Abstract
γδ T cells act as a first line of defense against invading pathogens. However, despite their abundance in mucosal tissue, little information is available about their functionality in this compartment in the context of HIV/SIV infection. Here we evaluated the frequency, phenotype and functionality of Vδ1 and Vδ2 T cells from blood, rectum, and the female reproductive tract (FRT) of Rhesus macaques to determine whether these cells contribute to control of SIV infection. No alteration in the peripheral Vδ1/Vδ2 ratio in SIV-infected macaques was observed. However, CD8+ and CD4+CD8+Vδ1 T cells were expanded along with upregulation of NKG2D, CD107, and Granzyme B (Grz B), suggesting cytotoxic function. In contrast, Vδ2 T cells showed a reduced ability to produce the inflammatory cytokine IFN-γ. In the FRT of SIV+ macaques Vδ1 and Vδ2 showed comparable levels across vaginal, ectocervical and endocervical tissues, however endocervical Vδ2 T cells showed higher inflammatory profiles than the two other regions. No sex difference was seen in the rectal Vδ1/Vδ2 ratio. Several peripheral Vδ1 and/or Vδ2 T cell subpopulations expressing IFN-γ, and/or NKG2D were positively correlated with decreased plasma viremia. Notably, Vδ2 CD8+ T cells of the endocervix were negatively correlated with chronic viremia. Overall our results suggest that a robust Vδ1 and Vδ2 T cell response in blood and the FRT of SIV-infected macaques contributes to control of viremia.
Introduction
As a major component of the mucosal immune system, γδ T cells may play a crucial role in early events during HIV transmission. Mucosal surfaces are the main entry point for HIV, and γδ T cells represent a key constituent of gut-associated lymphoid tissue (1). Compared to αβ T cells, γδ T cells have few available V gene segments in the TCR, with 3 main Vδ segments and 7 functional Vγ segments (2). Most studies have addressed two γδ T cell subsets, Vδ1 and Vδ2. Vδ1+ T cells are mainly found in epithelial tissues of the intestine (2) and are efficiently triggered by host ligands, including stress-induced self-antigens, glycolipids presented by CD1c (3) and MIC A/B molecules (4). In contrast, Vγ9Vδ2 T cells, the Vδ2 subset, are mainly found in peripheral blood and are activated by non-peptidic molecules, such as phosphoantigens, produced by many microbial pathogens and stressed cells (5, 6). Activated γδ T cells exhibit a multiplicity of effector functions including direct killing of infected cells, antibody dependent cellular cytotoxicity (ADCC), and production of cytokines such as IFN-γ (7) and IL17 (8, 9). γδ T cells have antigen presenting and regulatory functions and through cross-talk enhance the cytotoxic effector function of NK cells (10). Activated γδ T cells can acquire B cell helper activity and thus might modify adaptive immunity by regulating antibody responses (11, 12). Interaction of γδ T cells with dendritic cells also impacts responses of both cell types (13).
γδ T cells in HIV infection have received only limited attention. HIV affects several lymphocyte subsets and impairs both acquired and innate immunity. Both Vδ1 and Vδ2 T cells are altered during HIV infection. Disease progression is associated with depletion of Vδ2 T cells and expansion of Vδ1 T cells, leading to an inversion of the normal Vδ2/Vδ1 ratio in peripheral blood and mucosal tissues (6, 14-18). This alteration may result from infected cells accumulating phosphoantigens, leading to a brief period of activation and rapid expansion of Vδ2 T cells which subsequently decline and become dysfunctional by an unknown mechanism (18- 20). In contrast, microbial translocation across the gut epithelium may induce expansion of peripheral Vδ1 T cells (8). The HIV/SIV-mediated changes in γδ T cells appear to be part of a strategy for evading antiviral immunity and establishing persistent infection with chronic disease.
In addition to the blood and intestinal tissue, γδ T cells are also present in uterine tissue (21) and the endocervix (17). In the FRT, the upper tract (fallopian tubes, uterus and endocervix) is lined by a single layer of columnar epithelial cells joined by tight junctions. The lower tract (ectocervix and vagina) lining is composed of stratified squamous epithelium that, unlike the upper reproductive tract, relies primarily on the presence of multiple layers to provide a protective barrier against the entry of organisms such as HIV/SIV (22, 23). Recently, HIV-1 transmission was shown to occur in both the upper and lower FRT (24). This compartment exhibits different immune microenvironments which influence HIV infection. Increased activation and factors important for immune responses have been reported in the ectocervix/endocervix, while the endometrium has shown high expression of factors that support HIV infectivity and favor HIV replication (25). Vδ1 is the predominant γδ T cell subset in the endocervix of uninfected women, but its frequency decreases in HIV positive women, along with that of Vδ2 T cells in both the endocervix and blood (17). However, no functionality of γδ T cells in the FRT has been assessed, and in general innate and adaptive immune responses in the FRT to HIV/SIV infection have not been completely defined because of complex regulation by female sex hormones and the degree of compartmentalization (22, 23, 26 - 29). Elucidating immunological mechanisms operative in the FRT and determining their impact on HIV transmission and control will be important for developing better strategies for prevention and treatment, as approximately 50% of HIV infections worldwide are in women (30).
Most of the studies characterizing lymphocytes in mucosal tissue, including the FRT and rectum, have focused on αβ T cells, DCs, B cells and NK cells (31 -34). Although the impact of SIV infection on peripheral and mucosal γδ T cells has been reported in some studies (8, 16), γδ T cells from the upper and lower FRT have been poorly explored in non-human primate models. In this study we focus on the distribution and functional properties of Vδ1 and Vδ2 T cells in peripheral blood of naïve and SIV-infected rhesus macaques and in mucosal tissues: rectal, ectocervix, endocervix and vagina, of chronically SIV-infected macaques in order to define the relationship of these cells to disease progression. In view of previously observed sex differences in HIV pathogenesis (35, 36) and SIV vaccine outcome (37) we also explored potential phenotypic and functional differences of peripheral and mucosal γδ T cells between males and females.
Material and Methods
Animals
Freshly isolated PBMCs from 11 naïve and viably frozen PBMC from 14 (4 females, 10 males) SIVmac251-infected Indian rhesus macaques (Macaca mulatta) were used for the assays. Eleven of the infected macaques had been previously vaccinated with various vectored SIV vaccines followed by boosts with SIV envelope protein whereas 3 had served as unvaccinated controls (Suppl Table I). Blood was collected between 14 and 76 weeks post-infection, and viral loads ranged from <50 to 1 ×107 SIV RNA copies/ml plasma (geometric mean of <3.1 × 104). Additionally, vaginal, ectocervical, endocervical, and/or rectal tissues were collected at necropsy (40 to 52 weeks post-infection) from16 (10 females and 6 males) SIVmac251-infected macaques, part of a previous vaccine study (37). Their viral loads ranged from <50 to 7.3 × 106 SIV RNA copies/ml plasma (geometric mean of <1.3 × 104). Although the 14 blood and 16 mucosal tissues were collected from 26 different SIV-infected animals, no differences were observed in the chronic viral load between both groups (data not shown).
Tissue processing and lymphocyte isolation
Peripheral blood samples were collected into EDTA-treated collection tubes. PBMCs were obtained by centrifugation on Ficoll-Paque PLUS gradients according to the product insert (GE Healthcare, Piscataway, NJ). Cells were washed with PBS and resuspended in R10 medium (RPMI 1640 containing 10% FBS, 2 mM L-glutamine (Invitrogen) and antibiotics). Rectal pinches (20 per animal) and pinch biopsies of the vagina, ectocervix and endocervix (40 per tissue) were obtained from each macaque following euthanasia and rinsed with pre-warmed R10. The pinches were minced in 5 ml of 40μg/ml Liberase TM (Roche) solution using a scalpel, transferred to a 50 ml tube, and brought up to10 ml with Liberase solution. Tissues were digested for 25 min (rectal tissue) or 45 min (FRT tissue) at 37°C with pulse vortexing every 5 min. The dissociated cells and tissue fragments were passed 5 times through a blunt end cannula using a 20 ml syringe. Cell suspensions were finally passed through a 70 μm cell strainer and washed with 40 ml of R10. Cell pellets were resuspended in R10, and cells were counted and distributed for ex vivo phenotypic and/or in vitro functional analyses by FACS.
γδ T cell immunophenotyping
γδ T cells subsets were identified using a fixable aqua blue dead cell stain (Life Technologies), and a combination of antibodies including CD3-APC-Cy7 (SP34-2), pan γδ TCR-PE (B1), CD4-Pac blue (L200) (all from BD Bioscience); Vδ2-FITC (15D;Thermofisher); and CD8-Ax700 (RPA-T8;eBioscience). CD3+ T cells were divided into Vδ1+ and Vδ2+ populations as described by Harris et al. (8) and as illustrated for a mucosal sample (Suppl Fig. 1), and were further subdivided by their CD4 and CD8 expression patterns. NKG2 receptor expression was assessed using combinations of anti-NKG2A-APC (Z99; Beckman Coulter) and anti-NKG2D-PE-Cy7 (1D11; BioLegend). For homing receptor expression and activation markers the following antibodies were used: α4β7-APC (NIH NHP Reagent Resource) and CCR7-PE-Cy7 (3D12 (BD BioScience) and CD69-Pac blue (FN50; BioLegend). For representative staining see Suppl Fig.2). For intracellular staining cells were fixed and permeabilized using a Perm/fix solution (BD Biosciences) prior to incubation with IFN-γ- PE-Cy7 (4S.B3; BDBioscience), TNF-α- PerCpCy5.5 (Mab11; BioLegend), CD107-PE-Cy5 (H4A3; BD BioScience) and Granzyme B-APC (GB12; Invitrogen). At least 50,000 CD3+ T cell events were acquired on a LSRII (BD Biosciences) and analyzed using FlowJo software version 9.8.5 (TreeStar Inc).
Functional and ex vivo analysis of γδ T cells
Peripheral and mucosal lymphocytes were mitogenically stimulated with PMA (50ng/ml) and ionomycin (250ng/ml). Mononuclear cells were incubated with monensin (golgi stop; BD Biosciences) according to the manufacturer's instructions and added at the start of the incubation. After 6h, cells were washed and stained for γδ T cell subsets and cytokines and cytotoxicity markers as described above. Values reported are after subtraction of non-stimulated control values. For ex vivo analysis, after 6h resting without stimulation each γδ T cell subset was further interrogated for the expression of NKG2 receptors, α4β7 (GALT homing), CCR7 (lymph node (LN) homing), and CD69 (activation). In some cases a limited number of mucosal cells were obtained and only some analyses could be performed.
Statistical analysis
The Wilcoxon rank-sum analysis was used for the comparison of phenotypic and functional data between naïve and SIV-infected macaques. The Wilcoxon signed-rank test was used to test for differences in paired samples within groups. The Spearman rank correlation test was used to assess the relationships of γδ T cell phenotype and function with viral loads. Figures display means with or without SEM or medians with or without interquartile ranges (IR). All p values are two-sided. Corrections for multiple comparisons have been addressed as follows: In panels with three or four sets of values to compare, the p values shown are not corrected, but marginal p values greater than 0.025 were considered non-significant. In panels with eight or more sets of values, the p values shown have been corrected by the Hochberg method for the number of unpaired or paired comparisons in the panel. Statistical analysis was performed using GraphPad Prism V6.01 (GraphPad Prism Software, La Jolla, CA) and SAS/STAT software version 9.3 (SAS Institute Inc., Cary, NC).
Results
Frequency of peripheral and mucosal-resident γδ T cells in SIV-infected macaques
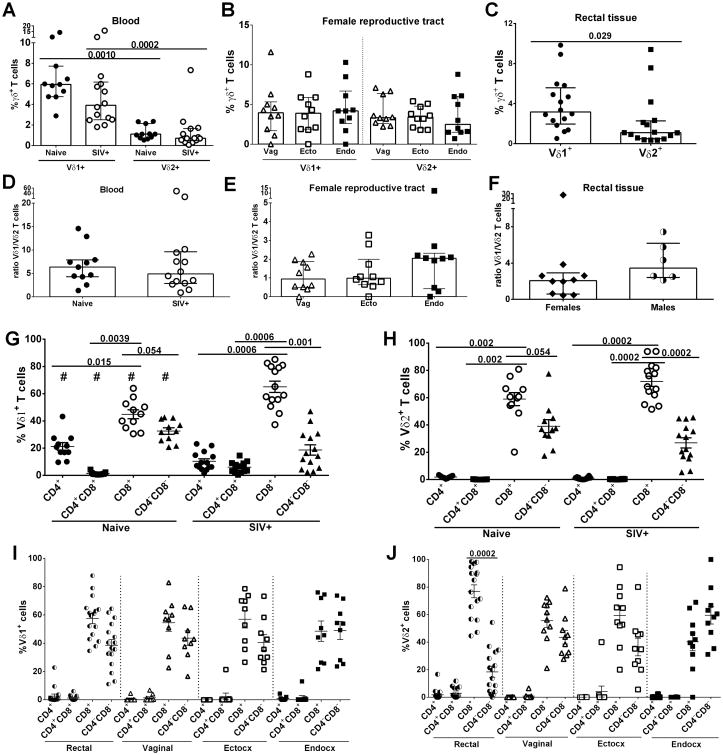
In view of the increasing interest in defining protective immune mechanisms against HIV/SIV infection in both peripheral and mucosal compartments, we investigated γδ T cells in SIV infected and non-infected rhesus macaques, initially determining the distribution of various subpopulations. Unlike humans, the peripheral blood of naïve and SIV-infected macaques exhibited a higher frequency of Vδ1 T cells than Vδ2 T cells (p = 0.0010 and 0.0002, respectively; Fig. 1A) in agreement with Wang et al. (40). Within the FRT, Vδ1 and Vδ2 T cell frequencies were comparable across all three compartments (Fig. 1B). In rectal tissue of all macaques Vδ1 cells were more prevalent than Vδ2 cells (p = 0.029: Fig. 1C); no differences were seen when macaques were analyzed by sex (data not shown). The Vδ1/Vδ2 ratio was seen to be similar in peripheral blood of naïve and SIV-infected macaques (Fig. 1D). Unfortunately, no mucosal tissue was available from naïve macaques for this study. Examination of FRT tissue from SIV-infected animals showed a non-significant upward trend of the Vδ1/Vδ2 ratio in the endocervix compared to the other two tissues (Fig. 1E). The Vδ1/Vδ2 ratio in rectal tissue showed no differences between males and females (Fig. 1F).
Figure 1. γδ T cell distribution in naïve and SIV-infected macaques.
Peripheral blood, rectal tissue and FRT tissue: vagina, ectocervix and endocervix were collected, processed and used for phenotypic analysis. Percentage of γδ T cells subsets in blood (A), FRT (B) and rectal tissue (C). Ratio of Vδ1+/Vδ2+ T cells in blood (D), FRT (E) and rectal tissue (F) of RMs. CD4 and CD8 expression in Vδ1+ (G and I) and Vδ2+ (H and J) subsets in blood, rectal tissue and FRT respectively. Only chronically SIV-infected macaques were included in the analysis of rectal and FRT tissues. #p<0.02 naïve vs SIV+. Results are expressed as median with IR (A – F) and mean ± SEM (G – J).
Expression of CD4 and CD8 was next explored in both Vδ1 and Vδ2 T cells. Analysis of peripheral blood revealed that both Vδ1 and Vδ2 γδ T cells in naïve and SIV-positive macaques are mainly CD8+, and exhibit significant differences in prevalence compared to each of the other 3 CD4/CD8 subsets with the exception of the marginally non-significant differences between the CD8+ and CD4-CD8- subsets for both Vδ1 and Vδ2 populations in naïve macaques (Fig. 1G and H). Although no alteration in the Vδ1/Vδ2 ratio was observed, we found that in SIV infected macaques the frequency of Vδ1 T cells expressing CD4+ and CD4-CD8-decreased but CD8+ and CD4+CD8+subsets increased compared to naïve animals(p < 0.02 for all 4 comparisons; Fig. 1G). In the rectal mucosa the majority of Vδ1 cells were CD8+ or CD4-CD8- (Fig. 1I) while rectal Vδ2 T cells predominantly expressed CD8+ compared to the CD4-CD8- population (p=0.0002; Fig. 1J). In the different FRT compartments statistically significant differences in the CD8+ and CD4-CD8- subpopulations were not observed in eitherγδ T cell subset (Fig. 1I and J). Frequencies of Vδ1 and Vδ2 CD8+ and CD4-CD8-T cell subsets were similar across the mucosal tissues, and few CD4+ and CD4+CD8+ cells were observed (Fig. 1I and J). No differences were found between females and males in the different rectal Vδ1 and Vδ2 CD4 and CD8 T cell subsets (data not shown), so results of both sexes were plotted together.
Homing and activation of peripheral γδ T cells in SIV infection
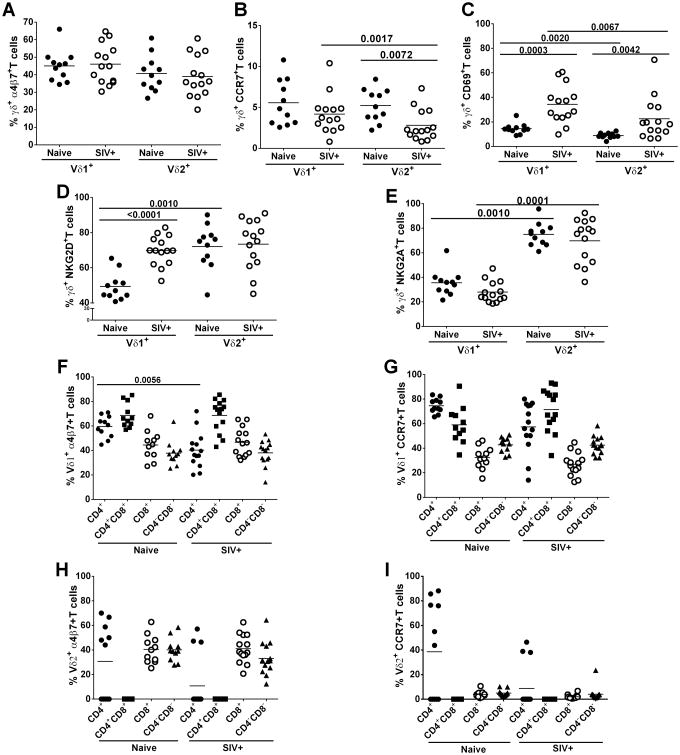
To elucidate potential trafficking of γδ T cells, we next investigated peripheral blood γδT cells for expression of the homing markers: α4β7 (GALT) and CCR7 (Lymph Node, LN). We also explored activation status by examining the expression of CD69, the inhibitory NK receptor NKG2A, and the activating NK receptor NKG2D.γδ T cell subsets, Vδ1 and Vδ2, from both naïve and SIV-infected macaques exhibited similar expression levels of α4β7 (Fig. 2A), while CCR7 expression on Vδ2 cells was lower in SIV-infected macaques compared to naïve (p=0.0072; Fig. 2B). Vδ2 also exhibited lower expression of CCR7 than Vδ1 T cells (p=0.0017). However, levels of the activation marker, CD69, in Vδ1 (p=0.0003) and Vδ2 (p=0.0042) cells were higher in SIV-infected compared to uninfected macaques (Fig.2C). Additionally, the Vδ1 subset exhibited a more highly activated profile than Vδ2 T cells regardless of SIV infection (p = 0.0020 and 0.0067 for naïve and SIV+ cells respectively; Fig. 2C). Vδ1 cells of SIV-infected macaques had increased expression of the activating receptor NKG2D (p<0.0001; Fig.2D). For the Vδ2 T cell subsets, no differences were observed in expression of NKG2D or NKG2A receptors between infected and uninfected rhesus macaques (Fig.2D and E). For both naïve and SIV-infected macaques, peripheral Vδ2 cells compared to Vδ1 cells showed higher expression of NKG2A (p = 0.0010 and 0.0001, respectively; Fig. 2E). However, higher expression of NKG2D was seen on Vδ2 compared to Vδ1 T cells from naïve macaques (p=0.0010; Fig. 2D), suggesting a different regulatory mechanism in infected animals.
Figure 2. γδ T cells expressing homing markers, activation markers and NK receptors.
Unstimulated cells from blood were analyzed for the frequency of Vδ1 and Vδ2 cells expressing α4β7 (A), CCR7 (B), CD69 (C), NKG2D (D) and NKG2A (E) in all groups of animals. Vδ1+ (F and G) and Vδ2+ (H and I) cells expressing CD4 and CD8 receptors were assessed for the homing receptors: α4β7 and CCR7. Naïve and chronically SIV infected macaques were included in all analyses. All results expressed as means.
We next examined homing markers on peripheral blood CD4 and CD8 γδ T cell subsets to assess potential trafficking to GALT and LN. Vδ1 CD4+ T cells of SIV infected macaques showed a lower expression of α4β7 (p=0.0056; Fig. 2F) and a similar although non-significant trend for CCR7 (Fig. 2G) than naïve macaques. Also Vδ2 CD8+ and CD4-CD8- T cells from SIV infected macaques showed marginally decreased expression of CCR7 compared to naïve animals (p=0.06 for both; Fig. 2I.) Regardless of SIV infection, α4β7 is mainly expressed by CD4+CD8+ and CD8+ subpopulations in Vδ1 and Vδ2 T cells respectively (Fig. 2F and H). On the other hand, CCR7 expression was high in CD4+Vδ1cells from naïve macaques, whereas expression was high in the CD4+CD8+ subset from SIV-positive macaques (Fig. 2G). Overall, however, fewer Vδ2 T cells expressed CCR7 in both naïve and SIV+ animals (Fig. 2I). Because only a limited number of cells was obtained from mucosal biopsies, homing, NK receptors and activation markers could not be assessed in those tissues.
Functionality of γδ T cells in chronically SIV-infected macaques
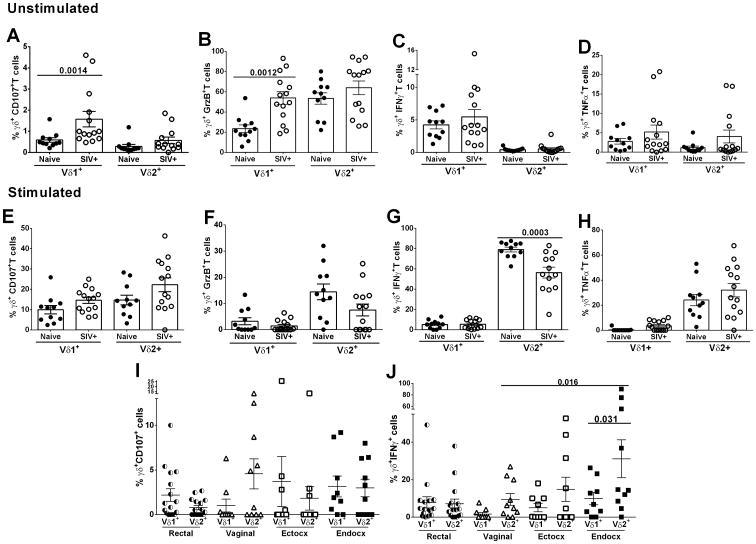
During HIV/SIV infection peripheral blood γδ T cell functions are dysregulated (6 - 8, 18). Therefore, the functionality of peripheral and mucosal Vδ1 and Vδ2 subsets from naïve and SIV-infected macaques was of interest. Lymphocytes from peripheral blood and mucosal tissues were left unstimulated or were mitogenically stimulated with PMA/ionomycin for 6h and then evaluated for cytokine production. Unstimulated, resting peripheral blood Vδ1 T cells from SIV-infected macaques exhibited higher expression of both CD107 (p=0.0014; Fig.3A) and Grz B (p=0.0012; Fig.3B) compared to naïve macaques. However, no difference in IFN-γor TNF-α expression between infected and uninfected macaques was observed (Fig.3C and D). When the response of Vδ1 and Vδ2 T cells to PMA/ionomycin stimulation was evaluated, the ability of both subsets of γδ T cells to express CD107, GrzB, and TNF-α was comparable to naïve macaques (Fig.3E, F and H). However, in SIV-infected macaques the ability of Vδ2 T cells to secrete IFN-γ (p=0.0003; Fig.3G) was lower compared to naïve macaques.
Figure 3. Expression of cytokines and degranulation markers by peripheral and mucosal γδ T cells.
Expression of CD107 (A), Grz B (B) and secretion of IFN-γ (C) and TNF-α (D) in Vδ1+ and Vδ2+ T cells after 6h resting (without stimulation) and after 6h PMA/ionomycin stimulation in peripheral blood (E - H) and rectal and FRT tissues (I and J). In I and J only SIV infected macaques were included in the analysis. Values of unstimulated cells were subtracted from values of stimulated cells. All results expressed as mean ± SEM.
Due to a low cell yield from mucosal biopsies, Vδ1 and Vδ2 T cells of SIV-infected macaques were only analyzed following PMA/ionomycin stimulation. No differences in CD107 expression levels were seen between Vδ1 and Vδ2 T cells of the rectal or FRT compartments (Fig. 3I). However, endocervical Vδ2 T cells exhibited greater IFN-γ secretion compared to vaginal Vδ2 T cells (p=0.016; Fig.3J) and also higher levels of IFN-γ expression compared to endocervical Vδ1T cells (p=0.031; Fig. 3J). This might suggest that γδ T cells from the endocervix exhibit a higher inflammatory profile compared to cells from the other compartments within the FRT. No difference was found between females and males in expression of CD107 or IFN-γ by rectal Vδ1 or Vδ2 T cells after stimulation (data not shown) so the results of both sexes were plotted together.
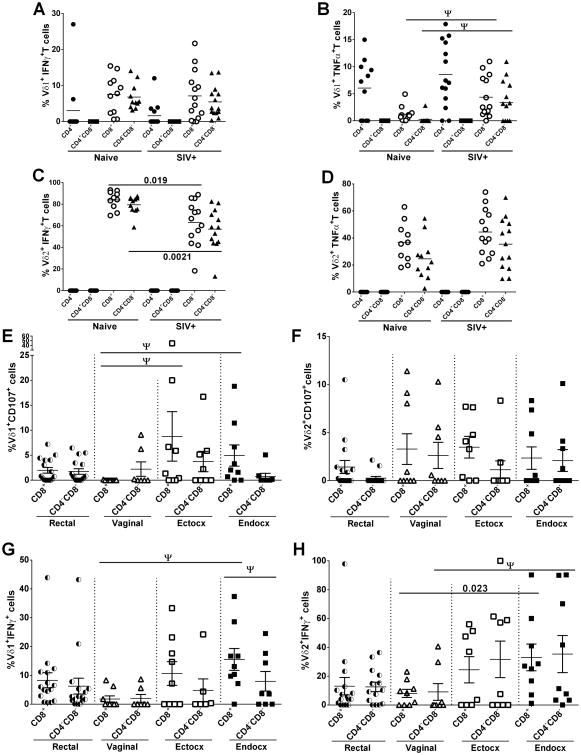
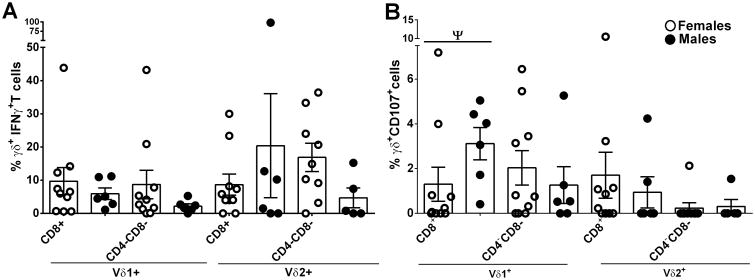
We also evaluated the response of peripheral CD4 and CD8 Vδ1 and Vδ2 T cell subpopulations to PMA/ionomycin stimulation (Fig. 4A-D). In view of the large number of tests, few of the differences observed remain significant after correction for multiple comparisons. We have called those that exhibited a p value <0.05 but were not significant after correction “tentative” (see legend to Figure 4) indicating that they are of interest but require additional confirmation. Thus, no differences of note are seen among the various CD4/CD8 subpopulations of Vδ1+IFNγ+ (Fig. 4A) or Vδ2+TNFα+ T cells (Fig. 4D) of naïve and SIV+ macaques. However, following stimulation, circulating Vδ1 CD8+ and CD4-CD8- T cells of infected macaques produced higher levels of TNF-α(tentatively significant; Fig.4B) compared to naïve, while CD8+ and CD4-CD8-Vδ2 T cells produced lower levels of IFN-γ (p = 0.019 and p = 0.0021, respectively; Fig.4C) than naïve macaques. No differences in CD107 and Grz B expression in SIV-infected or naïve macaques were seen in the various subpopulations of γδ T cells expressing CD4 or CD8 (data not shown).
Figure 4. Expression of cytotoxicity markers and cytokines by peripheral and mucosal CD8+ and CD4-CD8- γδ T cells subsets.
Blood from naïve and chronically SIV infected macaques was collected, processed and analyzed for CD8+ and CD4-CD8- γδ T cells expressing IFN-γ (A, C) and TNF-α (B, D) after 6h of PMA/ionomycin stimulation. Rectal and FRT tissues from only SIV infected macaques (E - H) were also analyzed. Values of unstimulated cells were subtracted from values of stimulated cells. A – D: results expressed as mean and E – H: as mean ± SEM. Ψ = tentatively significant after correction for multiple comparisons.
In mucosal tissue from SIV-infected macaques, Vδ1CD8+ T cells from both ectocervix and endocervix showed higher expression of CD107 compared to vaginal γδ T cells (tentatively significant; Fig 4E). No differences were observed in the expression of CD107 by Vδ2 T cells across the different mucosal tissues analyzed (Fig. 4F). Endocervical Vδ1 CD8+ T cells also exhibited higher IFN-γ production compared to vaginal cells (tentatively significant; Fig.4G) and they were also significantly elevated above the endocervical CD4-CD8- cells (tentatively significant; Fig. 4G). For Vδ2 T cells, both CD8+ and CD4-CD8- endocervical subpopulations exhibited higher frequencies of IFN-γ+ cells (p=0.023 and tentatively significant, respectively; Fig. 4H) compared to vaginal cells.
Sex difference in mucosal resident γδ T cells
Sex-related differences have been documented for various immunological parameters in mucosal tissues (41) as well as for their impact on AIDS disease progression (18, 35, 36). However, a potential sex bias in γδ T cell distribution and functionality has not been explored in HIV/SIV infection. Here we interrogated γδ T cells in rectal tissue from SIV-infected female and male macaques for the expression of IFN-γ and the CD107 degranulation marker. No difference was seen between sexes in IFN-γ production after stimulation with PMA/ionomycin (Fig.5A). While chronically SIV-infected males maintained higher levels of Vδ1 CD8+ T cells expressing CD107 compared to females suggesting that viremia control in rectal tissue of males might be associated with cytolytic activity of Vδ1 T cells, this difference was only tentatively significant due to the number of tests (Fig. 5B).
Figure 5. Sex difference in the expression of CD107 on γδ T cells from rectal tissue.
Rectal biopsies from chronically SIV-infected female and male macaques were collected, processed and assessed for the expression of IFN-γ (A) and CD107 (B) after 6h of PMA/ionomycin stimulation. Values of unstimulated cells were subtracted from values of stimulated cells. All results expressed as mean ± SEM. Ψ = tentatively significant after correction for multiple comparisons.
Viremia control is associated with preserved peripheral and mucosal γδ T cell levels
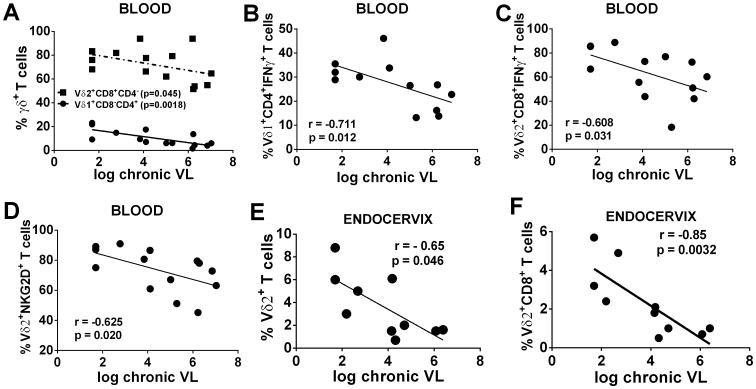
Natural virus suppressors that control virus replication maintain levels of Vδ2 T cells equivalent to those of healthy controls (42-44). We explored whether the control of viral load in chronically SIV-infected macaques was associated with better preservation of γδ T cells in peripheral blood and/or in mucosal tissue. A significant negative correlation was observed between levels of circulating Vδ1+CD4+ T cells and chronic viremia (p = 0.0018; Fig 6A) and a marginally significant negative correlation between Vδ2+ CD8+ T cells and chronic viremia (p = 0.045; Fig. 6A). Also significant negative correlations were seen between Vδ1 CD4+IFN-γ+ and Vδ2 CD8+IFN-γ+ T cells and chronic viremia (p=0.012 and p = 0.031 respectively; Fig.6B and C). Circulating NKG2D+ Vδ2 T cells were also negatively correlated with chronic viral loads (p = 0.020; Fig. 6D). No significant correlations between γδ T cells in rectal tissue and viremia of either males or females were observed (data not shown). In contrast, in mucosal tissue of the FRT, total endocervical Vδ2 T cells correlated negatively with chronic viremia (p=0.046;Fig.6E) as did CD8+ Vδ2 T cells (p=0.0032; Fig.6F).
Figure 6. Chronically SIV infected macaques with low plasma viral load maintain high γδ T cells levels in peripheral blood and mucosal tissue.
Correlation of peripheral Vδ1+ and Vδ2+ cells (A), Vδ1+expressing CD4 and IFNγ (B), Vδ2+ expressing CD8 and IFNγ(C), and Vδ2+ expressing the NKG2D receptor (D) with plasma viral loads. Mucosal Vδ2+ T cells from endocervix (E and F) correlated with chronic plasma viral load.
Discussion
While effects of HIV/SIV infection on peripheral blood γδ T cells have been reported, few studies have examined the more populous mucosal γδ T cells during HIV/SIV infection, and to our knowledge, none have investigated the effects of SIV infection on γδ T cells in the different regions of the FRT of Rhesus macaques. Our interest in this area was stimulated by the known sex bias in HIV pathogenesis (35, 36), and our recent observation of a sex bias in vaccine-induced protective efficacy (37). Sex differences in viral pathogenesis are associated with differences in immune responses (45). The observed sex bias in vaccine-induced protection was correlated with viral-specific mucosal IgA and mucosal memory B cells (37). These findings suggested that differences might also occur between males and females in γδ T cell populations at the mucosal sites of SIV infection and replication. Therefore, in this study we investigated effects of SIV infection on phenotypic and functional characteristics of γδ T-cell populations in the rectum and the FRT in comparison to those in peripheral blood.
To obtain a comprehensive picture of the γδ T cell populations in our Rhesus macaques, we initially characterized the cells in the peripheral blood of naïve and SIV-infected animals. Unlike humans, macaques exhibit a predominance of Vδ1 T cells in peripheral blood (40). A further expansion of peripheral Vδ1 T cells in SIV infection has been attributed to microbial translocation across the mucosal epithelium (8). Although here we did not observe a change in the Vδ1/Vδ2 ratio in blood of SIV-positive macaques compared to naïve animals (Fig. 1D) we did see changes in Vδ1+CD4+ and CD8+ T cell subsets (Fig. 1G). The decreases in CD4+ and CD4-CD8- γδ T cells of SIV-infected macaques might reflect CD4 depletion occurring by a direct or indirect mechanism. Concomitantly, an increase in the Vδ1 CD8+ and CD4+CD8+ subsets suggests the expansion of cytotoxic cells in response to infection. While we observed no depletion of Vδ2 T cells in SIV-infected macaques in comparison to the naïve animals, this population of cells has been reported to be lost and/or to become dysfunctional early following HIV/SIV infection (6-8, 16-18). Alteration in γδ T cells subsets has been widely shown during HIV infection (5, 17, 18, 44). In contrast only a single report concerning Vδ1/Vδ2 ratio alterations in SIV infected Rhesus macaques has appeared (8), showing a marginally significant difference in blood between naïve and SIV- infected macaques. Unfortunately, we were unable to obtain mucosal tissues from naïve macaques in order to determine if we could confirm a similar increase in Vδ1/Vδ2 ratio in gut tissue following SIV infection as seen previously (8). Future longitudinal studies following SIV infection of rhesus macaques might help to explain the lack of alteration of the Vδ1/Vδ2 ratio seen in our study. Analysis of the microbiome might elucidate the finding as well, as the altered ratio observed previously was attributed to microbial translocation.
The trafficking of γδ T cells between peripheral blood and secondary lymphoid organs has not been explored to any extent. Here, we investigated the impact of chronic SIV infection on the homing profile of peripheral γδ T cells to gut and LN. No alteration in the frequency of total α4β7+ Vδ1 or Vδ2 T cells was observed (Fig. 2A), while the frequency of CCR7+Vδ2 T cells was decreased during SIV infection (Fig. 2B), suggesting diminished active trafficking of Vδ2 T cells to LN. The proportions of Vδ1+α4β7+ were decreased in the blood of infected macaques (Fig. 2F, along with a downward trend in Vδ1+CCR7+CD4+ T cells, Fig. 2G). We speculate that a decline in Vδ2+ T cells trafficking to the LN might influence B cell responses. Human peripheral blood Vδ2+ T cells have been reported to help B cells secrete antibody (46), and mouse splenic γδ T cells have been shown to modulate pre-immune B cell function (47). Moreover, a recent finding in mice has shown that in the absence of αβ T cells, γδ T cells are localized in close proximity to B cells within germinal centers (48). Whether the Vδ2 T cell subset in macaques can perform functions similar to those of LN T follicular helper cells, that are lost or dysregulated during SIV infection (49) will require further investigation.
The cytolytic function of γδ T cells is tightly regulated by receptors such as NKG2A, NKG2D and NKG2C (50, 51). HIV/SIV infection activates γδ T cells as shown here by the increase in CD69+ γδ T cells (Fig. 2C) consistent with previous reports (52). Activated γδ T cells also express the NKG2C receptor which triggers cytotoxic function in Vδ1 T cells (53). Unfortunately, we lacked an antibody reactive with NKG2C for macaque cells, but observed an increased frequency in SIV-infected animals of γδ T cells expressing NKG2D (Fig. 2D), another activating receptor. Potent in vitro antiviral activity of Vδ1 T cells from HIV-infected patients has been described involving engagement of the NKp30 receptor and the combined effect of NKp30-induced CC-chemokines: CCL3, CCL4, and CCL15 (54). Cytotoxicity of Vδ1 T cells against multiple myeloma cells has been reported to be mediated in part by the TCR receptor, also involving NKG2D (55). Here, support for a role of Vδ1 cells as cytotoxic effectors includes expression of CD107a and Grz B (Fig. 3A and B). Vδ2 cells were also shown to express IFN-γ (Fig. 3G). However, activated Vδ1 cells may also function as regulatory T cells, as they have been reported to have strong suppressive activity (56 - 58). Clearly this subpopulation of γδ T cells requires further in depth investigation to elucidate which role it plays in HIV/SIV infection and whether it would be a useful therapeutic target.
Our main goal was to explore the distribution and functionality of γδ T cells in mucosal tissues as the primary site of HIV entry. Mucosal immune responses to HIV/SIV are likely to be critical for providing protection against infection and disease progression (37, 59 -61). Unfortunately, we did not have access to mucosal tissues of naïve macaques, but were only able to examine mucosal samples (rectal and FRT biopsies) of SIV-infected animals. Nevertheless, we were able to acquire a generalized overview of mucosal γδ T cells in the SIV-infected rhesus macaque. As previously reported in non-human primates (8), the intestinal mucosa of SIV-positive macaques exhibits predominantly Vδ1 T cells. We confirmed that here showing that Vδ1/Vδ2 ratios in rectal tissue (Fig. 1F) were somewhat elevated compared to the three tissues of the FRT (Fig. 1E). In both rectal tissue and the FRT Vδ1 and Vδ2 CD8+ and CD4-CD8- were the main subsets of γδ T cells. CD4-CD8- γδ T cells are particularly important during pregnancy, as they exhibit regulatory function by secreting TGF-β and IL-10 to support tolerance and avoid maternal rejection of the fetus, thereby maintaining the pregnancy (62). This illustrates the importance of defining the roles of different CD4 and CD8 γδ T cell subsets in different tissues. Here we saw elevated CD8+ CD107+ and IFN-γ+ Vδ1 cells in the ectocervix and endocervix of the FRT (Fig. 4E and G), suggesting cytotoxic function at sites of SIV infection. IFN-γ+ Vδ2 cells also appeared to be elevated in the endocervix compared to the vagina (Fig. 4H), perhaps enhancing recruitment of other immune cells and mediating effector functions (7, 63). Overall, γδ T cells in the endocervix exhibited a high inflammatory profile compared to the other two compartments within the FRT. An examination of rectal γδ T cells revealed no differences between males and females. Both sexes showed similar Vδ1/Vδ2 ratios (Fig. 1F). Therefore, these cell populations did not seem responsible for the sex bias we observed previously in which female macaques exhibited delayed SIV acquisition following repeated low dose rectal challenges (37). We noticed however, that males tended to have higher frequencies of rectal CD107+ Vδ1 T cells (Fig. 5B) suggesting that they might be able to better control local viremia, again highlighting the potential cytotoxic function of this cell population. This potential sex difference in mucosal γδ T cells in SIV infection requires confirmation. In general, several subpopulations of both Vδ1 and Vδ2 cells in peripheral blood were correlated with decreased chronic viremia (Fig. 6A-D), strengthening the presumed protective role of these cells. However, in the FRT, it was the Vδ2 subpopulation in the endocervix that was significantly correlated with control of plasma viremia (Fig. 6E and F).
The potential role of peripheral γδ T cells in viremia control was previously reported in natural virus suppressors (42). However, this is the first report concerning a protective role for Vδ2 T cells of the FRT. Results of clinical studies have supported a protective role of peripheral Vδ2 T cells. HIV suppressors maintain equivalent levels of Vδ2 T cells compared to healthy controls (42 - 44). While we also observed here in SIV-infected macaques significant correlations of Vδ1 subpopulations with decreased viremia (Fig. 6A and B), no in vivo protective role of these cells has been demonstrated in HIV disease. However, the frequency of peripheral Vδ1 CD4+ T cells was positively correlated with the CD4 T cell count in HIV-infected individuals (44).
This first study of phenotypic and functional characteristics of γδ T cells in the FRT has revealed a potentially protective role of Vδ2 T cells in controlling viremia. Further studies examining γδ T cells in the FRT early in SIV infection and in naïve animals are needed to determine if these cells contribute to protection against acquisition (by control of infectious foci) and/or to control of acute viremia. Although we did not track menstrual cycle phases in this study, it will be important to include this parameter in future investigations to determine if the complex regulation of the FRT by female sex hormones impacts γδ T cell frequencies and function. Additionally, the contribution of peripheral γδ T cells, although a small population of cells, should not be overlooked in overall control of chronic viremia. Efforts to strengthen the innate γδ T-cell immune response against HIV/SIV may have important implications for both treatment strategies and prevention of transmission through mucosal surfaces.
Supplementary Material
Acknowledgments
We thank the veterinarians and their staff at the NIH Animal Facility for expert care of the macaques and implementation of the research protocols; Kathy McKinnon and Sophia Brown for flow cytometric support; and Ranajit Pal and Maria Grazia Ferrari (Advanced BioScience Laboratories Inc.) for quantification of SIV RNA viral loads.
Footnotes
This study was supported by the Intramural Research Program of the NIH, National Cancer Institute.
References
- 1.Meresse B, Cerf-Bensussan N. Innate T cell responses in human gut. Semin Immunol. 2009;21:121–9. doi: 10.1016/j.smim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Adams EJ, Havran WL. Introduction to Cellular Immunology Special Issue on γδ T cells. Cell Immunol. 2015;296:1–2. doi: 10.1016/j.cellimm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–48. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegers GM, Lamb LS., Jr Cytotoxic and regulatory properties of circulating Vδ1+ γδ T cells: a new player on the cell therapy field? Mol Ther. 2014;22:1416–22. doi: 10.1038/mt.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedellec S, Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: from signals to functions. Semin Immunol. 2010;22:199–206. doi: 10.1016/j.smim.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Chaudhry S, Poonia B, Shao Y, Pauza CD. Depletion and dysfunction of Vγ2Vδ2 T cells in HIV disease: mechanisms, impacts and therapeutic implications. Cell Mol Immunol. 2013;10:42–9. doi: 10.1038/cmi.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrati C, D'Offizi G, Gougeon ML, Malkovsky M, Sacchi A, Casetti R, Bordoni V, Cimini E, Martini F. Innate gamma/delta T-cells during HIV infection: Terra relatively Incognita in novel vaccination strategies? AIDS Rev. 2011;13:3–12. [PubMed] [Google Scholar]
- 8.Harris LD, Klatt NR, Vinton C, Briant JA, Tabb B, Ladell K, Lifson J, Estes JD, Price DA, Hirsch VM, Brenchley JM. Mechanisms underlying γδ T-cell subset perturbations in SIV-infected Asian rhesus macaques. Blood. 2010;116:4148–57. doi: 10.1182/blood-2010-05-283549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournié JJ, Dieli F. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood. 2011;118:129–38. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 10.Cairo C, Surendran N, Harris KM, Mazan-Mamczarz K, Sakoda Y, Diaz-Mendez F, Tamada K, Gartenhaus RB, Mann DL, Pauza CD. Vγ2Vδ2 T cell Costimulation Increases NK cell Killing of Monocyte-derived Dendritic Cells. Immunology. 2014 Sep 16; doi: 10.1111/imm.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caccamo N, Todaro M, La Manna MP, Sireci G, Stassi G, Dieli F. IL-21 regulates the differentiation of a human γδ T cell subset equipped with B cell helper activity. PLoS One. 2012;7:e41940. doi: 10.1371/journal.pone.0041940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Bansal RR, Mackay CR, Moser B, Eberl M. IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur J Immunol. 2012;42:110–9. doi: 10.1002/eji.201142017. [DOI] [PubMed] [Google Scholar]
- 13.Cardone M, Ikeda KN, Varano B, Gessani S, Conti L. HIV-1-induced impairment of dendritic cell cross talk with γδ T lymphocytes. J Virol. 2015;89:4798–808. doi: 10.1128/JVI.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poles MA, Barsoum S, Yu W, Yu J, Sun P, Daly J, He T, Mehandru S, Talal A, Markowitz M, Hurley A, Ho D, Zhang L. Human immunodeficiency virus type 1 induces persistent changes in mucosal and blood gammadelta T cells despite suppressive therapy. J Virol. 2003;77:10456–67. doi: 10.1128/JVI.77.19.10456-10467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunnari G. Do Vgamma2Vdelta2 T cells influence HIV disease progression? Clin Infect Dis. 2008;46:1473–5. doi: 10.1086/587108. [DOI] [PubMed] [Google Scholar]
- 16.Kosub DA, Lehrman G, Milush JM, Zhou D, Chacko E, Leone A, Gordon S, Silvestri G, Else JG, Keiser P, Jain MK, Sodora DL. Gamma/Delta T-cell functional responses differ after pathogenic human immunodeficiency virus and nonpathogenic simian immunodeficiency virus infections. J Virol. 2008;82:1155–65. doi: 10.1128/JVI.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strbo N, Alcaide ML, Romero L, Bolivar H, Jones D, Podack ER, Fischl MA. Loss of Intra-Epithelial Endocervical Gamma Delta (GD) 1 T Cells in HIV-Infected Women. Am J Reprod Immunol. 2015 Dec 15; doi: 10.1111/aji.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimini E, Agrati C, D'Offizi G, Vlassi C, Casetti R, Sacchi A, Lionetti R, Bordoni V, Tumino N, Scognamiglio P, Martini F. Primary and Chronic HIV Infection Differently Modulates Mucosal Vδ1 and Vδ2 T-Cells Differentiation Profile and Effector Functions. PLoS One. 2015;10:e0129771. doi: 10.1371/journal.pone.0129771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali Z, Yan L, Plagman N, Reichenberg A, Hintz M, Jomaa H, Villinger F, Chen ZW. Gammadelta T cell immune manipulation during chronic phase of simian-human immunodeficiency virus infection [corrected] confers immunological benefits. J Immunol. 2009;183:5407–17. doi: 10.4049/jimmunol.0901760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauza CD, Poonia B, Li H, Cairo C, Chaudhry S. γδ T Cells in HIV Disease: Past, Present, and Future. Front Immunol. 2015;5:687. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88:185–94. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, Patel MV. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol. 2014;72:236–58. doi: 10.1111/aji.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen PV, Kafka JK, Ferreira VH, Roth K, Kaushic C. Innate and adaptive immune responses in male and female reproductive tracts in homeostasis and following HIV infection. Cell Mol Immunol. 2014;11:410–27. doi: 10.1038/cmi.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, Rothwangl KB, Veazey RS, Hope TJ. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog. 2014;10:e1004440. doi: 10.1371/journal.ppat.1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgener A, Tjernlund A, Kaldensjo T, Abou M, McCorrister S, Westmacott GR, Mogk K, Ambrose E, Broliden K, Ball B. A systems biology examination of the human female genital tract shows compartmentalization of immune factor expression. J Virol. 2013;87:5141–50. doi: 10.1128/JVI.03347-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goode D, Aravantinou M, Jarl S, Truong R, Derby N, Guerra-Perez N, Kenney J, Blanchard J, Gettie A, Robbiani M, Martinelli E. Sex hormones selectively impact the endocervical mucosal microenvironment: implications for HIV transmission. PLoS One. 2014;9:e97767. doi: 10.1371/journal.pone.0097767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J Reprod Immunol. 2013;97:74–84. doi: 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadzic SV, Wang X, Dufour J, Doyle L, Marx PA, Lackner AA, Paulsen DB, Veazey RS. Comparison of the vaginal environment of Macaca mulatta and Macaca nemestrina throughout the menstrual cycle. Am J Reprod Immunol. 2014;71:322–9. doi: 10.1111/aji.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kersh EN, Henning T, Vishwanathan SA, Morris M, Butler K, Adams DR, Guenthner P, Srinivasan P, Smith J, Radzio J, Garcia-Lerma JG, Dobard C, Heneine W, McNicholl J. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol. 2014;43:310–6. doi: 10.1111/jmp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Report on the global AIDS epidemic. 2012 www.UNAIDS.org.
- 31.Ahmed SM, Al-Doujaily H, Johnson MA, Kitchen V, Reid WM, Poulter LW. Immunity in the female lower genital tract and the impact of HIV infection. Scand J Immunol. 2001;54:225–38. doi: 10.1046/j.1365-3083.2001.00927.x. [DOI] [PubMed] [Google Scholar]
- 32.Trifonova RT, Lieberman J, van Baarle D. Distribution of immune cells in the human cervix and implications for HIV transmission. Am J Reprod Immunol. 2014;71:252–64. doi: 10.1111/aji.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultheiss T, Stolte-Leeb N, Sopper S, Stahl-Hennig C. Flow cytometric characterization of the lymphocyte composition in a variety of mucosal tissues in healthy rhesus macaques. J Med Primatol. 2011;40:41–51. doi: 10.1111/j.1600-0684.2010.00446.x. [DOI] [PubMed] [Google Scholar]
- 34.Stevceva L, Kelsall B, Nacsa J, Moniuszko M, Hel Z, Tryniszewska E, Franchini G. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J Virol. 2002;76:9–18. doi: 10.1128/JVI.76.1.9-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, Quinn TC, Vlahov D. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–4. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 36.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis. 2014;209(3):S86–92. doi: 10.1093/infdis/jiu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuero I, Mohanram V, Musich T, Miller L, Vargas-Inchaustegui DA, Demberg T, Venzon D, Kalisz I, Kalyanaraman VS, Pal R, Ferrari MG, La Branche C, Montefiori DC, Rao M, Vaccari M, Franchini G, Barnett SW, Robert-Guroff M. Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIVmac251 Rectal Challenge. PLoS Pathog. 2015;11:e1005101. doi: 10.1371/journal.ppat.1005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, Di Pasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–57. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV (mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87:1708–19. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, Nam KH, Morita CT. Conservation of nonpeptide antigen recognition by rhesus monkey V gamma 2V delta 2 T cells. J Immunol. 2003;170:3696–706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- 41.Sankaran-Walters S, Macal M, Grishina I, Nagy L, Goulart L, Coolidge K, Li J, Fenton A, Williams T, Miller MK, Flamm J, Prindiville T, George M, Dandekar S. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013;4:10. doi: 10.1186/2042-6410-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riedel DJ, Sajadi MM, Armstrong CL, Cummings JS, Cairo C, Redfield RR, Pauza CD. Natural viral suppressors of HIV-1 have a unique capacity to maintain gammadelta T cells. AIDS. 2009;23:1955–64. doi: 10.1097/QAD.0b013e32832ff1ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudová S, Li H, Sajadi MM, Redfield RR, Pauza CD. Impact of persistent HIV replication on CD4 negative Vγ2Vδ2 T cells. J Infect Dis. 2012;205:1448–55. doi: 10.1093/infdis/jis217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng NN, McElrath MJ, Sow PS, Mesher A, Hawes SE, Stern J, Gottlieb GS, De Rosa SC, Kiviat NB. Association between peripheral γδ T-cell profile and disease progression in individuals infected with HIV-1 or HIV-2 in West Africa. J Acquir Immune Defic Syndr. 2011;57:92–100. doi: 10.1097/QAI.0b013e318215a877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34:1050–9. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caccamo N, Battistini L, Bonneville M, Poccia F, Fournié JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, Dieli F, Salerno A. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol. 2006;177:5290–5. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Getahun A, Heiser RA, Detanico TO, Aviszus K, Kirchenbaum GA, Casper TL, Huang C, Aydintug MK, Carding SR, Ikuta K, Huang H, Wysocki LJ, Cambier JC, O'Brien RL, Born WK. γδ T Cells Shape Preimmune Peripheral B Cell Populations. Immunol. 2016;196:217–31. doi: 10.4049/jimmunol.1501064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pao W, Wen L, Smith AL, Gulbranson-Judge A, Zheng B, Kelsoe G, Mac Lennan IC, Owen MJ, Hayday AC. Gamma delta T cell help of B cells is induced by repeated parasitic infection, in the absence of other T cells. Curr Biol. 1996;6:1317–25. doi: 10.1016/s0960-9822(02)70718-5. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Wang X, Malam N, Lackner AA, Veazey RS. Persistent Simian Immunodeficiency Virus Infection Causes Ultimate Depletion of Follicular Th Cells in AIDS. J Immunol. 2015;195:4351–7. doi: 10.4049/jimmunol.1501273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angelini DF, Zambello R, Galandrini R, Diamantini A, Placido R, Micucci F, Poccia F, Semenzato G, Borsellino G, Santoni A, Battistini L. NKG2A inhibits NKG2C effector functions of γδ T cells: implications in health and disease. J Leukoc Biol. 2011;89:75–84. doi: 10.1189/jlb.0710413. [DOI] [PubMed] [Google Scholar]
- 51.Niu C, Jin H, Li M, Xu J, Xu D, Hu J, He H, Li W, Cui J. In vitro analysis of the proliferative capacity and cytotoxic effects of ex vivo induced natural killer cells, cytokine-induced killer cells, and gamma-delta T cells. BMC Immunol. 2015;16:61. doi: 10.1186/s12865-015-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gan YH, Pauza CD, Malkovsky M. Gamma delta T cells in rhesus monkeys and their response to simian immunodeficiency virus (SIV) infection. Clin Exp Immunol. 1995;102:251–5. doi: 10.1111/j.1365-2249.1995.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fausther-Bovendo H, Wauquier N, Cherfils-Vicini J, Cremer I, Debré P, Vieillard V. NKG2C is a major triggering receptor involved in the V[delta]1 T cell-mediated cytotoxicity against HIV-infected CD4 T cells. AIDS. 2008;22:217–26. doi: 10.1097/QAD.0b013e3282f46e7c. [DOI] [PubMed] [Google Scholar]
- 54.Hudspeth K, Fogli M, Correia DV, Mikulak J, Roberto A, Della Bella S, Silva-Santos B, Mavilio D. Engagement of NKp30 on Vδ1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood. 2012;119:4013–6. doi: 10.1182/blood-2011-11-390153. [DOI] [PubMed] [Google Scholar]
- 55.Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy. 2012;14:1110–8. doi: 10.3109/14653249.2012.700766. [DOI] [PubMed] [Google Scholar]
- 56.Kühl AA, Pawlowski NN, Grollich K, Blessenohl M, Westermann J, Zeitz M, Loddenkemper C, Hoffmann JC. Human peripheral gammadelta T cells possess regulatory potential. Immunology. 2009;128:580–8. doi: 10.1111/j.1365-2567.2009.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan DX, Duan J, Li MQ, Xu B, Li DJ, Jin LP. The decidual gamma-delta T cells up-regulate the biological functions of trophoblasts via IL-10 secretion in early human pregnancy. Clin Immunol. 2011;141:284–92. doi: 10.1016/j.clim.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Hua F, Kang N, Gao YA, Cui LX, Ba DN, He W. Potential regulatory role of in vitro-expanded Vδ1 T cells from human peripheral blood. Immunol Res. 2013;56:172–80. doi: 10.1007/s12026-013-8390-2. [DOI] [PubMed] [Google Scholar]
- 59.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, Pollard RB, Yee HF, Jr, Martin JN, Deeks SG, Shacklett BL. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–89. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultheiss T, Schulte R, Sauermann U, Ibing W, Stahl-Hennig C. Strong mucosal immune responses in SIV infected macaques contribute to viral control and preserved CD4+ T-cell levels in blood and mucosal tissues. Retrovirology. 2011;8:24. doi: 10.1186/1742-4690-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shacklett BL, Ferre AL. Mucosal immunity in HIV controllers: the right place at the right time. Curr Opin HIV AIDS. 2011;6:202–7. doi: 10.1097/COH.0b013e3283453e2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman JC, Chapman FM, Michael SD. The production of alpha/beta and gamma/delta double negative (DN) T-cells and their role in the maintenance of pregnancy. Reprod Biol Endocrinol. 2015;13:73. doi: 10.1186/s12958-015-0073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pauza CD, Riedel DJ, Gilliam BL, Redfield RR. Targeting γδ T cells for immunotherapy of HIV disease. Future Virol. 2011;6:73–84. doi: 10.2217/FVL.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.