Abstract
There is currently a lack of understanding how genetic background and sex differences attribute to the heterogeneity of obsessive-compulsive disorder (OCD). An animal model of compulsive-like behaviors has been developed through bidirectional selection of house mice (Mus musculus) for high (big cotton nests; BIG mice) and low levels (small nests; SMALL mice) of nest-building behavior. The BIG male strains have predictive and face validity as a spontaneous animal model of OCD. Here, we evaluated compulsive-, anxiety-, cognitive-, and depression-like behaviors among male and proestrus female replicate strains each of BIG (BIG1, BIG2) and SMALL (SML1, SML2) nest-builders, and randomly-bred Controls (C1, C2). BIG1 and BIG2 males and females had higher nesting scores when compared to SMALL and Control strains. Male BIG1 and BIG2 strains showed more compulsive-like nesting than BIG1 and BIG2 proestrus females, which was not observed among the other strains. Nesting scores were also different between BIG replicate male strains. A similar pattern was observed in the compulsive-like marble burying behavior with BIG strains burying more marbles than SMALL and Control strains. Significant replicate and sex differences were also observed in marble burying among the BIG strains. The open field test revealed replicate effects while the BIG strains showed less anxiety-like behavior in the elevated plus maze test compared to the SMALL strains. For novel object recognition only the Control strains showed replicate and sex differences. In the depression-like forced swim test proestrus females demonstrated less depression-like behavior than males. BIG and SMALL nest-building strains had a higher corticosterone stress response than the Control strains. Together these results indicate a strong interplay of genetic background and sex in influencing expression of behaviors in our compulsive-like mouse model. These results are in congruence with the clinical heterogeneity of OCD.
Keywords: OCD, non-induced mouse model, strain effects, sex effects, genetic background
1. Introduction
Obsessive-compulsive disorder (OCD) is a debilitating psychiatric condition characterized by invasive and persistent thoughts (obsessions) and repetitive behaviors (compulsions) [4]. OCD has an estimated lifetime prevalence of 2.3% in the United States [97]. The majority of patients suffering from OCD perform repetitive rituals to mitigate uncomfortable feelings of anxiety [32]. Others engage in repetitive behaviors due to subjective sensations also called sensory phenomena [79]. Obsessions can be associated with contamination, fear and symmetry, while compulsions include hand washing, checking or counting [88]. Such excessive ritualistic behaviors become distressing and significantly interfere with daily functioning [4]. OCD exhibits a large behavioral repertoire with high rates of repetition and provides an ethological basis for studying compulsive-like behaviors in animal models of OCD [11]. Though inappropriate for investigating the entire OCD spectrum because obsessions cannot readily be assessed, animal models can provide deep insight into various forms of compulsivity [74].
Sex differences in OCD [14, 29, 63] is thought to contribute to the heterogeneity of OCD [59]. Age of onset [14], phenomenology [59] and co-morbidity [69] are some of the gender related differences that have been observed. Males usually have an earlier onset for most symptom dimensions of OCD than females [23, 72, 82, 83]. Clinical studies have indicated that sexual, religious and symmetrical obsessions, and compulsive checking and ordering/arranging are more frequently seen in males than females, while females tend to exhibit more obsessions for contamination and compulsive cleaning than men [30, 62, 72, 108].
In females, ovarian hormones may play a critical role in modulating obsessions and compulsions [113]. Fluctuations in the female hormonal cycle may contribute to increased risk of onset and exacerbation of OCD symptoms at certain reproductive events, including premenstruum phase, menstrual cycle, pregnancy, and postpartum [1, 63, 73, 109, 113]. Few rodent studies have established that the female sex hormone estrogen can modulate compulsive-like behaviors [33, 37, 58]. The proestrus stage of the estrous cycle in female mice has higher circulating levels of estrogen influencing anxiety-, cognitive- and depression-like behaviors [106, 116] and, therefore, the comparison between males and proestrus females for compulsive-like behaviors may provide additional ways to gain important insights using animal models of OCD.
Additionally, inbred female mouse strains have shown significant differences in expression of drug induced compulsive-like behavior [27], indicating that strain comparisons can be valuable for understanding expression of compulsivity. This is important considering the compelling genetic basis of OCD from various human studies [25, 51, 52, 56, 76, 96, 105], which coupled with the sex differences can provide crucial clues about the complex interactions of these two elements in expression of compulsive and affective behaviors.
According to Maio et al. [74], comparing our compulsive-like mouse strains is valuable for understanding the disorder. Compulsive-like behavior in our mice is defined as excessive and repetitive expression of otherwise normal behaviors, i.e., nest-building and marble burying. For example, nest-building in the compulsive-like strains (BIG1 and BIG2) involves rapid and repeated movements of the front legs and mouth to pull excessive amount of cotton through the cage top metal bars over extended periods of time [50], which shows face validity with repetitive behaviors in OCD [79]. This rapid, excessive and repetitive nesting behavior is not observed in the Control and SMALL strains. The BIG strains also exhibit predictive validity as a spontaneous non-induced model of OCD-like behaviors through attenuation of these compulsive-like behaviors with fluoxetine and clomipramine treatment, which are first line treatments for OCD [50]. In addition, the tricyclic antidepressant desipramine, which is not effective in treating OCD, did not change the compulsive-like behaviors in the compulsive-like mice [50]. These strains were developed by bidirectional selection for high and low levels of nest-building behavior [19, 71] using a stock population, i.e., HS/Ibg outbred strain, that was derived from a cross among eight inbred house mouse, Mus musculus, strains, i.e., A, AKR, BLB/c, C3H/2, C57BL, DBA/2, Is/Bi, and RIII [71, 77].
Bidirectional selection resulted in three levels of nest-building behavior (with two replicate strains within each level). The replicates within each level of nest building were maintained as separate strains, i.e., not interbred with the other replicate, but subjected to the same selection regime. Using replicate strains is important to make sure that responses to artificial selection are due to selection and not the result of founder effects or random genetic drift when comparing the different levels of selection, i.e., selection for building big nests or small nests, or randomly bred [17, 19]. The two BIG strains consistently display high levels of nesting with a forty-fold difference in the amount of cotton used when compared to the two SMALL strains which display very low levels of nesting [19, 71]. The two randomly-bred strains serve as a selection Control and show intermediate levels of nesting [19, 71].
Compulsive-like nesting in our mice has a genetic factor with about 30% of the variation in behavioral expression among individuals due to additive genetic factors and about 70% of the variation due to environmental factors [19, 71]. This estimate of heritability of nest building falls within the range of heritability estimates of 0.36 [111], 0.37 [26], 0.23-0.58 [60], and similar estimates for different types of OCD [16]. In addition, quantitative genetic analyses have revealed nesting behavior to be a highly polygenic trait [19, 71], which is consistent with OCD in humans likely being influenced by many different genes [57, 89, 101].
Considering the heterogeneity and sex factors in OCD and the behavioral and genetic characteristics of our mouse model of OCD, we aimed to investigate the effects of genetic background and sex on the compulsive-, anxiety-, cognitive- and depression-like behaviors for a better understanding of factors influencing compulsivity.
2. Methods
The University of Alaska Fairbanks Institutional Animal Care and Use Committee approved the animal care and experimental procedures (IACUC assurance number 497513).
2.1. Mice
2.1.1. Husbandry
Mice were housed in polypropylene cages (27×17×12 cm) with wood shavings in a temperature (22±1°C) and humidity (60-80%) controlled room of the Biological Research and Diagnostics (BiRD) Facility vivarium on a 12:12h light-dark cycle. Pups were weaned at 19-21 days of age and housed with same-sex and same-strain littermates until the end of all experiments. Food (Purina Mills, Lab Diet Mouse Diet #5015, St. Louis, MO) and water were available ad libitum.
2.1.2. Mouse strains
Randomly-bred Control strains (C1, C2), BIG strains selected to build big nests (BIG1, BIG2) and SMALL strains selected to build small nests (SML1, SML2) [17, 18, 19] were used. The BIG strains express higher levels of compulsive-like behaviors, i.e., nest building and marble burying, when compared to the SMALL strains [50], while the Control strains are intermediate [19, 71]. Male and female mice (Mus musculus) of these six different mouse strains were used for the behavioral experiments (C1 males n=20; C1 females n=21; C2 males n=20; C2 females n=17; BIG1 males n=21; BIG1 females n=22; BIG2 males n=20; BIG2 females n=14; SML1 males n=19; SML1 females n=21; SML2 males n=19; SML2 females n=20).
2.1.3. Estrous cycle
Female mice in proestrus were used for the study. The proestrus stage was determined daily by both visual and vaginal cytology methods [20]. All females from all the strains were visually inspected daily. Vaginal cytology was performed on those females that showed visual signs of proestrus. The females for which proestrus was confirmed through cytology were subjected to behavioral testing the same day. This procedure was conducted daily until desired sample sizes were achieved for a specific test. When the females cycled to their next proestrus, which was typically on the 4th or the 5th day, they were subjected to the next behavioral test in the schedule and this cycle was continued until all behavioral tests were completed.
2.2. Experimental design
All mice were at least 60 days old at the start of the experiment. All behavioral tests were done in the light phase of the light-dark cycle. All males underwent behavioral tests once in every 5 days consisting of nest building (on day 1), marble burying (on day 6), open field (on day 11), elevated plus maze (on day 16), novel object recognition (on day 21) and forced swim test (on day 26). For females, behavioral tests were conducted every 4-5 days depending on their proestrus stage. Males and females were tested on this schedule until desired sample sizes were achieved. A gap of 4 days for males and 3-4 days for females between each behavioral test minimized the behaviors interfering with each other.
For nest-building, animals were housed individually in cages and, therefore, following data collection after 24 hours, they were returned to home cages with their littermates and kept for 4 days to negate any isolation effects that could influence performance in subsequent tests [55]. For marble burying, open field, elevated plus maze, and novel object recognition the mice were singly housed just before testing. After testing they were returned to their home cage with their littermates. Upon reintroduction to their littermates, some fighting was observed within the first hour, which was similar to fighting observed after a normal cage change, which should not have interfered with the subsequent behavioral assessment considering a 3-4 day gap between tests. Immediately after the forced swim test, trunk blood was collected to determine stress-induced plasma corticosterone levels. An observer blind to the conditions of experimental animals and the hypothesized outcome of the study collected all data.
2.3. Measuring compulsive-like behavior
2.3.1. Nest-building test
Nest-building behavior was used as a measure of the compulsive-like phenotype of the mice [50]. Male and female mice of all the strains were singly housed and provided with a pre-weighed cotton roll in the cage-top food hopper. After 24 hours, the cotton roll was removed and weighed [17, 18, 19, 71]. The total nesting score was defined as the amount of cotton used over the 24-hour period. Generally, all the mice incorporated the cotton into their nest. The more cotton was used, the more elaborate the nest was, which progressed from cotton on the bottom of the nest, to a bowl nest, and finally to a dome nest.
2.3.2. Marble burying test
Compulsive-like behavior was also assessed through the marble burying test [2, 7, 31, 50]. Mice were placed in a standard housing cage with 5 cm deep clean bedding and 9 glass marbles arranged in two rows. Testing was performed in the presence of white noise. The total number of marbles 2/3 buried was quantified. The current protocol was modified from existing protocols in our lab [50] and other studies [24, 31, 107]. The mouse strains were found to replicate the 20-minute protocol with 20 marbles in a 10-minute trial with 9 marbles. Our group has also published a study with rats where 9 marbles for 10 minutes provided conclusive findings [70].
2.4. Measuring anxiety-like behaviors
2.4.1. Open field test
Open field behavior was used to assess anxiety-like behavior of the mice [92]. BIG, SMALL and Control male and female mice were transported in their home cages, singly housed and placed on a rack outside the testing room just prior to testing. The open field apparatus consisted of an open field arena (40 × 40 × 30 cm) with 16 10×10 cm squares marked on its floor. For testing, mice were placed in the central 4 squares of the field and allowed to explore the arena for 5 minutes. Entries into the central (20 × 20 cm) and peripheral squares were recorded [40] by the ANYMaze video-tracking program (Stoelting Co., IL, USA). The apparatus was cleaned before each test.
2.4.2. Elevated plus maze
Assessment of anxiety-like behavior was also determined with the elevated plus maze test [114]. The plus maze consisted of two open arms (5 × 40 cm) and two closed arms (5 × 40 × 20 cm) at right angles to each other. Each mouse was placed in the central square facing an open arm, and was allowed to explore the maze for 5 minutes. The time spent on the open arms was determined [115] by the ANYMaze video tracking program (Stoelting Co., IL, USA). An entry was defined as all four paws being on the arm. The maze was cleaned before each test.
2.5. Measuring novel object recognition behavior
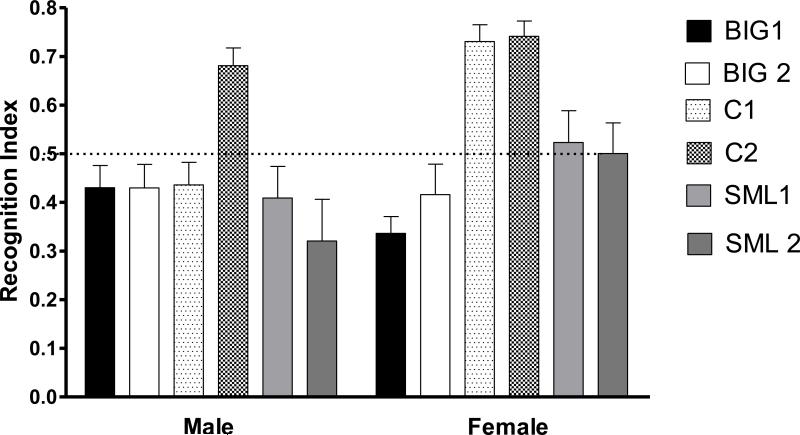
The novel object recognition test was performed to measure object recognition memory [8]. During day 1, mice were habituated to the open field arena (40 × 40 × 30 cm) for 3 minutes. Twenty-four hours later on day 2, the time spent on investigating two identical objects (plastic toys) within a 5 cm distance in the open field arena was recorded for 3 minutes with the ANYMaze video tracking program (Stoelting.co). Mice were then taken out of the arena and returned to their home cages for 4 hrs. After 4 hrs one of the objects was replaced with a novel object of different shape and size and animals were then reintroduced into the arena and allowed to explore the objects for 3 minutes. Time spent exploring the familiar and novel objects were recorded. The preference of one object over another was assessed through the Recognition Index (RI) which is the time spent on the novel object relative to the time spent on both novel and familiar objects: [RI = TN/(TN + TF)] where TN is time spent on the novel object and TF is time spent on the familiar object) [38, 67].
2.6. Measuring depression-like behavior
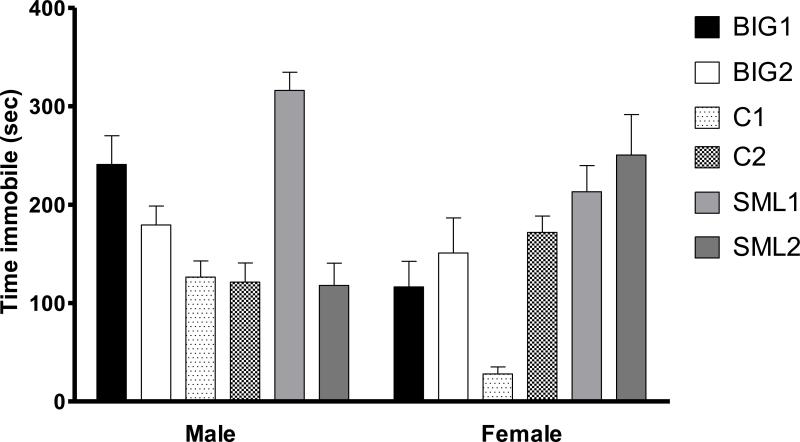
Time spent immobile in the forced swim test was used as a measure of depression-like behavior [41, 90, 91], where immobility is defined as the absence of no active behaviors like swimming, jumping or diving [12, 41, 91]. Mice were introduced in a glass cylinder, 20.5 cm in diameter and 21.5 cm in depth, which was filled with 18 cm of water maintained at 25 - 27°C for 10 minutes [44].
2.7. Corticosterone ELISA
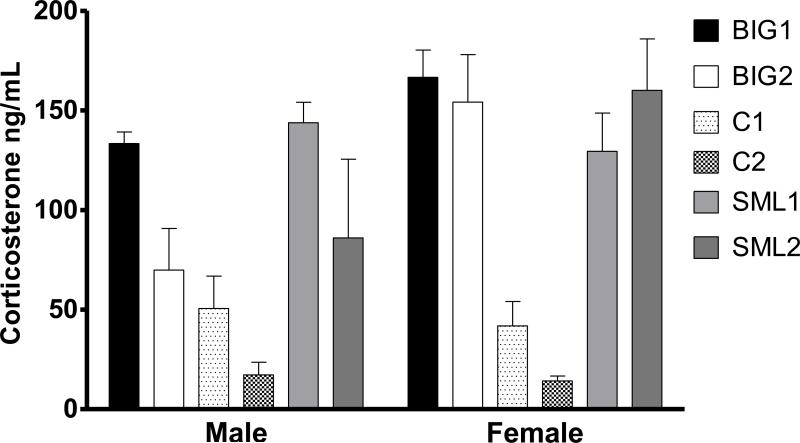
Mice were euthanized after the forced swim test through cervical dislocation to measure stress induced corticosterone levels. Trunk blood was collected randomly from each strain and sex (C1 males n=4; C1 females n=5; C2 males n=6; C2 females n=6; BIG1 males n=4; BIG1 females n=4; BIG2 males n=7; BIG2 females n=5; SML1 males n=5; SML1 females n=5; SML2 males n=6; SML2 females n=6) in chilled heparinized tubes and stored at -80°C. After thawing the blood was centrifuged at 1000 × g for 20 minutes. The plasma was extracted and placed in fresh tubes and stored at −80°C. The corticosterone levels were determined using a corticosterone ELISA kit from Cayman Chemicals (Catalogue ID: 500655) as per the manufacturer's instructions and as previously described [42]. The detection limit (80% B/B0) of the assay is approximately 80 pg/mL (Cayman) with inter- and intra-assay coefficient of variation of 2.2% and 8.9%, respectively.
2.8. Statistical analyses
All measurement values are expressed in mean ± standard error of the mean (SEM). Statistical Analysis System (SAS Version 9.4, Cary, NC) software was used for statistical analyses. All behavioral and biochemical measures were tested in a general linear model (GLM) analysis of variance (ANOVA) for effects of strain (BIG, SMALL, Control), sex (female, males), replicate (1, 2) nested within strain, and strain × sex interaction. If significant effects were found, appropriate post-hoc pair-wise comparisons were conducted using the Tukey's Studentized Range test. If the replicate effect was significant, the strain effect was tested over the replicate effect. If the replicate effect was not significant, the strain effect was tested over the error term. The total nesting score was square root transformed to obtain a more normal distribution [17, 18, 19], while the data are presented as non-transformed nesting scores. Significant correlations between behaviors within strains were determined through linear regression.
3. Results
3.1. Behavioral Data
3.1.1. Strain, replicate and sex differences in nest building
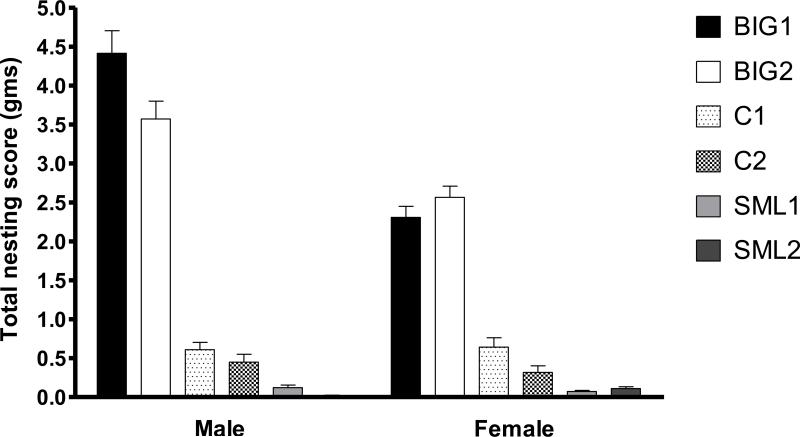
Significant strain (F2,3=175.41, p<0.001), replicate (F3,226=3.71, p<0.02) and sex (F1,226=15.61, p<0.0001) effects were observed for compulsive-like nest building. Both male and female big nest builders (BIG1 and BIG2) used more cotton to build a nest than the Control mice (C1 and C2) and the SMALL mice (SML1 and SML2), and the Control mice built bigger nests than the SMALL mice. Most SML2 male mice did not use any cotton. The replicate effect was due to significant differences between the BIG1 and BIG2 males and C1 and C2 females. Sex differences were attributed to BIG1 and BIG2 males using more cotton than proestrus females for nesting (Fig. 1). The significant interaction between strain and sex (F2,226=16.68, p<0.0001) was due to females and males not being different in total nesting scores in the C1 and C2, and SML1 and SML2 strains, while the BIG strains showed large differences.
Figure 1. Nest-building behavior.
Mean (± SEM) total nesting scores of male and female BIG, SMALL and Control replicate strains. BIG1 and BIG2 males had higher nesting score than C1 (t=15.28 p<0.001 and t=12.95 p<0.001), C2 (t=17.20 p<0.001 and t=14.86 p<0.001), SML1 (t=20.47 p<0.001 and t=18.16 p<0.001) and SML2 (t=22.72 p<0.001 and t=20.42 p<0.001) males. BIG1 males had higher nesting score than BIG2 males (t=2.365 p<0.05). BIG1 and BIG2 females had higher nesting score than C1 (t=9.564 p<0.001 and t=9.364 p<0.001), C2 (t=11.81 p<0.001 and t=11.43 p<0.001), SML1 (t=15.19 p<0.001 and t=14.34 p<0.001) and SML2 (t=14.20 p<0.001 and t= 13.49 p<0.001) females. BIG1 and BIG2 males had higher nesting score than BIG1 and BIG2 females (t=6.682 p<0.001 and t=2.886 p<0.05).
3.1.2. Replicate and sex differences in marble burying
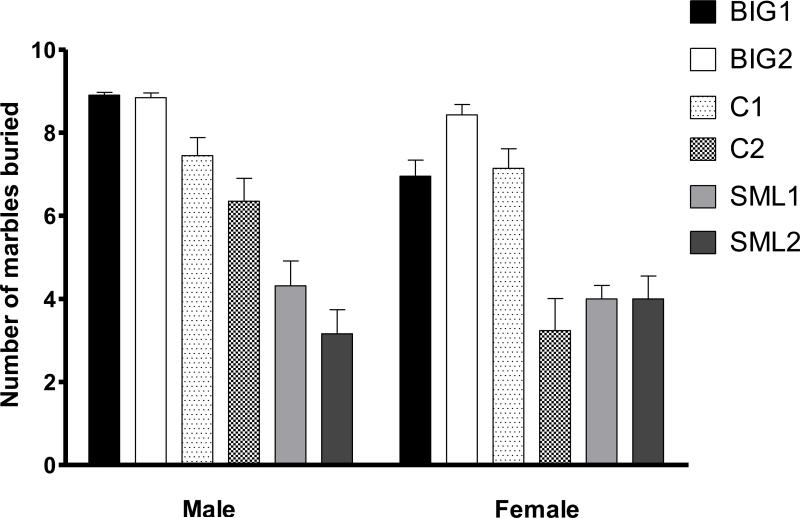
For compulsive-like marble burying, the strain differences were marginally significant (F2,3=9.02, p<0.054), predominantly due to the significant replicate effect (F3,226=10.21, p<0.0001), although the general trend of BIG mice burying more marbles than SMALL mice with Control mice having intermediate values was in line with previously found significant strain differences [53]. The replicate effect was predominantly due to C1 females burying more marbles than C2 females. The significant sex effect (F1,226=11.03, p<0.002) resulted from C2 and BIG1 males burying more marbles than their female counterparts (Fig. 2). The significant sex by strain interaction effect (F2,226=4.07, p<0.02) was due to the SMALL strains not showing sex or replicate effects while the other strains did.
Figure 2. Marble burying behavior.
Mean (± SEM) number of marbles buried in male and female BIG, SMALL and Control replicate strains. Replicate effect was due to C1 females burying more marbles than C2 females (t=5.850 p<0.001). BIG1 and C2 males buried more marbles than BIG1 (t=3.132 p<0.05) and C2 (t=4.201 p<0.001) females, contributing to the sex differences and strain by sex interactions.
3.1.3. Replicate differences in open field behavior
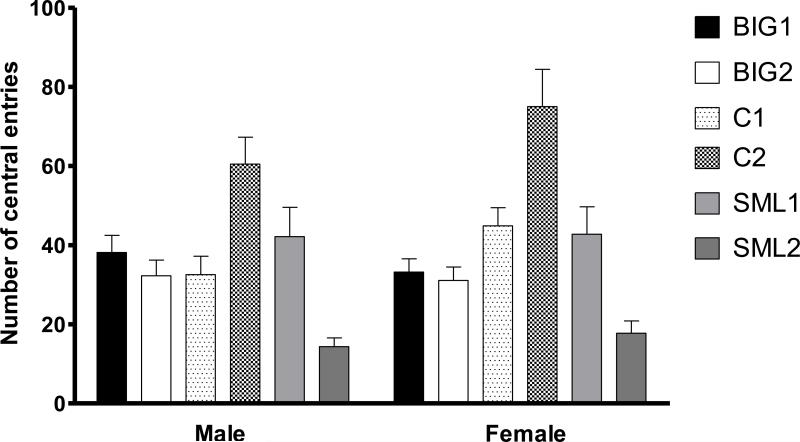
There were no significant strain (F2,3=1.16, p>0.40), sex (F1,226=1.73, p>0.15) and strain by sex interaction (F2,226=2.54, p= 0.08) effects on anxiety-like behavior in the open field, as measured by the number of central entries. Significant replicate effects (F3,226=17.87, p<0.0001) were due to female and male C2 mice making more central entries than C1 mice. Replicate effects were also due to female and male SML1 mice making more central entries than SML2 mice (Fig. 3).
Figure 3. Open field behavior.
Mean (± SEM) number of central entries in male and female BIG, SMALL and Control replicate strains. C2 males and females had more central entries than C1 males (t=3.739 p<0.001) and females (t=3.903 p<0.001), respectively. SML1 males and females had more central entries than SML2 males (t=3.624 p<0.001) and females (t=3.903 p<0.01), respectively.
3.1.4 Strain differences in elevated plus maze behavior
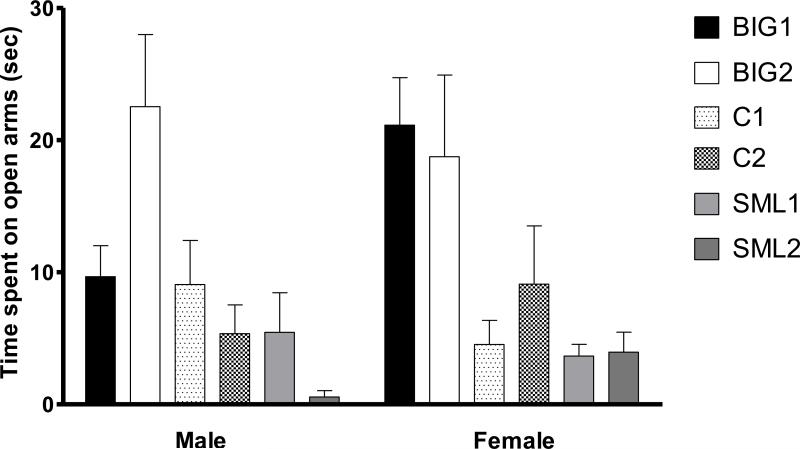
In the elevated plus maze test, anxiety-like behavior, as measured by the time spent on the open arms, was significantly influenced by strain (F2,226=22.11, p<0.0001) without a significant replicate effect (F3,226=1.11, p>0.30). The strain differences were due to BIG2 males spending more time on the open arms than C2, SML1 and SML2 males and the BIG1 and C1 males spending more time on the open arms than the SML2 males. For females, the C1, C2, SML1 and SML2 strains spent less time on open arms than the BIG1 and BIG2 females. No significant sex (F1,226=0.75, p>0.30) and strain by sex interaction (F2,226=0.74, p>0.40) effects were found (Fig. 4).
Figure 4. Elevated plus maze behavior.
Mean (± SEM) time spent on open arms of male and female BIG, SMALL and Control replicate strains. Significant strain differences were observed in time spent on open arms between male BIG2 with C2 (t=3.881, p<0.001), SML1 (t=3.807, p<0.001) and SML2 strains (t=4.899, p<0.001). The C1, C2, SML1 and SML2 females spent less time on open arms when compared to BIG1 females (t= 3.836, p<0.001; t=2.627, p<0.05; t=4.041, p<0.001; t=3.923, p<0.001, respectively). The C1, SML1 and SML2 females spent less time on open arms when compared to BIG2 females (t= 2.904, p<0.01; t=3.085, p<0.01; t=2.994, p<0.01, respectively).
3.1.5 Sex and replicate differences in novel-object recognition memory
No significant strain (F2,3=5.33, p>0.09) and strain by sex interaction (F2,226=2.80, p>0.05) effects were found for novel object recognition. The significant effects of sex (F1,226=6.87, p<0.01) on novel object recognition was mostly due to female C1 mice showing better performance than their male counterparts (Fig. 5). A significant replicate effect was found (F3,226=3.44, p<0.01), which was predominantly due to differences between C1 and C2 males.
Figure 5. Novel Object Recognition behavior.
Mean (± SEM) recognition index of male and female BIG, SMALL and Control replicate strains. The C2 males had higher RI values when compared to C1 males (t=3.340, p<0.01). The C1 females had higher RI when compared to C1 males (t=3.921, p<0.001).
3.1.6 Sex and replicate differences in the forced swim test
In the forced swim test, depression-like behavior, as measured by duration of immobility, revealed no significant strain (F2,3=1.78, p>0.25) and strain by sex interaction (F2,226=1.82, p>0.15) effects. The significant sex (F1,226=5.67, p<0.05) effect was due to proestrus females demonstrating less depression-like immobility behavior than did males, especially SML1, BIG1, C1, but not SML2 strains (Fig. 6). The significant replicate effect (F3,226=8.64, p<0.0001) was due to differences between the SML1 and SML2 males and the C1 and C2 females.
Figure 6. Forced Swim Test.
Mean (± SEM) immobility time of male and female BIG, SMALL and Control replicate strains. SML1 males had higher immobility times than SML2 males (t=5.796, p<0.001) while C2 females had higher immobility times than C1 females (t=4.229, p<0.001). BIG1, C1 and SML1 males had higher immobility times when compared to BIG1 (t=3.063, p<0.05), C1 (t=3.778, p<0.01) and SML1 (t=3.083, p<0.01) females. In the SML2 strain, females had higher immobility time than the males (t=3.827, p<0.01).
3.2. Strain differences in corticosterone plasma levels
Plasma corticosterone levels were significantly influenced by strain (F2,52=12.17, p<0.0001) without a replicate effect (F3,52=2.53, p>0.05). The BIG and SMALL nest-building strains generally mounted a higher corticosterone response to forced swim followed by euthanasia. Corticosterone levels in male BIG1 and SML1 strains were significantly higher than the C2 strain (Fig. 7). Also, BIG1 males had higher corticosterone levels than C1 males. In females, both the BIG and SMALL strains had higher corticosterone levels when compared to the Control strains. No significant sex (F3,52=3.68, p>0.05) and strain by sex interaction (F2,52=1.54, p>0.20) effects were observed.
Figure 7. Plasma Corticosterone Levels.
Mean (± SEM) corticosterone levels as a measure of HPA axis stress response of male and female BIG, SMALL and Control replicate strains. Levels in male BIG1 and SML1 strains were significantly higher than the Control C2 strain (t=3.773, p<0.001 and t=4.385, p<0.001, respectively). BIG1 (t=2.455, p<0.05) and SML1 (t=2.916, p<0.05) males had higher corticosterone levels than C1 males. Female BIG1, BIG2, SML1 and SML2 also had higher corticosterone levels than female C1 (t=3.90, p<0.001; t=3.73, p<0.001; t=2.91, p<0.05 and t=4.91, p<0.001, respectively) and C2 (t=4.95, p<0.001; t=4.85, p<0.001; t=3.99, p<0.001 and t=5.30, p<0.001, respectively).
4. Discussion
In this study we report for the first time that females of both the BIG strains displayed face validity as a non-induced compulsive-like model by using more cotton for nest building and burying more marbles compared to the females of the SMALL strains. For nest-building behavior, the female Control strains were intermediate but closer to the SMALL strains, while for marble burying one of the Control strains was similar to the BIG strains and the other Control strain was similar to the SMALL strains. BIG1 and BIG2 females had less compulsive-like nesting (48% and 28%, respectively), when compared to BIG1 and BIG2 males establishing a sex difference, which was specific to the compulsive-like mice because the SMALL and Control strains did not show differences between males and females. We hypothesize that this difference was due to using proestrus females in this study with higher estrogen levels. For marble burying a similar sex difference was also observed for the BIG1 strain, but not the BIG2 strain although the trend was in the same direction.
Prior studies have shown that compulsive-like behaviors were enhanced in the absence of estrogen, such as estrogen-deficient male mice that showed development of compulsive-like wheel running and grooming behavior. These behaviors were considerably reduced with estrogen replacement [58]. Estrogen administration in ovariectomized rats with 8-OH-DPAT induced compulsive-like traits caused significant reduction in the compulsive-like behavior [33]. Females in proestrus have high physiological circulating estrogen levels [22, 99], but whether estrogen played a direct role in reducing compulsive-like behaviors in proestrus female compared to male compulsive-like mice remains to be elucidated.
Cohort based clinical studies have found that overall males have higher vulnerability to obsessions and compulsions when compared to females [14, 53]. Although obsessions are difficult to model in animals, the severity of compulsions can be a suitable measure of it [34]. Therefore, the higher levels of compulsive-like behaviors in BIG males compared to BIG females may further add face validity to the mouse model for understanding sex differences that attribute to the complexity of OCD.
Open field and elevated plus maze tests were used to assess anxiety-like behavior in males and females of BIG, SMALL and Control strains. No significant strain effects were found for the number of central entries in the open field, which indicates that this measure of anxiety-like behavior did not correlate with the level of compulsive-like behavior. However, significant strain differences were observed in elevated plus maze tests for both males and females. Generally the BIG strains showed less anxiety-like behavior in the elevated plus maze test when compared to the SMALL strains, which is consistent with our previous findings [50], and the females showed this general pattern most clearly. The responses of the six strains in the open field and elevated plus maze varied, which may be due to these tests measuring different aspects of emotionality associated with anxiety [6, 93].
Although BIG mice overall were less anxious than the SMALL mice in the elevated plus maze, within each BIG strain the correlations were generally opposite. Elevated plus maze exploration had a negative correlation with levels of compulsive-like nesting (r = −0.708, p<0.005) and marble burying (r = −0.562, p<0.05) behavior in BIG2 females indicating that an increase in compulsive-like behavior was associated with more anxiety (less exploration of the open arms). A negative correlation was also found between marble burying (but not nesting) with open arm exploration in BIG1 males (r = −0.817, p<0.0001). This result could be due to a changing genetic correlation structure as selection progressed, similar to what we found in these mice for the relationship between food consumption and nest building previously [19]. Alternatively, genetic drift and founder effects [17, 19] might be responsible for the correlations observed in just the BIG2 females and the BIG1 males.
The association of OCD with general anxiety is deemed controversial [103]. Studies have shown that the ego-dystonic and intrusive nature of obsessions differ largely from general anxiety [64, 65]. There is also large heterogeneity and variability of anxiety in OCD, though general anxiety is a common comorbid psychiatric condition associated with the disorder [5, 84]. This makes it an ambiguous indicator for the disorder [85]. General anxiety and panic disorders have a late onset when compared to OCD, which has a very early onset [95]. In addition, neurocircuitry models proposed for anxiety and OCD differ. While anxiety and panic disorders have been linked to dysregulation of amygdala-ventromedial prefrontal cortex-hippocampus circuitry [98], OCD is thought to occur due to abnormal fronto-striatal circuitry [36, 49, 88, 94]. Consequently, findings from the current study will allow the BIG2 female and BIG1 male strains to be used for studying association between various forms of compulsivity and anxiety. The BIG1 females and BIG2 males on the other hand can provide understanding of various forms of compulsivity and how they vary based on sex and genetic background.
In the depression-like forced swim test, no significant relationship was found between the level of compulsive-like behavior and immobility times as the strain effect was not significant. However, significant sex differences were observed with the females generally showing less depression-like behavior than males. Rodent studies have shown sex and strain differences in depression-like behavior in the forced swim task [13, 102]. Proestrus female mice and rats with higher circulating estrogen have been found to be less immobile in forced swim task when compared to the male counterparts [43, 44, 47], which is similar to our findings. Depression is a common comorbidity associated with OCD [5, 87, 104], but has been found to vary considerably among studies [3]. Human studies have shown that anxiety and depressions are influenced by factors like race/ethnicity and gender differences [78]. Hence, an effect of genetic background coupled with sex differences could influence the comorbid depression disorders in OCD and present a complex set of interactions, also reflected in our six mouse strains with different compulsive-like phenotypes.
The novel object recognition test revealed no significant strain effects, although the Control strains tended to outperform the BIG and SMALL strains. Females significantly outperformed the males, mostly due to the Control strains and less so due to the SMALL strains. The novel object recognition task is useful for studying working memory [38, 46, 100]. Several clinical studies have reported deficits in working memory among OCD patients [75, 81, 110], while others have reported no significant difference [85]. Our findings in the mice may be due to the fact that differences in working memory capacity among OCD patients are linked to intrusive thoughts or obsessions [15], which simply cannot be assessed in animals. Alternatively, novel object recognition memory may not be equivalent to the working memory deficits measured in OCD patients.
The female Control mice had significantly lower plasma corticosterone levels than the BIG or SMALL strain females. This general pattern was also observed in the males, but to a lesser degree. Previous studies have shown that female rodents secrete higher levels of corticosterone than males [10, 21, 66], which the BIG1, BIG2, and SML2 mice tended to show as well, although the overall sex effect was not significant. Additionally, in proestrus, corticosterone levels in response to stress are higher [9, 21, 35, 112]. Coping with stress is promoted by the hypothalamic-pituitary-adrenal (HPA) axis [28, 106] and corticosterone is a primary end point of HPA axis activation in mice [54]. OCD is known to be stress responsive since symptoms not only increase during periods of stress but stressful events can also precede the onset of obsessive-compulsive symptoms [80]. Our results show that selection to behavioral extremes, both in the high and low direction of nesting, resulted in enhanced activation of the HPA axis due to stress imparted by the forced swim test. OCD patients have higher baseline urine [45] and blood [61] cortisol levels than healthy matched controls, which can be compared to the higher stress-induced corticosterone levels in the BIG strains compared to the Control strains.
Significant replicate effects nested within strain were found for nest building, marble burying, open field behavior, novel object recognition memory, and the forced swim test. These replicate effects were most likely due to differences in genetic background as a result of random genetic drift and founder effects within each strain [17, 19]. Of special interest are the replicate effects between the BIG strains as they represent the compulsive-like phenotype and may correspond to subtypes of compulsive-like phenotypes as seen in human OCD patients [39, 48, 68]. The male BIG1 strain nested more than the BIG2 strain, while the BIG2 strain showed more anxiety-like behavior in the elevated plus maze than the BIG1 strain. However, the females were not different from each other for either trait indicating a potential sex by genotype interaction. Further, sex differences in only the BIG1 but not the BIG2 strains in marble burying add heterogeneity based on specific compulsive-like traits that might significantly vary depending on the sex and genotype.
5. Conclusion
In the current study, we confirmed that females of the compulsive-like BIG strains also have face validity as a mouse model of OCD, enhancing our earlier findings in males [50]. Proestrus females also showed lower levels of compulsive-like behaviors than males, which suggest that physiological changes in females related to the estrous cycle might influence compulsive-like behavioral expression. A differential response was observed in anxiety-like behaviors with replicate effects in the open field and strain differences in the elevated plus maze. Sex differences were seen for depression-like behavior with an elevated HPA axis response in females compared to males. Overall, our mouse strains can be used to better understand how genetic and sex factors and behavioral correlates contribute to the compulsive-like phenotype. Replicate effects in compulsive-like expression also indicate symptom heterogeneity that is strongly associated with OCD making it a complex neuropsychiatric disorder. Future studies will aim at investigating sex and strain differences in first line therapy responses and the neurobiological mechanisms in the mouse model that could broaden the understanding of heterogeneity and drug unresponsiveness in certain OCD subtypes when compared to others.
Highlights.
Big (BIG) nest-building mice are a spontaneous animal model of OCD.
BIG male mice showed higher levels of compulsive-like behaviors than proestrus females.
Replicate effects were exhibited in anxiety-like open field behavior.
Compulsive-like strains showed less anxiety-like behavior than the non-compulsive-like strains on the elevated plus maze.
Novel object recognition memory was not differentially affected by level of compulsivity.
Depression-like behavior was greater among males than proestrus females.
Interplay of sex and strain (genetic background) affected the compulsive-, anxiety- and depression-like behaviors.
Acknowledgements
We thank the Biological Research and Diagnostics (BiRD) Facility animal quarters staff for excellent routine animal care. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395. The work was also supported by College of Natural Sciences & Mathematics (CNSM) and the Office of the Vice-Chancellor for Research. These funding sources did not have a role in the study design, collection, analysis, and interpretation of data, and submission of this paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramowitz JS, Schwartz S. a, Moore KM, Luenzmann KR. Obsessive-compulsive symptoms in pregnancy and the puerperium. J. Anxiety Disord. 2003;17:461–478. doi: 10.1016/s0887-6185(02)00206-2. doi:10.1016/S0887-6185(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 2.Albelda N, Joel D. Animal models of obsessive-compulsive disorder: Exploring pharmacology and neural substrates. Neurosci. Biobehav. Rev. 2012;36:47–63. doi: 10.1016/j.neubiorev.2011.04.006. doi:10.1016/j.neubiorev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Altintaş E, Taşkintuna N. Factors associated with depression in obsessive-compulsive disorder: A cross-sectional study. Noropsikiyatri Ars. 2015;52:346–353. doi: 10.5152/npa.2015.7657. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L607169035\ nhttp://dx.doi.org/10.5152/npa.2015.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association Diagnostic and statistical manual of mental disorders. 2000 doi:10.1016/B978-1-4377-2242-0.00016-X. [Google Scholar]
- 5.Anagnostopoulos DC, Korlou S, Sakellariou K, Kondyli V, Sarafidou J, Tsakanikos E, Giannakopoulos G, Liakopoulou M. Comorbid psychopathology and clinical symptomatology in children and adolescents with obsessive-compulsive disorder. Psychiatriki. 2016;27:27–36. http://www.ncbi.nlm.nih.gov/pubmed/27110880. [PubMed] [Google Scholar]
- 6.Anchan D, Clark S, Pollard K, Vasudevan N. GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav. 2014;4:51–59. doi: 10.1002/brb3.197. doi:10.1002/brb3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013:50978. doi: 10.3791/50978. doi:10.3791/50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. doi:10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: Sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. doi:10.1210/en.138.9.3842. [DOI] [PubMed] [Google Scholar]
- 10.Babb JA, Masini CV, Day HEW, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. doi:10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachus SE, Pellis SM, Rowland NE, Stellar JR, Szechtman H, Eilam D, Zor R, Fineberg N, Hermesh H. Animal behavior as a conceptual framework for the study of obsessive–compulsive disorder (OCD) Behav. Brain Res. 2012;231:289–296. doi: 10.1016/j.bbr.2011.06.033. http://www.sciencedirect.com/science/article/pii/S0166432811005079. [DOI] [PubMed] [Google Scholar]
- 12.Bastos CP, Pereira LM, Ferreira-Vieira TH, Drumond LE, Massensini AR, Moraes MFD, Pereira GS. Object recognition memory deficit and depressive-like behavior caused by chronic ovariectomy can be transitorialy recovered by the acute activation of hippocampal estrogen receptors. Psychoneuroendocrinology. 2015;57:14–25. doi: 10.1016/j.psyneuen.2015.03.020. doi: http://dx.doi.org/10.1016/j.psyneuen.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Bielajew C, Konkle ATM, Kentner AC, Baker SL, Stewart A, Hutchins AA, Santa-Maria Barbagallo L, Fouriezos G. Strain and gender specific effects in the forced swim test: effects of previous stress exposure. Stress. 2003;6:269–280. doi: 10.1080/10253890310001602829. doi:10.1080/10253890310001602829. [DOI] [PubMed] [Google Scholar]
- 14.Bogetto F, Venturello S, Albert U, Maina G, Ravizza L. Gender-related clinical differences in obsessive-compulsive disorder. Eur. Psychiatry. 1999;14:434–441. doi: 10.1016/s0924-9338(99)00224-2. doi:10.1016/S0924-9338(99)00224-2. [DOI] [PubMed] [Google Scholar]
- 15.Brewin CR, Smart L. Working memory capacity and suppression of intrusive thoughts. J. Behav. Ther. Exp. Psychiatry. 2005:61–68. doi: 10.1016/j.jbtep.2004.11.006. doi:10.1016/j.jbtep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Browne HA, Gair SL, Scharf JM, Grice DE. Genetics of obsessive-compulsive disorder and related disorders. Psychiatr. Clin. North Am. 2014;37:319–35. doi: 10.1016/j.psc.2014.06.002. doi:10.1016/j.psc.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bult A, Lynch CB. Multiple selection responses in house mice bidirectionally selected for thermoregulatory nest-building behavior: crosses of replicate lines. Behav. Genet. 1996;26:439–446. doi: 10.1007/BF02359488. doi:10.1007/BF02359488. [DOI] [PubMed] [Google Scholar]
- 18.Bult A, Lynch CB. Nesting and fitness: Lifetime reproductive success in house mice bidirectionally selected for thermoregulatory nest-building behavior. Behav. Genet. 1997;27:231–240. doi: 10.1023/a:1025610130282. doi:10.1023/A:1025610130282. [DOI] [PubMed] [Google Scholar]
- 19.Bult A, Lynch CB. Breaking through artificial selection limits of an adaptive behavior in mice and the consequences for correlated responses. Behav. Genet. 2000;30:193–206. doi: 10.1023/a:1001962124005. doi:10.1023/A:1001962124005. [DOI] [PubMed] [Google Scholar]
- 20.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035538. doi:10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey MP, Deterd CH, De Koning J, Helmerhorst F, De Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J. Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. doi:10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 22.Carswell HV, Dominiczak a F., Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am. J. Physiol. 2000;278:H290–4. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- 23.Castle DJ, Deale A, Marks IM. Gender differences in obsessive compulsive disorder. Aust. N. Z. J. Psychiatry. 1995;29:114–117. doi: 10.3109/00048679509075899. [DOI] [PubMed] [Google Scholar]
- 24.Chikahisa S, Sano A, Kitaoka K, Miyamoto KI, Sei H. Anxiolytic effect of music depends on ovarian steroid in female mice. Behav. Brain Res. 2007;179:50–59. doi: 10.1016/j.bbr.2007.01.010. doi:10.1016/j.bbr.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Cruz C, Camarena B, King N, Páez F, Sidenberg D, de la Fuente JR, Nicolini H. Increased prevalence of the seven-repeat variant of the dopamine D4 receptor gene in patients with obsessive-compulsive disorder with tics. Neurosci. Lett. 1997;231:1–4. doi: 10.1016/s0304-3940(97)00523-5. [DOI] [PubMed] [Google Scholar]
- 26.Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, Neale BM, Yang J, Lee SH, Evans P, Barr CL, Bellodi L, Benarroch F, Berrio GB, Bienvenu OJ, Bloch MH, Blom RM, Bruun RD, Budman CL, Camarena B, Campbell D, Cappi C, Cardona Silgado JC, Cath DC, Cavallini MC, Chavira DA, Chouinard S, Conti DV, Cook EH, Coric V, Cullen BA, Deforce D, Delorme R, Dion Y, Edlund CK, Egberts K, Falkai P, Fernandez TV, Gallagher PJ, Garrido H, Geller D, Girard SL, Grabe HJ, Grados MA, Greenberg BD, Gross-Tsur V, Haddad S, Heiman GA, Hemmings SMJ, Hounie AG, Illmann C, Jankovic J, Jenike MA, Kennedy JL, King RA, Kremeyer B, Kurlan R, Lanzagorta N, Leboyer M, Leckman JF, Lennertz L, Liu C, Lochner C, Lowe TL, Macciardi F, McCracken JT, McGrath LM, Mesa Restrepo SC, Moessner R, Morgan J, Muller H, Murphy DL, Naarden AL, Ochoa WC, Ophoff RA, Osiecki L, Pakstis AJ, Pato MT, Pato CN, Piacentini J, Pittenger C, Pollak Y, Rauch SL, Renner TJ, Reus VI, Richter MA, Riddle MA, Robertson MM, Romero R, Rosàrio MC, Rosenberg D, Rouleau GA, Ruhrmann S, Ruiz-Linares A, Sampaio AS, Samuels J, Sandor P, Sheppard B, Singer HS, Smit JH, Stein DJ, Strengman E, Tischfield JA, Valencia Duarte AV, Vallada H, Van Nieuwerburgh F, Veenstra-VanderWeele J, Walitza S, Wang Y, Wendland JR, Westenberg HGM, Shugart YY, Miguel EC, McMahon W, Wagner M, Nicolini H, Posthuma D, Hanna GL, Heutink P, Denys D, Arnold PD, Oostra BA, Nestadt G, Freimer NB, Pauls DL, Wray NR, Stewart SE, Mathews CA, Knowles JA, Cox NJ, Scharf JM. Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003864. doi:10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Haas R, Seddik A, Oppelaar H, Westenberg HGM, Kas MJH. Marked inbred mouse strain difference in the expression of quinpirole induced compulsive like behavior based on behavioral pattern analysis. Eur. Neuropsychopharmacol. 2012;22:657–663. doi: 10.1016/j.euroneuro.2012.01.003. doi:10.1016/j.euroneuro.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 28.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. doi:10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 29.de Mathis MA, de Alvarenga P, Funaro G, Torresan RC, Moraes I, Torres AR, Zilberman ML, Hounie AG. Gender differences in obsessive-compulsive disorder: a literature review. Rev. Bras. Psiquiatr. 2011;33:390–399. doi: 10.1590/s1516-44462011000400014. doi:10.1590/S1516-44462011000400014. [DOI] [PubMed] [Google Scholar]
- 30.de Mathis MA, do Rosario MC, Diniz JB, Torres AR, Shavitt RG, Ferrão YA, Fossaluza V, de Bragança Pereira CA, Miguel EC. Obsessive-compulsive disorder: Influence of age at onset on comorbidity patterns. Eur. Psychiatry. 2008;23:187–194. doi: 10.1016/j.eurpsy.2008.01.002. doi:10.1016/j.eurpsy.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Deacon RMJ. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. doi:10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- 32.Diniz J, Miguel E, Oliveira A. Outlining new frontiers for the comprehension of obsessive-compulsive disorder: a review of its relationship with fear and anxiety. Rev. Bras. 2012;34:81–103. doi: 10.1590/s1516-44462012000500007. doi: http://dx.doi.org/10.1590/S1516-44462012000500007. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Guasti A, Agrati D, Reyes R, Ferreira A. Ovarian steroids counteract serotonergic drugs actions in an animal model of obsessive-compulsive disorder. Psychoneuroendocrinology. 2006;31:924–934. doi: 10.1016/j.psyneuen.2006.05.003. doi:10.1016/j.psyneuen.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Fernando ABP, Robbins TW. Animal models of neuropsychiatric disorders. Annu. Rev. Clin. Psychol. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. doi:10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- 35.Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. doi:10.1210/en.143.7.2534. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol. Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. doi:10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 37.Flaisher-Grinberg S, Albelda N, Gitter L, Weltman K, Arad M, Joel D. Ovarian hormones modulate “compulsive” lever-pressing in female rats. Horm. Behav. 2009;55:356–365. doi: 10.1016/j.yhbeh.2008.10.002. doi:10.1016/j.yhbeh.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Fonseca CS, Gusmão ID, Raslan ACS, Monteiro BMM, Massensini AR, Moraes MFD, Pereira GS. Object recognition memory and temporal lobe activation after delayed estrogen replacement therapy. Neurobiol. Learn. Mem. 2013;101:19–25. doi: 10.1016/j.nlm.2012.12.016. doi:10.1016/j.nlm.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Fontenelle LF, Mendlowicz MV, Versiani M. Clinical subtypes of obsessive-compulsive disorder based on the presence of checking and washing compulsions. Rev. Bras. Psiquiatr. 2005;27:201–207. doi: 10.1590/s1516-44462005000300008. doi:/S1516-44462005000400008. [DOI] [PubMed] [Google Scholar]
- 40.Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, Pfaff DW, Rhodes ME. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. doi:10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA. Progesterone attenuates depressive behavior of younger and older adult C57/BL6, wildtype, and progesterone receptor knockout mice. Pharmacol. Biochem. Behav. 2011;99:525–531. doi: 10.1016/j.pbb.2011.05.024. doi:10.1016/j.pbb.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism. BTBR mice, Physiol. Behav. 2010;100:264–267. doi: 10.1016/j.physbeh.2010.03.005. doi:10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frye CA, Walf AA. Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiol. Behav. 2009;97:266–269. doi: 10.1016/j.physbeh.2009.02.022. doi:10.1016/j.physbeh.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 α-reductase. Brain Res. 2004;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. doi:10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Gehris TL, Kathol RG, Black DW, Noyes R. Urinary free cortisol levels in obsessive-compulsive disorder. Psychiatry Res. 1990;32:151–158. doi: 10.1016/0165-1781(90)90081-f. doi:10.1016/0165-1781(90)90081-F. [DOI] [PubMed] [Google Scholar]
- 46.Goulart BK, de Lima MNM, de Farias CB, Reolon GK, Almeida VR, Quevedo J, Kapczinski F, Schröder N, Roesler R. Ketamine impairs recognition memory consolidation and prevents learning-induced increase in hippocampal brain-derived neurotrophic factor levels. Neuroscience. 2010;167:969–973. doi: 10.1016/j.neuroscience.2010.03.032. doi:10.1016/j.neuroscience.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Gouveia A, Jr, Afonseca TL, Maximino C. Influence of gender and estrous cycle in the forced swim test in rats. Psychol. 2008;1:191–197. http://www.scielo.br/scielo.php?pid=S1983-32882008000200012&script=sci_arttextnpapers3://publication/uuid/BCA3B1B4-8845-4B5B-8756-437239D75DE1. [Google Scholar]
- 48.Grados M, Riddle MA. Do all obsessive-compulsive disorder subtypes respond to medication? Int. Rev. Psychiatry. 2008;20:189–193. doi: 10.1080/09540260801889153. doi:791891782 [pii]r10.1080/09540260801889153 [doi] [DOI] [PubMed] [Google Scholar]
- 49.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. doi: 10.1038/npp.2009.128. doi:10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greene-Schloesser DM, Van der Zee EA, Sheppard DK, Castillo MR, Gregg KA, Burrow T, Foltz H, Slater M, Bult-Ito A. Predictive validity of a non-induced mouse model of compulsive-like behavior. Behav. Brain Res. 2011;221:55–62. doi: 10.1016/j.bbr.2011.02.010. doi:10.1016/j.bbr.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, Leventhal BL, Cook EH. Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am. J. Med. Genet. - Neuropsychiatr. Genet. 2002;114:541–552. doi: 10.1002/ajmg.10519. doi:10.1002/ajmg.10519. [DOI] [PubMed] [Google Scholar]
- 52.Hanna GL, Veenstra-VanderWeele J, Cox NJ, Van Etten M, Fischer DJ, Himle JA, Bivens NC, Wu X, Roe CA, Hennessy KA, Dickel DE, Leventhal BL, Cook EH. Evidence for a Susceptibility Locus on Chromosome 10p15 in Early-Onset Obsessive-Compulsive Disorder. Biol. Psychiatry. 2007;62:856–862. doi: 10.1016/j.biopsych.2007.01.008. doi:10.1016/j.biopsych.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hantouche EG, Lancrenon S. [Modern typology of symptoms and obsessive-compulsive syndromes: results of a large French study of 615 patients]. L'Encéphale. 1996;22:9–21. Spec No, http://www.ncbi.nlm.nih.gov/pubmed/8767023. [PubMed] [Google Scholar]
- 54.Harbuz M. Neuroendocrine function and chronic inflammatory stress. Exp. Physiol. 2002;87:519–525. doi: 10.1113/eph8702411. doi:10.1113/eph8702411. [DOI] [PubMed] [Google Scholar]
- 55.Hawley WR, Grissom EM, Dohanich GP. The relationships between trait anxiety, place recognition memory, and learning strategy. Behav. Brain Res. 2011;216:525–530. doi: 10.1016/j.bbr.2010.08.028. doi:10.1016/j.bbr.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 56.Hemmings SMJ, Kinnear CJ, Niehaus DJH, Moolman-Smook JC, Lochner C, Knowles JA, Corfield VA, Stein DJ. Investigating the role of dopaminergic and serotonergic candidate genes in obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2003;13:93–98. doi: 10.1016/s0924-977x(02)00129-3. doi:10.1016/S0924-977X(02)00129-3. [DOI] [PubMed] [Google Scholar]
- 57.Hettema JM, Neale MC, Kendler KS, Ph D. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am. J. Psychiatry. 2001;158:1568–78. doi: 10.1176/appi.ajp.158.10.1568. doi:10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 58.Hill RA, McInnes KJ, Gong ECH, Jones MEE, Simpson ER, Boon WC. Estrogen Deficient Male Mice Develop Compulsive Behavior. Biol. Psychiatry. 2007;61:359–366. doi: 10.1016/j.biopsych.2006.01.012. doi:10.1016/j.biopsych.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Jaisoorya TS, Janardhan Reddy YC, Srinath S, Thennarasu K. Sex differences in Indian patients with obsessive-compulsive disorder. Compr. Psychiatry. 2009;50:70–75. doi: 10.1016/j.comppsych.2008.05.003. doi:10.1016/j.comppsych.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Katerberg H, Delucchi KL, Stewart SE, Lochner C, Denys DAJP, Stack DE, Andresen JM, Grant JE, Kim SW, Williams KA, Den Boer JA, Van Balkom AJLM, Smit JH, Van Oppen P, Polman A, Jenike MA, Stein DJ, Mathews CA, Cath DC. Symptom dimensions in OCD: Item-level factor analysis and heritability estimates. Behav. Genet. 2010;40:505–517. doi: 10.1007/s10519-010-9339-z. doi:10.1007/s10519-010-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kluge M, Schüssler P, Künzel HE, Dresler M, Yassouridis A, Steiger A. Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J. Psychiatr. Res. 2007;41:928–933. doi: 10.1016/j.jpsychires.2006.08.005. doi:10.1016/j.jpsychires.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Jaurrieta N, Leckman JF, Vallejo J. Gender differences in obsessive-compulsive symptom dimensions. Depress. Anxiety. 2008;25:832–838. doi: 10.1002/da.20332. doi:10.1002/da.20332. [DOI] [PubMed] [Google Scholar]
- 63.Labad J, Menchón JM, Alonso P, Segalàs C, Jiménez S, Vallejo J. Female reproductive cycle and obsessive-compulsive disorder. J. Clin. Psychiatry. 2005;66:428–435. doi: 10.4088/jcp.v66n0404. quiz 546. doi:10.4088/JCP.v66n0404. [DOI] [PubMed] [Google Scholar]
- 64.Langlois F, Freeston MH, Ladouceur R. Differences and Similarities Between Obsessive Intrusive Thoughts and Worries in Non-Clinical Population: Study 1. Behav. Res. Ther. 2000;38:157–173. doi: 10.1016/s0005-7967(99)00027-3. doi:10.1016/S0005-7967(99)00027-3. [DOI] [PubMed] [Google Scholar]
- 65.Langlois F, Freeston MH, Ladouceur R. Differences and similarities between obsessive intrusive thoughts and worry in a non-clinical population: Study 2. Behav. Res. Ther. 2000;38:175–189. doi: 10.1016/s0005-7967(99)00028-5. doi:10.1016/S0005-7967(99)00027-3. [DOI] [PubMed] [Google Scholar]
- 66.Larkin JW, Binks SL, Li Y, Selvage D. The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrenal axis responses to intracerebroventricular ethanol administration in the rat. J. Neuroendocrinol. 2010;22:24–32. doi: 10.1111/j.1365-2826.2009.01934.x. doi:10.1111/j.1365-2826.2009.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazaroni TLN, Raslan ACS, Fontes WRP, de Oliveira ML, Bader M, Alenina N, Moraes MFD, dos Santos RA, Pereira GS. Angiotensin-(1-7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol. Learn. Mem. 2012;97:113–123. doi: 10.1016/j.nlm.2011.10.003. doi:10.1016/j.nlm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Leckman JF, Bloch MH, King RA. Symptom dimensions and subtypes of obsessive-compulsive disorder: A developmental perspective. Dialogues Clin. Neurosci. 2009;11:21–33. doi: 10.31887/DCNS.2009.11.1/jfleckman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lensi P, Cassano GB, Correddu G, Ravagli S, Kunovac JL, Akiskal HS. Obsessive-compulsive disorder familial-developmental history, symptomatology, comorbidity and course with special reference to gender-related differences. Br. J. Psychiatry. 1996;169:101–107. doi: 10.1192/bjp.169.1.101. doi:10.1192/bjp.169.1.101. [DOI] [PubMed] [Google Scholar]
- 70.Llaneza DC, Frye CA. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol. Biochem. Behav. 2009;93:337–342. doi: 10.1016/j.pbb.2009.05.003. doi:10.1016/j.pbb.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch CB. Response to divergent selection for nesting behavior in Mus musculus. Genetics. 1980;96:757–765. doi: 10.1093/genetics/96.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahajan SN, Chopra A, Mahajan A,R. Gender differences in clinical presentation of obsessive compulsive disorder: a hospital based study. Delhi. Psychiatry J. 2014;17:284–290. [Google Scholar]
- 73.Maina G, Albert U, Bogetto F, Vaschetto P, Ravizza L. Recent life events and obsessive-compulsive disorder (OCD): The role of pregnancy/delivery. Psychiatry Res. 1999;89:49–58. doi: 10.1016/s0165-1781(99)00090-6. doi:10.1016/S0165-1781(99)00090-6. [DOI] [PubMed] [Google Scholar]
- 74.Maio TP, Filgueiras GB, Cunha DC, Estanislau C. Animal Models of Obsessive-Compulsive Disorder: Strain Differences. World J. Neurosci. 2014;4:240–246. doi:10.4236/wjns.2014.43027. [Google Scholar]
- 75.Martin A, Wiggs CL, Altemus M, Rubenstein C, Murphy DL. Working memory as assessed by subject-ordered tasks in patients with obsessive-compulsive disorder. J. Clin. Exp. Neuropsychol. 1995;17:786–792. doi: 10.1080/01688639508405167. doi:10.1080/01688639508405167. [DOI] [PubMed] [Google Scholar]
- 76.McDougle CJ, Epperson CN, Price LH, Gelernter J. Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive compulsive disorder. Mol. Psychiatry. 1998;3:270–273. doi: 10.1038/sj.mp.4000391. doi:10.1038/sj.mp.4000391. [DOI] [PubMed] [Google Scholar]
- 77.McClearn GE, Wilson JR, Meredith W. The use of isogenic and heterogenic mouse stocks in behavioral research. In: Lindzey G, Theissen DD, editors. Contributions to Behavior-Genetic Analysis: The Mouse as a Prototype. Appleton-Century-Crofts; NewYork: 1970. pp. 3–22. [Google Scholar]
- 78.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. doi:10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miguel EC, Do Rosário-Campos MC, Da Silva Prado H, Do Valle R, Rauch SL, Coffey BJ, Baer L, Savage CR, O'Sullivan RL, Jenike MA, Leckman JF. Sensory phenomena in obsessive-compulsive disorder and Tourette's disorder. J. Clin. Psychiatry. 2000;61:150–156. doi: 10.4088/jcp.v61n0213. doi:10.4088/JCP.v61n0213. [DOI] [PubMed] [Google Scholar]
- 80.Morgado P, Freitas D, Bessa JM, Sousa N, Cerqueira JJ. Perceived stress in obsessive-compulsive disorder is related with obsessive but not compulsive symptoms. Front. Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00021. doi:10.3389/fpsyt.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakao T, Nakagawa A, Nakatani E, Nabeyama M, Sanematsu H, Yoshiura T, Togao O, Tomita M, Masuda Y, Yoshioka K, Kuroki T, Kanba S. Working memory dysfunction in obsessive-compulsive disorder: A neuropsychological and functional MRI study. J. Psychiatr. Res. 2009;43:784–791. doi: 10.1016/j.jpsychires.2008.10.013. doi:10.1016/j.jpsychires.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 82.Narayanaswamy JC, Viswanath B, Veshnal Cherian A, Bada Math S, Kandavel T, Janardhan Reddy YC. Impact of age of onset of illness on clinical phenotype in OCD. Psychiatry Res. 2012;200:554–559. doi: 10.1016/j.psychres.2012.03.037. doi:10.1016/j.psychres.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 83.Nestadt G, Bienvenu OJ, Cai G, Samuels J, Eaton WW. Incidence of obsessive-compulsive disorder in adults. J. Nerv. Ment. Dis. 1998;186:401–406. doi: 10.1097/00005053-199807000-00003. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=9680040. [DOI] [PubMed] [Google Scholar]
- 84.Nestadt G, Lan T, Samuels J, Riddle M, Bienvenu OJ, Liang KY, Hoehn-Saric R, Cullen B, Grados M, Beaty TH, Shugart YY. Complex segregation analysis provides compelling evidence for a major gene underlying obsessive-compulsive disorder and for heterogeneity by sex. Am. J. Hum. Genet. 2000;67:1611–6. doi: 10.1086/316898. doi:10.1086/316898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nutt D, Malizia A. Anxiety and OCD –the chicken or the egg? J. Psychopharmacol. 2006;20:729–731. doi: 10.1177/0269881106068424. doi:10.1177/0269881106068424. [DOI] [PubMed] [Google Scholar]
- 86.Ornstein TJ, Arnold P, Manassis K, Mendlowitz S, Schachar R. Neuropsychological performance in childhood OCD: A preliminary study. Depress. Anxiety. 2010;27:372–380. doi: 10.1002/da.20638. doi:10.1002/da.20638. [DOI] [PubMed] [Google Scholar]
- 87.Pallanti S, Grassi G, Sarrecchia ED, Cantisani A, Pellegrini M. Obsessive-compulsive disorder comorbidity: Clinical assessment and therapeutic implications. Front. Psychiatry. 2011;2 doi: 10.3389/fpsyt.2011.00070. doi:10.3389/fpsyt.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014;15:410–424. doi: 10.1038/nrn3746. doi:10.1038/nrn3746. [DOI] [PubMed] [Google Scholar]
- 89.Pauls DL, Alsobrook JP. The inheritance of obsessive-compulsive disorder. Child Adolesc. Psychiatr. Clin. N. Am. 1999;8:481–496. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1999-03673-003&site=ehost-live. [PubMed] [Google Scholar]
- 90.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. doi:10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 91.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Thérapie. 1977;229:327–336. doi:10.1196/annals.1317.038. [PubMed] [Google Scholar]
- 92.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. doi:10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 93.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol. Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. doi:10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, Cannistraro PA, Wilhelm S. Functional Magnetic Resonance Imaging Study of Regional Brain Activation During Implicit Sequence Learning in Obsessive-Compulsive Disorder. Biol. Psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. doi:10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 95.Rosenbaum JF, Biederman J, Bolduc-Murphy E. a, Faraone SV, Chaloff J, Hirshfeld DR, Kagan J. Behavioral inhibition in childhood: a risk factor for anxiety disorders. Harv. Rev. Psychiatry. 1993;1:2–16. doi: 10.3109/10673229309017052. doi:10.3109/10673229309017052. [DOI] [PubMed] [Google Scholar]
- 96.Ross J, Badner J, Garrido H, Sheppard B, Chavira DA, Grados M, Woo JM, Doo P, Umaña P, Fournier E, Murray SS, Mathews CA. Genomewide linkage analysis in Costa Rican families implicates chromosome 15q14 as a candidate region for OCD. Hum. Genet. 2011;130:795–805. doi: 10.1007/s00439-011-1033-6. doi:10.1007/s00439-011-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. doi:10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. doi:10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shors TJ. Estrogen and learning: Strategy over parsimony. Learn. Mem. 2005;12:84–85. doi: 10.1101/lm.93305. doi:10.1101/lm.93305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Silvers J, Harrod S, Mactutus C, Booze R. Automation of the novel object recognition task for use in adolescent rats. J. Neurosci. Methods. 2007;166:99–103. doi: 10.1016/j.jneumeth.2007.06.032. doi:10.1016/j.jneumeth.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smoller JW, Block SR, Young MM. Genetics of anxiety disorders: The complex road from DSM to DNA. Depress. Anxiety. 2009:965–975. doi: 10.1002/da.20623. doi:10.1002/da.20623. [DOI] [PubMed] [Google Scholar]
- 102.Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchil GA, Takahashi JS, Redei EE. Sex-and lineage-specific inheritance of depression-like behavior in the rat. Mamm. Genome. 2004;15:648–662. doi: 10.1007/s00335-004-2326-z. doi:10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stein DJ, Fineberg NA, Bienvenu OJ, Denys D, Lochner C, Nestadt G, Leckman JF, Rauch SL, Phillips KA. Should ocd be classified as an anxiety disorder in DSM-V? Depress. Anxiety. 2010;27:495–506. doi: 10.1002/da.20699. doi:10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- 104.Sun J, Li Z, Buys N, Storch EA. Correlates of comorbid depression, anxiety and helplessness with obsessive-compulsive disorder in Chinese adolescents. J. Affect. Disord. 2015;174:31–37. doi: 10.1016/j.jad.2014.11.004. doi:10.1016/j.jad.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Taylor S. Molecular genetics of obsessive–compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol. Psychiatry. 2013;18:799–805. doi: 10.1038/mp.2012.76. doi:10.1038/mp.2012.76. [DOI] [PubMed] [Google Scholar]
- 106.Ter Horst JP, De Kloet ER, Schächinger H, Oitzl MS. Relevance of stress and female sex hormones for emotion and cognition. Cell. Mol. Neurobiol. 2012;32:725–735. doi: 10.1007/s10571-011-9774-2. doi:10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. doi:10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tükel R, Polat A, Genç A, Bozkurt O, Atlı H. Gender-related differences among Turkish patients with obsessive-compulsive disorder. Compr. Psychiatry. 2004;45:362–366. doi: 10.1016/j.comppsych.2004.06.006. doi:10.1016/j.comppsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Uguz F, Kaya V, Gezginc K, Kayhan F, Cicek E. Clinical correlates of worsening in obsessive-compulsive symptoms during pregnancy. Gen. Hosp. Psychiatry. 2011;33:197–199. doi: 10.1016/j.genhosppsych.2011.01.013. doi:10.1016/j.genhosppsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 110.Van Der Wee NJA, Ramsey NF, Jansma JM, Denys DA, Van Megen HJGM, Westenberg HMG, Kahn RS. Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. Neuroimage. 2003;20:2271–2280. doi: 10.1016/j.neuroimage.2003.05.001. doi:10.1016/j.neuroimage.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 111.Van Grootheest DS, Boomsma DI, Hettema JM, Kendler KS. Heritability of obsessive-compulsive symptom dimensions. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008;147:473–478. doi: 10.1002/ajmg.b.30622. doi:10.1002/ajmg.b.30622. [DOI] [PubMed] [Google Scholar]
- 112.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. doi:10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 113.Vulink NCC, Denys D, Bus L, Westenberg HGM. Female hormones affect symptom severity in obsessive-compulsive disorder. Int. Clin. Psychopharmacol. 2006;21:171–175. doi: 10.1097/01.yic.0000199454.62423.99. doi:10.1097/01.yic.0000199454.62423.99. [DOI] [PubMed] [Google Scholar]
- 114.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. http://dx.doi.org/10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav. Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. doi:10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav. Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. doi:10.1016/j. [DOI] [PMC free article] [PubMed] [Google Scholar]