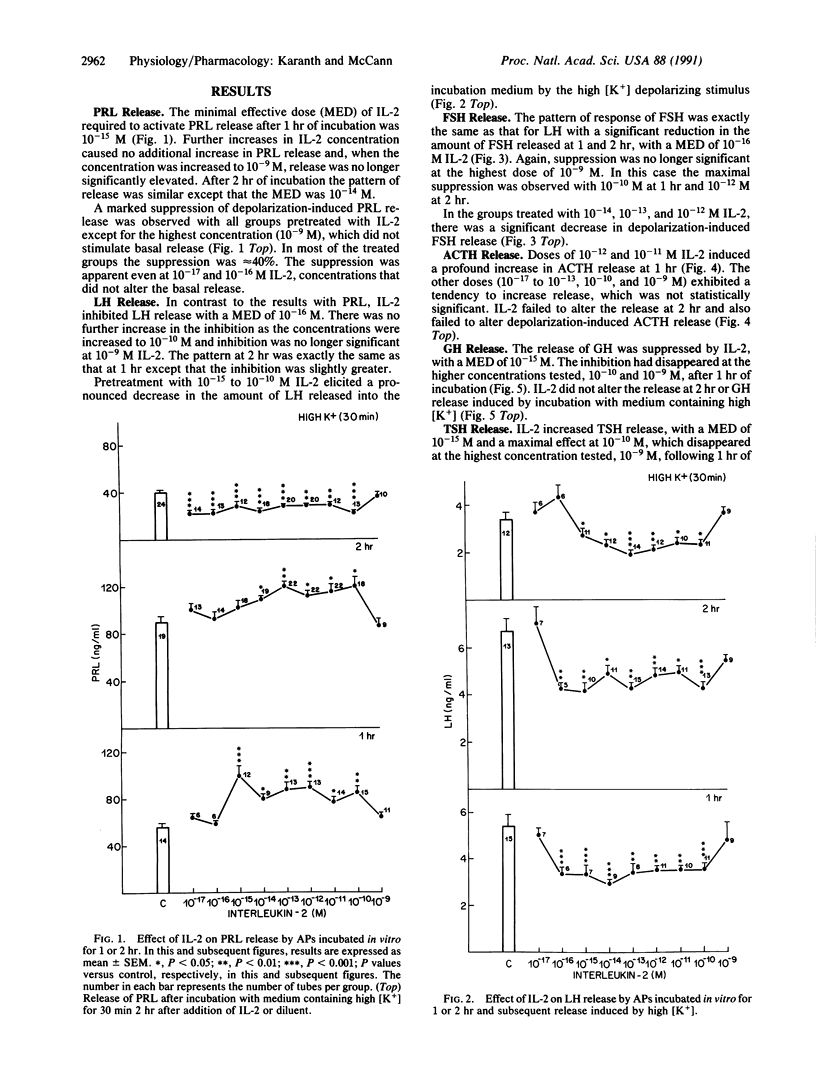
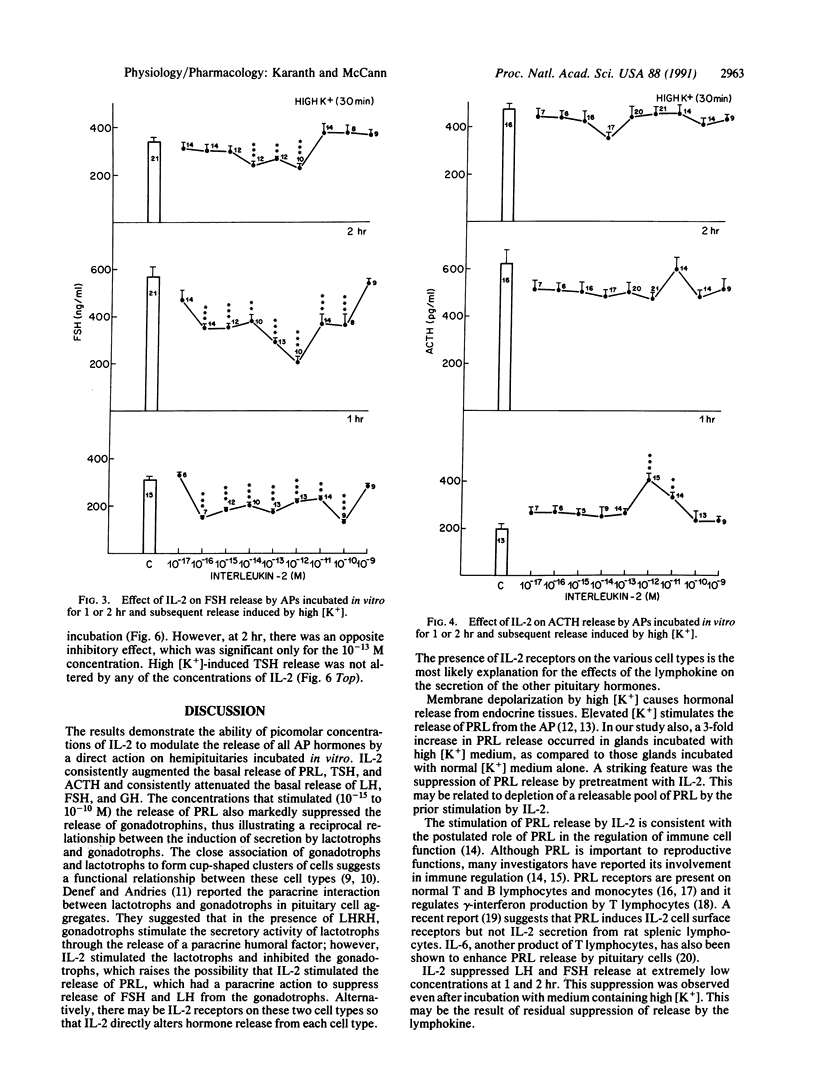
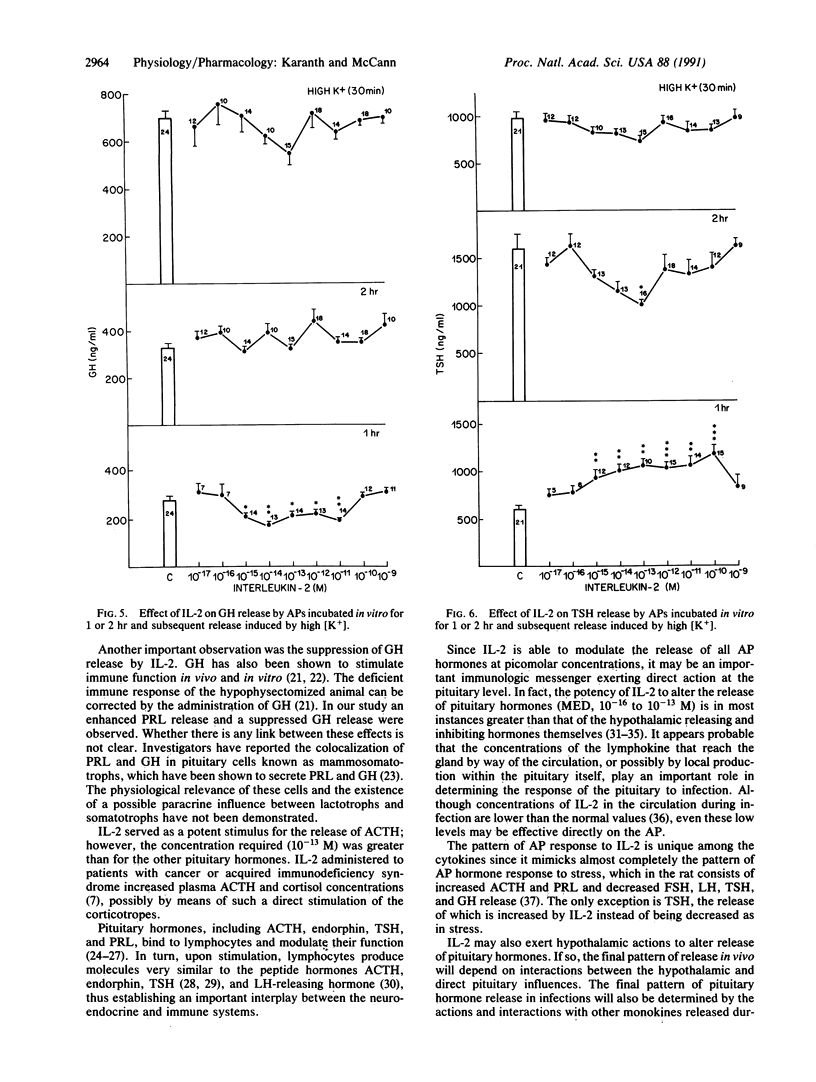
Abstract
Several monokines, proteins secreted by monocytes and macrophages, alter release of hormones from the anterior pituitary. We report here the ability of femtomolar concentrations of interleukin 2 (IL-2), a lymphokine released from T lymphocytes, to alter directly pituitary hormone release. The effects of concentrations of IL-2 ranging from 10(-17) to 10(-9) M on anterior pituitary hormone release were evaluated in vitro. Hemipituitaries were preincubated in 1 ml of Krebs-Ringer bicarbonate buffer (KRB) followed by incubation for 1 or 2 hr with KRB or KRB containing different concentrations of IL-2. This was followed by incubation for 30 min in 56 mM potassium medium to study the effect of pretreatment with IL-2 on subsequent depolarization-induced hormone release. Prolactin (PRL), luteinizing hormone (LH), follicle-stimulating hormone (FSH), corticotropin (ACTH), growth hormone (GH), and thyrotropic hormone (TSH) released into the incubation medium were measured by radioimmunoassay. IL-2 stimulated the basal release of PRL at 1 or 2 hr but suppressed the subsequent depolarization-induced PRL release, perhaps because the readily releasable pool of PRL was exhausted. The minimal effective dose (MED) was 10(-15) M. Conversely, IL-2 significantly suppressed the basal release of LH and FSH at 1 or 2 hr, with a MED of 10(-16) M, thus demonstrating a reciprocal action of the cytokine on lactotrophs and gonadotrophs. The subsequent depolarization-induced release of LH and FSH was suppressed, indicative of a persistent inhibitory action of IL-2. IL-2 stimulated ACTH and TSH release at 1 hr and the MEDs were 10(-12) and 10(-15) M, respectively. Conversely, IL-2 significantly lowered the basal release of GH at 1 hr, with a MED of 10(-15) M. The release of GH was not altered at 2 hr. The high potassium-induced release of ACTH, TSH, and GH was not affected. The results demonstrate that IL-2 at picomolar concentrations affects the release of anterior pituitary hormones. This cytokine may serve as an important messenger from lymphocytes exerting a direct paracrine action on the pituitary by its release from lymphocytes in the gland or concentrations in the blood that reach the gland may be sufficient to activate it.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman A., Singh A., Kral T., Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989 Feb;10(1):92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- Bellussi G., Muccioli G., Ghè C., Di Carlo R. Prolactin binding sites in human erythrocytes and lymphocytes. Life Sci. 1987 Aug 24;41(8):951–959. doi: 10.1016/0024-3205(87)90682-5. [DOI] [PubMed] [Google Scholar]
- Berczi I., Nagy E., Kovacs K., Horvath E. Regulation of humoral immunity in rats by pituitary hormones. Acta Endocrinol (Copenh) 1981 Dec;98(4):506–513. doi: 10.1530/acta.0.0980506. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Beach J. E., Holaday J. W., Smallridge R. C., Fein H. G. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987 Oct 23;238(4826):519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brown S. L., Smith L. R., Blalock J. E. Interleukin 1 and interleukin 2 enhance proopiomelanocortin gene expression in pituitary cells. J Immunol. 1987 Nov 15;139(10):3181–3183. [PubMed] [Google Scholar]
- Burgus R., Dunn T. F., Desiderio D., Ward D. N., Vale W., Guillemin R. Characterization of ovine hypothalamic hypophysiotropic TSH-releasing factor. Nature. 1970 Apr 25;226(5243):321–325. doi: 10.1038/226321a0. [DOI] [PubMed] [Google Scholar]
- Carrier M., Emery R. W., Wild-Mobley J., Perrotta N. J., Russell D. H., Copeland J. G. Prolactin as a marker of rejection in human heart transplantation. Transplant Proc. 1987 Aug;19(4):3442–3443. [PubMed] [Google Scholar]
- Coutelier J. P., Kehrl J. H., Bellur S. S., Kohn L. D., Notkins A. L., Prabhakar B. S. Binding and functional effects of thyroid stimulating hormone on human immune cells. J Clin Immunol. 1990 Jul;10(4):204–210. doi: 10.1007/BF00918653. [DOI] [PubMed] [Google Scholar]
- Denef C., Andries M. Evidence for paracrine interaction between gonadotrophs and lactotrophs in pituitary cell aggregates. Endocrinology. 1983 Mar;112(3):813–822. doi: 10.1210/endo-112-3-813. [DOI] [PubMed] [Google Scholar]
- Emanuele N. V., Emanuele M. A., Tentler J., Kirsteins L., Azad N., Lawrence A. M. Rat spleen lymphocytes contain an immunoactive and bioactive luteinizing hormone-releasing hormone. Endocrinology. 1990 May;126(5):2482–2486. doi: 10.1210/endo-126-5-2482. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Brazeau P., Böhlen P., Esch F., Ling N., Wehrenberg W. B. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982 Nov 5;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Campbell I. L. Cytokines: an expanding network of immuno-inflammatory hormones. Mol Endocrinol. 1988 Dec;2(12):1151–1156. doi: 10.1210/mend-2-12-1151. [DOI] [PubMed] [Google Scholar]
- Hartmann D. P., Holaday J. W., Bernton E. W. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 1989 Aug;3(10):2194–2202. doi: 10.1096/fasebj.3.10.2787766. [DOI] [PubMed] [Google Scholar]
- Heijnen C. J., Bevers C., Kavelaars A., Ballieux R. E. Effect of alpha-endorphin on the antigen-induced primary antibody response of human blood B cells in vitro. J Immunol. 1986 Jan;136(1):213–216. [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A., Smith E. M., Dion L. D., Blalock J. E. Regulation of lymphokine (gamma-interferon) production by corticotropin. J Immunol. 1984 Jan;132(1):246–250. [PubMed] [Google Scholar]
- Krulich L., Hefco E., Illner P., Read C. B. The effects of acute stress on the secretion of LH, FSH, prolactin and GH in the normal male rat, with comments on their statistical evaluation. Neuroendocrinology. 1974;16(5-6):293–311. doi: 10.1159/000122576. [DOI] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- Lotze M. T., Frana L. W., Sharrow S. O., Robb R. J., Rosenberg S. A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 1985 Jan;134(1):157–166. [PubMed] [Google Scholar]
- Mittler J. C., Arimura A., Schally A. V. Release and synthesis of luteinizing hormone and follicle-stimulating hormone in pituitary cultures in response to hypothalamic preparations. Proc Soc Exp Biol Med. 1970 Apr;133(4):1321–1325. doi: 10.3181/00379727-133-34681. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. The interleukins. FASEB J. 1989 Oct;3(12):2379–2388. doi: 10.1096/fasebj.3.12.2676681. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Mastro A. M., Hymer W. C. Prolactin induction of interleukin-2 receptors on rat splenic lymphocytes. Endocrinology. 1990 Jan;126(1):88–94. doi: 10.1210/endo-126-1-88. [DOI] [PubMed] [Google Scholar]
- Nikitovitch-Winer M. B., Atkin J., Maley B. E. Colocalization of prolactin and growth hormone within specific adenohypophyseal cells in male, female, and lactating female rats. Endocrinology. 1987 Aug;121(2):625–630. doi: 10.1210/endo-121-2-625. [DOI] [PubMed] [Google Scholar]
- Pandian M. R., Talwar G. P. Effect of growth hormone on the metabolism of thymus and on the immune response against sheep erythrocytes. J Exp Med. 1971 Nov 1;134(5):1095–1113. doi: 10.1084/jem.134.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V., Jurcovicova J., McCann S. M. Central action of interleukin-1 in altering the release of TSH, growth hormone, and prolactin in the male rat. J Neurosci Res. 1987;18(1):179–183. doi: 10.1002/jnr.490180125. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W. Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology. 1990 Aug;127(2):849–856. doi: 10.1210/endo-127-2-849. [DOI] [PubMed] [Google Scholar]
- Sato S. Postnatal development, sexual difference and sexual cyclic variation of prolactin cells in rats: special reference to the topographic affinity to gonadotroph. Endocrinol Jpn. 1980 Oct;27(5):573–583. doi: 10.1507/endocrj1954.27.573. [DOI] [PubMed] [Google Scholar]
- Schettini G., Judd A. M., MacLeod R. M. In vitro studies on basal and stimulated prolactin release by rat anterior pituitary: a possible role for calmodulin. Endocrinology. 1983 Jan;112(1):64–70. doi: 10.1210/endo-112-1-64. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Elsholtz H. P., Tanaka T., Friesen H. G., Gout P. W., Beer C. T., Noble R. L. Receptor-mediated mitogenic action of prolactin in a rat lymphoma cell line. Endocrinology. 1983 Jul;113(1):159–165. doi: 10.1210/endo-113-1-159. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Blalock J. E. A molecular basis for interactions between the immune and neuroendocrine systems. Int J Neurosci. 1988 Feb;38(3-4):455–464. doi: 10.3109/00207458808990706. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow E. C., Feldbush T. L., Oaks J. A. The effect of growth hormone and insulin upon MLC responses and the generation of cytotoxic lymphocytes. J Immunol. 1981 Jan;126(1):161–164. [PubMed] [Google Scholar]
- Spangelo B. L., Judd A. M., Isakson P. C., MacLeod R. M. Interleukin-6 stimulates anterior pituitary hormone release in vitro. Endocrinology. 1989 Jul;125(1):575–577. doi: 10.1210/endo-125-1-575. [DOI] [PubMed] [Google Scholar]
- Suda T., Tozawa F., Ushiyama T., Sumitomo T., Yamada M., Demura H. Interleukin-1 stimulates corticotropin-releasing factor gene expression in rat hypothalamus. Endocrinology. 1990 Feb;126(2):1223–1228. doi: 10.1210/endo-126-2-1223. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Voltage-dependent calcium channels in pituitary cells in culture. II. Participation in thyrotropin-releasing hormone action on prolactin release. J Biol Chem. 1984 Jan 10;259(1):427–434. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]


