Abstract
Rationale
Brainstem apolipoprotein AII (apoa2) mRNA expression correlates with apnea in breathing present in the adult C57Bl/6J (B6) sleep apnea model.
Objectives
To test the hypothesis that the B6 apoa2 gene contributes to the trait, we performed plethysmographic testing in apoa2 knock out (KO: −/−) mice, an in situ brainstem-spinal cord preparation comparing KO to WT (+/+) mice, and B6xDBA recombinant inbred strains (RISs).
Measurements and Main Results
Apoa2 WT do, but KO and heterozygote (+/−) mice do not exhibit apnea during post-hypoxic breathing, measured in vivo. In the in situ model, pauses and instability in fictive phrenic bursting are substantially reduced in KO vs. WT preparations. In 24 RISs, apnea number in vivo was higher in strains with B6 apoa2 than with DBA apoa2 alleles.
Conclusions
The B6 apoa2 polymorphism is directly involved in breath production, and its identification suggests a novel pathway influencing risk for adult sleep apnea
For disorders like obstructive sleep apnea, identification of genetic risk remains elusive given the complex interactions along genes, environment, and lifestyle present in adult-onset human disorders. As a result, we and others study intermediate traits in animal models. Ventilatory responses to hypoxia and hypercapnia differ among strains of mice and are traits linked to various chromosomes, indicating the presence of naturally occurring genetic variations affecting ventilatory control (Tankersley, 1999; Tankersley and Broman, 2004; Tankersley et al., 1994). In the B6 strain, there occur apneas in breathing during wakefulness and the different stages of sleep (Moore et al., 2014); and more apnea and periodic breathing are provoked upon reoxygenation after brief exposure to hypoxia (Han et al., 2002). Apnea during resting breathing and provoked by hypoxic exposure respond to medications used to treat human central apnea (Yamauchi et al., 2010).
In a comparison of A/J to B6 mice intercross breeding and a genomic approach placed the trait of B6 post-hypoxic apneas to a region on mouse Chromosome 1 (Gillombardo et al., 2012). The linkage was to a region identified previously as having an interaction effect of hypoxia and hypercapnia (Tankersley and Broman, 2004). Using gene expression methods, we discovered a very high mRNA expression for apolipoprotein A2 (apoa2) present in B6 vs. A/J mice. This gene resides in this region of Chromosome 1, and is polymorphic between A/J and B6 strains. The rationale for the study concerns the specificity of suspected apoa2 expression differences.
We tested for the biologic role of this gene by recreating an apoa2 knock-out (KO) mouse and using this strain in in vivo tests of breathing and in an in situ brainstem model of fictive breathing. These tests address apoa2 relevance in the B6 genome and in the absence of the lungs and heart, the cortex, and the cerebellum, respectively. Next, we tested B6 and DBA mice, which differ in a number of neurocognitive traits (Crabbe, 2014) but as well differ in SNP structure and expression of the apoa2 gene; and we tested commercially available B6xDBA recombinant inbred strains (RISs) to test an hypothesis that the actions of genes other than apoa2 might obscure post-hypoxic pause expression. If it did, findings would mean that the remaining variation in genetic background is more important, and diminish the potential relevance of polymorphic differences in apoa2 genes, which also is present in human populations. Results confirm and extend a robust effect of apoa2 expression on breathing on neural processes that produce respiratory rhythms in the presence of genetic heterogeneity.
Methods
Animal Husbandry
Procedures were approved by the Louis Stokes Cleveland DVA Medical Center (LSDVAMC) IACUC committee (protocols- current: 11-058-MS-14-013; past: 11-058-MS-006), and are in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. C57BL/6J (B6) mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). A B6 mouse colony has been housed in the LSDVAMC Animal Research Facility for many years. Periodically the phenotype was confirmed additional B6 mice purchased from this vendor. Mice selected for this study were group housed in colonies in the LSDVAMC Animal Research Facility (laboratory chow and water ad libitum; with a 7AM to 7 PM and 7 PM to 7 AM light-dark cycle).
Strain Derivation and KO characterization
The apoa2 knock out (KO) mice were re-derived at Jackson Laboratory from mice donated originally by Dr. Jan Breslow (B6;129S4-Apoa2<tm1Bres>/J; Stock# 006258, Jackson Laboratory, Bar Harbor ME, USA), originally constructed to examine the role of Apoa2 in the liver and exhibiting dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity (Weng and Breslow, 1996). In this KO (−/−), the genomic fragment containing apoa2 exons 1-3 and part of exon 4 was replaced with a neomycin selection cassette (Jackson Laboratory, Bar Harbor, ME, USA). Northern blot analysis on total liver RNA demonstrated that the apoa2 transcript was not detectable was not detected in the liver in KO mice. We back bred the animals to B6 for several generations and found the trait consistently present. WT and KO animals were genotyped and brother-sister mated for 3 generations before being entered into trials. On the ARC diet of the time (2018SX Teklad Global 18% Protein Rodent Diet, Teklad Harlan Sprague Dawley, Indianapolis IN, USA), weights from the time of weaning (3-4 wks.) (+/+ : −/− :: 6.2±4.0 gms: 6.5 ±4.2 gms, M±SD) to the age limit of testing (12 wks) (+/+ : −/− :: 24.1±4.3 gms: 24.2±4.7 gms) were similar (p=0.55 for trend). Between KO and WT strains, a battery tests (open field testing, rotorod agility and strength, hot plate responses, and navigation of a visual maze), and 2-wks of activity-inactivity profiling disclosed no significant differences. Thus, the KO was viable, and without major developmental deficits compared to WT mice.
DBA/6J and B6xDBA recombinant inbred strains (RISs) were derived at and obtained from Jackson Laboratories (Bar Harbor ME). We selected 24 RIS strains 1:1 matched for the B6 apoa2 genotype (Crabbe et al., 1994) for testing of breathing pattern. Each RIS strain was provided as one male and 2 females, and were bred in the Animal Resource Center to produce progeny which were raised for testing between 8-12 wks. of age.
Genomic Testing
Apoa2 genotyping was by end-point PCR. Tissue was obtained from mouse tail (birth genotyping), and/or liver or brainstem (obtained after phenotyping or from the in situ preparation) was lysed in 40uL 0.2M NaOH at 80 degrees C for 15 minutes. The reaction was stopped with the addition of 360uL 0.04M Tris-HCL pH 7.5. Extracted DNA was used as a template for PCR following the Jackson Labs protocol using nested primers: oIMR6118 5'-GAC CTT GGA CTC CAG TCT GC-3', oIMR6119 5'-CGG TTT CTC CTC AAG GTT CA-3', and oIMR8444 5'-GCC TGA AGA ACG AGA TCA GC-3'. The PCR product was run on a 1% agarose gel, and the genotype was determined by size analysis of the resultant bands (WT: 287bp, HET: 143bp/287bp, and KO: 143bp).
For genotyping of the B6xDBA RISs, we relied on the information from the Mouse Phenome Database (http://phenome.jax.org/SNP), which reports genetic polymorphisms for all these inbred strains. For the RIS strains there was available coverage of 3776 markers (98% coverage over 20 chromosomes: Build 37). These genotypes were predominantly homozygotic [(%): AA:49.7 AB:2.4 BB:47.9 not BB:0 not AA:0)], and included markers specific for the known apoa2 gene allelic differences between the parental strains. Markers were linked to trait values using quantitative trait linkage methods (Crabbe et al., 1994; Gillombardo et al., 2012).
For detection of Apoa2 message, RNA was extracted from flash-frozen mouse brainstem or liver using either Ribozol RNA Extraction Reagent (Amresco) or a Qiagen RNAeasy kit. One microliter (5% cDNA reaction volume) was used for a standard, 60 cycle quantitative PCR with an APOA2 TaqMan Gene Expression Assay (ThermoFisher Scientific, Mm00442687_m1, NM_013474.2), with the FAM reporter 5’-TCTGTAGCCTGGAAGGAGCTTTGGT. Mouse GAPDH Endogenous Control (4352339E, Applied Biosystems, USA) was used as an internal control.
Ventilatory Testing
Breathing in vivo was measured by whole body plethysmography using the pressure change in the chamber, referenced to a comparably sized chamber (Han et al., 2001; Han et al., 2002). The variations of pressure in the chamber are expressed as voltage swings by the transducer output, and recorded using Lab View 7.1 (National Instruments, Austin, TX, USA). The transducer output was analyzed and scored using a custom written program (Breath Detect, Cleveland OH, USA). This program identifies each individual breath according to the crest and trough of the transducer’s voltage output, and permits visual artifact identification and rejection, and manual corrections of breath contours in the record for the identification of sniffing and sighs, or movement. Frequency (f) is determined by the cycle length from the onset of one inspiration to the next. While an estimate of relative tidal volume (VT) can be determined, frequency is the reliable measure for determination of rhythmogenesis, apnea, and unstable breathing, and it was the trait linked to a QTL region on Chromosome 1 (Gillombardo et al., 2012). Pauses (apneas) were defined as absence of breathing lasting longer than 2.5 breathing cycles (a metric used in human phenotyping) (Gillombardo et al., 2012). There was a moderately high correlation (r2 = 0.85) to this value and the frequency of breathing lower than the mean plus two standard deviations. In this report, pause number in the post-hypoxic period is the metric reported; however, illustrations of frequency over time in Poincaré plots (Gonsenhauser et al., 2004) are provided to visualize the distribution of breath times with frequency by frequency + 1 breath, with a greater dispersion around a line of identity showing greater variability.
Brainstem in situ Preparation (fictive breathing)
The working heart-brainstem preparation is an in situ preparation first described by Paton (Paton, 1996b) and developed in our group (Dhingra et al., 2013). Phrenic (PNA) is the index of fictive breathing, recorded via suction electrodes, filtered (0.003–3 kHz) and amplified (5–20 K; Grass P511, West Warwick, RI, USA), digitized (Power 1401, CED, Cambridge, UK), and stored (10 kHz sampling frequency) on a computer using Spike2 acquisition software (CED, Cambridge, UK). After the tuned respiratory rhythm stabilized (15–20 min), baseline activity is recorded for at least 5 min to assess respiratory patterning.
Phrenic nerve onset and offset times are derived from a threshold crossing algorithm and visually inspected for artifacts. Respiratory period and variability are calculated from the recorded baseline epoch of at least consecutive 200 breaths (n = 6 wild-type; 5 KO). Fictive frequency is determined along with measures of variability- the coefficient of variation and the standard deviation of successive differences. While sniffs, sighs and body movement artifacts are absent, fictive breathing rates are slower than in vivo rates (Dutschmann et al., 2000; Paton, 1996b). Apnea are quantitatively identified in the in situ preparation using phrenic nerve activity. Criteria for identification of long cycles here is a TTOT greater than the mean plus one standard deviation, and a subset of longer cycles, characterized as pauses, is when TTOT was greater than the mean plus two or three standard deviations. The frequency and variability in pauses (mean plus 2SD or 3SD) are described as a percentage of the long cycles (mean plus 1SD).
Recombinant Inbred Strains
For the testing of differences among the B6 and DBA/6J strains, B6 mice (n=24) and DBA/6J mice (n=6,), and mice from 24 DBAxB6 RISs were examined for post-hypoxic pause expression along with variables collected during the hypoxic exposure, and during a subsequent exposure to 5 minutes of 5% CO2/balance oxygen. Results were compared among strains according to known B6 or DBA ApoA2 genotype. We utilized published genotypes for the DBA, B6, and RIS strains to perform in silico quantitative trait linkage (QTL) analysis. The analysis was performed using pause trait number to not only the apoa2 genotype but also on other portions of the genome for co-occurrence of linkage with respiratory frequency at rest, respiratory frequency during the hypoxic period, and respiratory frequency during exposure to 5 minutes of 5% CO2/balance oxygen. The LOD thresholds were determined by 10000 permutations, and was significant at 20% level at an LOD level of 4.77 and for 5%, 5.81.
Results
Ventilatory in vivo phenotype of KO vs WT mouse
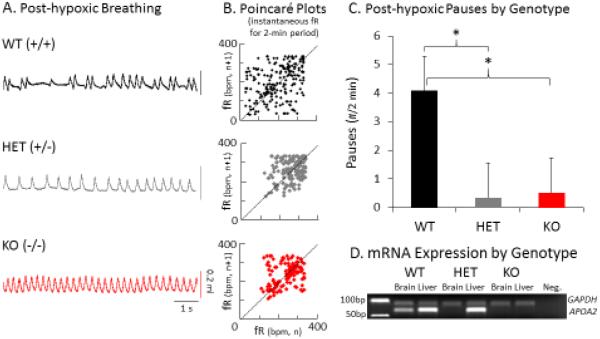
Second generation mice from a KO/WT reciprocal intercross were tested for ventilatory behavior. The breathing phenotypes of WT mice were similar to prior observations in the B6, with pauses during resting breathing and prominent post-hypoxic apneas; such events were reduced in Apoa2 heterozygotes (HET=B6+/−) and KO mice (Figure 1A). As depicted by Poincaré plots, the WT mouse exhibits a larger dispersion of values in the first 2-min following hypoxia than either HET or KO mice (Figure 1B). Mean number of apnea in the 2-min following reoxygenation differed among genotypes (P<0.001) (Figure 1C). The instability trait is attenuated by the presence of one null allele. Apoa2 mRNA expression is present in the WT in both the brain and liver (Figure 1D), and correlated to trait expression. Gene expression, present in the liver in HET mice, was not present in the brainstem, consistent with brain-specific regulation of expression.
Figure 1. Respiratory stability correlates with Apoa2 mRNA expression.
A. Three representative traces (8-s) of breathing patterns are presented from individual wild type (WT, HET- heterozygote, and KO mice. Vertical scale bar represents 0.2 ml. B. Poincaré plots display the instantaneous frequency of one breath (fR n on X-axis) against the frequency of the next breath (fR n+1 on the Y axis) for the 2-min in reoxygenation following hypoxia, and illustrate greatest dispersion in the WT mouse. C. The bar represents the mean and SD of values for the number (#) of apnea in the 2 min following reoxygenation, from WT (n=14), HET (n=27), and KO (n=17) mice in an intercross study of inheritance. D. D. Gel electrophoresis showing mRNA levels of Apoa2 gene vs. a housekeeping gene (GAPDH) in representative WT, HET, and KO mice showing 1) WT: relative reduction of Apoa2 mRNA in the brainstem vs. the liver, 2) HET: Apoa2 mRNA present in the liver but not in the brainstem, 3) KO: Apoa2 mRNA absent in both liver and brainstem. The results from HET mice indicate the necessity for both B6 alleles to express the trait of apnea. ANOVA differences: p<0.001 and * indicates differences in unpaired t-tests: p<0.01)
Brainstem in situ phenotype of KO vs WT mice
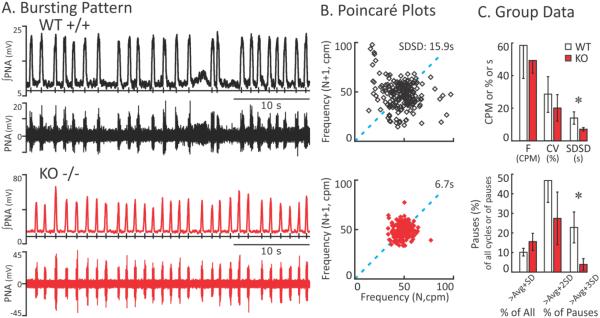
The brainstem-spinal cord model approximates in vivo like respiratory motor activity (Paton, 1996b) but is isolated from vagal and chemoreceptive inputs, and circulatory, cortical and cerebellar influences. Examples of fictive breathing from representative WT and KO preparations illustrate representative differences in rhythmogenesis (Figure 2A). A previously recognized irregular pattern of breathing in the B6 (Stettner et al., 2008b) is confirmed (Stettner et al., 2008a). The new finding is that the irregularity in breath patterning is reduced in the KO mouse. The dispersion of breath-by-breath respiratory cycle duration (described by Poincaré plots, Figure 2B) was greater in WT than in KO mice, reminiscent of the in vivo patterning shown in Figure 1 for these genotypes.
Figure 2. The fictive respiration pattern in the in situ preparation of KO less variable than that of WT mice.
A. Bursting Patterns. Representative traces of integrated and raw phrenic nerve activity (∫PNA and PNA respectively) showing the ‘fictive’ breathing pattern from WT (top) and KO (bottom) in situ preparations. B. Poincaré plots. Representative Poincaré plots, showing the distribution of instantaneous respiratory frequency (cycles per minute) for a WT (top) and KO (bottom) mice, with points representing a successive cycle (N+1) plotted against the current cycle (N) for 200 cycles. The dotted line is the line of identity. C. Group Data. The top graph shows the mean and standard deviation for frequency (F), its coefficient of variation (CV), and the standard deviation of successive differences (SDSD) in TTOT (s) for WT (open box) and KO (solid box) mice. The mean frequencies and its CV were not significantly different in KO and WT mice. Successive differences (SD) in TTOT is the average of absolute differences in duration between cycle (N) and cycle (N+1) and the SDSD is simply the standard deviation of that average. The greater the standard deviation the greater the distribution of point in the Poincaré plot. The bottom graph shows pauses in the pattern. The first pair of bars reflects the percentage of cycles that were greater than the average plus one standard deviation of cycle length. The percentages of long cycles were not significantly different in WT and KO rats. The next two pairs of bars represent the percentage of long cycles that are pauses; in that the cycles were greater than the average plus 2 and 3 standard deviations respectively. In WT rats, approximately 50% and 25% of the long cycles were pauses (avg +2SD or +3SD respectively) whereas KO expressed few cycles that were as long the avg+3SD.
Composite data from KO (n=6) and WT (n=8) preparations indicate a significantly stabilized respiratory rhythm in the former, as determined by a lower standard deviation of successive differences (Figure 2C). The mean and the percentage of breaths that were slower than the mean plus one standard deviation are similar between the two strain, indicating similar overall distributions in the cycle lengths over two minutes in the in situ preparation. However, pauses defined as a breathing time slower than the mean and two or three standard deviations were proportionately higher in WT preparations, with 47% and 23% of long cycles had durations greater than the mean plus two or three standard deviations, respectively; in contrast the KO preparations had fewer, only 27% and 4% of cycles meeting the definitions of pauses. In terms relative to the in vivo preparation where the definition is a cessation of breathing for 2 respiratory cycles, the rate of long pauses was ~7.6 in WT and 0.5 pauses in KO per 2 min periods of recordings, respectively (p<0.001).
Ventilatory Phenotype in RISs
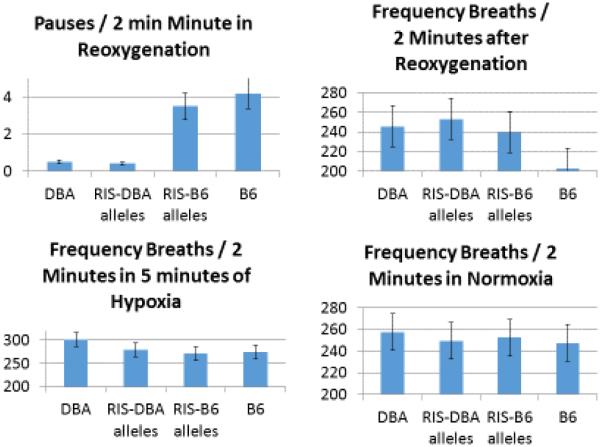
Post-hypoxic pause expression was lower in DBA than in B6 mice, and pause expression in RISs differed in relation to whether the apoa2 genotype was B6 or DBA (Figure 3). Only one of twelve RISs with a B6 genotype did not consistently exhibit unstable breathing and apnea after hypoxia. On the other hand, the frequency during resting breathing, and responses following hypoxia and during hypoxia could be different but did not track with strain results of the post-hypoxic pause phenotype. a result found consistently over time (Gillombardo et al., 2012; Moore et al., 2014; Moore et al., 2012). Similar findings were observed with hypercapnic exposure (data not shown).
Figure 3. Pause Generation and Frequency in Recombinant Inbred Strains.
The four graphs illustrate responses in DBAxB6 recombinant inbred strains (RISs) according to the apoa2 polymorphism (n=56 with DBA apoa2 SNPs; 78 with B6 apoa2 SNPs), and the parental strains (DBA/6J, n=6; B6, n =100). Top left: Pause number in the first 2 minutes of reoxygenation show significant differences among strains (P<0.001), being lower in the DBA and RIS strains with a DBA apoa2 polymorphisms. Top Right: The respiratory frequency during the same 2 min period shows significant differences (P<0.001). Bottom Left: Frequency is presented during the last 2 minutes of hypoxia (P<0.001). Bottom Right: Frequency at rest in the strains (P<0.06).
The LOD score calculated for the apoa2 regional SNPs was 15.2, providing additional support for the importance of the B6 apoa2 genotype in the determination of pause trait. Two other regions on Chromosome 3 and 18 reached some linkage: 5.85 and 14, respectively, to the pause phenotype after consideration of multiple comparisons, consistent with the trait as having a complex architecture, i.e. more than one gene contributes to the trait value. None of these regions with significant linkage to the pause trait co-localized with traits of resting frequency, frequency during hypoxia or hypercapnia. Therefore, there is a strong association between the presence of the B6 apoa2 polymorphism both physiologically and computationally and breathing stability, as measured by the number of apnea following hypoxic exposure.
Discussion
We report a deterministic role for the B6 apoa2 gene polymorphism in respiratory rhythmogenesis. The in vivo inheritance pattern discloses a recessive effect of the B6 allele on the apnea phenotype, as the presence of both B6 alleles in the brain is required for transcript and trait expression; in contrast, apoa2 mRNA expression in the liver requires only one allele. Pauses present in the WT in situ preparation are not present in the KO mouse and variations among fictive breaths is less, consistent with an action on the central controller. In RISs, the B6 apoa2 SNP structure showed a strong correlation with the expression of post-hypoxic apnea despite substantial genetic variation in the rest of the genome. Thus, the impact of this B6 polymorphism is robust and active in the generation of respiratory patterning over time.
The in situ brainstem-spinal cord model maintains a central pattern exhibited by activation of the phrenic nerve, while cortical, cerebellar, and afferent neural input are absent (Dutschmann and Dick, 2013; Paton, 1996a, b). The production of fictive breaths as measured, by mean and 1SD values, are similar between WT and KO animals, but the a pause trait is present in WT but not in KO preparations. Our results do not point to any particular anatomic area where the apoa2 gene or B6 polymorphism might act. The synaptic and cellular adaptive mechanisms for breath patterning influenced by this gene are as likely to function in pontine as much as medullary centers, or both (Dutschmann et al., 2004) .
Results among the in-bred B6 and DBA/6J strains indicates that strain differences exist beyond that of a B6 vs. A/J background comparison previously reported. The use of the B6xDBA RISs tests for the relative impact of the B6 apoa2 polymorphism on post-hypoxic breathing patterning despite diversity in B6 and DBA alleles in the rest of the genome. Results overall confirm a strong effect in that if the apoa2 SNP sequence is like that of the B6 apnea are more likely to be present than when the DBA SNP sequence is present. However, there is substantial variability among RISs with the B6 apoa2 SNPs, a >10 fold difference gene in the quantitative expression of the trait. Some of this variation is due to the testing, and some might be due to recombination in the apoa2 gene itself, which may occur across the entire genome (Jackson Laboratory, Bar Harbor ME). Another reasonable conclusion is that the mitigation or amplification of the trait is the result of other genes or regulatory factors, which may affect B6 apoa2 gene expression or modulate its functions. Such a genetic effect was found in an investigation of apoa2 polymorphisms regulating fatty acid composition, fatness and growth traits in the pig (Ballester et al., 2016). In our prior study in mice we found 2 regions on mouse Chromosome 1 other than the apoa2 that produced a statistical association with the trait (Gillombardo et al., 2012). In that study we focused only on Chromosome 1; in this study we took advantage of the published genomes for the RISs to do in silico trait linkage across 20 chromosomes and there is the suggestion that regions on 2 other chromosomes might contribute to trait variation among strains. We did not examine these regions further. A finding of multiple loci contributing to a physiologic trait is not unusual (Crabbe, 2014); however, it does raise two issues relevant to translational research. First, to determine the impact of apoa2 on breathing in human populations one may need to account not only for the polymorphisms of the apoa2 gene but for polymorphic genes on other chromosomes. Second, the expression of apnea as a genetically determined trait could vary among individuals according to a specific polymorphism (e.g. epigenetic effects or multiple cis-acting polymorphisms) with a recessive effect, i.e. requiring SNPs sequencing in both alleles.
There are prior physiologic studies in the sleep apnea B6 model indicating that the traits of pauses (apnea) are attenuated by serotonin agonists given in situ (Stettner et al., 2008a) and in vivo (Yamauchi et al., 2008), and by acetazolamide (Yamauchi et al., 2007). When sleep-wake monitoring is performed, apnea are present in the B6 mouse during quiet wakefulness and during NREM and REM sleep, and in all states apnea are attenuated by the orexin agonist (Moore et al., 2014). Thus, pharmacologically the gene actions are not directly tied to a singular neurotransmitter pathway. Also suggestive of a more general property, the presence of apnea in the B6 vs A/J strain was associated with differences in stability, a general term encompassing regularity of a signal over time. There is a greater dispersion in frequency in vivo in the B6 vs. A/J, that is preserved during anesthesia (Gonsenhauser et al., 2004). This feature is illustrated as wider dispersions around the line of identity in the WT than KO strains in the representative Poincaré plots (Figures 1 and 2). Structures in a string of breaths can also be examined by non-linear analyses; while less able to identify the physiologic origin, studies show differences in entropy and complexity as a result of a sigh or drug in the B6 compared to the A/J strain (Yamauchi et al., 2010). The pause trait, as recapitulated by a prolonged time between two cycles, is seen in the in situ preparation. The network property could represent an active inhibition, like that seen in RETT syndrome mice (Abdala et al., 2016), or a failure of breath generation (Del Negro et al., 2005), or a combination of interactions (Abdala et al., 2009) influenced by the B6 apoa2 polymorphism.
In humans, variations in respiratory control around eupnea are observed in the pathogenesis of sleep apnea, and genes, environment, and aging, and co-morbidities. Small reductions in carbon dioxide contribute to the production of repetitive apneas during sleep in adult disease (Dempsey et al., 2013; Eckert et al., 2013). Moreover, a greater variability in resting breathing, as measured by the coefficient of variation, in patients during wakefulness predicts that more central and mixed apneas will appear during sleep for the types of apneas during sleep, and a greater variation in breathing may predict lower acceptance of continuous positive pressure therapy for sleep apnea (Yamauchi et al., 2013; Yamauchi et al., 2011). Breathing (in)stability might be an additional trait to measure.
Polymorphisms of the apolipoprotein A2 gene in humans associate with apolipoprotein A-II deficiency, hypercholesterolemia, metabolic disease, and obesity (Martin-Campos et al., 2004; Smith et al., 2013). An interaction of a specific human apoa2 SNP polymorphism and exposure to high-fat diet appears to predispose to obesity in three human populations (Corella et al., 2011). Similar metabolic phenotypes are caused by polymorphisms in the mouse gene (Warden et al., 1993), and one may select certain inbred mice strains as models for human disorders of dyslipidemia and hypercholesterolemia (Svenson et al., 2007). Another apoa2 KO reconstituted recently reported effects of the gene on lipids in the circulation (McLeod et al., 2014). In the B6 but not in the A/J obesity is induced a high-fat diet (Nadeau et al., 2000). The present report finds a novel role for the apoa2 gene on breathing, and uncovers a need to known how the HDL-cholesterol pathway at a genomic level affects neural as well as metabolic pathways.
Finding the Apoa2 gene pathway links one gene with effects on obesity and cardiovascular risk, present in sleep apnea syndromes, to apnea generation. One might imagine that a putative polymorphism that confers breathing instability which also enhances weight gain could lead over time to an increase in upper airway critical closing pressure and thus be a proximate risk for a person to develop adult sleep apnea syndrome.
Highlights.
Apolipoprotein A2, an HDL-cholesterol carrier and a risk factor for obesity in humans, as a gene influences the production of apneas in the C57BL/6J sleep apnea mouse model.
The deletion of apoa2 in the mouse leads to breathing pauses (apnea) in both animal recordings and in a reduced brain preparation.
The action of the B6 polymorphism is strong even in the presence of a variable genetic background.
Acknowledgements
We wish to thank Gjinovefa (Gina) Kola for her diligence and skill with the in situ preparation. This work is supported by Merit Award from the VA Research Service I01BX001464 to KPS, and by the NIH (R21NS052452 to KPS and T32HL07913 to SMA). We thank the Louis Stokes Cleveland DVA Medical Center Animal Resource Center for the assistance in the breeding and care of animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conception and design: KPS, CBG, FH, MY
Analysis and interpretation: NK, MJD, RD, MM, CBG, SA, TED
Drafting the manuscript for important intellectual content: KPS, CBG, MD, TED, MJD
References
- Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respiratory physiology & neurobiology. 2009;168:19–25. doi: 10.1016/j.resp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, Paton JF. Deficiency of GABAergic synaptic inhibition in the Kolliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome. The Journal of physiology. 2016;594:223–237. doi: 10.1113/JP270966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester M, Revilla M, Puig-Oliveras A, Marchesi JA, Castello A, Corominas J, Fernandez AI, Folch JM. Analysis of the porcine APOA2 gene expression in liver, polymorphism identification and association with fatty acid composition traits. Animal genetics. 2016;47:552–559. doi: 10.1111/age.12462. [DOI] [PubMed] [Google Scholar]
- Corella D, Tai ES, Sorli JV, Chew SK, Coltell O, Sotos-Prieto M, Garcia-Rios A, Estruch R, Ordovas JM. Association between the APOA2 promoter polymorphism and body weight in Mediterranean and Asian populations: replication of a gene-saturated fat interaction. Int J Obes (Lond) 2011;35:666–675. doi: 10.1038/ijo.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Rodent models of genetic contributions to motivation to abuse alcohol. Nebr Symp Motiv. 2014;61:5–29. doi: 10.1007/978-1-4939-0653-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Buck KJ, Metten P. Use of recombinant inbred strains for studying genetic determinants of responses to alcohol. Alcohol Alcohol Suppl. 1994;2:67–71. [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Xie A, Patz DS, Wang D. Physiology in Medicine: Obstructive Sleep Apnea Pathogenesis and Treatment - Considerations Beyond Airway Anatomy. J Appl Physiol (1985) 2013 doi: 10.1152/japplphysiol.01054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra RR, Zhu Y, Jacono FJ, Katz DM, Galan RF, Dick TE. Decreased Hering-Breuer input-output entrainment in a mouse model of Rett syndrome. Front Neural Circuits. 2013;7:42. doi: 10.3389/fncir.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Comprehensive Physiology. 2013;2:2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Morschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kolliker-Fuse nucleus. Respiratory physiology & neurobiology. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Wilson RJ, Paton JF. Respiratory activity in neonatal rats. Auton Neurosci. 2000;84:19–29. doi: 10.1016/S1566-0702(00)00177-6. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillombardo CB, Yamauchi M, Adams MD, Dostal J, Chai S, Moore MW, Donovan LM, Han F, Strohl KP. Identification of novel mouse genes conferring posthypoxic pauses. J Appl Physiol. 2012;113:167–174. doi: 10.1152/japplphysiol.01394.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsenhauser I, Wilson CG, Han F, Strohl KP, Dick TE. Strain differences in murine ventilatory behavior persist after urethane anesthesia. J Appl Physiol. 2004;97:888–894. doi: 10.1152/japplphysiol.01346.2003. [DOI] [PubMed] [Google Scholar]
- Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J Appl Physiol. 2001;91:1962–1970. doi: 10.1152/jappl.2001.91.5.1962. [DOI] [PubMed] [Google Scholar]
- Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol. 2002;92:1133–1140. doi: 10.1152/japplphysiol.00785.2001. [DOI] [PubMed] [Google Scholar]
- Martin-Campos JM, Escola-Gil JC, Ribas V, Blanco-Vaca F. Apolipoprotein A-II, genetic variation on chromosome 1q21-q24, and disease susceptibility. Current opinion in lipidology. 2004;15:247–253. doi: 10.1097/00041433-200406000-00003. [DOI] [PubMed] [Google Scholar]
- McLeod JF, Leempoels JM, Peng SX, Dax SL, Myers LJ, Golder FJ. GAL-021, a new intravenous BKCa-channel blocker, is well tolerated and stimulates ventilation in healthy volunteers. British journal of anaesthesia. 2014;113:875–883. doi: 10.1093/bja/aeu182. [DOI] [PubMed] [Google Scholar]
- Moore MW, Akladious A, Hu Y, Azzam S, Feng P, Strohl KP. Effects of orexin 2 receptor activation on apnea in the C57BL/6J mouse. Respiratory physiology & neurobiology. 2014;200:118–125. doi: 10.1016/j.resp.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Chai S, Gillombardo CB, Carlo A, Donovan LM, Netzer N, Strohl KP. Two weeks of buspirone protects against posthypoxic ventilatory pauses in the C57BL/6J mouse strain. Respiratory physiology & neurobiology. 2012;183:35–40. doi: 10.1016/j.resp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Paton JF. The ventral medullary respiratory network of the mature mouse studied in a working heart-brainstem preparation. The Journal of physiology. 1996a;493:819–831. doi: 10.1113/jphysiol.1996.sp021425. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996b;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Smith CE, Tucker KL, Arnett DK, Noel SE, Corella D, Borecki IB, Feitosa MF, Aslibekyan S, Parnell LD, Lai CQ, Lee YC, Ordovas JM. Apolipoprotein A2 polymorphism interacts with intakes of dairy foods to influence body weight in 2 u.s. Populations. J Nutr. 2013;143:1865–1871. doi: 10.3945/jn.113.179051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Hilaire G, Dutschmann M. 8-OH-DPAT suppresses spontaneous central apneas in the C57BL/6J mouse strain. Respiratory physiology & neurobiology. 2008a;161:10–15. doi: 10.1016/j.resp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Huppke P, Gartner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respiratory physiology & neurobiology. 2008b;160:21–27. doi: 10.1016/j.resp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Svenson KL, Von Smith R, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol. 2007;102:2369–2378. doi: 10.1152/japplphysiol.01077.2006. [DOI] [PubMed] [Google Scholar]
- Tankersley CG. Genetic control of ventilation: What are we learning from murine models? Curr Opin Pulm Med. 1999:5. doi: 10.1097/00063198-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Broman KW. Interactions in hypoxic and hypercapnic breathing are genetically linked to mouse chromosomes 1 and 5. J Appl Physiol. 2004;97:77–84. doi: 10.1152/japplphysiol.01102.2003. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol. 1994;267:R1371–1377. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- Warden CH, Daluiski A, Bu X, Purcell-Huynh DA, De Meester C, Shieh BH, Puppione DL, Gray RM, Reaven GM, Chen YD, et al. Evidence for linkage of the apolipoprotein A-II locus to plasma apolipoprotein A-II and free fatty acid levels in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10886–10890. doi: 10.1073/pnas.90.22.10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng W, Breslow JL. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14788–14794. doi: 10.1073/pnas.93.25.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Dostal J, Kimura H, Strohl KP. Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J Appl Physiol. 2008;105:518–526. doi: 10.1152/japplphysiol.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Dostal J, Strohl KP. Acetazolamide protects against posthypoxic unstable breathing in the C57BL/6J mouse. J Appl Physiol. 2007;103:1263–1268. doi: 10.1152/japplphysiol.01287.2006. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Jacono FJ, Fujita Y, Yoshikawa M, Ohnishi Y, Nakano H, Campanaro CK, Loparo KA, Strohl KP, Kimura H. Breathing irregularity during wakefulness associates with CPAP acceptance in sleep apnea. Sleep & breathing = Schlaf & Atmung. 2013;17:845–852. doi: 10.1007/s11325-012-0775-2. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Kimura H, Strohl KP. Mouse models of apnea: strain differences in apnea expression and its pharmacologic and genetic modification. Advances in experimental medicine and biology. 2010;669:303–307. doi: 10.1007/978-1-4419-5692-7_62. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Tamaki S, Yoshikawa M, Ohnishi Y, Nakano H, Jacono FJ, Loparo KA, Strohl KP, Kimura H. Differences in breathing patterning during wakefulness in patients with mixed apnea-dominant vs obstructive-dominant sleep apnea. Chest. 2011;140:54–61. doi: 10.1378/chest.10-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]