Abstract
Purpose
HSP90 inhibition is well known to sensitize cancer cells to radiation. However, it is currently unknown if additional radiosensitization could occur in the more clinically relevant setting of chemoradiation (CRT). We used the potent HSP90 inhibitor ganetespib to determine if it can enhance CRT effects in NSCLC.
Experimental Design
We first performed in vitro experiments in various NSCLC cell lines combining radiation with or without ganetespib. Some of these experiments included clonogenic survival assay, DNA damage repair and cell cycle analysis, and Reverse Phase Protein Array. We then determined if chemotherapy affected ganetespib radiosensitization by adding carboplatin-paclitaxel to some of the in vitro and in vivo xenograft experiments.
Results
Ganetespib significantly reduced radiation clonogenic survival in a number of lung cancer cell lines, and attenuated DNA damage repair with irradiation. Radiation caused G2/M arrest that was greatly accentuated by ganetespib. Ganetespib with radiation also dose-dependently up-regulated p21 and down-regulated pRb levels that were not apparent with either drug or radiation alone. However, when carboplatin-paclitaxel was added, ganetepsib was only able to radiosensitize some cell lines but not to others. This variable in vitro CRT effect was confirmed in vivo using xenograft models.
Conclusions
Ganetespib was able to potently sensitize a number of NSCLC cell lines to radiation but has variable effects when added to platinum-based doublet CRT. For optimal clinical translation, our data emphasizes the importance of preclinical testing of drugs in the context of clinically-relevant therapy combinations.
Keywords: Hsp90 inhibitor, non-small cell lung cancer, chemoradiotherapy
Introduction
Lung cancer is the leading cause of cancer death in the United States and has a 5-year relative survival rate of only 16% (1). Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancer cases and the lack of significant treatment advance is related to the highly resistant nature of this disease. While chemotherapy provides only useful palliation for stage IV NSCLC, the treatment of locally advanced, unresectable NSCLC is with curative intent using concomitant chemotherapy and radiotherapy (chemoradiotherapy, CRT), which produces longer overall survival than sequential chemotherapy and radiation therapy (2–6), but the outcomes remain poor. The median survival ranges from 17–28 months, despite significantly increased toxicity of the combination therapy. There is a strong need to improve therapy efficacy in NSCLC without substantially increasing normal tissue toxicity. Indeed, recent clinical trials have investigated the combination of CRT with molecularly targeted agents, either with angiogenesis inhibitors or with EGFR targeting agents. Unfortunately, either due to intolerable toxicities (7) or to lack of efficacy (8), these trials have not advanced the management of this disease.
Hsp90 is a molecular chaperone protein ubiquitously present in cells; however its function is critically important for the maintenance of cancer cell growth (9, 10). Inhibiting its function has been extensively studied for its potent antitumor effect (9, 11, 12). An attractive feature of targeting Hsp90 is that the cytotoxicity of Hsp90 inhibitors is tumor selective (13). Hsp90 inhibition has also been known to be radiation sensitizing on tumor cells (14–25). Some of these studies indicated that the radiation sensitization is also tumor selective since normal cells are not affected (14, 15, 17). However, the clinical development of Hsp90 inhibitors has been hampered by the severe toxicities of first generation inhibitors, including severe ocular and hepatic toxicities (26, 27).
Ganetespib, a second generation Hsp90 inhibitor with little to no ocular or hepatic toxicities, has been safely used in thousands of patients in over 60 clinical trials internationally. A completed phase II randomized trial in stage IV NSCLC combining ganetespib with docetaxel compared to docetaxel alone has demonstrated efficacy signal in a subgroup of patients (28), and therefore it was further tested in a phase III randomized trial (GALAXY II) (NCT01798485). Despite the safety and promising efficacy of this drug in advanced NSCLC, the experience of combining ganetespib with CRT is limited. While radiation sensitizing effect is well known for this class of inhibitors, one simply cannot assume that synergy could be seen with CRT. This was once assumed for EGFR inhibitors when preclinical studies demonstrated synergy with radiotherapy alone (29, 30), but ultimately failed in a number of phase III clinical trials when EGFR targeting agents were combined with CRT in oropharyngeal cancer, esophageal cancer, and NSCLC(8, 31, 32). While this manuscript was under preparation, Gomez-Casal et al. reported the HSP90 inhibitor ganetespib radiosensitizes human lung adenocarcinoma cells(33), however whether ganetespib enhanced therapeutic effects of chemoradiotherapy was not demonstrated. The purpose of the current study is to evaluate the cytotoxic action of the combination of radiation with ganetespib and test its potential to synergize with CRT for the treatment of NSCLC.
Materials and Methods
Cell culture, reagents and irradiator
The human non-small lung cancer cell lines H460, A549, H1299, H1650, H358 and H2087 cells were all obtained from the American Type Culture Collection (ATCC) and routinely maintained in RPMI-1640 medium supplemented with 10% FBS, and 10,000 U/mL of penicillin-streptomycin. Cell lines were authenticated at Characterized Cell Line Core Facility at MD Anderson Cancer Center using the Short Tandem Repeat method every 6 months of use in the lab. Ganetespib [3-(2,4-dihydroxy-5-isopropylphenyl)-4-(1-methyl-1H-1,2,4-triazol-5(4H)-1] was provided by Synta Pharmaceuticals Corp. Cells and animals were irradiated with a JL Shepherd Mark I-68A 137Cs irradiator with 137Cs sources at the doses from 0–6 Gy. The Cesium-Irradiator output (cGy/min) was measured in-air using an ADCL (Accredited-Dosimetry-Calibration-Laboratory) calibrated ion-chamber. Dosimetry in Simulated-Irradiation-Geometry was performed employing Gafchromic-Film “EBT3”. For Film-Dosimetry, mouse was simulated by dosimetrically-equivalent Gel “SuperFlab”. EBT3 response in Simulated-Geometry versus in-Air Reference-Calibration-Geometry provided dose-rate in mouse. The treatment set-up employed table, cerrobend-block and mouse-restrainer on top. Cesium beam pointed up. All these pieces were provided with mutually inter-locking pins to ensure set-up reproducibility. For reduced penumbra, the block was provided with appropriate divergence.
Clonogenic survival assay (CSA) to determine ganetespib radiation sensitization effect
The effectiveness of the combination of ganetespib and ionizing radiation was assessed by CSA. H460, A549, H1299, and H1650 cells were seeded (100–2000 cells/well) in duplicate in 6-well plates. The medium was changed 16 hours after plating and the cells were treated with either vehicle (DMSO) or ganetespib (30 nM). Five hours following ganetespib treatment, the cells were subjected to irradiation at doses from 0–6 Gy. Twenty-four hours after ganetespib treatment, media was changed and the cells were maintained in normal culture conditions. On about 12th–20th day, the medium was removed and cell colonies were stained with crystal violet (0.1% in 20% methanol) (Sigma-Aldrich, St. Louis, MO, USA). Colony numbers were assessed visually and colonies containing > 50 normal-appearing cells were counted. The surviving fraction was calculated using SigmaPlot 10.0 (San Jose, CA, USA).
Clonogenic survival assay for CRT with ganetespib
The combination effect of chemo radiation and ganetespib was assayed in vitro using clonogenic survival assay. Cells were seeded in 6-well plates 16 hours prior to treatment. The cells were treated by combination chemotherapy (Paclitaxel 3.51 nM with Carboplatin 24.23 nM) and/or ganetespib (30 nM) followed by radiation (2 Gy) after 4–5 hours. Drugs were washed out 24 hours after the treatment and the cells were maintained in normal cultural conditions for 12–15 days. The colonies were stained by crystal violet (0.1% in 20% methanol) (Sigma-Aldrich, St. Louis, MO, USA). Colony numbers were assessed visually and colonies containing > 50 normal-appearing cells were counted. The surviving fraction was calculated using GraphPad Prism 6 (La Jolla, CA, USA).
DNA repair foci formation assay
H460 and A549 cells were grown as monolayers on chamber slides with plastic bottom (Nunc Lab-Tek, Roskilde, Denmark) and were treated with DMSO or ganetespib (25 nM, 50 nM) 24 hours after seeding into culture chambers. Five hours after ganetespib treatment, the cells were subjected to irradiation at dose of 2 Gy. Thirty minutes, 4 hours, 24 hours and 48 hours after irradiation, cells were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature and washed in PBS. The cells were then permeabilized in 0.5% Triton X-100 for 10 min, and blocked in PBS with 3% BSA (Bovine serum albumin) for 20 min at room temperature. The cells were sequentially incubated with anti-53BP1 antibody (Cell Signaling Technology, Danvers, MA, USA) at 1:100 dilution overnight at 4 °C and Alexa Fluor® 488 Conjugate secondary antibody (Cell Signaling Technology, Danvers, MA, USA) at a 1:1000 dilution for 1 hour at 37°C in PBS with 1.5% BSA and washed three times in PBS. Nuclei were counterstained with 1:500 4-diamidino-2-phenylindole dihydrochloride (DAPI) in PBS. Cover glasses were mounted in Vectashield (Vector Laboratories). Fluorescence images were captured with Leica fluorescence microscope equipped with a CCD camera and images were imported into Advanced Spot Image analysis software. DNA repair foci were quantified using ImageJ software (NIH public domain).
Flow cytometry cell cycle analysis
H460 and A549 cells were treated with vehicle (DMSO) or ganetespib (50 nM) with or without irradiation as described above and were harvested 4 hours, 16 hours, 24 hours, 48 hours, 72 hours and 96 hours after irradiation and fixed in 70% ethanol for 1 hour at 4°C. The cells were stained with propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) for 15 min in the dark and FACS analysis was performed using a Becton-Dickinson FACS Calibur flow cytometer (BD Biosciences, Heidelberg, Germany) per the manufacturer’s instructions. Assays were performed in triplicates. The percentage of cells in each phase of the cell cycle (sub-G1, G1, S and G2/M) was determined with Flow-Jo (TreeStar Inc., Ashland, OR).
Flow cytometry apoptosis assay
Cells were treated with vehicle (DMSO) or ganetespib (50 nM) followed by irradiation (2 Gy) after 4 hours. Cells were trypsinized 24 hours post irradiation and stained by AnnexinV-FITC and propidium iodide using the Abcam apoptosis detection kit (ab14085, Abcam, Cambridge, United Kingdom) according to manufacturer’s instructions. The flow analysis was performed on Becton-Dickinson FACS Calibur flow cytometer (BD Biosciences, Heidelberg, Germany) and analyzed with Flow-Jo (TreeStar Inc., Ashland, OR). The assays were done in triplicate.
Reverse phase protein array (RPPA)
H460 and A549 cell lines were treated with vehicle (DMSO) or ganetespib (10 nM, 25 nM, 50 nM and 100 nM) with or without irradiation as described above and were harvested 24 hours after ganetespib treatment. Cell lysates were prepared using lysis buffer containing 1% Triton X-100, 50mM HEPES, pH 7.4, 150mM NaCl, 1.5mM MgCl2, 1mM EGTA, 100mM NaF, 10mM Na pyrophosphate, 1mM Na3VO4, 10% glycerol, containing freshly added protease and phosphatase inhibitors and 4× SDS sample buffer containing 40% Glycerol, 8% SDS, 0.25M Tris-HCL, pH 6.8. and 10% volume of 2-mercaptoethanol, arrayed on nitrocellulose-coated FAST slides (Whatman), and probed for a standard list of antibodies (34).
Immunoblot analysis
H460 cell lines were treated with vehicle (DMSO) or ganetespib (25 nM, 50 nM) with or without irradiation (2 Gy, 4 Gy) as described above and were harvested 24 hours after genetespib treatment. After washing with ice-cold PBS, cell lysates were collected in RIPA Buffer (Life Technologies, Grand Island, NY, USA) with protease inhibitor cocktail (Roche, Eugene, OR USA). Protein concentrations were measured by protein assay kit (Life Technologies, Grand Island, NY, USA). Total cellular lysates were loaded onto 4–12% Bis-Tris gel (Bio-Rad, Hercules, CA, USA), separated by electrophoresis and electro-transferred onto nitrocellulose membranes. Membranes were blocked for 1 h at room temperature in 5% nonfat dry milk in Tris-Buffer saline containing 0.1% Tween20 (TBST). The membrane was incubated with primary antibodies (p21, phospho-CDC2, CDC2, phospho-p90RSK, p90RSK, phospho-AKT, AKT, phospho-mTOR, mTOR, phospho-EGFR, EGFR, pS6, cleaved caspase 9 and caspase 7, phospho-histone γH2AX, GAPDH, β-actin antibodies are all from Cell Signaling Technology, Danvers, MA, USA) at 4 °C overnight and subsequently incubated with a secondary antibody for 1 hour at room temperature. After washing the membrane with TBST, HRP activity was detected using Clarity Western ECL substrate (Bio-Rad, Hercules, CA, USA) and analyzed by ChemiDoc imager (Bio-Rad, Hercules, CA, USA).
In vivo NSCLC xenograft model
Female NCr nu/nu mice (5 weeks old, from Taconic Biosciences, Germantown, NY, USA) were used for tumor studies. Animals were maintained in an Association for the Assessment and Accreditation of Laboratory Animal Care approved facility in accordance with current regulations of the U.S. Department of Agriculture and Department of Health and Human Services. Experimental methods were approved by and in accordance with institutional guidelines established by the Institutional Animal Care and Use Committee. For H460 xenografts, mice were subcutaneously inoculated with a total of 1 × 106 H460 cells in 20 ul PBS into their right hind legs to prepare the tumor model. For A549 xenografts, mice were subcutaneously inoculated with a total of 5 × 106 cells in 50 ul PBS into the right hind legs. When the tumor reached 100 mm3 (H460) or 150 mm3 (A549), mice were randomized into different treatment groups (4–10 animals per group). Mice were treated with Carboplatin (30mg/kg)-Paclitaxel (10mg/kg) intraperitoneally once weekly for two weeks and/or intravenously via the tail vein with 100 mg/kg ganetespib dissolved in 200 ul 10/18 DRD (10% DMSO, 18% Cremophore RH 40, 3.8% dextrose) once weekly for two weeks. Mice not included in the ganetespib treatment groups received the same volume of solvent. Five hours after drug treatment, irradiation was applied locally to the tumor-bearing legs of unanesthetized mice at 2 Gy once daily for 5 days. Tumor volumes (V) were calculated by caliper measurements of the width (W), and length (L) of each tumor using the formula: V=0.5 × (L × W2). The tumor growth curve was calculated using GraphPad Prism 6 (La Jolla, CA, USA).
Immunohistochemistry
Tumors were fixed in 1:10 diluted formalin (Fisher scientific, Cat# 245-684, Waltham, MA USA) overnight and embedded in paraffin. Tissue sections (2 mm) were deparaffinized in 100% xylene and rehydrated through incubation in descending ethanol dilutions (100–60%) followed by boiling at 125 °C for 2 min in Citrate buffers (10 mM Sodium Citrate pH 6). To reduce the endogenous peroxidase activity, slides were treated with 3% H2O2 for 10 min and subsequently probed with the primary antibody anti-cleaved caspase 7 (Cell Signaling Technology, Danvers, MA, USA) at 1:1000 dilution and secondary antibody SignalStain® Boost IHC Detection Reagent (HRP, Rabbit) (Cell Signaling Technology, Danvers, MA, USA). DAB (SignalStain® DAB Substrate Kit, Cell Signaling Technology, Danvers, MA, USA) was used as chromogen. Tissue sections were imaged using a PerkinElmer Vectra 2 microscope (PerkinElmer, Waltham, MA, USA) and analyzed with PerkinElmer Inform 2.1 analysis software (PerkinElmer, Waltham, MA, USA) at MD Anderson Cancer Center Flow Cytometry & Cellular Imaging Core Facility.
Statistical analysis
Statistical significance was assessed by Student’s t-test (2 sample assuming unequal variances) and expressed as standard mean error. A difference was considered significant if p < 0.05. RPPA statistical analysis used two-way analysis of variance (ANOVA) performed on a marker-by-marker base to test for the interaction between radiation and drug treatment and one-way ANOVA for drug only effect. Pairwise comparisons between different drug doses were done using Tukey's honest significance (HSD) Test with 95% family-wise confidence level. To control for multiple testing, the resulting p-values, computed from test statistics applied, were modeled using a beta-uniform mixture (BUM) model in order to select p-value cutoffs according to pre-defined false discovery rates (FDRs). All statistical analyses of RPPA data were performed using R (version R3.1.0) and Bioconductor packages (http://www.r-project.org/).
Results
Ganetespib sensitizes NSCLC cells to radiation
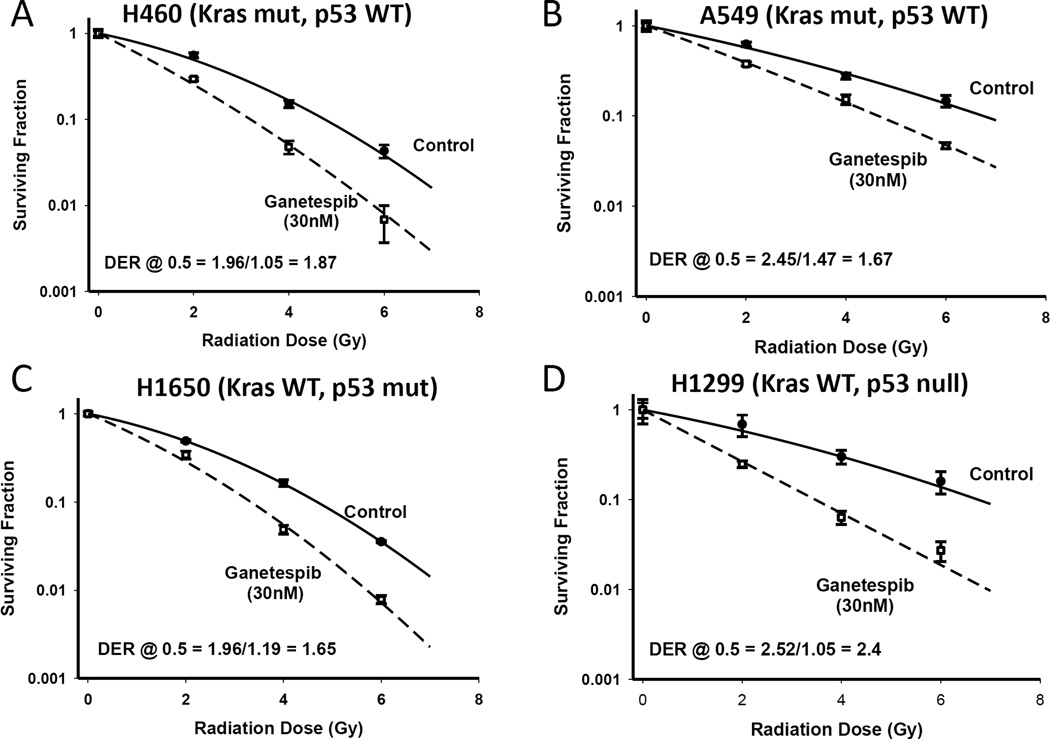
We assessed the ability of ganetespib to radiosensitize human NSCLC cells of varying genetic backgrounds using clonogenic survival curve assays. We evaluated the Kras mutant/p53 wild type cells H460 and A549, Kras wild type/p53 mutant cells H1650 cells, and the Kras wild type/p53 null H1299 cell lines. Cells were exposed to ganetespib at 30 nM for 5 hours, subsequently irradiated with gamma-rays and incubated for a further 19 hours in the presence of ganetespib. Irradiation in combination with ganetespib had a strong radiosensitizing effect on H460 and H1299 cells. Moderate sensitizing effects of ganetespib were observed for the A549 and H1650 cells (Figure 1 A–D). The radiosensitivity enhancement ratios at a survival rate of 50% were 1.87 in the H460 cell, 1.67 in the A549, 1.65 in H1650 and 2.4 in H1299. The results indicated that ganetespib can potentiate the radiation effect in different NSCLC cells.
Figure 1. Ganetespib is a potent radiation sensitizer in non-small cell lung cancer cell lines.
Clonogenic survival curves for H460 and A549 (Kras mutant, p53 WT) (A–B), H1650 (Kras WT, p53 mutant) (C) and H1299 (Kras WT and p53 null) (D), cells treated with or without 30 nM ganetespib for 5 hours prior to irradiation followed by an additional 19 hours of post-irradiation incubation in ganetespib containing medium.
Ganetespib inhibits radiation-induced DNA damage repair foci in NSCLC cancer cells
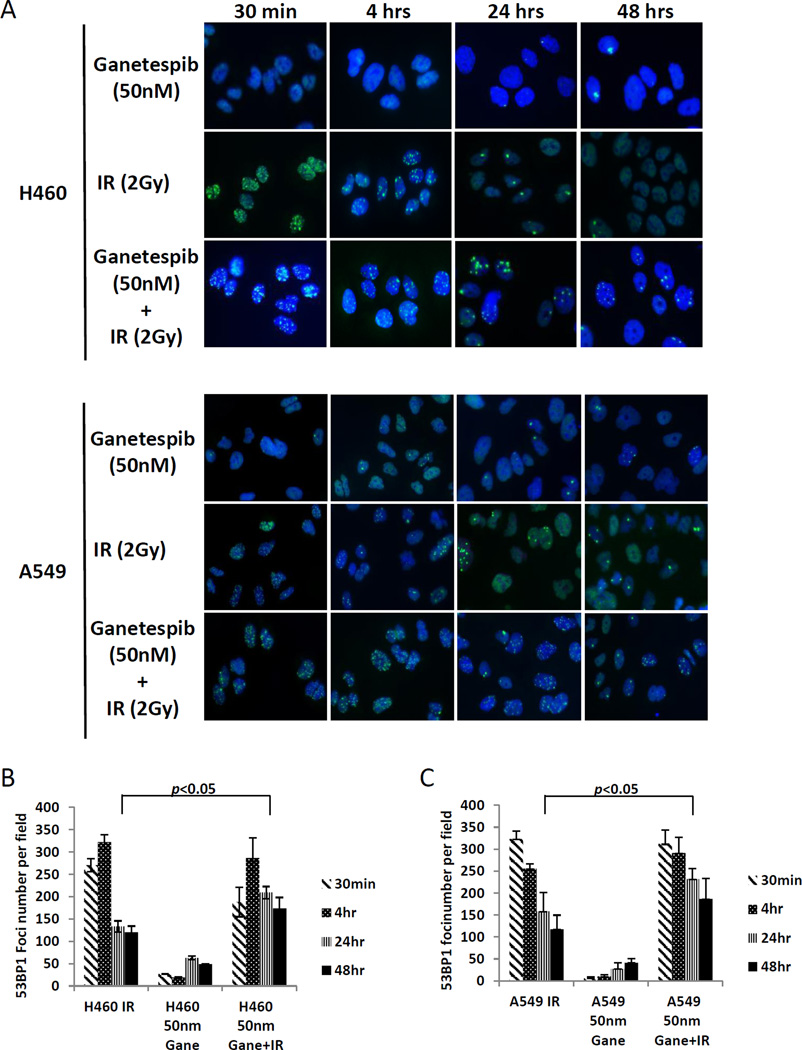
Double-strand DNA breaks induced by radiation, if unrepaired, can lead to genomic instability and cell death. To determine if radiation induced DNA damage repair can be hampered by ganetespib, we measured the DNA damage repair response by evaluating 53BP1 foci formation (Figure 2A–C). In both the H460 and A549 cells, a substantial rise in 53BP1 foci formation is seen after 30 minutes of 2 Gy radiation for both the IR alone group and the IR+ganetespib group, but not apparent with ganetespib alone. While this effect is sustained at 4 hours, by 24 hours, the number of foci was reduced in the IR alone group but not to the same level as the ganetespib alone group. However, in both cell lines, the number of foci in the cells exposed to both ganetespib and radiation was sustained significantly longer compared with radiation alone at both the 24 and 48 hour time points. This suggests that ganetespib impairs the repair of radiation-induced double-strand DNA breaks.
Figure 2. Ganetespib attenuate DNA damage repair in H460 and A549 cells assessed by p53BP1 foci formation.
Detection of p53BP1 foci formation was performed 30 mins, 4 hrs, 24 hrs and 48 hrs after irradiation (2 Gy) with or without 50 nM ganetespib pre-treatment prior to irradiation (A). Statistical data are presented as average foci number of 12 high power (20×) fields (B-C). Asterisks indicate p < 0.05.
G2/M arrest induced by irradiation is further intensified by ganetespib pretreatment
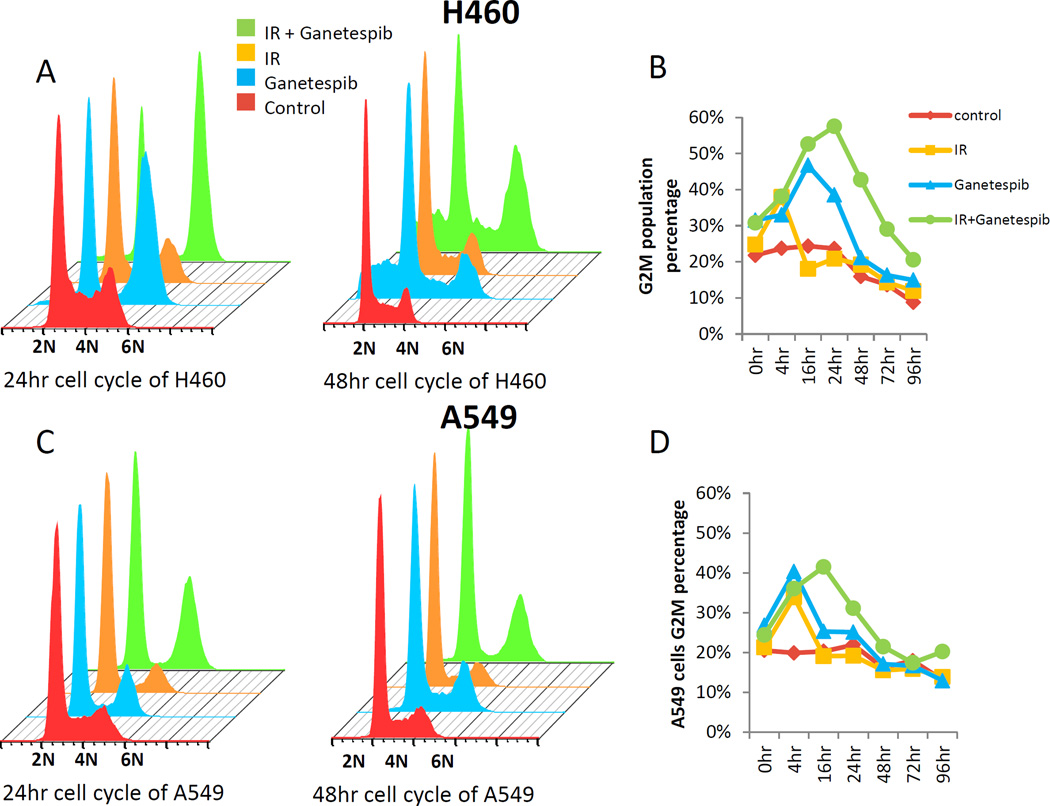
As Hsp90 inhibitors are known to affect the cell cycle checkpoint (19, 21–24, 35, 36), as does irradiation, which causes a G1/S arrest in P53 WT cells (37), we next determined the interaction of radiation with ganetespib on the cell cycle effects in both H460 and A549 cells (Figure 3A–D). G2/M arrest was induced by ganestepib, radiation, and the combination, at 4 hours postirradiation. For both H460 and A549, the irradiation effect normalized by 16 hours, but for ganetespib alone the effect continued for 16 hours, but is mostly restored to baseline by 48 hours. The addition of ganetespib appeared to further augment the radiation-induced G2/M arrest in both H460 and A549 cells at 16–24 hour, an effect that is not fully restored to baseline in H460 until nearly 96 hours post-irradiation (Figure 3B).
Figure 3. Ganetespib increases radiation induced G2M arrest.
H460 (A–B) and A549 (C–D) cells were treated with or without ganetespib (50 nM) for 5 hrs and then irradiated with 2 Gy. Cells were collected at each time point thereafter and analyzed by flow cytometry for the percentage of cells in G2/M. Drug treatment was continued after irradiation in the ganetespib-treated groups.
Ganetespib pretreatment accentuates the radiation effect by altering expression level of checkpoint proteins and kinase activities
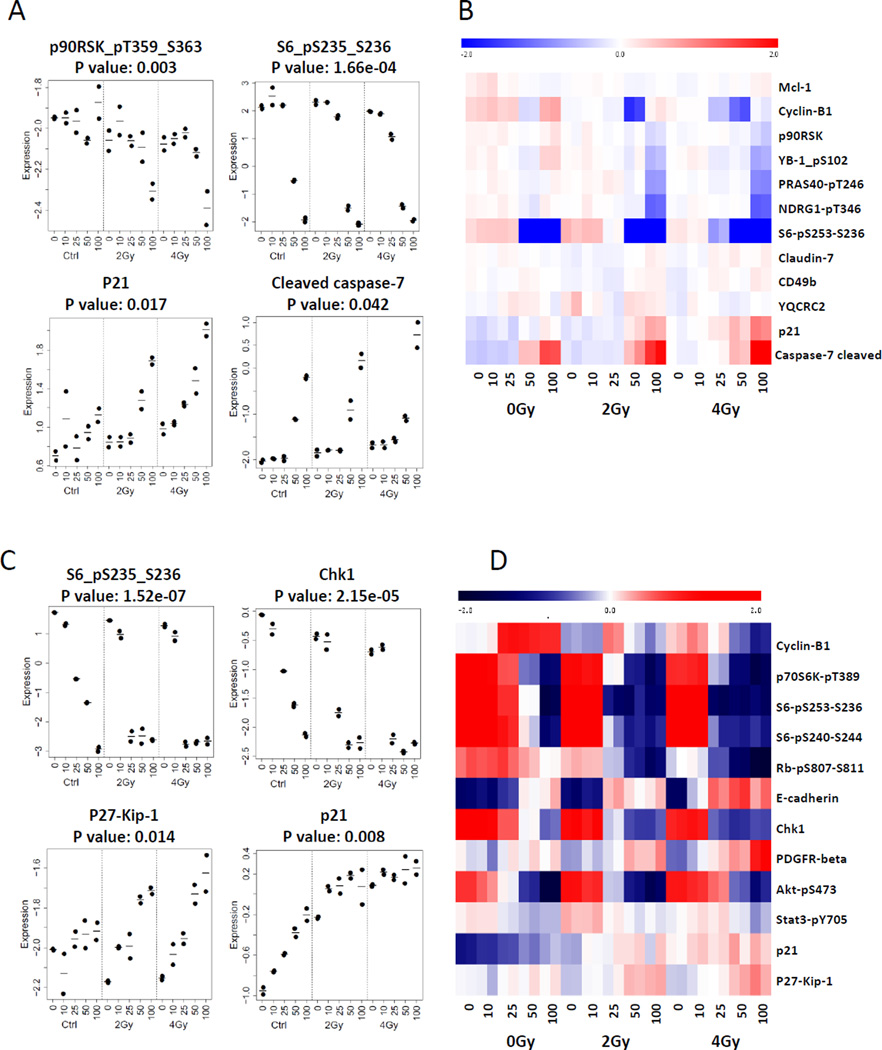
We next sought to define the mechanisms by which ganetespib reduced clonogenic survival and sensitized NSCLC cells to radiation. Cell lysate was prepared from H460 cells and A549 cells treated with ganetespib at 10 nM, 25 nM, 50 nM and 100 nM, with or without 2 and 4 Gy radiation for 24 hours, and RPPA was performed. As shown in Supplemental Figures 1 and 2, many oncogenic proteins were downregulated by treatment of ganetespib alone including phospho-Akt, phospho-mTOR, phospho-p90RSK, and EGFR. Although radiation can induce the activation of Akt and mTOR at the doses of 2–4 Gy, as previously reported(38, 39), the combination of ganetespib suppressed this activation along with other phosphokinases in general (Supplemental Figure 3A). In addition to oncogenic proteins, ganetespib pretreatment followed by radiation also altered the expression levels of checkpoint proteins (Figure 4A–D, Supplemental Figure 3B). Ganetespib dose-dependently upregulated pCDC2 (pCDK1), but in combination with radiation there was instead a downregulation of pCDC2 at higher ganetespib levels. CDC2 is known to be involved in the G2/M checkpoint by regulating the activity of cyclin-dependent kinase inhibitor p21 which regulates G2/M transition. Ganetespib combined with radiation reduced the activity of phospho-p90RSK and phospho-S6, which is known as a regulator of CDC2 (Figure 4A–D, Supplemental Figure 3B) at the dose of radiation (2–6 Gy) and ganetespib (25–100 nM). In A549 cells, cell cycle checkpoint proteins p21, p27, Chk1, Rb and phospho-S6 are also altered after the combined treatment (Figure 4C–D). Ganetespib combined with radiation also increased cleaved caspase 7 and caspase 9 and DNA damage repair protein phospho-γH2AX that were not apparent with radiation alone (Supplemental Figure 3B). Apoptosis was also determined by Annexin V-PI staining and flow cytometry analysis (Supplemental Figure 4 A–B). There was a significant increase in the percentage of apoptotic cells with ganetespib treatment alone, an effect that was not enhanced with radiation.
Figure 4. Ganetespib altered expression level and activity of client growth factors and cell cycle progression proteins.
H460 and A549 cells were treated with ganetespib at 0 nM, 10 nM, 25 nM, 50 nM and 100 nM 5 hrs prior to irradiation (0 Gy, 2 Gy and 4 Gy) followed by an additional 19 hours of post-irradiation incubation in ganetespib containing medium. H460 and A549 cell lysates were collected for RPPA analysis (A–D). Heat map of RPPA results indicated altered activities or expression level of proteins (B and D).
Ganetespib demonstrated variable sensitizing effects with CRT in vitro and in vivo
Since CRT is the standard treatment for unresectable locally advanced NSCLC, adding a radiation sensitizer like ganetespib may improve clinical outcomes. In order to assay whether ganetespib enhances the treatment efficacy of CRT, we performed clonogenic survival assay using A549 and H460 (Figure 5A–B). Robust clonogenic survival reduction was again seen in both cell lines when treated with ganetespib and IR. When chemotherapy was added, ganetespib was able to further enhance treatment efficacy for both chemotherapy and CRT in H460 cells but not in A549 cells. In A549, there was even a paradoxical increase in clonogenic survival when ganetespib was added to chemotherapy or CRT. Since A549 and H460 are both p53 wild-type cells, we tested two cell lines that harbored p53 mutations, H2087 and H358 (Figure 5 C–D). While ganetespib was again able to sensitize both of the cell lines to radiation, this was no longer apparent in the H2087 cells when co-treated with chemotherapy.
Figure 5. Ganetespib demonstrates variable effects with CRT in vitro.
Clonogenic survival assay results of H460, A549 (A–B) and H2087, H358 (C–D) tested with radiation alone, chemotherapy alone, or in combination with ganetespib.
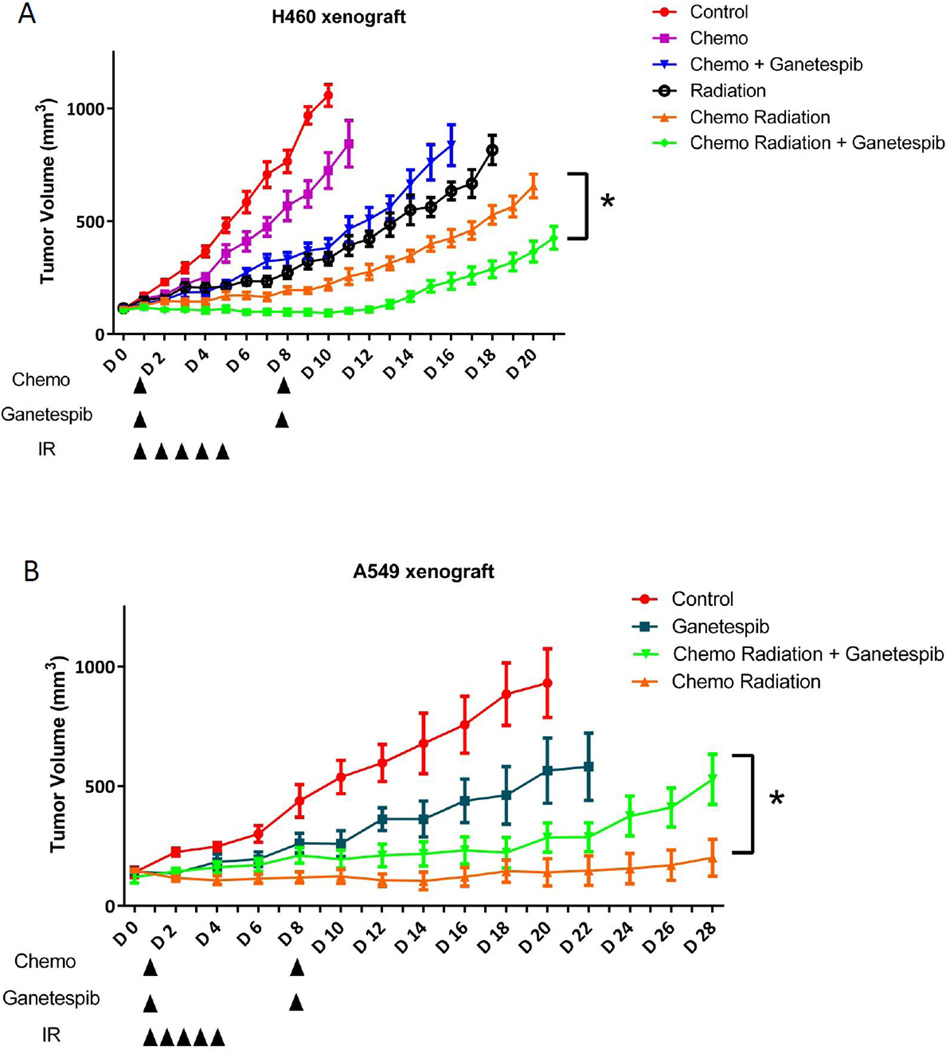
Next, we assessed the effect of ganetespib on anti-tumor treatment sensitivity in vivo compared to CRT alone. For this, we established a treatment protocol that reflected a shortened version of what’s done clinically for unresectable NSCLC using fractionated daily 2 Gy radiation and concurrent chemotherapy with carboplatin and paclitaxel for 5 days, followed by consolidation chemotherapy alone for the second week. We compared this “standard therapy” to chemotherapy alone or radiation alone, with or without two doses of once-weekly ganetespib. As shown in Figure 6A, in H460 xenografts, chemotherapy alone showed only minimal tumor growth inhibition, but when combined with ganetespib, the anti-tumor effect of chemotherapy was significantly increased. As expected, combined CRT had much stronger anti-tumor effect than either chemotherapy or radiation alone. However, ganetespib added to CRT produced the greatest tumor growth delay (Figure 6A). The addition of ganetespib to CRT delayed tumor growth by 7 days compared with CRT and by 17 days compared with non-treated control.
Figure 6. Ganetespib shows differential effects with CRT in H460 and A549 xenografts.
Ncr nude mice were injected with 1 × 106 H460 cells or 5 × 106 A549 subcutaneously in the right leg. When tumor volume reached around 100 mm3 for H460 or 150 mm3 for A549, mice bearing established xenograft (n=4–10/group) were exposed to radiation at 2 Gy daily for 5 days, with or without i.p. 30 mg/kg Carboplatin + 10 mg/kg Paclitaxel 1×/week for 2 weeks and combined with i.v. 100 mg/kg ganetespib 1×/week for 2 weeks, either alone or in combination. Tumor volumes are indicated as average of each treatment group and the error bars are the SEM.
To determine if the DNA damage and cytotoxic effects of treatment in vivo could be compared to what we saw in vitro, we performed immunohistochemical staining for 53BP1 foci and cleaved caspase 7 on tumors removed at the end of the first 5 days of treatment. Compared to all the treatment groups, the tumors treated with the combination of ganetespib with CRT had significantly higher levels of P53BP1 foci and cleaved caspase 7 levels (Supplemental Figure 5). These results demonstrate that the enhanced DNA damage and cytotoxic effect of ganetespib with radiation seen in vitro could be further enhanced with concurrent CRT.
Given the paradoxical in vitro clonogenic survival assay results of the A549 cell line, we determined if such results could be recapitulated in vivo (Figure 6B). Ganetespib alone has antitumor effects by causing tumor growth delay for nearly a week. CRT had an even stronger effect on preventing tumor regrowth, but when ganetespib was added to CRT it caused rapid tumor progression after an initial period of tumor control.
Discussion
In clinical radiotherapy, tumor radioresistance is one of the causes of local failure after radiotherapy. The development of drugs that can enhance the sensitivity of tumor cells to radiation is of great importance to improve the outcomes of lung cancer therapy. Although there are many studies that have focused on the development of radiosensitizers, the targeted agents that have been tested clinically, namely vascular targeting drugs and EGFR inhibitors, have so far not been clinically useful when combined with CRT for NSCLC. Unfortunately most of rationale for the combination has been based on preclinical studies using radiation alone. The best example was the EGFR targeting drugs. It has been well established in preclinical studies that EGFR inhibition sensitizes radiation for multiple tumor types, including head and neck cancer and NSCLC(29, 40). The approach seemed promising when a survival benefit was demonstrated for cetuximab when it was combined with radiotherapy compared to radiotherapy alone in head and neck cancer in a phase III randomized trial(32). Unfortunately, the benefit of cetuximab disappeared when it was combined with CRT, as shown in two large randomized trials for head and neck cancer (RTOG 0522 (41)) and unresectable NSCLC (RTOG 0617 (8)). This experience highlights the importance of critically evaluating radiosensitizers in preclinical models that at least modestly reflect the treatment regime used in the clinical setting.
Several studies have shown that Hsp90 inhibitors can enhance radiation sensitivity of human cancer cell lines of different origin(14–25). These sensitizing effects are the result of the Hsp90 inhibitor-mediated abrogation of the G2 checkpoint, apoptosis and the inhibition of DNA repair. Studies have shown that one of the causes of sensitization could be inhibition of DNA double strand break (DSB) repair (17–21, 36). Checkpoint arrest mainly at G2/M phase has also been suggested as a cause of radiosensitization with Hsp90 inhibitors (19–24, 36). Radiosensitization effect in vivo by Hsp90 inhibitors has also been demonstrated (14, 21, 23). These data strongly suggest that targeting Hsp90 with its inhibitors represents a promising strategy for enhancing the sensitivity of cancer cells to radiation (19–24). Ganetespib is an investigational small molecule inhibitor of Hsp90 with favorable pharmacologic properties that distinguishes it from other first- and second-generation Hsp90 inhibitors in terms of potency, safety, and tolerability (27, 42). Ganetespib has also been shown to possess robust antitumor activity against a variety of cancer types in preclinical studies, including lung, breast, and prostate (38, 43–47). In addition, it has been shown in NSCLC cell lines that a synergistic combinatorial benefit was seen with the taxanes such as paclitaxel or docetaxel (43).
As expected, we demonstrated that ganetespib significantly reduced clonogenic survival of various lung cancer cell lines, attenuated DNA damage repair, induced cell cycle arrest by the enhanced upregulation of negative cell cycle regulators (p21 and/or p27) and/or downregulation of positive regulators (cyclins D1 and E, CDK1, CDK2 and CDK4) that was greater than either treatment alone. Since CRT with carboplatin and paclitaxel is the standard treatment regime for unresectable NSCLC, adding ganetespib to standard CRT may have synergistic effects that need preclinical validation. Importantly, we have demonstrated that ganetespib has variable effects when combined with CRT in vitro and in two xenograft NSCLC models in vivo. Our in vitro clonogenic survival assay results showed that ganetespib alone enhances radiation effects in a panel of cell lines, but it demonstrated variable effects when combined with chemo or CRT. The xenograft models of H460 and A549 confirmed what we observed in vitro. H460 xenograft tumors gained additional benefits by adding ganetespib when treated with CRT, showing delayed tumor growth and increased DNA damage and apoptosis. On the other hand, A549 xenograft tumors was very well controlled by CRT alone but progressed rapidly when ganetespib was added. The xenograft results are consistent with what we saw in vitro, where there was also an increase in clonogenic cell survival when ganetespib was added to either chemotherapy or CRT. On the surface, the cell lines that do not respond to ganetespib when combined to CRT do not appear to be related to Kras or p53 mutation statuses. The mechanism for the variable effects of ganetespib in the various cell lines needs further investigation.
Our findings imply that only a subgroup of lung cancer patients may benefit from HSP90 inhibition when receiving CRT. This may warrant the need for a predictable biomarker that could potentially identify patients to receive or avoid HSP90 inhibitors in combination with chemotherapy or CRT. It is important to emphasize the fact that drugs which sensitize cancer cells to radiation may not have the same effect when added with chemotherapy or CRT. This may explain the failure of previous trials using EGFR inhibitors in combination with CRT as well as the recent futility closure of the GALAXY-II docetaxel-ganetespib trial in lung cancer. Future development of the optimal drug and radiation combination needs to be tested in rigorous preclinical models within the context of clinically-relevant therapy combinations.
Supplementary Material
Translational Relevance.
The current study shows that ganetespib, a selective and highly potent Hsp90 inhibitor, inhibits clonogenic survival in NSCLC cells, and synergizes the efficacy of irradiation. This provided the motivation to explore the combination of ganetespib with CRT to improve the clinical outcomes of unresectable NSCLC. When it was tested with CRT, ganetespib was able to sensitize some cell lines, but in others it had either no radiation sensitization effect or even abrogated the CRT effect. Our data cautions the assumption that drugs that sensitize to radiation would also sensitize to CRT. Rigorous and systematic preclinical testing of drugs must be done in the clinical context in which the disease is being treated. Identifying ways to circumvent this variable effect or predictive biomarkers will be needed for optimal translation of radiation sensitizing drugs to the clinical setting.
Acknowledgments
Research Support: Funding was provided in part by The Mabuchi Program in Targeted Radiotherapy, United Against Lung Cancer, and The University of Texas MD Anderson Cancer Center and by the National Cancer Institute Cancer Center Support Grant CA016672.
We thank Dr. Jared Burks, director of MD Anderson Cancer Center Flow Cytometry & Cellular Imaging Core Facility, for expert assistance with quantification of immunohistochemistry staining.
SHL receive research funding support from STCube Pharmaceuticals, Roche/Genentech, and is the principle investigator of a clinical trial combining Ganetespib with chemoradiotherapy in esophageal cancer (NCT02389751) but does not receive financial support from Synta Pharmaceuticals, the manufacturer of Ganetespib. DAP was an employee of Synta Pharmaceuticals.
S. H. Lin is funded in part through a research contract with STCube Pharmaceuticals, which does not manufacture or market any of the drugs discussed. D.A. Proia was director at Synta Pharmaceuticals Corp.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: S.H. Lin, Y. Wang, H. Liu
Development of methodology: H. Liu, Y. Wang, A. Potter, J. Zhang, Y. Qiao
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H. Liu, Y. Wang, A. Potter, J. Zhang, Y. Qiao
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): H. Liu, L. Diao, J. Wang
Writing, review, and/or revision of the manuscript: S.H. Lin, Y. Wang, H. Liu, D.A. Proia,
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S.H. Lin, H. Liu, Y. Wang, J. Zhang, Y. Qiao, D.A. Proia
Study supervision: S.H. Lin
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 3.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 4.Curran WJ, Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komaki R, Scott CB, Sause WT, Johnson DH, Taylor SGt, Lee JS, et al. Induction cisplatin/vinblastine and irradiation vs. irradiation in unresectable squamous cell lung cancer: failure patterns by cell type in RTOG 88-08/ECOG 4588. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1997;39:537–544. doi: 10.1016/s0360-3016(97)00365-9. [DOI] [PubMed] [Google Scholar]
- 6.Rowell NP, O'Rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD002140.pub2. CD002140. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. The Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. The Lancet. 2013;14:e358–e369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 10.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel HJ, Modi S, Chiosis G, Taldone T. Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert Opin Drug Discov. 2011;6:559–587. doi: 10.1517/17460441.2011.563296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 14.Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. [PubMed] [Google Scholar]
- 15.Russell JS, Burgan W, Oswald KA, Camphausen K, Tofilon PJ. Enhanced cell killing induced by the combination of radiation and the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin: a multitarget approach to radiosensitization. Clin Cancer Res. 2003;9:3749–3755. [PubMed] [Google Scholar]
- 16.Machida H, Nakajima S, Shikano N, Nishio J, Okada S, Asayama M, et al. Heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin potentiates the radiation response of tumor cells grown as monolayer cultures and spheroids by inducing apoptosis. Cancer Sci. 2005;96:911–917. doi: 10.1111/j.1349-7006.2005.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi M, Yu D, Hirayama R, Ninomiya Y, Sekine E, Kubota N, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 18.Koll TT, Feis SS, Wright MH, Teniola MM, Richardson MM, Robles AI, et al. HSP90 inhibitor, DMAG, synergizes with radiation of lung cancer cells by interfering with base excision and ATM-mediated DNA repair. Mol Cancer Ther. 2008;7:1985–1992. doi: 10.1158/1535-7163.MCT-07-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stingl L, Stuhmer T, Chatterjee M, Jensen MR, Flentje M, Djuzenova CS. Novel HSP90 inhibitors, NVP-AUY922 and NVP-BEP800, radiosensitise tumour cells through cell-cycle impairment, increased DNA damage and repair protraction. Br J Cancer. 2010;102:1578–1591. doi: 10.1038/sj.bjc.6605683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha K, Fiskus W, Rao R, Balusu R, Venkannagari S, Nalabothula NR, et al. Hsp90 inhibitor-mediated disruption of chaperone association of ATR with hsp90 sensitizes cancer cells to DNA damage. Mol Cancer Ther. 2011;10:1194–1206. doi: 10.1158/1535-7163.MCT-11-0094. [DOI] [PubMed] [Google Scholar]
- 21.Zaidi S, McLaughlin M, Bhide SA, Eccles SA, Workman P, Nutting CM, et al. The HSP90 inhibitor NVP-AUY922 radiosensitizes by abrogation of homologous recombination resulting in mitotic entry with unresolved DNA damage. PLoS One. 2012;7:e35436. doi: 10.1371/journal.pone.0035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djuzenova CS, Blassl C, Roloff K, Kuger S, Katzer A, Niewidok N, et al. Hsp90 inhibitor NVP-AUY922 enhances radiation sensitivity of tumor cell lines under hypoxia. Cancer Biol Ther. 2012;13:425–434. doi: 10.4161/cbt.19294. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi N, Wild AT, Chettiar ST, Aziz K, Kato Y, Gajula RP, et al. Novel Hsp90 inhibitor NVP-AUY922 radiosensitizes prostate cancer cells. Cancer Biol Ther. 2013;14:347–356. doi: 10.4161/cbt.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milanovic D, Firat E, Grosu AL, Niedermann G. Increased radiosensitivity and radiothermosensitivity of human pancreatic MIA PaCa-2 and U251 glioblastoma cell lines treated with the novel Hsp90 inhibitor NVP-HSP990. Radiat Oncol. 2013;8:42. doi: 10.1186/1748-717X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enomoto A, Fukasawa T, Takamatsu N, Ito M, Morita A, Hosoi Y, et al. The HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin modulates radiosensitivity by downregulating serine/threonine kinase 38 via Sp1 inhibition. Eur J Cancer. 2013;49:3547–3558. doi: 10.1016/j.ejca.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 26.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 27.Zhou D, Liu Y, Ye J, Ying W, Ogawa LS, Inoue T, et al. A rat retinal damage model predicts for potential clinical visual disturbances induced by Hsp90 inhibitors. Toxicol Appl Pharmacol. 2013;273:401–409. doi: 10.1016/j.taap.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Ramalingam S, Goss G, Rosell R, Schmid-Bindert G, Zaric B, Andric Z, et al. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1) Ann Oncol. 2015;26:1741–1748. doi: 10.1093/annonc/mdv220. [DOI] [PubMed] [Google Scholar]
- 29.Milas L, Fan Z, Andratschke NH, Ang KK. Epidermal growth factor receptor and tumor response to radiation: in vivo preclinical studies. Int J Radiat Oncol Biol Phys. 2004;58:966–971. doi: 10.1016/j.ijrobp.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Nasu S, Ang KK, Fan Z, Milas L. C225 antiepidermal growth factor receptor antibody enhances tumor radiocurability. Int J Radiat Oncol Biol Phys. 2001;51:474–477. doi: 10.1016/s0360-3016(01)01671-6. [DOI] [PubMed] [Google Scholar]
- 31.Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. The Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 32.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Casal R, Bhattacharya C, Epperly MW, Basse PH, Wang H, Wang X, et al. The HSP90 Inhibitor Ganetespib Radiosensitizes Human Lung Adenocarcinoma Cells. Cancers. 2015;7:876–907. doi: 10.3390/cancers7020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 35.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nature reviews Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 36.Niewidok N, Wack LJ, Schiessl S, Stingl L, Katzer A, Polat B, et al. Hsp90 Inhibitors NVP-AUY922 and NVP-BEP800 May Exert a Significant Radiosensitization on Tumor Cells along with a Cell Type-Specific Cytotoxicity. Transl Oncol. 2012;5:356–369. doi: 10.1593/tlo.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He S, Zhang C, Shafi AA, Sequeira M, Acquaviva J, Friedland JC, et al. Potent activity of the Hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expression. Int J Oncol. 2013;42:35–43. doi: 10.3892/ijo.2012.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan M, Han ZC. Phosphatidylinositide 3-kinase/AKT in radiation responses. Histol Histopathol. 2004;19:915–923. doi: 10.14670/HH-19.915. [DOI] [PubMed] [Google Scholar]
- 40.Raben D, Helfrich B, Bunn PA., Jr Targeted therapies for non-small-cell lung cancer: biology, rationale, and preclinical results from a radiation oncology perspective. Int J Radiat Oncol Biol Phys. 2004;59:27–38. doi: 10.1016/j.ijrobp.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 41.Adkins D, Ley J, Wildes TM, Michel L. RTOG 0522: huge Investment in patients and resources and no benefit with addition of cetuximab to radiotherapy--why did this occur? J Clin Oncol. 2015;33:1223–1224. doi: 10.1200/JCO.2014.59.6908. [DOI] [PubMed] [Google Scholar]
- 42.Ying W, Du Z, Sun L, Foley KP, Proia DA, Blackman RK, et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther. 2012;11:475–484. doi: 10.1158/1535-7163.MCT-11-0755. [DOI] [PubMed] [Google Scholar]
- 43.Proia DA, Sang J, He S, Smith DL, Sequeira M, Zhang C, et al. Synergistic activity of the Hsp90 inhibitor ganetespib with taxanes in non-small cell lung cancer models. Invest New Drugs. 2012;30:2201–2209. doi: 10.1007/s10637-011-9790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acquaviva J, Smith DL, Sang J, Friedland JC, He S, Sequeira M, et al. Targeting KRAS-mutant non-small cell lung cancer with the Hsp90 inhibitor ganetespib. Mol Cancer Ther. 2012;11:2633–2643. doi: 10.1158/1535-7163.MCT-12-0615. [DOI] [PubMed] [Google Scholar]
- 45.Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, et al. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18:4973–4985. doi: 10.1158/1078-0432.CCR-11-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedland JC, Smith DL, Sang J, Acquaviva J, He S, Zhang C, et al. Targeted inhibition of Hsp90 by ganetespib is effective across a broad spectrum of breast cancer subtypes. Invest New Drugs. 2014;32:14–24. doi: 10.1007/s10637-013-9971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proia DA, Zhang C, Sequeira M, Jimenez JP, He S, Spector N, et al. Preclinical activity profile and therapeutic efficacy of the HSP90 inhibitor ganetespib in triple-negative breast cancer. Clin Cancer Res. 2014;20:413–424. doi: 10.1158/1078-0432.CCR-13-2166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.