Abstract
Background
Serum fibrosis markers are useful in staging chronic hepatitis B (HBV) and C (HCV) but have not been evaluated in chronic hepatitis D (HDV).
Aims
We evaluated the utility of serum fibrosis markers (fibrosis-4 score [FIB-4], AST to ALT ratio [AAR], age-platelet index [API], AST-to-platelet-ratio-index [APRI] and Hui score) in HDV infection.
Methods
Clinical and histologic laboratory data from HBV, HCV and HDV patients were evaluated and serum fibrosis markers were calculated. The ability of fibrosis markers to detect advanced fibrosis (Ishak ≥4) and cirrhosis (Ishak =6) were evaluated and compared between viral infections.
Results
1003 subjects (HCV=701, HBV=240 and HDV=62) with mean age of 46 ±11 and 66% male were evaluated. HDV subjects had higher ALT and AST than HCV and lower platelets than both HBV and HCV. Histologically, HDV had the greatest percentage of Ishak≥4 and necroinflammation. FIB-4 performed best in detecting advanced fibrosis and cirrhosis in all viral cohorts. In HDV, area under the receiver operator curve (AUROC) 95% confidence intervals for detecting advanced fibrosis were: FIB-4=0.70 (0.55–0.84), API=0.69 (0.55–0.82), APRI=0.68 (0.54–0.82), Hui score=0.63 (0.49–0.78), AAR=0.63 (0.48–0.77). The AUROC for detecting cirrhosis in HDV were: FIB-4=0.83(0.69–0.97), API=0.80(0.66–0.95), APRI=0.75(0.61–0.89), Hui score=0.70(0.49–0.91) and AAR=0.70(0.48–0.93). Adjustment of published cut-offs led to marginal improvements in FIB4 for advanced fibrosis and of APRI for cirrhosis in HDV.
Conclusions
Serum fibrosis markers have lower performance accuracy in chronic HDV infected patients compared to HBV and HCV patients. Other noninvasive fibrosis markers should be explored to assist in the management of these patients.
Keywords: Non-invasive markers, APRI, FIB4, chronic hepatitis D, fibrosis
Introduction
Chronic hepatitis delta virus (HDV) infection affects an estimated 15–20 million people worldwide and is considered the most severe form of chronic viral hepatitis(1,2). With increased serological testing and awareness of HDV, its prevalence in Western Europe and North America has decreased (3,4), however, the disease burden remains high in Africa, the Mediterranean basin, Central Asia, Mongolia and Russia (2,5). The clinical course of chronic HDV infection has been described to be accelerated compared to hepatitis B virus (HBV) mono-infection with rates of fibrosis progression to cirrhosis within 2–6 years(6). The risk of hepatocellular carcinoma and mortality due to liver disease is also increased compared to other viral hepatitis infections(7). Treatment of this devastating disease remains unsatisfactory. Interferon-based therapies result in a clearance rate of 25–30% at most, and only after prolonged treatment (8). New potential therapies are still in various stages of clinical development (9–12).
Hepatic fibrosis is a consequence of chronic viral liver diseases, which left unabated leads to structural and functional changes (13). The final common pathway of increasing hepatic fibrosis is the development of cirrhosis, cirrhosis-related complications and ultimately death from liver failure (13,14). Although liver biopsy is the gold-standard method of assessing hepatic fibrosis, it is expensive, invasive and has procedural risks (15). In chronic viral hepatitis, identification of advanced fibrosis and cirrhosis remains important, especially for clinical decision-making purposes such as therapy, screening for hepatocellular carcinoma (HCC), monitoring cirrhosis-related complications, and referral for liver transplantation.
Over the past two decades, several clinically useful non-invasive markers for hepatic fibrosis have been developed and found to be accurate in both viral and non-viral liver diseases. In chronic hepatitis B and C, the AST-to-ALT ratio (AAR) (16) AST-to-Platelet Ratio Index (APRI) (17,18), Fibrosis-4 (FIB-4) Test (19,20), Age-Platelet index (API) (21) and the Hui score (22) have been extensively studied their performance accuracies for predicting hepatic fibrosis have been reasonably well defined. While commercially available algorithms and transient elastography have also been studied, they are expensive, use uncommon parameters and are not routinely available worldwide, especially for analysis of large populations or in underserved areas (23,24).
Although chronic HDV infection has been reported worldwide, its highest prevalence has been described in areas with limited resources, which often makes liver biopsy, commercial fibrosis serologic testing and transient elastography inaccessible. The utility of a simple non-invasive fibrosis biomarker for staging of disease, clinical decision-making and education of patients from routinely performed laboratory tests has not been explored in chronic delta hepatitis. Thus, we sought to evaluate and compare the performance accuracy of readily available and published non-invasive fibrosis markers in subjects with chronic HDV infection.
Methods
Study population
This retrospective, cross-sectional study was performed using a cohort of patients with chronic hepatitis B, C and D who underwent liver biopsy at the Clinical Center of the National Institutes of Health between 1985 and 2015 (NCT00001971). The analysis included consecutive adult subjects with chronic HBV, HCV and HDV infection who underwent an initial pre-treatment liver biopsy and had concurrent laboratory assessments that included routine liver tests and complete blood counts.
Subject data were collected using the NIH Biomedical Translational Research Information System (BTRIS) including age (at time of liver biopsy), gender and self-reported race and ethnicity. In some instances, laboratory values from external sources were captured through data abstraction. As some subjects had multiple laboratory values around the time of the liver biopsy, component laboratory results were chosen that were taken closest to the time of the procedure and within the previous 2 months.
Chronic HCV infection was defined as presence of HCV-RNA in serum for 6 months before the liver biopsy or presence of histologic and clinical features of non-A non-B hepatitis in patients undergoing biopsy before 1991 who were later proven to have HCV infection based upon testing of stored serum for HCV RNA. Chronic HBV infection was defined as presence of hepatitis B surface antigen (HBsAg) in serum at time of liver biopsy and positive staining for HBsAg or HBcAg in hepatocytes. Chronic HDV infection was defined as presence of anti-HDV and HDV RNA in serum or positive staining for HDAg in hepatocytes histologically.
All patients were enrolled in clinical research protocols that had been approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board and gave written, informed consent for participation.
Liver Histopathology
Liver biopsy specimens were read and scored by an expert hepatopathologist (D.E.K.). Only biopsies with adequate tissue were scored, which was defined as biopsy length of 15 mm or a minimum of 10 portal tracts visualized. The modified histology activity index (HAI)(25) was used for grading of necroinflammation and the Ishak fibrosis score(26) for staging of fibrosis. The total necroinflammatory HAI score comprised the individual scores for periportal inflammation and necrosis (0–10), lobular inflammation and necrosis (0–4), and portal inflammation (0–4). The Ishak fibrosis (Ishak) score scale ranged from 0 for no fibrosis to 6 for cirrhosis.
Serum Fibrosis Markers
The non-invasive serum markers selected for evaluation have been reported and validated largely in chronic hepatitis C and B with cut-offs that have a high positive predictive value for identifying advanced fibrosis or cirrhosis. For the purposes of this study, the most widely published algorithms that could be calculated with routine laboratory testing were evaluated. These scoring systems included the AST to ALT ratio (AAR), the Age-Platelet Index (API), the AST-to-Platelet-Ratio-Index (APRI), the Fibrosis-4 (FIB-4) score and the Hui score (Supplemental Table S1).
The AST to ALT Ratio
Perhaps the earliest non-invasive surrogate marker utilized in predicting hepatic fibrosis was the ratio of the absolute value for AST and ALT. The AAR was created and validated for use in chronic hepatitis C where an AAR ≥1 was predictive of cirrhosis (16). Further analyses showed that it was also helpful in chronic hepatitis B and nonalcoholic steatohepatitis (27,28). The formula for calculating the AAR is:
The Age-Platelet Index
The API was initially developed for use in chronic hepatitis C with set ranges to predict cirrhosis(21). Using a cutoff of ≥6 to identify cirrhosis resulted in an AUROC of 0.91 (29). It has also been studied in chronic HBV with AUROC being 0.89 for identifying cirrhosis in one study (30). The formula for calculating the API is:
The AST-Platelet-Ratio-Index
The APRI was developed in a cohort of patients with chronic hepatitis C with cut-offs studied for significant fibrosis, defined as Ishak≥3 and for cirrhosis, defined as Ishak≥5 (31). In the original paper, the AUROC for significant fibrosis was 0.80 using a cutoff of ≥1.5 and for cirrhosis was 0.89 using a cutoff of ≥2.0. APRI has also been studied in a large meta-analysis in chronic hepatitis B (32) with an AUROC value of 0.79 for significant fibrosis at a cutoff of 1.5 and 0.75 for cirrhosis using a cutoff of 2.0. The formula for calculating the APRI is:
Fibrosis-4 Index
FIB-4 was originally developed in cohorts of chronic HCV/HIV patients(20) and later validated in mono-infected chronic HCV(33). It yielded an AUROC of 0.85 for severe fibrosis (METAVIR F≥3) and 0.91 for cirrhosis (METAVIR F4). The cutoff of FIB-4 ≥3.25 was used for estimating advanced fibrosis (Ishak≥4) in the initial mono-infected HCV study. A recent meta-analysis(34) using FIB-4 in chronic hepatitis B revealed the mean AUROC for cirrhosis of 0.84. The formula for calculating the FIB-4 index is:
The Hui score
The Hui score (22) was developed exclusively for chronic hepatitis B. This scoring system yielded an AUROC of 0.79 for significant fibrosis in the initial publication. As most of the scoring systems for predicting fibrosis prediction were initially developed in hepatitis C, the Hui score was an attractive prediction model to evaluate in chronic hepatitis D as well as hepatitis C. The formula for calculating the Hui score is:
Statistical Methods
Statistical analysis was performed by SAS software (Statistical Analysis Software, version 9.4; SAS Institute, Cary, NC). The data was stratified according to two outcomes, advanced fibrosis (Ishak ≥4) and cirrhosis (Ishak =6). Univariate analysis was performed for the following variables: FIB-4, AAR, APRI, Hui score and API. Logistic transformation was applied for normality. The area under the receiver operating characteristic (AUROC) curve was used to measure the diagnostic accuracy of the biomarkers in HCV, HBV, and HDV for detecting advanced fibrosis and cirrhosis.
Sensitivity, specificity, positive and negative predictive values were also calculated for advanced fibrosis and cirrhosis based on cutoffs predefined in the literature. To compare the differences between different models, the DeLong method was employed to compare the highest performing model to others for calculation of the standard error of the AUC and of the difference between two AUCs(35).
Lastly, adjustment of cutoff points for detecting advanced fibrosis using FIB-4 and cirrhosis using APRI were performed using Youden-Indices to evaluate for improvement in the diagnostic performance of these markers in chronic hepatitis D(36).
Results
Subject demographics and laboratory evaluation
A total of 1452 subjects were identified of whom, 307 were excluded as they had a co-existing non-viral hepatitis liver diseases and 142 because of incomplete clinical documentation. The final study population consisted of 1003 adult subjects with chronic viral hepatitis; 701 due to HCV, 240 to HBV and 62 to HDV/HBV coinfection (Figure 1).
Figure 1.
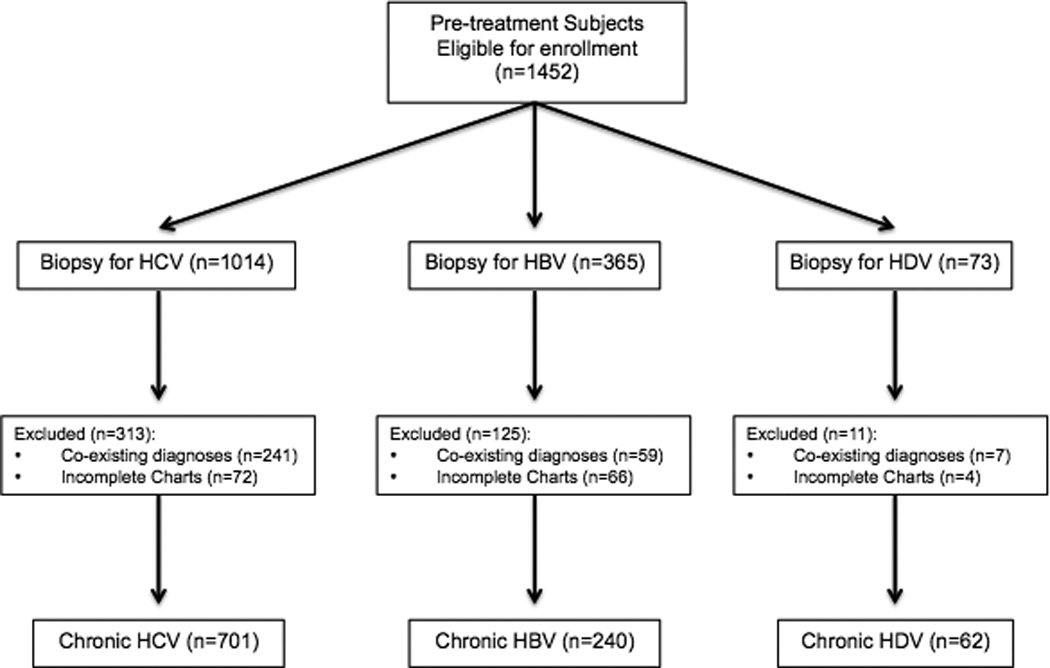
Study Flow Diagram
Of the 1452 subjects with pre-treatment baseline liver biopsies, 1003 were evaluated for this study. This figure shows development of this study population.
The mean age for all subjects was 46 ± 11 years with a predominance of male sex (n=664, 66%) and Caucasian race (n=615, 61%) in the overall study cohort (Table 1). No subjects had hepatocellular carcinoma or decompensated liver disease at the time of enrollment and liver biopsy. Age distribution was different among the three groups, the HDV (39 years) and HBV (43 years) cohorts being younger than the HCV cohort (47 years, p<0.0001). The HDV and HBV cohorts were also more likely to be men (78% and 79%) than the HCV cohort (63%). Patients with chronic hepatitis B were more likely to be Asian (35%) than those with HDV (18%) or HCV (7%) infection, but the relative distribution of non-Asian races and ethnic groups tended to be similar.
Table 1.
Demographics of the Study Population
| HDV (n=62) |
HBV (n=240) |
HCV (n=701) |
HDV vs HBV |
HDV vs HCV |
|
|---|---|---|---|---|---|
| Mean Age (SD) | 39 (10) | 42 (13) | 47 (10) | 0.03 | <0.0001 |
| Male Gender (%) | 49 (79%) | 188 (78%) | 427 (61%) | 0.33 | 0.06 |
| Ethnicity | |||||
| White | 58% | 52% | 65% | 0.18 | 0.2 |
| Asian | 18% | 33% | 7% | ||
| Black/African American | 16% | 10% | 17% | ||
| Other | 3% | -- | 0.50% | ||
| Unknown | 5% | 6% | 10% | ||
| Laboratory Data (Mean and SD) | |||||
| ALT (U/L) | 134 (147) | 124 (95) | 95 (77) | 0.65 | 0.04 |
| AST (U/L) | 86 (72) | 76 (60) | 65 (49) | 0.28 | 0.03 |
| Platelets (K/uL) | 160 (70) | 182 (59) | 194 (67) | 0.02 | 0.0002 |
| Total Bilirubin (mg/dL) | 0.84 (0.62) | 0.85 (0.48) | 0.79 (0.44) | 0.88 | 0.55 |
| Albumin (g/dL) | 3.7 (0.6) | 3.8 (0.5) | 3.9 (0.4) | 0.17 | 0.003 |
| Alkaline Phosphatase (U/L) | 98 (45) | 83 (34) | 79 (36) | 0.02 | 0.003 |
| Modified HAI Inflammation Scores* | |||||
| Mean (SD) | 10.2 (3.3) | 7.7 (3.5) | 7.9 (2.8) | <0.0001 | <0.0001 |
| 0–4 | 2 (3.2%) | 39 (16.2%) | 67 (9.5%) | ||
| 5–8 | 19 (30.6%) | 105 (43.8%) | 334 (47.6%) | ||
| 9–12 | 18 (29.0%) | 70 (29.2%) | 262 (37.4%) | ||
| 13–18 | 21 (33.9%) | 26 (10.8%) | 38 (5.4%) | ||
| Ishak Fibrosis Stage | |||||
| Mean (SD) | 3.6 (1.5) | 2.4 (1.8) | 2.2 (1.8) | <0.0001 | <0.0001 |
| 0 | 1 (2%) | 126 (18%) | 29 (12%) | ||
| 1 | 6 (10%) | 159 (23%) | 74 (31%) | ||
| 2 | 2 (3%) | 136 (19%) | 24 (10%) | ||
| 3 | 27 (43%) | 107 (15%) | 57 (24%) | ||
| 4 | 8 (13%) | 97 (14%) | 18 (8%) | ||
| 5 | 8 (13%) | 26 (4%) | 17 (7%) | ||
| 6 | 10 (16%) | 51 (7%) | 23 (9%) | ||
2 HDV biopsies were not scored with HAI scoring system
HBV: Hepatitis B; HCV: Hepatitis C; HDV: Hepatitis D; ALT: alanine aminotransferase; AST: aspartate aminotransferase; SD: standard deviation; HAI: Histologic activity score
Routine laboratory test results in the three cohorts are also shown in Table 1. In general, serum aminotransferase levels were higher in patients with chronic hepatitis B and D than in hepatitis C and these results were statistically significant for ALT values (HDV = 134±147 U/L, HBV = 124±95 U/L, HCV = 95±77 U/L). In contrast, serum AP and bilirubin were only minimally higher among those with HDV than those with HBV or HCV infection. Strikingly, platelet counts were lowest in the HDV cohort (160±70 K/µL) compared to the HBV cohort (182±59 K/µL, p<0.02) and HCV cohort (194±67 K/µL, p<0.0002).
In subjects with HDV, genotype 1 infection was the predominant infection (genotype 1=41, genotype 6=1, unknown genotype=20). Quantitative HBV DNA level was detectable in 20 of 24 chronic HDV subjects who were not on nucleoside analogs and was significantly lower at 1.9 ± 0.9 log IU/mL compared to chronic HBV subjects at 6.5 ± 2.1 log IU/mL (p<0.001).
Histopathologic evaluation
Histological scoring showed that patients with HDV infection had on average higher necroinflammatory scores (HAI) and more advanced fibrosis that those with HBV or HCV infection (Table 1). Thus, 34% of those with chronic delta hepatitis had severe activity (HAI 13–18) compared to 11% with hepatitis B and only 5% with hepatitis C. The HBV cohort had the broadest range of HAI scores, having higher proportion with mild inflammation and necrosis (16%) compared to HCV (10%) and HDV (3%). On the other hand, the HBV cohort had a higher rate of more severe activity than the HCV cohort. These differences were reflected in the mean HAI scores for the three cohorts, being highest with HDV infection (10.1±3.3), intermediate with HBV (7.7±3.5) and lowest with HCV infection (7.9±2.8, p<0.0001).
Fibrosis scores were also higher in the HDV cohort, 16% having cirrhosis (Ishak=6), compared to only 7% of those with HBV and 9% with HCV infection. Conversely, the HDV cohort had a lower percentage (15%) of patients with no or only mild fibrosis (IF≤2) compared to 53% of patients with HBV and 60% with HCV infection. These differences were also reflected in the mean fibrosis scores in the three cohorts, being highest with HDV (3.6±1.5), intermediate with HBV (2.4±1.8) and lowest with HCV (2.2±1.8: p<0.0001).
Biomarker performances in identifying advanced fibrosis (Ishak ≥4)
In identifying advanced fibrosis, all biomarkers (AAR, API, FIB-4, APRI and Hui score) were evaluated in all three viral hepatitis cohorts. The ROC analysis for each virus and scoring methods were analyzed (Figure 2) and area under receiver operator curve (AUROC) was calculated (Table 2). In all viral cohorts, FIB-4 consistently performed the best of all of the biomarkers with an AUROC of 0.86 in HCV, 0.81 in HBV and 0.70 in HDV. AAR performed the worst of all biomarkers with an AUROC of 0.71 in HCV and HBV and 0.63 in HDV.
Figure 2.
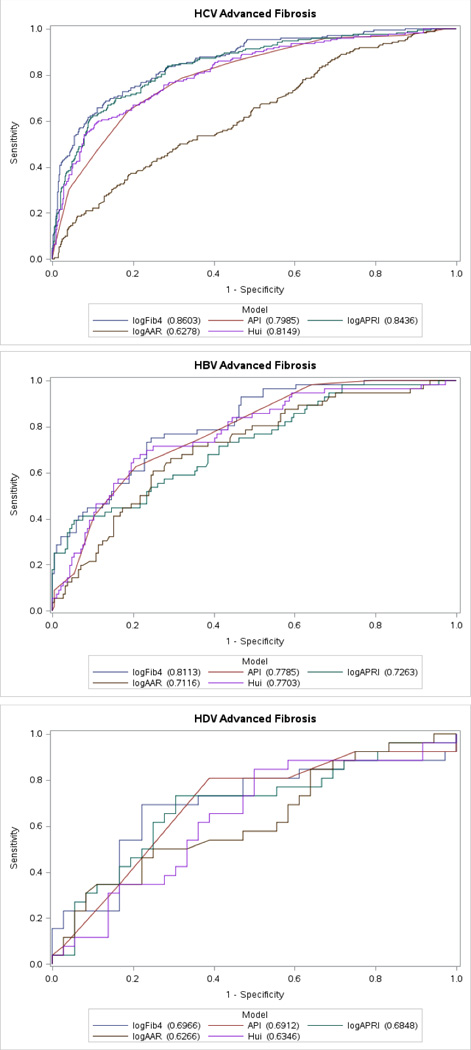
Receiver operating characteristic curves of fibrosis markers for the diagnosis of advanced fibrosis (F≥4) in chronic viral hepatitis B, C and D.
Table 2.
Area Under The Receiver Operating Curve (AUROC) describing the performance of Non-invasive fibrosis predictive models in chronic viral hepatitis B, C and D
| Advanced Fibrosis AUROC by Virus (Ishak Fibrosis Score ≥4) | |||
|---|---|---|---|
| Model | HDV | HBV | HCV |
| Area (Confidence Interval) | |||
| logFib4 | 0.70 (0.55–0.84) | 0.81 (0.75–0.87) | 0.86 (0.83–0.89) |
| logAPRI | 0.68 (0.54–0.82) | 0.73 (0.65–0.80) | 0.84 (0.81–0.88) |
| logAAR | 0.63 (0.48–0.77) | 0.71 (0.63–0.79) | 0.71 (0.58–0.68) |
| logAPI | 0.69 (0.55–0.89) | 0.78 (0.72–0.84) | 0.80 (0.76–0.84) |
| logHui | 0.63 (0.49–0.78) | 0.77 (0.70–0.84) | 0.81 (0.78–0.85) |
| Cirrhosis AUROC by Virus (Ishak Fibrosis Score =6) | |||
| Area (Confidence Interval) | |||
| logFib4 | 0.83 (0.69–0.97) | 0.83 (0.75–0.91) | 0.91 (0.87–0.95) |
| logAPRI | 0.75 (0.61–0.89) | 0.69 (0.55–0.82) | 0.87 (0.83–0.92) |
| logAAR | 0.70 (0.48–0.93) | 0.78 (0.69–0.86) | 0.68 (0.61–0.76) |
| logAPI | 0.80 (0.66–0.95) | 0.80 (0.71–0.89) | 0.88 (0.83–0.93) |
| logHui | 0.70 (0.49–0.91) | 0.77 (0.65–0.89) | 0.83 (0.76–0.89) |
Similar to previously published data in HCV, the APRI was the second best performing noninvasive biomarker in detecting advanced fibrosis with an AUROC of 0.84. However, the APRI then demonstrated a large performance decline in HBV (AUROC=0.73) and performed even worse in HDV (AUROC=0.68). The Hui score, as previously discussed, has only been studied in chronic hepatitis B. Interestingly, its performance was better in HCV (AUROC=0.81) compared to HBV (AUROC=0.77) and performed poorly in HDV (AUROC=0.63). The API performed similarly to the Hui score with an AUROC of 0.80 in HCV, 0.78 in HBV and 0.69 in HDV.
Since FIB-4 performed the best across all chronic viral hepatitis cohorts in detecting advanced fibrosis, the FIB-4’s AUROC performance was then compare to the performance of the other noninvasive biomarkers (Supplemental Table S2). In HCV, FIB-4 demonstrated statistically superior performance to AAR (p<0.0001), API (p<0.0001) and Hui score (p<0.05) but failed to demonstrate an improvement over APRI (p=0.13). In HBV, FIB-4 was superior to APRI (p<0.01) and AAR (p<0.01) but failed to demonstrate a performance improvement over the Hui score (p=0.21) and the API (p=0.14). Interestingly, in HDV, FIB-4 did not demonstrate any significant improved diagnostic performance over any another other model.
The sensitivity, specificity, positive and negative predictive values of FIB-4 in chronic Hepatitis D was evaluated utilizing the published standard cut-off ≥ 3.25 for the detection of advanced fibrosis. Overall it performed the worst in HDV (Supplemental Table S3). The overall sensitivity and specificity were low in HDV at 56% and 65% respectively. The positive predictive value of FIB-4 was also low in HDV (26%) and HBV (33%) compared to HCV (52%). The negative predictive values was also lowest in HDV at 83% compared to 94% in HBV and 95% in HCV.
Adjustment of FIB-4 cutoffs for diagnosing advanced fibrosis (Ishak ≥4)
Since FIB-4 is widely used and has an established cutoff for diagnosing advanced fibrosis in hepatitis C, an attempt was made to adjust the cutoff values to ascertain if diagnostic accuracy could be improved in HDV (Table 3). A lower cutoff value of ≥2.03 improved the positive predictive value to 69% with an incremental improvement in sensitivity and specificity at 69% and 78% respectively. However, the negative predictive value declined to 83% from 78%.
Table 3.
Evaluation of published and adjusted cut-offs for FIB-4 in advanced fibrosis and APRI in cirrhosis in Chronic HDV
| Adjustment of FIB-4 for advanced fibrosis | |||||
|---|---|---|---|---|---|
| Biomarker | Cutoffs | Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
| FIB-4 | ≥3.25* | 56 | 65 | 33 | 83 |
| ≥2.03 | 69 | 78 | 69 | 78 | |
| Adjustment of APRI for cirrhosis | |||||
| APRI | ≥2.0* | 25 | 92 | 73 | 60 |
| ≥1.67 | 90 | 60 | 30 | 97 | |
Validated Cut-offs for Chronic Hepatitis B and C.
APRI: Aspartate aminotransferase to platelet ratio index; FIB-4: Fibrosis score using 4 factors.
Optimal cutoff adjustment performed by Youden-Indices.
Biomarker performances in identifying cirrhosis (Ishak =6)
In subjects with cirrhosis (Ishak=6), all biomarkers were evaluated for their performance accuracy (Figure 3). Similar to the advanced fibrosis analysis, FIB-4 outperformed all other biomarkers in all cohorts with an AUROC in HCV of 0.91, in HBV and HDV at 0.83 (Table 2). Similarly, AAR again performed consistently poor in all viral hepatitis cohorts with an AUROC of 0.68 in HCV, 0.78 in HBV and 0.70 in HDV.
Figure 3.
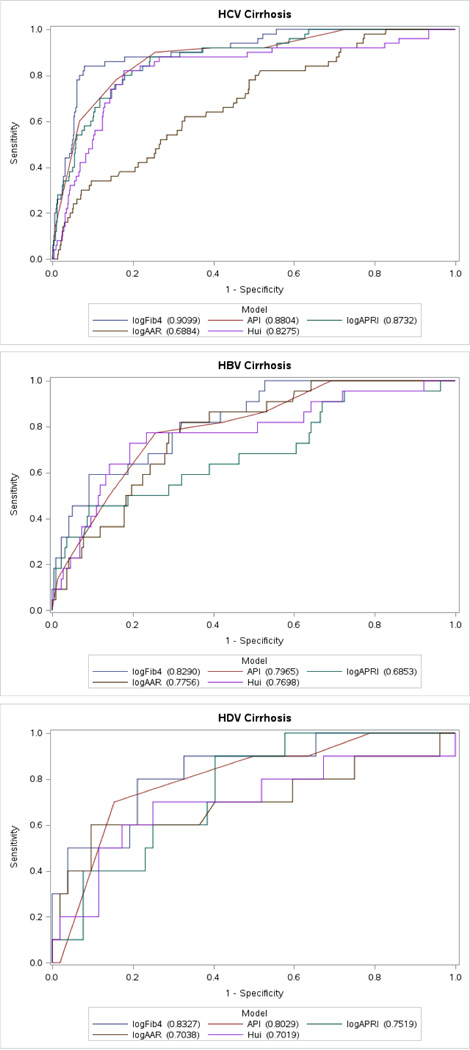
Receiver operating characteristic curves of fibrosis markers for the diagnosis of cirrhosis (F=6) in chronic viral hepatitis B, C and D.
Interestingly, in cirrhosis, the API was the second best performing noninvasive biomarker. In HCV, the AUROC was 0.88 and in both HBV and HDV the AUROC was 0.80. The APRI performed similarly to the API in HCV (AUROC=0.87) but then experienced a drastic performance decline in both HBV (AUROC=0.69) and in HDV (AUROC=0.75). Similar to the findings in the advanced fibrosis analysis, the Hui score performed the best in HCV (AUROC of 0.83), and with decay in performance in chronic HBV (AUROC=0.77) and in chronic HDV (AUROC=0.70).
Since FIB-4 once again performed the best across all chronic viral hepatitis infections, a direct comparison of FIB-4 in cirrhosis to other noninvasive biomarkers was performed (Supplemental Table S4). In HCV, FIB-4’s performance was statistically superior to AAR (p<0.0001), APRI (p<0.005) and Hui score (p<0.01) but only trended towards improved performance over API (p=0.08). Interestingly, in HBV, FIB-4 only performed statistically better than APRI (p<0.001). In HDV related cirrhosis, FIB-4 failed to significantly outperform any other biomarker and only demonstrated a trend towards significance with AAR (p=0.06) and the Hui score (p=0.07).
Adjustment of cutoffs for APRI in HDV related cirrhosis (Ishak =6)
As APRI has been validated in other viral hepatitis for diagnosis of cirrhosis, an attempt was made to improve the diagnostic accuracy for the detection of HDV cirrhosis (Table 3). At the published cutoff of ≥2.0, APRI demonstrated a low sensitivity of 25% but an excellent specificity of 92% in detecting HDV cirrhosis. By Youden-Indices, adjustment to a cutoff of ≥1.67 yielded a higher sensitivity at 90% but lower specificity at 60%. At ≥1.67, the negative predictive value improved from 60% to 97% but the positive predictive value declined from 73% from 30%.
Discussion
In this study of 1003 well-characterized subjects with viral hepatitis, the role of several validated non-invasive fibrosis models for predicting the spectrum of liver disease from advanced fibrosis to cirrhosis were evaluated, with focus on the performance of these models in chronic hepatitis D infection. With the exception of the Hui Score, all of these biomarkers were initially created for use in hepatitis C but have also been evaluated in HBV(37). While there is increasing evidence that these scores perform worse in HBV compared to HCV, the utility in HDV is less well defined(38). The results from this study not only add to the growing body of evidence that these biomarkers perform worse in HBV compared to HCV, but in the identification of hepatic fibrosis extending beyond advanced fibrosis, these biomarkers perform the worst in HDV. In identifying Ishak fibrosis ≥4 in HDV, the best performing non-invasive fibrosis marker performed worse than the lowest performing non-invasive fibrosis marker in HBV and HCV. In detecting HDV related cirrhosis, the performance of these biomarkers do not match those of HBV and HCV.
Similar to the recent description by Lutterkorn and colleagues from HDV infected subjects in the HIDIT-2 cohort, we add to the paucity of existing evidence that non-invasive fibrosis markers perform poorly in HDV (38). In contrast, the data presented in this cohort compares three unique cohorts of chronic viral hepatitis patients with comparisons of noninvasive fibrosis biomarker performance across HBV, HCV and HDV infection. Additionally, our analysis utilizes differing histologic fibrosis cutoffs (Ishak ≥4 and Ishak >6) which can be utilized with equivalencies of Metavir F≥3 and F=4(39). Of interest, we performed an analysis utilizing their cutoff of Ishak >2 and >4 which yielded similar results (Supplemental Tables S5 and S6). Finally, we provide direct histologic comparisons across chronic viral hepatitis infections related not only to fibrosis but also necroinflammation, which may provide insight for the poor performance of the compared models in HDV.
There are several plausible reasons that may explain the poor performance of these markers in HDV. First, it has been described that HDV is often more rapidly progressive, has higher transaminase levels and greater thrombocytopenia compared to HBV and HCV monoinfection (4,40). Much of this is due to the high levels of activity in HDV, which is manifested as hepatic inflammation and is identified on histology(41,42). Similar to other descriptions, in our cohort, patients with HDV demonstrated significantly higher levels of necroinflammation on biopsy. Therefore, since all of the evaluated biomarkers utilize either a component of the transaminases or platelets in their model, this could explain the poor performance in HDV compared to HBV and HCV. Second, these biomarkers were initially designed for use in single viral infections such as HBV or HCV and not dual viral infections such as HDV. Interestingly, in dual viral infections such as HIV/HCV, these models perform worse(20,33). Thus, while it appears more likely that the poor performance of these biomarkers are a result of disease severity, the dual viral component in HDV/HBV may play a contribution.
In all viral cohorts evaluated in this study, FIB-4 consistently outperformed all other biomarkers while the AAR consistently performed the worst. The data presented in this cohort adds to the growing body of evidence that FIB-4 may be the most clinically accurate biomarker that utilizes commonly available tests (43,44). The major components utilized in calculating the biomarkers examined in this study include platelets (4 of 5 biomarkers), transaminases (4 of 5 biomarkers) and age (2 of 5 biomarkers) that may provide a rationale for these performances. It has been well described that platelet counts decline with increasing hepatic fibrosis, portal hypertension and differing levels of thrombopoietin synthesis (45). Additionally, hepatic transaminases are often elevated until end stage liver disease (46) and ALT-to-AST ratios will change with advanced liver disease (47). Finally, it has been suggested that advanced age is often associated with more advanced fibrosis due to the duration of viral exposure (48). As such, of all of the biomarkers evaluated, FIB-4 utilized the greatest number of variables (age, AST, ALT and platelet) whereas AAR utilized only two variables (AST and ALT), which are hepatocellular in nature and are significantly intertwined. Thus, it is plausible that biomarkers that utilize more variables that represent different aspects of liver disease may provide a better representation of the extent of hepatic fibrosis. Various studies utilizing HDV RNA quantitation have produced varying results, perhaps differing by genotype (41,42,49). While there may be a future role of HDV RNA in fibrosis biomarkers, there is still much to be understood about HDV RNA quantitation, especially across different genotypes and with a lack of standardization between assays. Additionally, given the current lack of availability of the HDV RNA quantitation, it’s utility as a biomarker in clinical medicine is limited.
In this study, we evaluated the ability of fibrosis biomarkers to identify patients with advanced fibrosis and cirrhosis. While other comparative biomarker studies have evaluated other aspects, namely significant fibrosis (Ishak ≥3), the intent of this study was to ascertain the ability of these biomarkers to identify HDV patients with significantly advanced fibrosis who might require additional medical interventions (50). In the clinical setting, an Ishak >F3 would warrant consideration/discussion of interferon-based therapies. Additionally, subjects with Ishak 6 (equivalent to METAVIR 4 fibrosis stage) would utilize additional healthcare including variceal screening(51). Given that interferon-based therapies result in sustained loss of HDV RNA in only 20–30 % of patients, and is currently the only recommended therapy for this devastating disease, it is important to identify patients who would be eligible candidates and worth treating (8,52,53). The utility of these biomarkers, or improved ones, that utilize simple routine tests could play a significant clinical role in identifying individuals who could be worth the gamble of interferon-based therapy, especially in countries without advanced medical capabilities. However, given the poor performance of the biomarkers examined in this study, specific HDV fibrosis biomarkers should continue to be sought.
To improve the performance of established noninvasive fibrosis biomarkers for HDV, we explored adjusting published cut-off values for identifying advanced fibrosis in FIB-4 and cirrhosis in APRI, which have the most extensive body of literature, and are the most commonly used of the models tested (54). Interestingly, while FIB-4 performed the best prior to any adjustment (≥3.25), the adjustment of FIB-4 to ≥2.03 in HDV resulted in an incremental improvement in detecting advanced fibrosis. In cirrhosis, the effect of adjusting the cut-offs was more drastic with significant improvement in sensitivity and negative predictive value with new cut-off of ≥1.67 for APRI. The mild improvement after adjusting to a lower cutoff in FIB-4 may be a result of the AST and ALT components of the calculation given that the transaminases are typically more elevated in patients with HDV compared to those with HBV monoinfection or HCV. Given these findings, it would be interesting to explore these new FIB-4 and APRI cutoffs in a separate cohort of HDV patients to see if these findings remain significant. However, it should noted that the improvement in performance in APRI and FIB-4 in HDV still does not rival that of HCV and HBV and thus, remains inferior in the detection of advanced fibrosis and cirrhosis.
This study, while strong for its pre-treatment baseline disease activity characterization, is limited by the small number of HDV patients and the lack of a validation cohort in evaluating adjustment cutoff values for FIB4 and APRI. Additionally, the low number of HDV subjects with cirrhosis may have impacted the performance of noninvasive biomarkers in identifying cirrhosis. In the US, studies on the prevalence of HDV are limited. It is believed that HDV prevalence is about 3–5% in those infected with HBV, thus limiting the number of HDV patients that can be studied (55). In a recent study evaluating the prevalence of HDV antibody, only 73 were identified after evaluating >25,000 HBV surface antigen positive subjects (56). Thus, future exploration can be performed in regions where HDV is endemic. Additionally, studies incorporating the evaluation of transient elastography in HDV may be considered though the high degrees of hepatic inflammation may need to be accounted for with higher cutoffs for significant fibrosis and cirrhosis(57). However, there is clinical utility in continued evaluation of these non-invasive models given several endemic areas of Africa, Central Asia and Mongolia are under-developed with scarce healthcare resources.
In conclusion, existing non-invasive biomarkers of hepatic fibrosis have lower performance accuracy in chronic HDV infected patients compared to mono-infected HBV and HCV patients. In areas with advanced medical technology, other noninvasive modalities of fibrosis quantification can be explored, whereas in medically underserved areas, these readily available noninvasive markers of fibrosis may provide insight into the staging of liver disease.
Supplementary Material
Acknowledgments
Financial support: This research was supported by the Intramural Division of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute of the National Institutes of Health.
The authors would like to thank Dr. Jay Lozier, MD, PhD for his assistance in obtaining hematologic parameters for the patient cohort and Andrea Beri, for BTRIS support.
Abbreviations
- HDV
hepatitis delta virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AP
alkaline phosphatase
- TBili
total bilirubin
- Alb
serum albumin
- APRI
aspartate aminotransferase to platelet ratio index
- AAR
aspartate aminotransferase to alanine aminotransferase ratio
- FIB4
fibrosis index based on four factors
- API
age platelet index
- HBsAg
hepatitis B surface antigen
- HDAg
hepatitis delta antigen
- HCC
hepatocellular carcinoma
- Ishak
Ishak fibrosis score
- HAI
histology activity index
- FDA
Food and Drug Administration
- AASLD
American Association for the Study of Liver Disease
- EASL
European Association for the Study of the Liver
- NIH
National Institutes of Health
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- DNA
deoxyribonucleic acid
- RNA
ribonucleic acid
- SD
standard deviation
- SE
standard error
- ROC
receiver operating curve
- AUROC
Area under the receiver operating curve
Footnotes
Conflicts of interest: None of the authors has financial interests or conflicts of interest related to this research.
Authors’ Contributions:
Study concept and design: Koh, Takyar
Acquisition of data: Takyar, Surana
Analysis and interpretation of data: Surana, Takyar, Koh, Heller, Wilkins, Hoofnagle, Liang
Drafting of the manuscript: Takyar, Koh, Surana, Heller
Critical revision of the manuscript for important intellectual content: Takyar, Koh, Surana, Heller, Kleiner, Wilkins, Hoofnagle, Liang
Statistical Analysis: Surana, Takyar, Koh, Wilkins
Study supervision: Koh
All authors approved the final version of the manuscript.
References
- 1.De Paschale M, Manco MT, Belvisi L, et al. Epidemiology of hepatitis D virus (HDV) infection in an urban area of northern Italy. Infection. 2012;40:485–491. doi: 10.1007/s15010-012-0247-4. [DOI] [PubMed] [Google Scholar]
- 2.Rizzetto M, Ciancio A. Epidemiology of hepatitis D. Seminars in liver disease. 2012;32:211–219. doi: 10.1055/s-0032-1323626. [DOI] [PubMed] [Google Scholar]
- 3.Servant-Delmas A, Le Gal F, Gallian P, Gordien E, Laperche S. Increasing prevalence of HDV/HBV infection over 15 years in France. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2014;59:126–128. doi: 10.1016/j.jcv.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Gish RG, Yi DH, Kane S, et al. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. Journal of gastroenterology and hepatology. 2013;28:1521–1525. doi: 10.1111/jgh.12217. [DOI] [PubMed] [Google Scholar]
- 5.Tsatsralt-Od B, Takahashi M, Nishizawa T, Endo K, Inoue J, Okamoto H. High prevalence of dual or triple infection of hepatitis B, C, and delta viruses among patients with chronic liver disease in Mongolia. Journal of medical virology. 2005;77:491–499. doi: 10.1002/jmv.20482. [DOI] [PubMed] [Google Scholar]
- 6.Fattovich G, Giustina G, Christensen E, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep) Gut. 2000;46:420–426. doi: 10.1136/gut.46.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136:1629–1638. doi: 10.1053/j.gastro.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Wedemeyer H, Yurdaydin C, Dalekos GN, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. The New England journal of medicine. 2011;364:322–331. doi: 10.1056/NEJMoa0912696. [DOI] [PubMed] [Google Scholar]
- 9.Volz T, Allweiss L, Ben MM, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. Journal of hepatology. 2013;58:861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. Journal of hepatology. 2016;65:490–498. doi: 10.1016/j.jhep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. The Lancet Infectious diseases. 2015;15:1167–1174. doi: 10.1016/S1473-3099(15)00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivacik D, Ely A, Ferry N, Arbuthnot P. Sustained inhibition of hepatitis B virus replication in vivo using RNAi-activating lentiviruses. Gene therapy. 2015;22:163–171. doi: 10.1038/gt.2014.94. [DOI] [PubMed] [Google Scholar]
- 13.Ghany MG, Kleiner DE, Alter H, et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 15.Bravo AA, Sheth SG, Chopra S. Liver biopsy. The New England journal of medicine. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 16.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. The American journal of gastroenterology. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 17.Teshale E, Lu M, Rupp LB, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS) Journal of viral hepatitis. 2014;21:917–920. doi: 10.1111/jvh.12279. [DOI] [PubMed] [Google Scholar]
- 18.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg SD, Lu M, Rupp LB, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:240–246. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 21.Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. Journal of viral hepatitis. 1997;4:199–208. doi: 10.1046/j.1365-2893.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.Hui AY, Chan HL, Wong VW, et al. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. The American journal of gastroenterology. 2005;100:616–623. doi: 10.1111/j.1572-0241.2005.41289.x. [DOI] [PubMed] [Google Scholar]
- 23.Adams LA, Bulsara M, Rossi E, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clinical chemistry. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 25.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 26.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. Journal of hepatology. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 27.Haukeland JW, Schreiner LT, Lorgen I, et al. ASAT/ALAT ratio provides prognostic information independently of Child-Pugh class, gender and age in non-alcoholic cirrhosis. Scandinavian journal of gastroenterology. 2008;43:1241–1248. doi: 10.1080/00365520802158614. [DOI] [PubMed] [Google Scholar]
- 28.Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Archives of internal medicine. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 29.Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376–1382. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 30.Kim BK, Kim SA, Park YN, et al. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver international : official journal of the International Association for the Study of the Liver. 2007;27:969–976. doi: 10.1111/j.1478-3231.2007.01519.x. [DOI] [PubMed] [Google Scholar]
- 31.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 32.Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC gastroenterology. 2012;12:14. doi: 10.1186/1471-230X-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 34.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 35.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 36.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Shin WG, Park SH, Jang MK, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2008;40:267–274. doi: 10.1016/j.dld.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Lutterkort GL, Wranke A, Yurdaydin C, et al. Non-invasive fibrosis score for hepatitis delta. Liver international : official journal of the International Association for the Study of the Liver. 2016 doi: 10.1111/liv.13205. [DOI] [PubMed] [Google Scholar]
- 39.Rozario R, Ramakrishna B. Histopathological study of chronic hepatitis B and C: a comparison of two scoring systems. Journal of hepatology. 2003;38:223–229. doi: 10.1016/s0168-8278(02)00357-4. [DOI] [PubMed] [Google Scholar]
- 40.Liao B, Zhang F, Lin S, et al. Epidemiological, clinical and histological characteristics of HBV/HDV co-infection: a retrospective cross-sectional study in Guangdong, China. PloS one. 2014;9:e115888. doi: 10.1371/journal.pone.0115888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braga WS, de Oliveira CM, de Araujo JR, et al. Chronic HDV/HBV co-infection: predictors of disease stage--a case series of HDV-3 patients. Journal of hepatology. 2014;61:1205–1211. doi: 10.1016/j.jhep.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 42.Zachou K, Yurdaydin C, Drebber U, et al. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2010;30:430–437. doi: 10.1111/j.1478-3231.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- 43.Houot M, Ngo Y, Munteanu M, Marque S, Poynard T. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Alimentary pharmacology & therapeutics. 2016;43:16–29. doi: 10.1111/apt.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallet V, Dhalluin-Venier V, Roussin C, et al. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Alimentary pharmacology & therapeutics. 2009;29:409–415. doi: 10.1111/j.1365-2036.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 45.Giannini E, Botta F, Borro P, et al. Relationship between thrombopoietin serum levels and liver function in patients with chronic liver disease related to hepatitis C virus infection. The American journal of gastroenterology. 2003;98:2516–2520. doi: 10.1111/j.1572-0241.2003.08665.x. [DOI] [PubMed] [Google Scholar]
- 46.Hoofnagle JH, Ghany MG. Approach to the Patient with Liver Disease. In: Longo, Fauci, Kasper, Hauser, Jameson, Loscalzo, editors. Harrison's Principles of Internal Medicine. United States of America: The McGraw-Hill Companies, Inc; 2012. [Google Scholar]
- 47.Nalpas B, Vassault A, Le Guillou A, et al. Serum activity of mitochondrial aspartate aminotransferase: a sensitive marker of alcoholism with or without alcoholic hepatitis. Hepatology. 1984;4:893–896. doi: 10.1002/hep.1840040517. [DOI] [PubMed] [Google Scholar]
- 48.Westbrook RH, Dusheiko G. Natural history of hepatitis C. Journal of hepatology. 2014;61:S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Romeo R, Foglieni B, Casazza G, Spreafico M, Colombo M, Prati D. High serum levels of HDV RNA are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PloS one. 2014;9:e92062. doi: 10.1371/journal.pone.0092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Man RA, Sprey RP, Niesters HG, et al. Survival and complications in a cohort of patients with anti-delta positive liver disease presenting in a tertiary referral clinic. Journal of hepatology. 1995;23:662–667. doi: 10.1016/0168-8278(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 51.Shiha GE, Zalata K. Ishak versus METAVIR: Terminology, Convertibility and Correlation with Laboratory Changes in Chronic Hepatitis C. In: Takahashi H, editor. Liver Biopsy: InTech. 2011. [Google Scholar]
- 52.Heller T, Rotman Y, Koh C, et al. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Alimentary pharmacology & therapeutics. 2014;40:93–104. doi: 10.1111/apt.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbas Z, Khan MA, Salih M, Jafri W. Interferon alpha for chronic hepatitis D. The Cochrane database of systematic reviews. 2011 doi: 10.1002/14651858.CD006002.pub2. CD006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.European Association for Study of L, Asociacion Latinoamericana para el Estudio del H. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. Journal of hepatology. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Terrault N, Ghany MG, Kang C, et al. Seroprevalence and Clinical Features of Hepatitis D Virus (HDV) Infection in a North American Cohort. Liver Meeting. San Francisco. 2015 [Google Scholar]
- 56.Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. Journal of hepatology. 2015;63:586–592. doi: 10.1016/j.jhep.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tapper EB, Cohen EB, Patel K, et al. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:932–937. e1. doi: 10.1016/j.cgh.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


