roughly the median age at ALS death among minorities) since effects of competing risks would be expected to be less at younger ages.
SES and ALS mortality.
To investigate which, if any, measures of SES were associated with ALS mortality, we first estimated HRs using proportional hazards models with age as the time metameter, adjusted for sex, for each SES measure separately (educational attainment, household income, household percent of poverty line, and home ownership). We next added race/ethnicity to these models to determine the extent to which race/ethnicity accounted for possible differences in ALS mortality by SES factors. To determine whether SES measures were associated with ALS independently of one another, and as health insurance may affect ALS ascertainment, we mutually adjusted for all SES measures and health insurance in a final model. As smoking has been associated with increased risk of ALS,23 we considered smoking as a possible confounder. Smoking was queried only in a subset of surveys and data were available on 501,233 participants in our sample (23.2%). We ascertained the association of race and SES with ALS adjusted for smoking status in this subsample.
We created missing data indicators for missing data (<5% missing for all covariates except type of health insurance, for which 28.2% was missing). Follow-up time was measured from the time of the survey or, for those surveyed before 1979, from January 1, 1979 (the start of electronic NDI data), until death or until the last date of NDI linkage. All analyses were conducted using weighted data in SAS (SAS Institute, Cary, NC). NLMS weights are derived from the original CPS weights adjusted so that the survey records in the NLMS represent the noninstitutionalized US population at the time of the survey. For specific analytic purposes, these weights are first adjusted using a ratio adjustment to account for the different survey sizes among the NLMS cohorts and then adjusted to represent the sample size. In this way, we maintain the proper record interrelationships for adjustments of oversampling and undersampling for age, sex, race, Hispanic origin, and state of origin, established in the original CPS weights, but adjusted so that proper estimates of variance can be obtained.
RESULTS
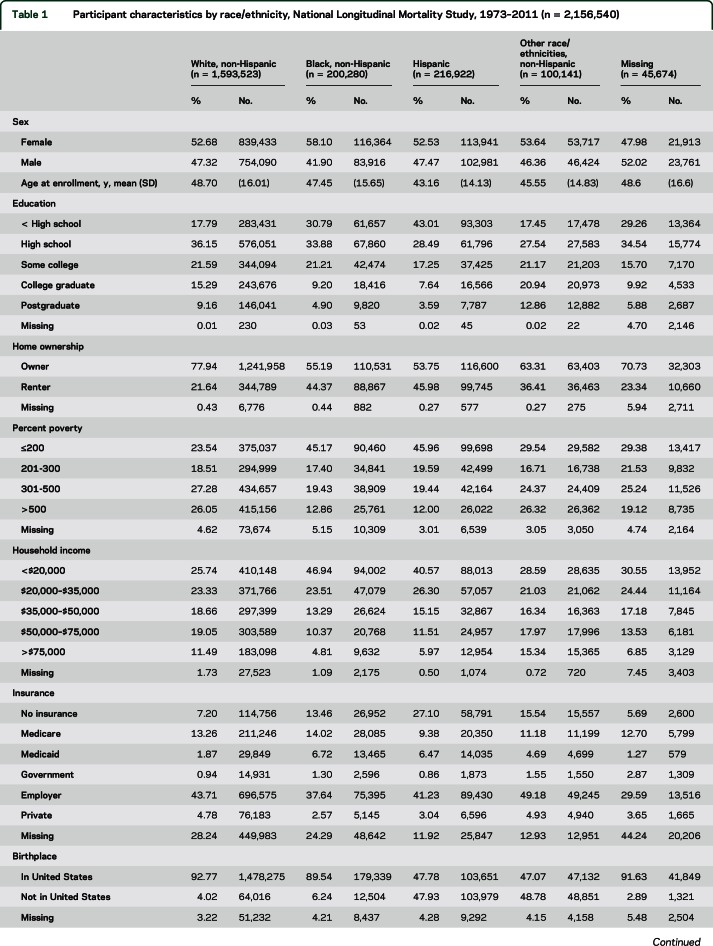
White participants in our sample were slightly older at enrollment, had higher levels of education, were more likely to be home owners, and had higher incomes compared with minority race/ethnicities (table 1). Black and Hispanic participants were substantially more likely to have no health insurance compared with white participants. Hispanic participants and persons of other race/ethnicities were born outside the United States far more often than white participants (47.9% and 47.7% vs 4.0%, table 1).
Table 1.
Participant characteristics by race/ethnicity, National Longitudinal Mortality Study, 1973–2011 (n = 2,156,540)
Race/ethnicity and ALS mortality.
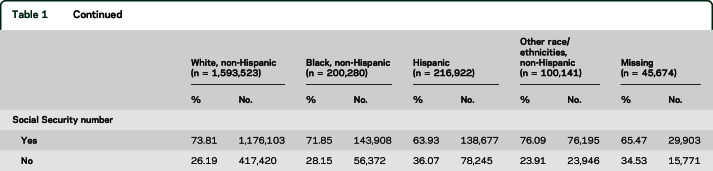
Race/ethnicity was strongly associated with risk of ALS. Compared with non-Hispanic white participants, non-Hispanic black, Hispanic, and non-Hispanic persons of other race/ethnicities were at substantially lower risk of ALS mortality (table 2, model 1). This association did not differ by sex (χ2df = 4 = 2.7, p = 0.60). In models further adjusted for all SES measures and type of health insurance, risk for Hispanic and Black participants were slightly attenuated (table 2, model 2). In models additionally adjusted for birthplace and presence of a Social Security number, risk for Hispanic and non-Hispanic persons of other race/ethnicities were further attenuated but remained substantial (table 2, model 3).
Table 2.
Adjusted hazard ratios (HR)a (95% confidence intervals [CIs]) for amyotrophic lateral sclerosis (ALS) mortality by race/ethnicity, National Longitudinal Mortality Study, women and men age 25 years or older, 1973–2011
In the sample with follow-up limited to age <75 years, as expected, the mean age of participants was younger in all race/ethnic groups and the difference in age between groups was somewhat attenuated compared to the full sample (white, mean = 46.2; black, mean = 45.4; Hispanic, mean = 41.9; other race/ethnicities, mean = 43.9 years). Associations of race/ethnicity with ALS mortality were very similar in models restricting follow-up to age <75 years (n = 2,001,071, no. cases = 829), in persons 35–75 years at enrollment (n = 1,486,746, no. cases = 1,145), and excluding the first 5 and 8 years of follow-up (n = 1,703,980, no. cases = 1,033; n = 1,430,181, no. cases = 871, respectively). Race/ethnic differences in ALS mortality were similar in participants with complete SES data, and, as in the main analyses, adjusting for SES accounted for very little of the race/ethnic differences in ALS mortality in this subgroup. The association of ALS with race/ethnicity was nearly unchanged after further adjustment for military service. In a model restricted to US-born respondents with Social Security numbers used in matches to the NDI, risk estimates were similar to those in the whole sample, though confidence intervals (CIs) were wider due to the reduced sample size (non-Hispanic black, HR 0.48, 95% CI 0.33–0.70; Hispanic, HR 0.66, 95% CI 0.39–1.12; non-Hispanic other race/ethnicities, HR 0.32, 95% CI 0.10–1.11). Further adjustment for smoking did not alter the association of race/ethnicity with ALS in the subsample with data on smoking. In analyses limiting follow-up to age 67, the HR for non-Hispanic black was somewhat greater (0.73, 95% CI 0.53–1.02), but HRs for other minorities were even lower than with full follow-up.
To explore differences over time, we investigated risk of ALS by race/ethnicity in 2 time periods, 1973–1999 and 2000–2011. Risk for Hispanic participants was slightly higher in the earlier vs later time period, though CIs overlapped considerably (HR1973–1999 0.78, 95% CI 0.46–1.31; HR2000–2011 0.55, 95% CI 0.37–0.83). Risk for other groups differed only slightly in the 2 time periods.
SES and ALS mortality.
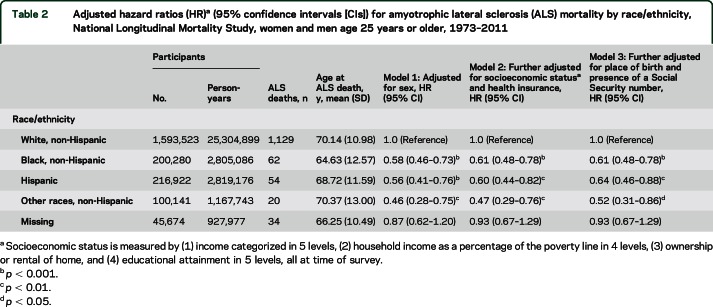
Measures of SES were moderately associated with ALS mortality rate in models adjusted only for sex, with lower ALS risk among respondents reporting low income and high household poverty compared to respondents with the highest income and least impoverishment (table 3, column 4). Type of health insurance was associated with ALS mortality, with lower rate of ALS for persons with private insurance and for persons missing insurance data. After adjusting for race/ethnicity, associations of income and poverty with ALS were attenuated, while educational attainment remained substantially associated with ALS mortality, with high school, some college, and postgraduate education all associated with elevated rate of ALS compared with less than high school education (table 3, column 5). In a model further adjusted for all other SES measures as well as type of health insurance, educational attainment remained statistically significantly associated with ALS (table 3, column 6). Further adjustment for smoking did not change the association of educational attainment with ALS.
Table 3.
Adjusted hazard ratios (HR)a (95% confidence intervals) for amyotrophic lateral sclerosis (ALS) mortality by indicators of socioeconomic status, National Longitudinal Mortality Study, women and men age 25 years or older, 1973-2011
DISCUSSION
In this large, US-representative sample, we identified a higher risk of ALS among white participants compared with race/ethnic minority participants that is not accounted for by multiple measures of SES, type of health insurance, or immigrant status. Moreover, adjustment for the 4 measures of SES available in the NLMS data scarcely changed associations of race/ethnicity with ALS, suggesting that further confounding due to imprecise measurement of SES is likely to be small. Prior studies that report ALS risk by race/ethnicity in US samples have in general found that risk of ALS in black participants is substantially less than that of white participants,11,24 with studies using national data finding risks in black participants to be approximately half that of white participants.1,10,12,13 However, all previous US-wide studies and most regional studies have not adjusted for any socioeconomic factors, health insurance status, or immigrant status.10,12,13
We found a slight association of low SES, as measured by income, household poverty, educational attainment, and home ownership, with lower ALS risk. However, these associations were attenuated and most were not statistically significant after adjusting for race/ethnicity, with the exception of educational attainment. Less than high school education in comparison with higher levels of education remained associated with lower ALS risk (with the exception of college education, for which the association was smaller and not statistically significant), even after adjustment for other indicators of SES and health insurance. We did not find a monotonic association between educational attainment and ALS risk, or with any other measure of SES and ALS. It seems somewhat unlikely that some protective factor was more prevalent only among the lowest education level or that some risk factor was more prevalent among most of the higher education levels.
While to our knowledge, no systematic examination of SES in association with ALS has been conducted, prior studies have suggested that certain jobs, including military service,2 heavy manual labor,3 and agricultural work,4 and job-related exposures, including electric fields or electric shocks, heavy metals,25 formaldehyde,26,27 hypoxia, and pesticides,27,28 may be risk factors for ALS, although findings have been inconsistent25,27,29 and reviews have concluded that the evidence for an association of ALS with a variety of occupational risk factors is not strong.30 It is possible that the association we found is due to underascertainment of ALS among persons with low education, even after accounting for their income and type of health insurance. Some evidence suggests that low education can lead to underascertainment of certain diseases, including parkinsonism,31 autism, and systemic lupus erythematosus.
The lower risk of ALS we found among US non-Hispanic black, Hispanic, and non-Hispanic persons of other race/ethnicities may be due to genetic differences between these groups vs white participants. This possibility raises 2 important issues. First, to what extent does self-identified race/ethnicity correspond to genetic ancestry in the United States? Self-identified race/ethnicity is associated with genetic variation to varying degrees in individuals in the United States. Persons identifying as black or African American, for example, have been found to be on average 69%–74% of West African origin and 11%–19% European–Middle Eastern genetic ancestry, and percentages for individuals vary very widely.32,33 African Americans have been found to have more heterozygosity in single-nucleotide polymorphisms and fewer probably damaging alleles compared with US whites, due to the genetic bottleneck experienced among European ancestral populations around the time of migration out of Africa,34 which may be protective against ALS. Ancestral genetic admixture of self-identified Hispanics in the United States has been estimated in different samples as being 62%–71% European, 21%–33% Amerindian, and 6% African genetic ancestry.33 Thus, on average, US Hispanics have a genetic ancestry that is heavily European, suggesting that genetics alone may not explain the lower risk of ALS we found among Hispanics. It is also possible that having genetic admixture is protective against ALS. A population study of Cuba, a country in which access to health care does not differ by race/ethnicity, found that persons of mixed race/ethnicity (estimated to be of 55% European and 45% African ancestry) were at substantially lower risk of ALS mortality compared with whites (85% European, 15% non-European ancestry) or blacks (75% West African ancestry).35 However, we know of no other studies that find genetic admixture protective against disease.
Second, does our understanding of the genetics of ALS support a hypothesis that genetic differences among race/ethnic groups could yield the substantially different rates of ALS by race/ethnicity that we found? Although little ALS genetic research has been conducted in non-European populations, results suggest that the genetic architecture may differ in different geographic regions.36 Moreover, the heritability of ALS has been estimated at 0.61 (0.38–0.78),37 indicating that genetics play an important role in ALS risk.
To the extent that our findings of race/ethnic differences in ALS reflect environmental causes of ALS, such as chemical exposures,25–27 lifestyle and health-related behaviors,23,38 and stressors,3 these factors would have to be associated with race/ethnicity and not with the SES measures we included in our analyses. Specific exposures that fit these criteria are challenging to identify, but may include diet, which is heavily patterned by race/ethnicity,39 although it is also patterned by SES.40
Our study has important limitations. We could not validate the ascertainment of ALS in the NDI nor were we able to determine whether ascertainment differs by race/ethnicity or SES. While we cannot rule out ascertainment bias, we adjusted for multiple possible sources of ascertainment bias, including insurance coverage and immigrant status. Furthermore, differences by race/ethnicity were largely similar in the earlier and later decades of the study. As differences in ascertainment by race/ethnicity are likely to have moderated over time, these results may suggest that ascertainment bias is not a primary driver of race/ethnic differences found here. In addition, ALS is more frequently underdiagnosed at older vs younger ages, which may introduce bias. We conducted sensitivity analyses restricted to persons age <75 years, among whom age-related underdiagnosis of ALS would be less prevalent compared with the whole sample. Results were very similar in this subsample.
Our results provide evidence that higher ALS mortality in US whites vs minority race/ethnicities is due to actual differences in risk of ALS in these groups, rather than reflecting socioeconomic differences in ALS risk or underascertainment due to health insurance or immigrant status in race/ethnic minority groups. These differences in race/ethnic risk may in turn reflect underlying differences in genetic risk factors for ALS in US race/ethnic groups.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- CI
confidence interval
- CPS
Current Population Surveys
- HR
hazard ratio
- ICD-9
International Classification of Diseases–9
- ICD-10
International Classification of Diseases–10
- NDI
National Death Index
- NLMS
National Longitudinal Mortality Study
- SES
socioeconomic status
AUTHOR CONTRIBUTIONS
Dr. Roberts contributed to designing the analytic plan and led the writing. Dr. Johnson conducted the data analyses and edited the manuscript. Dr. Chen contributed to designing the analytic plan and edited the manuscript. Dr. Cudkowicz contributed to the study design and edited the manuscript. Dr. Weisskopf conceived the study, contributed to designing the analytic plan, and edited the manuscript. All authors approved the submitted manuscript.
STUDY FUNDING
Funding was provided by Muscular Dystrophy Association grant MDA239243, NIH grant NS 082105, and the Agency for Toxic Substances and Disease Registry's National ALS Registry program.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology 2007;68:1002–1007. [DOI] [PubMed] [Google Scholar]
- 2.Weisskopf MG, O'Reilly EJ, McCullough ML, et al. Prospective study of military service and mortality from ALS. Neurology 2005;64:32–37. [DOI] [PubMed] [Google Scholar]
- 3.Beghi E, Logroscino G, Chiò A, et al. Amyotrophic lateral sclerosis, physical exercise, trauma and sports: results of a population-based pilot case-control study. Amyotroph Lateral Scler 2010;11:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furby A, Beauvais K, Kolev I, Rivain J-G, Sébille V. Rural environment and risk factors of amyotrophic lateral sclerosis: a case-control study. J Neurol 2010;257:792–798. [DOI] [PubMed] [Google Scholar]
- 5.Singh GK, Kogan MD, Van Dyck PC, Siahpush M. Racial/ethnic, socioeconomic, and behavioral determinants of childhood and adolescent obesity in the United States: analyzing independent and joint associations. Ann Epidemiol 2008;18:682–695. [DOI] [PubMed] [Google Scholar]
- 6.Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: results from the Americans' changing lives study. J Health Social Behav 2005;46:274–288. [DOI] [PubMed] [Google Scholar]
- 7.Krieger N, Waterman PD, Hartman C, et al. Social hazards on the job: workplace abuse, sexual harassment, and racial discrimination: a study of black, Latino, and white low-income women and men workers in the United States. Int J Health Serv 2006;36:51–85. [DOI] [PubMed] [Google Scholar]
- 8.Gundogdu B, Al-Lahham T, Kadlubar F, Spencer H, Rudnicki SA. Racial differences in motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degeneration 2014;15:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology 2003;61:742–749. [DOI] [PubMed] [Google Scholar]
- 10.Leone M, Chandra V, Schoenberg BS. Motor neuron disease in the United States, 1971 and 1973–1978 patterns of mortality and associated conditions at the time of death. Neurology 1987;37:1339. [DOI] [PubMed] [Google Scholar]
- 11.McGuire V, Longstreth W, Koepsell TD, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology 1996;47:571–573. [DOI] [PubMed] [Google Scholar]
- 12.Mehta P, Antao V, Kaye W, et al. Prevalence of amyotrophic lateral sclerosis: United States, 2010–2011. MMWR Surveill Summ 2014;63:1–14. [PubMed] [Google Scholar]
- 13.Noonan CW, White MC, Thurman D, Wong L-Y. Temporal and geographic variation in United States motor neuron disease mortality, 1969–1998. Neurology 2005;64:1215–1221. [DOI] [PubMed] [Google Scholar]
- 14.Rogot E, Sorlie PD, Johnson NJ, Schmidt C. A Mortality Study of 1.3 Million Persons by Demographic, Social and Economic Factors: 1979–1985 Follow-up. Report No. 92–3297. Bethesda, MD: National Institutes of Health; 1992. [Google Scholar]
- 15.Sorlie PD, Backlund E, Keller JB. US mortality by economic, demographic, and social characteristics: the National Longitudinal Mortality Study. Am J Public Health 1995;85:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Bureau of the Census. The Current Population Survey: Design and Methodology. Report No. 40. Washington, DC: US Department of Commerce; 1978. [Google Scholar]
- 17.Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis 1986;39:719–734. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. User's Manual: The National Death Index. Report No. 90–1148. Washington, DC: USGPO; 1990. [Google Scholar]
- 19.Hoffman PM, Brody JA. The reliability of death certificate reporting for amyotrophic lateral sclerosis. J Chronic Dis 1971;24:5–8. [DOI] [PubMed] [Google Scholar]
- 20.Weisskopf MG, Cudkowicz ME, Johnson N. Military service and amyotrophic lateral sclerosis in a population-based cohort. Epidemiology 2015;26:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kioumourtzoglou MA, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population: validation of a scoring system and a model for survival prediction. Brain 1995;118:707–719. [DOI] [PubMed] [Google Scholar]
- 23.Alonso A, Logroscino G, Hernan MA. Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2010;81:1249–1252. [DOI] [PubMed] [Google Scholar]
- 24.Annegers JF, Appel S, Lee JR-J, Perkins P. Incidence and prevalence of amyotrophic lateral sclerosis in Harris County, Texas, 1985–1988. Arch Neurol 1991;48:589–593. [DOI] [PubMed] [Google Scholar]
- 25.Callaghan B, Feldman D, Gruis K, Feldman E. The association of exposure to lead, Mercury, and selenium and the development of amyotrophic lateral sclerosis and the epigenetic implications. Neurodegenerative Dis 2011;8:1–8. [DOI] [PubMed] [Google Scholar]
- 26.Roberts AL, Johnson NJ, Cudkowicz ME, Eum K-D, Weisskopf MG. Job-related formaldehyde exposure and ALS in the United States. J Neurol Neurosurg Psychiatry 2015;87:786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisskopf MG, Morozova N, O'Reilly EJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2009;80:558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malek AM, Barchowsky A, Bowser R, Youk A, Talbott EO. Pesticide exposure as a risk factor for amyotrophic lateral sclerosis: a meta-analysis of epidemiological studies: pesticide exposure as a risk factor for ALS. Environ Res 2012;117:112–119. [DOI] [PubMed] [Google Scholar]
- 29.Weisskopf MG, McCullough ML, Morozova N, Calle EE, Thun MJ, Ascherio A. Prospective study of occupation and amyotrophic lateral sclerosis mortality. Am J Epidemiol 2005;162:1146–1152. [DOI] [PubMed] [Google Scholar]
- 30.Sutedja NA, Fischer K, Veldink JH, et al. What we truly know about occupation as a risk factor for ALS: a critical and systematic review. Amyotroph Lateral Scler 2008;10:1–19. [DOI] [PubMed] [Google Scholar]
- 31.Hemming JP, Gruber-Baldini AL, Anderson KE, et al. Racial and socioeconomic disparities in parkinsonism. Arch Neurol 2011;68:498–503. [DOI] [PubMed] [Google Scholar]
- 32.Tishkoff SA, Reed FA, Friedlaender FR, et al. The genetic structure and history of Africans and African Americans. Science 2009;324:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollenbach JA, Saperstein A, Albrecht M, et al. Race, ethnicity and ancestry in unrelated transplant matching for the National Marrow Donor Program: a comparison of multiple forms of self-identification with genetics. Plos One 2015;10:e0135960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohmueller KE, Indap AR, Schmidt S, et al. Proportionally more deleterious genetic variation in European than in African populations. Nature 2008;451:994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaldivar T, Gutierrez J, Lara G, Carbonara M, Logroscino G, Hardiman O. Reduced frequency of ALS in an ethnically mixed population a population-based mortality study. Neurology 2009;72:1640–1645. [DOI] [PubMed] [Google Scholar]
- 36.Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 2014;17:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Chalabi A, Fang F, Hanby MF, et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. J Neurol Neurosurg Psychiatry 2010;81:1324–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jong SW, Huisman MHB, Sutedja NA, et al. Smoking, alcohol consumption, and the risk of amyotrophic lateral sclerosis: a population-based study. Am J Epidemiol 2012;176:233–239. [DOI] [PubMed] [Google Scholar]
- 39.Young RP, Hopkins RJ. A review of the Hispanic paradox: time to spill the beans? Eur Respir Rev 2014;23:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J Acad Nutr Diet 2012;112:624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]