Significance
Defective prelamin A processing causes cardiovascular alterations and premature death in Hutchinson–Gilford progeria syndrome (HGPS) patients and also occurs during physiological aging. We found overt repolarization abnormalities in HGPS patients at advanced disease stages. Similar alterations were present in progeroid Zmpste24−/− mice, which had cardiomyocytes that exhibited prolonged calcium transient duration and reduced sarcoplasmic reticulum calcium loading capacity and release, consistent with absence of isoproterenol-induced ventricular arrhythmias. Zmpste24−/− mice developed age-dependent bradycardia and PQ interval/QRS complex prolongation, likely contributing to premature death. These defects correlated with mislocalization of connexin43, which was also noted in heart tissue from HGPS patients. These results reveal molecular alterations that might cause cardiac rhythm alterations and premature death in HGPS.
Keywords: Hutchinson–Gilford progeria syndrome, progerin, prelamin A, connexin43, calcium handling
Abstract
Hutchinson–Gilford progeria syndrome (HGPS) is a rare genetic disease caused by defective prelamin A processing, leading to nuclear lamina alterations, severe cardiovascular pathology, and premature death. Prelamin A alterations also occur in physiological aging. It remains unknown how defective prelamin A processing affects the cardiac rhythm. We show age-dependent cardiac repolarization abnormalities in HGPS patients that are also present in the Zmpste24−/− mouse model of HGPS. Challenge of Zmpste24−/− mice with the β-adrenergic agonist isoproterenol did not trigger ventricular arrhythmia but caused bradycardia-related premature ventricular complexes and slow-rate polymorphic ventricular rhythms during recovery. Patch-clamping in Zmpste24−/− cardiomyocytes revealed prolonged calcium-transient duration and reduced sarcoplasmic reticulum calcium loading and release, consistent with the absence of isoproterenol-induced ventricular arrhythmia. Zmpste24−/− progeroid mice also developed severe fibrosis-unrelated bradycardia and PQ interval and QRS complex prolongation. These conduction defects were accompanied by overt mislocalization of the gap junction protein connexin43 (Cx43). Remarkably, Cx43 mislocalization was also evident in autopsied left ventricle tissue from HGPS patients, suggesting intercellular connectivity alterations at late stages of the disease. The similarities between HGPS patients and progeroid mice reported here strongly suggest that defective cardiac repolarization and cardiomyocyte connectivity are important abnormalities in the HGPS pathogenesis that increase the risk of arrhythmia and premature death.
The LMNA gene encodes A-type lamins (lamin A and lamin C), key components of the mammalian nuclear envelope with important structural and regulatory functions that affect signaling, transcription, and chromatin organization among other processes (1). Mature lamin A is produced from the precursor prelamin A through a series of posttranslational modifications, consisting of sequential farnesylation at the cysteine in the Cysteine-Serine-Isoleucine-Methionine motif, cleavage of the Serine-Isoleucine-Methionine residues, carboxymethylation of the newly accessible cysteine, and a final proteolytic cleavage by the zinc metallopeptidase STE24 (ZMPSTE24, also called FACE-1) (2).
Mutations in the human LMNA gene or defective processing of prelamin A cause a group of diseases termed laminopathies, including the premature aging disorder Hutchinson–Gilford progeria syndrome (HGPS), a very rare genetic disorder with an estimated prevalence of 1 in 21 million people (www.progeriaresearch.org). HGPS patients exhibit accelerated atherosclerosis and arterial stiffness, leading to premature death at an average age of 14.6 y, predominantly from myocardial infarction, heart failure, or stroke (3, 4). Most HGPS patients carry a noninherited de novo heterozygous synonymous mutation in the LMNA gene (c.1824C > T: GGC > GGT; p.G608G), which activates the use of an internal 5′ splicing site in exon 11 that causes the synthesis of progerin. This unprocessed form of prelamin A lacks 50 amino acids encompassing the ZMPSTE24 cleavage site and therefore, remains permanently farnesylated (2). ZMPSTE24 mutations have also been linked to several other human progeroid syndromes (5, 6), reinforcing the notion that accumulation of progerin or prelamin A accelerates cellular aging. Moreover, progerin and prelamin A are both expressed in cells and tissues of normally aging non-HGPS individuals, suggesting their involvement in physiological aging (reviewed in refs. 2 and 7).
Genetically modified mice expressing prelamin A or progerin have enabled the study of mechanisms underlying progeria (8) and testing of the efficacy of various therapies (9, 10). Here, we examined cardiac electrical alterations in 15 HGPS patients with the classical LMNA c.1824C > T mutation, representing ∼5% of the world population (www.progeriaresearch.org). We then correlated the observed alterations in HGPS patients to the underlying molecular processes in Zmpste24-null mice; these mice accumulate farnesylated prelamin A in the nuclear envelope and phenocopy several other defects observed in HGPS, including cardiovascular alterations and premature death (average lifespan ∼20 wk vs. >2 y in WT mice) (9, 11).
Results
Electrocardiographic Alterations in HGPS Patients.
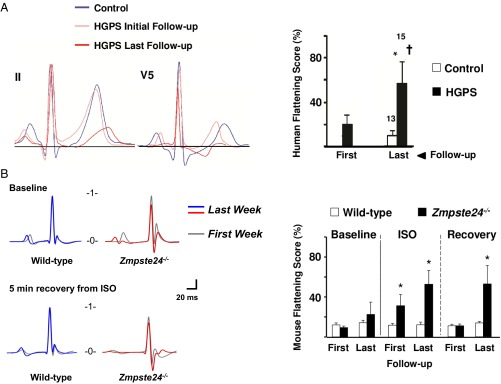
SI Appendix, Tables S1 and S2 show electrocardiographic measurements from HGPS patient carriers of the LMNA c.1824C > T mutation (n = 15, age range 2–19 y old) and controls (n = 13, age range 4–19 y old), respectively. A glossary of electrocardiographic parameters can be found in SI Appendix. As they aged, seven patients showed overt repolarization abnormalities in at least one electrocardiogram (ECG) that were compatible with coronary artery disease (ST segment depression/elevation and negative and biphasic T waves) (a representative example is in SI Appendix, Fig. S1A). One patient showed significant QTc interval prolongation (537 ms) in the context of ischemia at advanced stages of the disease (18 y of age). These alterations were not observed in controls (a representative example is in SI Appendix, Fig. S1B). Repolarization abnormalities in HGPS patients were highly evident at advanced disease stages. Compared with age-matched controls, HGPS patients exhibited significant T-wave flattening, which was exacerbated as disease progressed (Fig. 1A, initial: initial ECG; last: last ECG during follow-up). All patients and controls had sinus rhythm and normal PR interval and QRS complex duration (SI Appendix, Tables S1 and S2). Interestingly, although all HGPS patients exhibited cardiac rhythm within physiological values, the heart rate and PR interval tended to be slower and larger, respectively, in older HGPS patients (6 of 15) at the end of the follow-up period (26.8 ± 2.3 mo follow-up): heart rates on first and last ECGs were 114 ± 7 and 101 ± 7 beats per minute (bpm), respectively, P = 0.36; PRs on first and last ECGs were 115 ± 2 and 128 ± 11 ms, respectively, P = 0.22.
Fig. 1.
Age-related exacerbation of repolarization abnormalities in HGPS patients and Zmpste24−/− mice. (A, Left) Extracted averaged ECG leads II and V5 from human control participants (blue traces) and HGPS patients at initial stage (Initial; light red trace) and advanced stages (Last; dark red trace). (A, Right) Quantification of mean flattening scores. (B, Left) Representative T-wave abnormalities in Zmpste24−/− mice comparing the first and the last isoproterenol (ISO) challenge (SI Appendix, SI Materials and Methods). (B, Right) Mean effective flattening scores at baseline, after ISO exposure and during 5-min recovery. *P < 0.05 vs. control; †P < 0.05 vs. first follow-up. First: 11-wk-old mice; Last: 19-wk-old mice or last week before death.
Time Course of ECG Abnormalities in Progeroid Zmpste24−/− Mice.
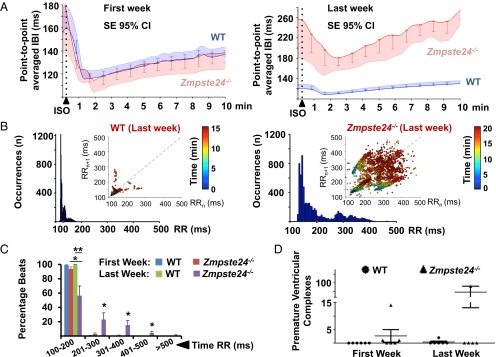
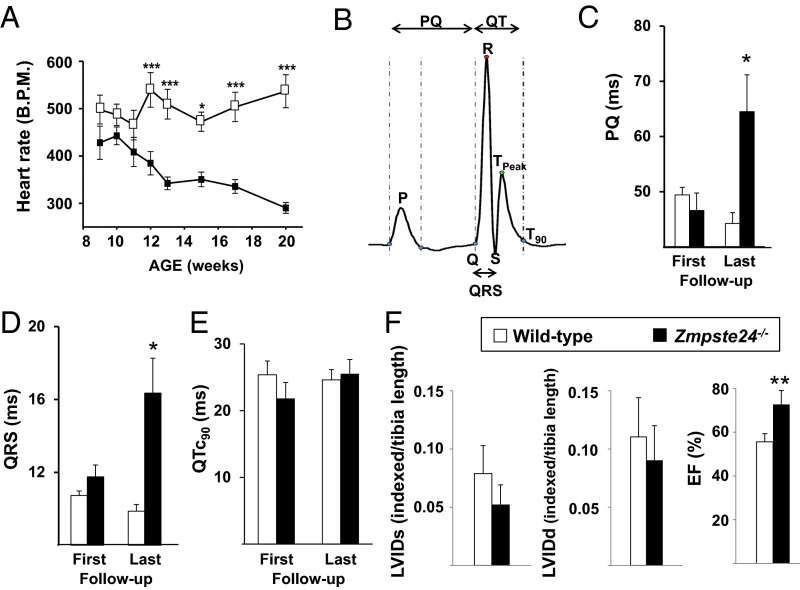
We next analyzed the electrocardiographic phenotype of Zmpste24−/− mice, an established preclinical model of HGPS (9, 11). A glossary of electrocardiographic parameters can be found in SI Appendix. To study repolarization, we quantified T-wave flattening by measuring T-wave morphology changes, including sharpness, by excess kurtosis of the T-wave peak in relation to its geometrical change from the isoelectric line and the area under the T wave (SI Appendix, SI Materials and Methods). Similar to HGPS patients, progeroid mice showed repolarization abnormalities manifested as significant T-wave flattening. To determine the risk of ventricular arrhythmia associated with T-wave alterations, we used the β-adrenergic agonist isoproterenol, which can trigger calcium-related alterations in cardiac repolarization. Weekly isoproterenol challenge, based on its sympathomimetic effect and ability to induce Ca2+-related alterations in repolarization, further exacerbated the repolarization alterations as Zmpste24−/− mice aged, although acute isoproterenol did not cause significant ventricular arrhythmias in Zmpste24−/− or controls (Fig. 1B). Although both groups responded normally in the first week of follow-up, older Zmpste24−/− mice developed a very marked reduction in the heart rate (bradycardia) during recovery from isoproterenol (Fig. 2A). In addition, compared with WT controls, Zmpste24−/− mice progressively developed longer RR intervals as they aged (Fig. 2 B and C). The variability of the RR interval, measured as the SD, increased linearly as a function of the average RR interval (SI Appendix, Fig. S2) and was associated with increased incidence of premature ventricular complexes (Fig. 2D and SI Appendix, Fig. S3) and a short lifespan. During both isoproterenol challenge (SI Appendix, Fig. S4) and recovery (SI Appendix, Fig. S5), Zmpste24−/− mice showed increased prolongation of PQ and QRS intervals. Progeroid Zmpste24−/− mice also developed age-dependent bradycardia in the absence of isoproterenol (Fig. 3A). In addition, at 18–20 wk of age, Zmpste24−/− mice showed significant signs of defective cardiac conduction compared with age-matched controls manifested as prolonged PQ interval and QRS complex, without alterations to the QTc interval (Fig. 3 B–E and SI Appendix, Table S3).
Fig. 2.
Severe bradycardia in postisoproterenol (post-ISO) administration recovery is associated with an increase in premature ventricular complexes in Zmpste24−/− mice. First: 11-wk-old mice; Last: 19-wk-old mice or last week before death. (A) β-Adrenergic response [interbeat interval (IBI)] in WT and Zmpste24−/− mice from the first to the last ISO challenge. 95% CI, 95% confidence interval. (B) RR histograms in WT and Zmpste24−/− mice during ISO time course challenge. The Insets show correlation plots between consecutive cardiac beats (RRn vs. RRn + 1; n = cardiac beat number within the entire registration period; color-coded timescale). A significant increase in the percentage of long RR intervals during the last week of follow-up (C) was also associated with a significant increase in bradycardia-related premature ventricular complexes (D). *P < 0.05; **P < 0.01 (SI Appendix, SI Materials and Methods).
Fig. 3.
Zmpste24−/− mice show preserved cardiac function but develop severe bradycardia and cardiac conduction abnormalities. (A) Heart rate (bpm) in conscious mice. (B) Template ECG showing the intervals used to quantify (C) PQ, (D) QRS, and (E) heart rate-corrected QT at 90% of repolarization from Tpeak (QTc90) duration measured on the baseline ECG at the first follow-up (week 11; First) and the last follow-up (Last) (SI Appendix, SI Materials and Methods). (F) LVIDs, LVIDd, and EF determined by transthoracic echocardiography in 18- to 20-wk-old mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Preserved Cardiac Function in Zmpste24−/− Mice.
Transthoracic echocardiography revealed no abnormalities in left ventricle end diastolic [left ventricular internal diameter at diastole (LVIDd)] or left ventricle end systolic [left ventricular internal diameter at systole (LVIDs)] parameters in progeroid Zmpste24−/− mice (Fig. 3F). Likewise, Zmpste24−/− mice had normal left ventricle mass, posterior wall and interventricular septal thickness at diastole (SI Appendix, Fig. S6A), and diastolic function but had a slightly higher left ventricle ejection fraction (EF) and fractional shortening than controls (Fig. 3F and SI Appendix, Fig. S6A), although within the normal range. The higher EF in progeriod mice might be explained by bradycardia, because longer diastolic intervals may favor such an increase. In fact, together, bradycardia and smaller body surface area in Zmpste24−/− mice explain the significantly lower cardiac output compared with WT controls (SI Appendix, Fig. S6B). However, cardiac index, which relates the cardiac output of the left ventricle per minute to body surface area, was not different between progeroid and WT mice (SI Appendix, Fig. S6B). Time course studies showed no between-genotype differences in systolic or diastolic blood pressure (SI Appendix, Fig. S6C).
Ex Vivo Cellular Electrophysiology.
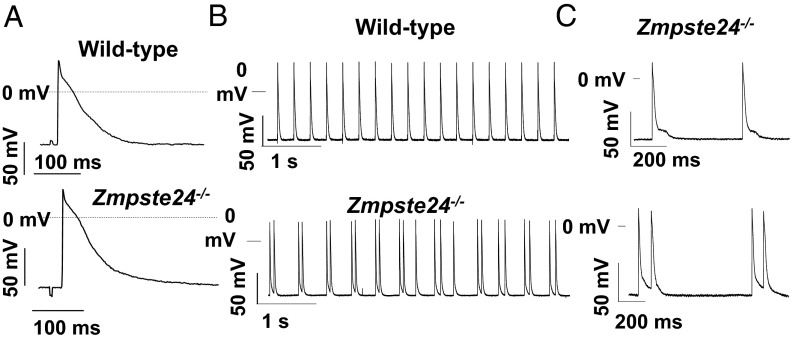
We next conducted electrophysiology studies in cardiac multicellular preparations from Zmpste24−/− mice to identify potential mechanisms underlying the arrhythmia risk associated with abnormal repolarization. No significant between-genotype differences were found in resting membrane potential, action potential (AP) amplitude, maximum velocity of depolarization (Vmax), or AP duration in right or left ventricular samples (SI Appendix, Table S4). Moreover, APs recorded in right and left ventricular preparations were indistinguishable between genotypes (Fig. 4A). Interestingly, most Zmpste24−/− ventricular preparations exhibited slow spontaneous automatic activity at ∼3 Hz (Fig. 4B). Also, Zmpste24−/− left ventricle preparations displayed afterdepolarizations that led to triggered APs, contrasting with normal AP in controls (Fig. 4 B and C).
Fig. 4.
Transmembrane APs recorded in multicellular left ventricular preparations. (A) Representative APs (3 Hz) from WT and Zmpste24−/− mice. (B) Left ventricular preparations from Zmpste24−/− mice displayed afterdepolarizations that occasionally yielded triggered APs (C, Lower).
We next analyzed transcription of genes encoding ion channel proteins involved in the different phases of the AP. Real-time quantitative PCR (qPCR) analysis in Zmpste24−/− hearts revealed no significant between-group differences in mRNA expression of Scn5A (encoding the cardiac sodium channel NaV1.5; involved in cell depolarization) or Kcna5, Kcnj2, Kcnd3, and Kcnq1 (encoding the potassium channels Kv1.5, Kir2.1, Kv4.3, and Kv7.1, respectively; all involved in AP repolarization); in contrast, Zmpste24−/− hearts showed significant up-regulation of Kcnh2 (encoding the potassium channel Kv11.1, also known as human ether-à-go-go related gene (hERG; involved in AP repolarization) (SI Appendix, Fig. S7).
Zmpste24−/− Mice Show Defective Sarcoplasmic Reticulum Ca2+ Handling.
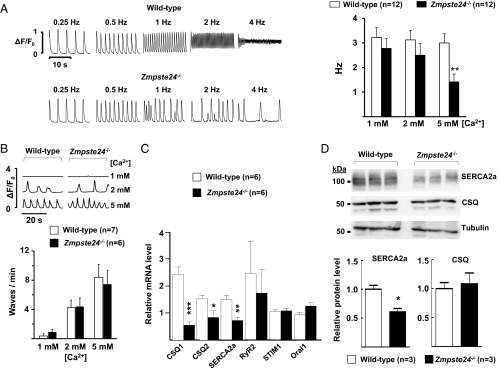
We next investigated the role of intracellular Ca2+ handling in the observed in vivo afterdepolarizations and triggered APs in multicellular left ventricle Zmpste24−/− preparations. Unlike WT cardiomyocytes, Zmpste24−/− cardiomyocytes were unable to maintain stable Ca2+ transients at higher stimulation frequencies, an effect that was more prominent at 5 mM extracellular Ca2+ (Fig. 5A). To test whether this defect in Zmpste24−/− cardiomyocytes was caused by unstable calcium release from the sarcoplasmic reticulum (SR) through the ryanodine receptor type 2 (RyR2) channel, we measured the frequency of spontaneous calcium waves at rest. However, we found no differences in the calcium wave frequency at any of the calcium concentrations examined (Fig. 5B). In line with these results, Zmpste24 deficiency did not affect the transcript level of RyR2 (Fig. 5C), which is responsible for calcium release from the SR (12).
Fig. 5.
Defective Ca2+ transients in Zmpste24−/− cardiomyocytes. (A, Left) Analysis of beat to beat response stability in isolated mouse ventricular myocytes subjected to increasing stimulation frequencies. Representative examples of calcium transients recorded in the presence of 5 mM extracellular Ca2+are shown. Irregular beat to beat responses start at lower frequencies in Zmpste24−/− cardiomyocytes. (A, Right) The graph shows threshold frequencies (in hertz) for the induction of nonuniform beat to beat responses at the indicated Ca2+ concentrations. Responses were recorded in myocytes isolated from WT and Zmpste24−/− mice (12 cells from six mice of each genotype). (B, Upper) Typical recordings of spontaneous calcium waves at the indicated Ca2+ concentrations. (B, Lower) Calcium dependency of the calcium wave frequency. Values are from seven WT mice (n = 15 cells) and six Zmpste24−/− mice (n = 14 cells). (C) qPCR of heart tissue. (D) Western blot analysis of heart tissue. Representative blots are shown, and relative band intensity was quantified as described in SI Appendix, SI Materials and Methods. *P < 0.05; **P < 0.01; ***P < 0.001.
These findings suggested possible impairment of L-type Ca2+ currents (ICa) or Ca2+ uptake by the SR in Zmpste24−/− cardiomyocytes as alternative causes for unstable calcium transients. Compared with WT controls, Zmpste24−/− hearts expressed significantly lower transcript and protein levels of SR Ca2+ ATPase, whereas there was no alteration in transcript levels of STIM1 (stromal interaction molecule component of the store-operated Ca2+ entry 1) and Orai1 (component of the store-operated Ca2+ entry), essential regulators of Ca2+ release-activated Ca2+ channels (Fig. 5 C and D). We also analyzed the expression of calsequestrin 1 (CSQ1) and CSQ2, calcium-binding proteins of the SR that help this organelle store a very high amount of Ca2+ during relaxation in each contraction–relaxation cycle. Although CSQ1 and CSQ2 transcript levels were significantly lower in Zmpste24−/− hearts (Fig. 5C), no between-genotype differences were observed at the protein level (Fig. 5D). Likewise, peak ICa did not differ significantly between cardiomyocytes of the two genotypes (SI Appendix, Fig. S8).
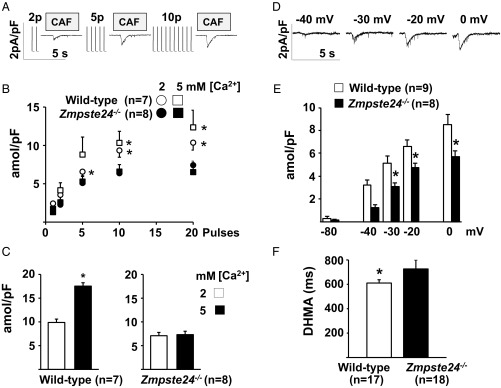
To determine SR Ca2+ reloading function in ventricular cardiomyocytes, we loaded the SR by exposing cells to an increasing number of stimulation pulses and estimated the resulting Ca2+ load from caffeine-elicited inward current traces (Fig. 6A). SR reloading function was significantly weaker in Zmpste24−/− cardiomyocytes than in WT cells after 5, 10, and 20 stimulation pulses (Fig. 6B), and the defective response was still more apparent after ≥30 stimulation pulses (Fig. 6C). Significantly lower SR Ca2+ loading in Zmpste24−/− cardiomyocytes was also evident from caffeine-elicited current traces recorded after loading the SR by depolarizing the membrane potential for 5 s to −40, −30, −20, or 0 mV (Fig. 6 D and E). In addition, intracellular Ca2+ transients recorded in isolated cardiomyocytes paced at 0.5 Hz revealed a significantly longer Ca2+-transient duration (at half-maximal amplitude) in Zmpste24−/− cells (Fig. 6F).
Fig. 6.
Reduced maximal SR Ca2+ uptake in Zmpste24−/− cardiomyocytes. SR calcium loading capacity in isolated ventricular myocytes. (A–C) Calcium loading measured as a function of the number of stimulation pulses used for SR loading. (A) Representative caffeine (CAF)-induced currents recorded after SR reloading with the indicated number of stimulation pulses. Transient exposure to CAF was used to release SR calcium content before reloading and measure loading after the train of stimulation pulses. (B) Time integral of CAF-induced currents recorded after SR reloading with the indicated stimulation pulses. Data were obtained from seven Zmpste24−/− and eight WT myocytes (from n = 5 mice) exposed consecutively to 2 and 5 mM extracellular Ca2+. Time integrals were converted to amoles and normalized to cellular capacitance (in picofarads). (C) Effect of extracellular calcium concentration on the time integral of CAF-induced current at steady state (after ≥30 stimulation pulses). (D–F) Calcium loading measured as a function of membrane potential. (D) Representative CAF-induced currents recorded after SR loading with a 5-s depolarization to the indicated membrane potentials. (E) Time integral of CAF-induced current recorded in the presence of 2 mM extracellular Ca2+ after SR reloading at rest (−80 mV) and after depolarizing to the indicated voltage. Data were obtained from eight Zmpste24−/− myocytes (n = 5 mice) and nine WT myocytes (n = 6 mice). (F) Mean calcium transient duration at half-maximal amplitude (DHMA) recorded in myocytes paced at 0.5 Hz (17 WT and 18 Zmpste24−/− myocytes from n = 6 mice per group). *P < 0.05.
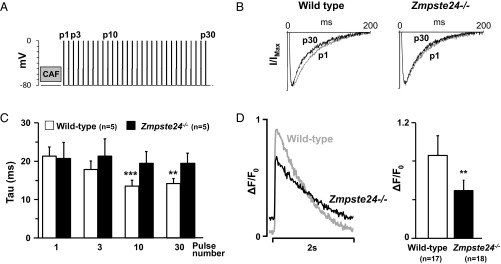
Finally, we measured the SR Ca2+ release-dependent inactivation of the ICa in response to consecutive stimulation pulses given after clearance of SR Ca2+ (Fig. 7A) to assess the feedback of SR calcium release on ICa inactivation. As expected, current traces recorded in WT cardiomyocytes showed a faster rate of ICa inactivation as the number of pulses used for SR reload increased from 1 (p1) to 30 (p30) (Fig. 7B). In contrast, the effect of SR loading on the ICa inactivation rate in Zmpste24−/− cardiomyocytes was very modest (Fig. 7B). Accordingly, the time constant for ICa inactivation decreased progressively with increasing pulse number in WT but not Zmpste24−/− cardiomyocytes (Fig. 7C). Moreover, the amplitude of the intracellular Ca2+ transient induced by repeated stimulation at 0.5 Hz was significantly lower in Zmpste24−/− cardiomyocytes (Fig. 7D). Together, these findings indicate that SR Ca2+ uptake and release are blunted in Zmpste24−/− cardiomyocytes, which also explains the failure of acute isoproterenol treatment to induce ventricular arrhythmia, despite abnormal repolarization.
Fig. 7.
Impaired SR calcium release-dependent inactivation of ICa in isolated Zmpste24−/− myocytes. (A) Protocol to measure the effect of SR calcium loading on ICa inactivation. SR calcium content was released with caffeine (CAF) and reloaded with a train of stimulation pulses. (B) Representative superimposed ICa recordings on p1 and p30. Currents were normalized to their peak values and fitted to a double-exponential equation. (C) Dependency of time constant (tau) for fast ICa inactivation on indicated pulses. (D) Representative calcium transient (ΔF/F0) recordings from myocytes paced at 0.5 Hz (Left) and calcium transient amplitude quantification (Right). Indicated cardiomyocytes are from n = 6 mice per genotype. **P < 0.01; ***P < 0.001.
Abnormal Connexin43 Localization in the Hearts of Zmpste24−/− Mice and HGPS Patients.
To further investigate conduction alterations in progeria, we performed immunohistopathological studies in heart tissue. Cardiomyocyte size was estimated by quantifying their cross-sectional area in heart sections as well as the membrane capacitance, which is linearly proportional to the plasma membrane area and cell size. Because Zmpste24−/− mice are smaller than age-matched controls (9, 11), we normalized results by tibia length. Normalized cross-sectional area and membrane capacitance were similar in cardiomyocytes of both genotypes (SI Appendix, Fig. S9). We also performed Mallory’s trichrome staining, which revealed normal chambers, ventricular walls, and myocardial fiber arrangement in Zmpste24−/− hearts (SI Appendix, Fig. S10 A and B). Moreover, Mallory’s trichrome staining did not reveal increased fibrosis in the ventricular interstitium (SI Appendix, Fig. S10B) or key structures involved in cardiac conduction, such as the atrioventricular node and the main His bundles (SI Appendix, Fig. S10C), which might have explained conduction abnormalities in Zmpste24−/− mice. However, consistent with previous studies in HGPS patients (13), Zmpste24−/− mice showed extensive fibrosis in the tunica media of the major coronary arteries (SI Appendix, Fig. S11A) and discontinuous and weak expression of smooth muscle actin within the medial arterial layer (SI Appendix, Fig. S11B).
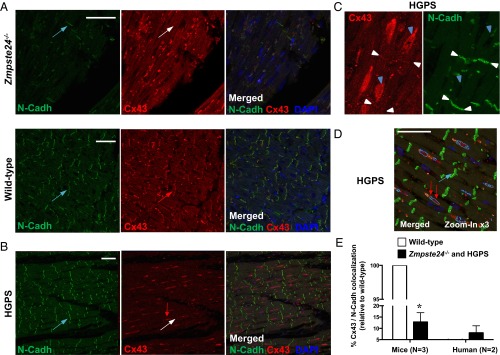
Another possible explanation for the observed cardiac conduction anomalies is altered expression of intercalated disk proteins that play a key role in intercellular connectivity, such as the gap junction protein connexin43 (Cx43) and plakoglobin, as well as cytoskeletal desmosome-interacting proteins, such as desmin, a class III intermediate filament connected to A-type lamins through Nesprin/Sun protein complexes. Western blot analysis in progeroid hearts revealed a significant approximately twofold increase in Cx43 expression without changes in plakoglobin or desmin (SI Appendix, Fig. S12). Remarkably, compared with WT controls, Zmpste24−/− cardiomyocytes exhibited marked mislocalization of Cx43 to the cytoplasm and the lateral long axis as revealed by double immunofluorescence to simultaneously detect Cx43 and N-Cadherin (Fig. 8A). Quantification of digital images revealed less Cx43/N-Cadherin colocalization in the hearts of progeroid mice than in WT controls (Fig. 8E).
Fig. 8.
Abnormal localization of Cx43 in hearts of progeroid Zmpste24−/− mice and HGPS patients. (A and B) Double immunofluorescence of left ventricle sections from mice of the indicated genotype and HGPS patients to detect N-Cadherin (N-Cadh; green in Left) and Cx43 (red in Center). Right shows merged images, with DAPI staining of nuclei (blue). (A) Cx43 lateralization was evident in Zmpste24−/− mice. Blue arrows mark examples of intercalated disk areas (N-Cadh positive). The white arrow in the Zmpste24−/− image marks an intercalated disk without Cx43 expression, and the red arrow in the WT image marks an intercalated disk with abundant Cx43 expression. (B) Representative images illustrating loss of Cx43/N-Cadh colocalization in HGPS heart. Blue arrow and white arrow are as in A. The red arrow marks a Cx43-positive area, which does not colocalize with N-Cadh. (C) Magnified view of an HGPS heart section. Blue arrowheads mark examples of predominant Cx43 expression near nuclei. White arrowheads mark examples of scant Cx43/N-Cadh colocalization at intercalated disks. (D) Automatic image segmentation of an HGPS heart section used for quantification of Cx43/N-Cadh colocalization. Green, red, and dark blue blob boundaries correspond to positive staining for N-Cadh, Cx43, and nuclei, respectively. Cyan blob boundaries represent areas showing Cx43/nuclei colocalization. (Scale bars: 50 µm.) (E) Percentage of Cx43/N-Cadh colocalization at the intercalated disks estimated by quantifying automatically segmented images. Results are represented relative to WT (=100); n = 3 WT and Zmpste24−/− mice and n = 2 HGPS patients were analyzed (n = 5 sections per individual). *P < 0.001.
Profound Cx43 mislocalization was also revealed by immunostaining of left ventricle specimens obtained from two HGPS patients at autopsy. In these patients, Cx43 was abundantly associated with the perinuclear rim (Fig. 8 B–D), which is its trafficking origin for subsequent targeting to the intercalated disks (14) (Discussion). Quantification of digital images confirmed a low percentage of Cx43/N-Cadherin colocalization in HGPS hearts (Fig. 8E). Defective cardiomyocyte connectivity associated to Cx43 mislocalization may contribute to cardiac conduction defects in progeria (Discussion).
Discussion
HGPS is a devastating genetic disease resulting from abnormal processing of prelamin A, which is characterized by premature cardiovascular disease and death at an average age of 14.6 y old (4). Here, we provide a comprehensive analysis of electrocardiographic abnormalities and underlying molecular changes at different stages of HGPS. Our findings may have implications for the risk of premature death in HGPS patients and potentially, normal aging, because defective prelamin A processing has also been revealed in cells and tissues of normally aging non-HGPS individuals (reviewed in refs. 2 and 7). A previous study identified repolarization abnormalities in 3 of 15 HGPS patients with the “classical” LMNA c.1824C > T mutation (3). Here, we confirm and extend these findings through a rigorous examination of a new cohort of 15 HGPS patients carrying the same mutation. One-half of the patients showed overt repolarization abnormalities in at least one ECG compatible with coronary artery disease (ST depression/elevation and negative and biphasic T waves). Repolarization abnormalities in HGPS patients were strongly evident at advanced disease stages. We also detected prominent mislocalization of Cx43 in left ventricle specimens obtained at autopsy from diseased HGPS patients; Cx43 mislocalization was also observed in the hearts of progeroid Zmpste24−/− mice and is indicative of defective cardiac conduction in progeria (see below).
Animal models resembling the clinical phenotype of HGPS patients are central to understanding the underlying mechanisms of the disease and developing novel therapies. Our analysis of the well-established Zmpste24−/− mouse model of progeria caused by prelamin A accumulation (9, 11) revealed the progressive development of cardiac rhythm alterations that can lead to premature death. Zmpste24−/− hearts and cardiomyocytes showed the following specific alterations: (i) T-wave repolarization abnormalities, which were also present in one-half of HGPS patients; (ii) prolonged PQ interval and wide QRS complex; (iii) development of bradycardia-related premature ventricular complexes and slow-rate polymorphic ventricular rhythms at late disease stages in the absence of ventricular arrhythmias during isoproterenol challenge; (iv) automatic spontaneous ventricular activity and afterdepolarizations ex vivo, which were associated with significantly slowed ICa inactivation and reduced amplitude of the intracellular Ca2+ transient; and (v) mislocalization of Cx43 in the heart.
Notably, some characteristics of the progeroid Zmpste24−/− mouse heart are also present in HGPS patients and frequently observed in normal aging; these characteristics include extensive fibrosis and loss of smooth muscle cells in coronary arteries, defective calcium homeostasis, progressive development of repolarization defects, and Cx43 mislocalization (15–17). Overt repolarization abnormalities in ST T waves and T-wave flattening in mice did not increase the risk of isoproterenol-triggered ventricular arrhythmia. However, T-wave alterations are well-known to increase the risk of lethal ventricular arrhythmias during ischemia (18), which might contribute to premature death in HGPS patients from myocardial infarction, one of the main causes of death in this population. T-wave abnormalities might be explained at least in part by an altered repolarization pattern brought about by two opposing ion channel changes observed in the hearts of Zmpste24−/− mice: on the one hand, the significant up-regulation of Kcnh2, which encodes the hERG channel responsible for IKr, would help terminate the AP plateau and shorten repolarization (19), and on the other hand, the observed slow ICa inactivation would tend to prolong the plateau and delay repolarization (20). Importantly, an abnormally long QTc interval was observed in only 1 of 51 ECGs from 15 HGPS patients and occurred in the context of ischemia and significant repolarization anomalies. Consistently, we observed no significant QTc prolongation in Zmpste24−/− mice, in agreement with the normal AP duration observed in Zmpste24−/− ventricular cardiomyocytes and the majority of ECG traces from HGPS patients. Although we documented significant QRS complex widening in progeroid mice, it is important to note that the high-amplitude QRS complex in the mouse ECG represents not only the spread of depolarization across the ventricle but also, the early phase of repolarization (21). We, therefore, carefully examined QRS complex and QT duration (QT90) (Fig. 3 B–E) to detect any significant increases in both parameters. However, mouse and human QT intervals must be compared with caution.
The occurrence of bradycardia-related premature ventricular complexes in Zmpste24−/− mice during recovery after isoproterenol shows a significant suppression of normal pacemaker activity, with emergence of ventricular ectopic escape discharges. Isoproterenol-induced heart rate increase in vivo is associated with increases in intracellular Na+ and Ca2+ concentration (22); these changes, in the presence of the postisoproterenol-induced bradycardia, likely contributed to the escape discharges and premature ventricular complexes arising from the Purkinje system (SI Appendix, Fig. S3). Our ex vivo experiments with multicellular ventricular preparations showed afterdepolarizations during spontaneous ventricular activity at ∼3 Hz, which is similar to the cycle length after isoproterenol treatment in vivo. Although we were unable to establish the exact origin of these ventricular afterdepolarizations, the in vivo data support involvement of the Purkinje system, consistent with slow idioventricular discharges (cycle length = 266.38) (SI Appendix, Fig. S3) arising from varying Purkinje locations.
Connexins are the pore-forming subunits of gap junctions and essential for proper intercellular electrical coupling between cardiomyocytes and AP spread during each cardiac cycle (23, 24). Cx43 is the major connexin expressed in the ventricles. Its abnormal expression, typically involving down-regulation and heterogeneous redistribution to the lateral cardiomyocyte membrane, is associated with different forms of chronic heart disease (hypertrophic, dilated, and ischemic cardiomyopathy) and even aging (24–26). Defective Cx43 expression results in electrical defects in the myocardium and contributes to arrhythmogenesis. Therefore, Cx43 mislocalization in the heart may explain, at least partly, the prolongation of PQ interval and QRS complex in Zmpste24−/− mice. Interestingly, reduced gap junction coupling accompanied by Cx43 lateralization has been linked to reduced functional expression of the alpha subunit (NaV1.5) of the cardiac sodium channel at the intercalated disk (27), and the reduced sodium current is likely to have contributed to the significantly impaired atrioventricular and intraventricular conduction in Zmpste24−/− mice. Previous computer simulations (28) suggest that, even in the presence of Cx43 mislocalization and low NaV1.5 expression, a normal peak ICa with slow inactivation and reduced Ca2+ transients could maintain relatively safe conduction in the ventricles of Zmpste24−/− mice, albeit at a reduced velocity. However, a high intracellular Ca2+ concentration would have compromised propagation safety and caused earlier block as observed for reduced intercellular coupling (28). Because the intercalated disk is regarded as a functional unit, with gap junction formation requiring the presence of neighboring mechanical junctions (29), we tested the expression of desmin and plakophilin-2. However, our Western blot analysis in Zmpste24−/− hearts revealed no alterations in the expression of these proteins.
Consistent with the findings in Zmpste24−/− mice, immunofluorescence studies in left ventricle specimens from deceased HGPS patients revealed abnormal cellular distribution of Cx43 to a predominantly perinuclear localization. Because Cx43 is packaged into vesicles at the perinuclear trans-Golgi network and then transported to the intercalated disk (14), our findings suggest that abnormal prelamin A processing causes defective Cx43 targeting to its distinctive microdomain at the gap junctions. Additional LMNA mutations might also cause cardiomyopathy by altering connexin expression and/or cellular localization. For example, transgenic mice expressing the lamin A N195K mutant, which causes dilated cardiomyopathy with conduction system disease in humans, die at an early age because of arrhythmia, and such a phenotype correlates with cardiac Cx43 and Cx40 misexpression and/or mislocalization (30). Future studies are warranted to elucidate the mechanism causing defective Cx43 microdomain targeting to gap junctions and their contribution to abnormal cardiac conduction in progeria.
Some of the nuclear envelope alterations in the heart associated with prelamin A or progerin expression also occur during normal aging (15–17), suggesting that shared mechanisms might cause cardiac alterations in HGPS patients and the geriatric population. Consistent with this idea, prelamin A and progerin are both produced in the cells of normally aging individuals, thus raising the possibility that altered lamin A processing contributes to normal aging and associated cardiovascular disease (reviewed in refs. 2 and 7). Much like in normal human aging, progeroid Zmpste24−/− mice develop coronary fibrosis, bradycardia, and severe conduction abnormalities. Cardiac conduction abnormalities also arise during aging in WT mice and are associated with an increased incidence of arrhythmias (31). However, Ca2+ transients differ between Zmpste24−/− mice and normally aging WT mice (32), with Zmpste24−/− cardiomyocytes being unable to maintain stable Ca2+ transients at 4 Hz. Ventricular cardiomyocytes from aged WT mice also have a significantly higher incidence of spontaneous Ca2+ sparks than cells from young animals; however, we did not observe a similar difference between progeroid mice and age-matched controls. Zmpste24−/− mice show weakened ICa inactivation and defective SR Ca2+ uptake and release, features also observed in aging human atrial myocytes (15). In line with these findings, Ca2+ transient amplitudes are reduced in progeroid mice (Fig. 6), aged WT mice (32), and aged human atrial cardiomyocytes (15).
The progressively developing bradycardia and deteriorating cardiac conduction in progeriod mice also resemble clinical rhythm abnormalities observed in the elderly (16, 17). Although the most common age-related cardiac conduction abnormality in humans is degenerative fibrosis (33), Zmpste24−/− mice did not show abnormal fibrosis in the ventricular interstitium or the major conduction structures. However, in line with observations in HGPS patients (13), the coronary arteries of Zmpste24−/− mice showed extensive fibrosis and reduced accumulation of smooth muscle cells, potential causes of vascular stiffening, reduced coronary flow, and abnormal impulse generation in the sinoatrial node and conduction in the atria and ventricles. The latter may have also contributed to T-wave alterations, especially during isoproterenol challenge, as well as progressive development of bradycardia in progeroid Zmpste24−/− mice. In fact, human patients with coronary artery disease show a high prevalence of conduction abnormalities, such as atrioventricular or sinoatrial block (34).
We provide a comprehensive characterization of cardiac abnormalities in HGPS patients and progeroid Zmpste24−/− mice, identifying a number of cellular and molecular alterations in both species that are likely to contribute to defective cardiac repolarization and conduction in progeria. Future studies are warranted to establish direct causal connections between the presence of unprocessed prelamin A or progerin and the cardiac abnormalities associated with HGPS, with the goal of identifying novel targets for therapeutic intervention. Based on our findings, major efforts should be placed into elucidating the mechanisms causing Cx43 mislocalization in the heart of progeroid mice and HGPS patients. These studies may pave the way to developing efficient therapies to improve cardiac conduction in progeria.
Although ion channels are highly conserved between humans and mice, significant electrophysiological differences exist (35), making it difficult to translate mouse findings to the clinical arena. For example, although we observed a tendency toward a slower heart rate in older HGPS patients with longer follow-up, HGPS patients did not show the bradycardia and other conduction abnormalities that appeared progressively in Zmpste24−/− mice. This difference might be due to the lack of sequential ECG assessment in humans until very advanced disease stages. Indeed, human left ventricle specimens obtained by autopsy revealed reduced localization of Cx43 at gap junctions, consistent with altered ventricular conduction velocity.
Continuous telemetry and other invasive electrophysiological measurements would have been desirable in Zmpste24−/− mice. However, these animals show severe body weight loss (body weight of ∼9 g at late stages compared with ∼30 g in age-matched WT controls) and are physically extremely fragile, precluding the use of commercially available telemetry systems or any other invasive approaches to register specific arrhythmias forecasting premature death. The physical deterioration of HGPS patients also limits implementation of any invasive measurements (e.g., implantable loop recordings) to further study rhythm abnormalities.
Materials and Methods
Clinical information from children with HGPS was obtained from The Progeria Research Foundation Medical and Research Database (principal investigator L.B.G.) and approved by the Rhode Island Hospital and Brown University Institutional Review Boards (Providence, RI). All participants or parents provided written informed consent in the primary language of the participant or parent. When appropriate, interpreters were used for consenting. At least one ECG recorded no more than 3 y before death was obtained from each of 15 HGPS patients. Thirteen gender- and age-matched control volunteers were weighed, and ECG traces were recorded for comparison. All control volunteers or parents provided written informed consent. Animal studies were carried out in male Zmpste24−/− mice (11) and age-matched WT male littermates (all C57BL/6). Mice were reared and housed in accordance with institutional guidelines and regulations and all procedures with mice were approved by the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) Ethical Committee. All other detailed materials and methods are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank María Jesús Andrés for technical assistance and help with art work, Susan Campbell for assistance with patient information, Maite Dubraska for assistance with blood sampling and ECG in control subjects, Inés Ortega and Virginia Zorita for animal maintenance and care, and Simon Bartlett for English editing. This work was supported by Spanish Ministry of Economy and Competitiveness (MINECO) Grants SAF2010-16044 and SAF2013-46663-R (to V.A.), SAF2011-30312 and SAF2014-58286-C2-1-R (to L.H.-M.), SAF2011-30088 (to E.D.), and SAF2014-52413-R (to C.L.-O.) and Fondo de Investigación Sanitaria del Instituto de Salud Carlos III Grants RD12/0042/0028 (to V.A.), RD12/0042/0011 (to J.T.), and RD12/0042/0002 (to L.H.-M.), with cofunding from the Fondo Europeo de Desarrollo Regional and the Progeria Research Foundation. J.A.G. is the recipient of a U-Mobility Grant from the Marie Curie cofunding of Regional, National and International Programme (Grant 246550). The Instituto Universitario de Oncología is supported by Obra Social Cajastur. The CNIC is supported by the MINECO and the Pro CNIC Foundation, and it is a Severo Ochoa Center of Excellence (MINECO Award SEV-2015-0505).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603754113/-/DCSupplemental.
References
- 1.Andrés V, González JM. Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol. 2009;187(7):945–957. doi: 10.1083/jcb.200904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trigueros-Motos L, González JM, Rivera J, Andrés V. Hutchinson-Gilford progeria syndrome, cardiovascular disease and oxidative stress. Front Biosci (Schol Ed) 2011;3:1285–1297. doi: 10.2741/226. [DOI] [PubMed] [Google Scholar]
- 3.Merideth MA, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358(6):592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon LB, et al. Progeria Clinical Trials Collaborative Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation. 2014;130(1):27–34. doi: 10.1161/CIRCULATIONAHA.113.008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrowman J, Wiley PA, Hudon-Miller SE, Hrycyna CA, Michaelis S. Human ZMPSTE24 disease mutations: Residual proteolytic activity correlates with disease severity. Hum Mol Genet. 2012;21(18):4084–4093. doi: 10.1093/hmg/dds233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osorio FG, et al. Cell autonomous and systemic factors in progeria development. Biochem Soc Trans. 2011;39(6):1710–1714. doi: 10.1042/BST20110677. [DOI] [PubMed] [Google Scholar]
- 7.Gordon LB, Rothman FG, López-Otín C, Misteli T. Progeria: A paradigm for translational medicine. Cell. 2014;156(3):400–407. doi: 10.1016/j.cell.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Kieckhaefer JE, Cao K. Mouse models of laminopathies. Aging Cell. 2013;12(1):2–10. doi: 10.1111/acel.12021. [DOI] [PubMed] [Google Scholar]
- 9.Varela I, et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437(7058):564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- 10.Villa-Bellosta R, et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2013;127(24):2442–2451. doi: 10.1161/CIRCULATIONAHA.112.000571. [DOI] [PubMed] [Google Scholar]
- 11.Pendás AM, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31(1):94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- 12.Willis BC, et al. Constitutive intracellular Na+ excess in purkinje cells promotes arrhythmogenesis at lower levels of stress than ventricular myocytes from mice with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2016;133(24):2348–2359. doi: 10.1161/CIRCULATIONAHA.116.021936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olive M, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30(11):2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyth JW, et al. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res. 2012;110(7):978–989. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herraiz-Martínez A, et al. Ageing is associated with deterioration of calcium homeostasis in isolated human right atrial myocytes. Cardiovasc Res. 2015;106(1):76–86. doi: 10.1093/cvr/cvv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen PN, et al. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64(6):531–538. doi: 10.1016/j.jacc.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8(1):143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verrier RL, Ikeda T. Ambulatory ECG-based T-wave alternans monitoring for risk assessment and guiding medical therapy: Mechanisms and clinical applications. Prog Cardiovasc Dis. 2013;56(2):172–185. doi: 10.1016/j.pcad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Perry M, Sanguinetti M, Mitcheson J. Revealing the structural basis of action of hERG potassium channel activators and blockers. J Physiol. 2010;588(Pt 17):3157–3167. doi: 10.1113/jphysiol.2010.194670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madhvani RV, et al. Targeting the late component of the cardiac L-type Ca2+ current to suppress early afterdepolarizations. J Gen Physiol. 2015;145(5):395–404. doi: 10.1085/jgp.201411288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274(3 Pt 2):H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 22.Vassalle M. The relationship among cardiac pacemakers. Overdrive suppression. Circ Res. 1977;41(3):269–277. doi: 10.1161/01.res.41.3.269. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya D, et al. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res. 2001;88(11):1196–1202. doi: 10.1161/hh1101.091107. [DOI] [PubMed] [Google Scholar]
- 24.Fontes MS, van Veen TA, de Bakker JM, van Rijen HV. Functional consequences of abnormal Cx43 expression in the heart. Biochim Biophys Acta. 2012;1818(8):2020–2029. doi: 10.1016/j.bbamem.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Peters NS. New insights into myocardial arrhythmogenesis: Distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts. Clin Sci (Lond) 1996;90(6):447–452. doi: 10.1042/cs0900447. [DOI] [PubMed] [Google Scholar]
- 26.Saffitz JE, Schuessler RB, Yamada KA. Mechanisms of remodeling of gap junction distributions and the development of anatomic substrates of arrhythmias. Cardiovasc Res. 1999;42(2):309–317. doi: 10.1016/s0008-6363(99)00023-1. [DOI] [PubMed] [Google Scholar]
- 27.Cerrone M, et al. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res. 2012;95(4):460–468. doi: 10.1093/cvr/cvs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81(5):727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 29.Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: From gene to disease. Circ Res. 2010;107(6):700–714. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- 30.Mounkes LC, Kozlov SV, Rottman JN, Stewart CL. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum Mol Genet. 2005;14(15):2167–2180. doi: 10.1093/hmg/ddi221. [DOI] [PubMed] [Google Scholar]
- 31.Signore S, et al. Late Na(+) current and protracted electrical recovery are critical determinants of the aging myopathy. Nat Commun. 2015;6:8803. doi: 10.1038/ncomms9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howlett SE, Grandy SA, Ferrier GR. Calcium spark properties in ventricular myocytes are altered in aged mice. Am J Physiol Heart Circ Physiol. 2006;290(4):H1566–H1574. doi: 10.1152/ajpheart.00686.2005. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer MI. The etiology and natural history of sinus node disorders. Arch Intern Med. 1982;142(2):371–372. [PubMed] [Google Scholar]
- 34.Hsueh CW, Lee WL, Chen YT, Ting CT. The incidence of coronary artery disease in patients with symptomatic bradyarrhythmias. Jpn Heart J. 2001;42(4):417–423. doi: 10.1536/jhj.42.417. [DOI] [PubMed] [Google Scholar]
- 35.London B. Cardiac arrhythmias: From (transgenic) mice to men. J Cardiovasc Electrophysiol. 2001;12(9):1089–1091. doi: 10.1046/j.1540-8167.2001.01089.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.