Abstract
Our aim was to determine the incidence of prediabetes and risk of developing cardiovascular disease (CVD) in women with polycystic ovary syndrome (PCOS). This prospective, observational study included 148 women with PCOS, without Type 2 diabetes mellitus (T2DM) and CVD present at baseline. In the fasting blood samples, we measured lipids, glucose, and insulin levels during oral glucose tolerance test, levels of C-reactive protein (CRP), steroids, 25-hydroxyvitamin D (25-OHD), prolactin, thyroid-stimulating hormone, and parathyroid hormone. The follow-up period was 3 years. At baseline, prevalent prediabetes was present in 18 (12%) of PCOS cases and it progressed to T2DM in 5 (3%) of the cases. Incident prediabetes during the follow-up was noted in 47 (32%) women or 4.7 per 1000 persons/year. Prediabetes was associated with elevated body mass index (BMI) (odds ratio [OR] = 1.089, confidence interval [CI]: 1.010; 1.174, p = 0.026), high baseline levels of CRP (OR = 3.286, CI: 1.299; 8.312, p = 0.012), homeostatic model assessment - insulin resistance (IR) (OR = 2.628, CI: 1.535; 4.498, p < 0.001), and high lipid accumulation product (LAP) (OR = 1.009, CI: 1.003; 1.016, p = 0.005). Furthermore, prediabetes was associated with low 25-OHD (OR = 0.795, CI: 0.724; 0.880, p ≤ 0.05). In addition, cardiovascular risk in PCOS women with prediabetes was high (hazard ratio = 1.092, CI: 1.036; 1.128, p < 0.001). We showed association of prediabetes with high BMI, IR, markers of inflammation, LAP, and low serum 25-OHD concentration. IR appears to be more relevant than the other predictors of prediabetes risk in this study. PCOS women are considered as a high-risk population for prediabetes.
KEY WORDS: Prediabetes incidence, cardiovascular disease, polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) affects between 5% and 8% of women and is characterized by androgen excess and increased risk of diabetes and heart disease [1,2]. In prospective trials, Moran et al. showed a prevalence of prediabetes of 35%, prevalence of Type 2 diabetes mellitus (T2DM) of 10%, 5-10 fold risk of progression from prediabetes to diabetes, and a 4-7 fold higher risk of T2DM in PCOS patients [3]. Insulin resistance (IR) appears to be central to the pathogenesis of androgen excess in this population and also contributes to dyslipidemia and endothelial dysfunction [4]. Therefore, it is not surprising that PCOS is frequently associated with an increased risk for the development of cardiovascular disease (CVD) and metabolic syndrome [5].
Accordingly, women with PCOS, in combination with obesity, cigarette smoking, dyslipidemia, hypertension, impaired glucose tolerance (IGT), and subclinical vascular disease are at moderate risk for developing CVD, while those with metabolic syndrome or T2DM are at high risk for CVD [6]. Data on prevalence and incidence of T2DM, and particularly data on CVD in PCOS patients, are very limited [7]. Inflammatory markers, such as C-reactive protein (CRP), were shown to be consistently associated with the incidence of T2DM in a population of otherwise healthy people [8] as well as in PCOS patients [9].
This study was designed to determine the risk factors and incidence of prediabetes, as well as cardiovascular risk in women with PCOS. An additional goal was to determine the metabolic profile, including body weight, lipid levels, parameters of insulin sensitivity, subclinical inflammation, lipid accumulation product (LAP), and 25-hydroxyvitamin D (25-OHD) levels in women with PCOS.
MATERIALS AND METHODS
Participants
All the patients in this follow-up cohort study were recruited from the Diagnostic and Policlinic Department of the Clinic for Endocrinology and Diabetes at the University Clinical Center of Sarajevo. The study was performed according to the principles outlined in the Declaration of Helsinki and in accordance with ethical recommendations of the local Ethics Committee.
The study included female patients diagnosed with PCOS, aged 20-40 years, without acute and chronic diseases, diabetes and CVD, and with normal levels of thyroid-stimulating hormone (TSH), prolactin (PRL), and parathyroid hormone (PTH). PCOS was diagnosed according to the Rotterdam criteria [10] with clinical evaluation, laboratory tests, and echo-sonography. All the patients signed an informed consent.
There were 239 women with PCOS, of which 148 women without diabetes and CVD at baseline were included in the study, while 91 were diagnosed with T2DM and therefore excluded (Figure 1). The mean follow-up time was 3 ± 1.6 years (years 2010-2015). Anthropometric and laboratory parameters were obtained annually. Out of the 148 non-diabetic women with PCOS, 130 were without prediabetes at the baseline, and were followed in order to obtain the incidence of prediabetes. The remaining 18 women with PCOS were diagnosed with prediabetes at baseline and were followed in order to obtain the incidence of T2DM.
FIGURE 1.
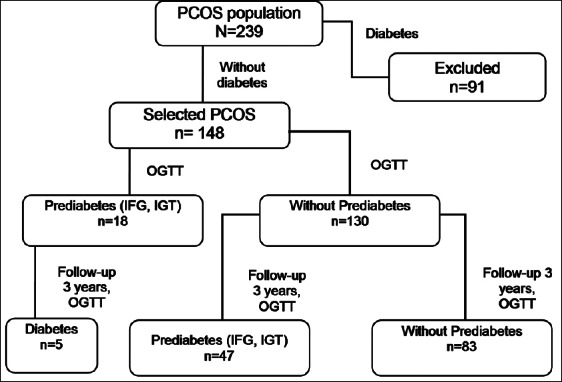
Flowchart of the study. PCOS: Polycystic ovary syndrome; OGTT: Oral glucose tolerance test; IFG: Impaired fasting glucose; IGT: Impaired glucose tolerance.
Hyperandrogenism was defined as sex hormone-binding globulin (SHBG) cutoff value <30 nmol/L and free androgen index test (FAI) >5. The cutoff value of testosterone was >3.0 nmol/L. We calculated FAI using formula as follows:
FAI = Total testosterone (nmol/L) × 100/SHBG (nmol/L) [11].
Lipid profile, CRP, 25-OHD, plasma glucose, and insulin levels during oral glucose tolerance test (OGTT), steroids, PRL, TSH, PTH were measured from morning fasting blood samples with standardized assays. Fasting samples were obtained annually.
Anthropometric and biochemical measurements
Anthropometric data were collected at each visit, including height, weight, blood pressure, the Ferriman–Gallwey score [12], and waist circumference. Hypertension was defined as systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg for daytime ambulatory blood pressure, or the use of anti-hypertensive medications [13]. Body mass index (BMI) was expressed as kg/height in meters squared. Waist circumference was measured at the midpoint between the lowest rib and the iliac crest. A waist circumference greater than 80 cm was considered as a factor for increased risk of metabolic complications [14].
Fasting serum 25-OHD concentration was measured in 88 of the 148 participants at the second visit. The reference range of 25-OHD was 20-50 ng/ml. Deficiency of 25-OHD was defined as 25-OHD ≤20 ng/ml.
Homeostasis model analysis (HOMA) [fasting glucose (mmol/L) × fasting insulin (µU/ml)/22.5] was also calculated [15], and IR was defined as HOMA-IR >2.5.
Additional information on smoking habits, physical activity, education, and employment status were collected by questionnaire. Education status was categorized as less or more than high school completed. Physical activity was designated as inactive or active. The participants were asked about current or former cigarette smoking habit, age at which smoking started and ended, and number of cigarettes consumed daily. Family history of diabetes was designated as negative, positive, or unknown.
Prediabetes criteria
Incident prediabetes was defined as an individual with fasting glucose <5.6 mmol/L at baseline examination and levels 5.6 to 6.9 mmol/L at the follow-up examination, thereby eliminating individuals with prevalent prediabetes [16]. Prediabetes is recognized through impaired fasting glucose (IFG) (glucose levels 5.6-6.9 mmol/L) or IGT (glucose levels 7.8-11 mmol/L) following a 2-hour 75 g OGTT given in the morning, after an appropriate overnight fast. Monitoring patients with prediabetes to assess their glycemic status should include at least an annual reassessment of fasting plasma glucose (FPG) or an OGTT [17,18]. Values of hemoglobin A1c (HbA1c) between 5.5% and 6.4% are criteria for prediabetes diagnosis.
The NCEP ATP III criteria [19] for the diagnosis of metabolic syndrome included abdominal obesity (waist circumference >80 cm), triglycerides (TGs) >1.7 mmol/L, high-density lipoprotein (HDL) <1.03 mmol/L, blood pressure >130/85 mmHg, and glucose >5.5 mmol/L. LAP was calculated using the formula [20]:
LAP for women = (waist circumference [cm] − 58) × (TG concentration [mmol/L]).
Statistical analysis
Descriptive statistics were utilized to compare the characteristics of participants according to BMI, and between participants who developed prediabetes compared to those who did not. Normal distribution was investigated using the Kolmogorov–Smirnov test. Data were expressed as means (standard deviation [SD]), medians (95% range), or absolute numbers and percentages, according to the type of variables and the normality of distributions. The continuous variables with skewed distribution were transformed using square root (weight, BMI, waist circumference, HDL, low-density lipoprotein [LDL], follicle-stimulating hormone [FSH], luteinizing hormone [LH], LH/FSH, CRP, androstenedione, dehydroepiandrosterone sulfate [DHEA-S], HOMA-IR), logarithm (cholesterol, LAP, progesterone, testosterone, fasting glucose, insulin), or inversion (TGs, estradiol, SHBG, 25-OHD) to normalize the distributions. Values of p less than 0.05 were considered statistically significant.
The unpaired Student’s t-test and the Chi-square test were used for comparisons between two groups of continuous and categorical variables, respectively. The Pearson correlation coefficients were used to examine the relationships between variables. Multivariate logistic analyses were performed to evaluate the association between investigated variables and incident prediabetes. Logistic models were adjusted for the potential confounding. Incidence rates were calculated using person-time analysis. The participants were censored at the last follow-up exam that they attended.
Cox proportional hazards modeling was utilized to estimate the hazard ratios (HRs) of prediabetes across quartiles of CRP, LAP and HOMA-IR, and tertiles of 25-OHD. Model 1 included the CRP, LAP, HOMA-IR, 25-OHD, age, BMI, physical activity, education, and smoking habits. Model 2 included Model 1 variables plus waist circumference. All covariates were defined as their values at baseline examination. We used Cox regression to assess the risk of developing CVD according to the prediabetes incidence in the women with PCOS. All models were adjusted for prevalent prediabetes.
Furthermore, a supplemental analysis was conducted upon classifying the study participants into two groups, according to their BMI: PCOS women with elevated BMI and PCOS women with normal body weight.
RESULTS
Out of the total 239 women diagnosed with PCOS, 148 (62%) were without diabetes and therefore included in the study (Figure 1). At baseline, prevalent prediabetes was found in 18 (12%) of the 148 women with PCOS, and it progressed to diabetes in 5 (3%) of these participants during the follow-up period. Out of the 148 eligible patients, 130 (88%) did not have prediabetes at baseline. During the 3-year follow-up period, incident prediabetes was noted in 47 (36%) of the 130 women with PCOS, or 4.7 per 1000 persons/year.
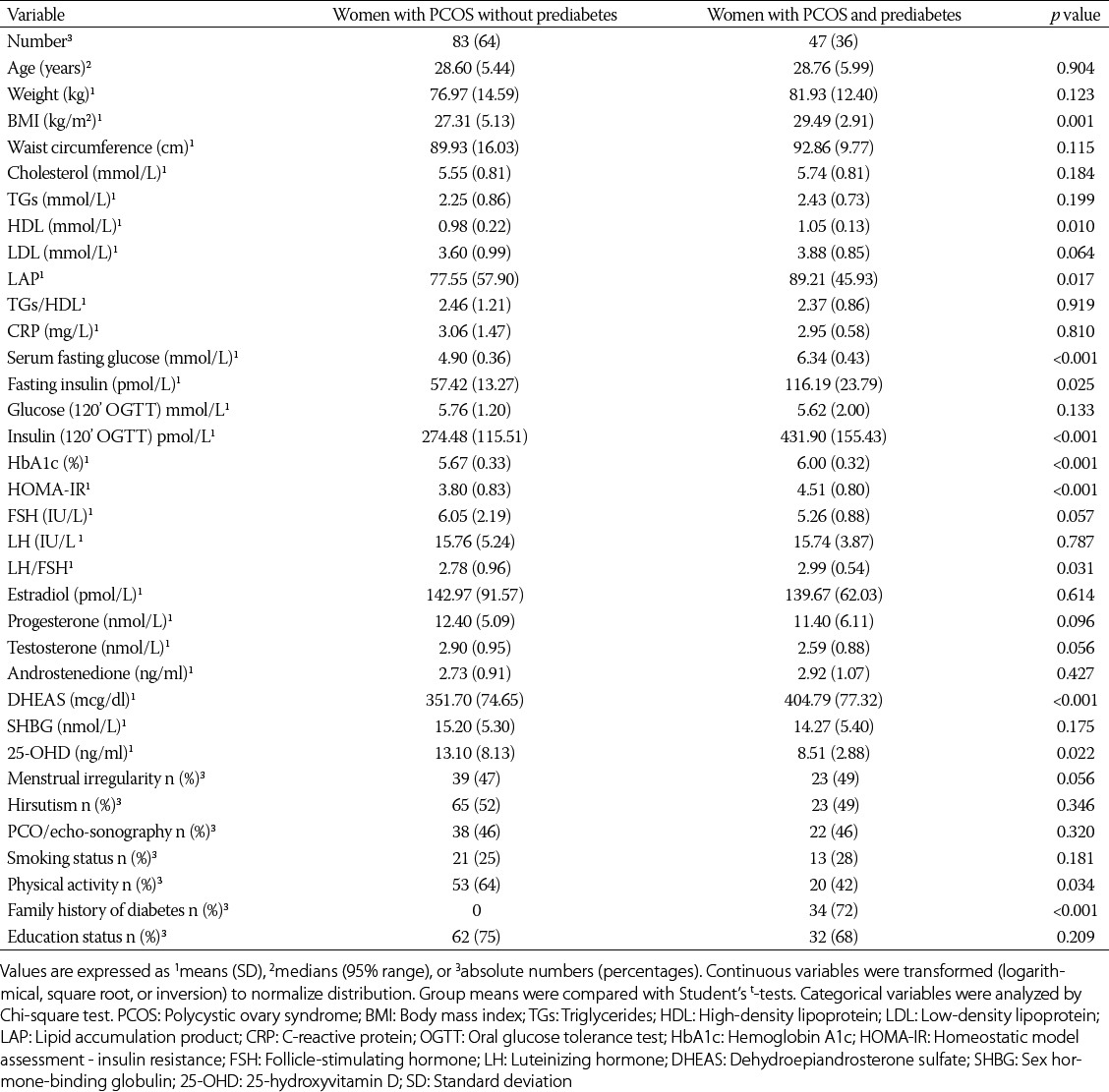
As shown in Table 1, the PCOS patients with incident prediabetes had similar frequencies of menstrual irregularity, hirsutism, echo-sonographic finding, smoking status, and education status as the PCOS participants without incident prediabetes. However, the women with PCOS with incident prediabetes had significantly higher levels of fasting glucose, fasting insulin, HOMA-IR, HbA1c, LAP, BMI, DHEA-S, and lower levels of 25-OHD than the women with PCOS without incident prediabetes (Table 1).
TABLE 1.
Characteristics of population with PCOS (n=130) according to incidence of prediabetes during the 3-year follow-up period
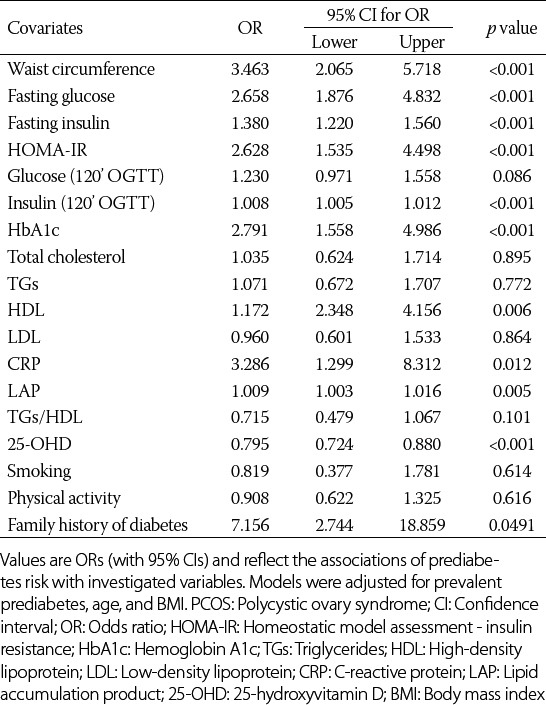
Among the 148 women with PCOS, the relationship between hormonal, metabolic, and inflammation status and incident prediabetes was assessed during the 3-year follow-up period (Table 2). The results showed that HOMA-IR (odds ratio [OR] = 2.628, confidence interval [CI]: 1.535; 4.498, p < 0.001), waist circumference (OR = 3.463, CI: 2.065; 5.718, p < 0.001), and family history of diabetes (OR = 7.156, CI: 2.744; 18.859, p = 0.049) are the most important predictors of prediabetes.
TABLE 2.
Predictors and diagnostic markers of incident prediabetes in women with PCOS (n=148)
The risk of developing CVD according to prediabetes incidence in the women with PCOS was significant (HR = 1.092, CI: 1.036; 1.128, p < 0.001).
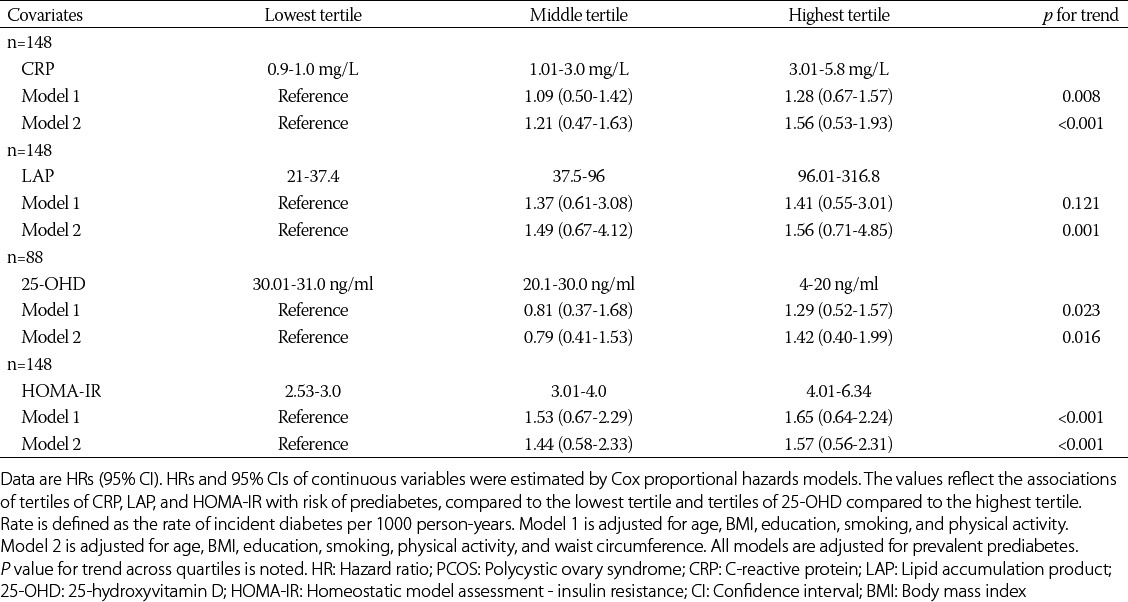
The Cox proportional hazard modeling showed that the prediabetes incidence was higher among the participants whose baseline levels of CRP, HOMA-IR, and LAP were in the highest versus the lowest tertile (Table 3). The relationship between 25-OHD and prediabetes incidence was also significant. The lowest and middle tertiles of 25-OHD were associated with prediabetes.
TABLE 3.
Hazard ratios for incident prediabetes in women with PCOS (n=148)
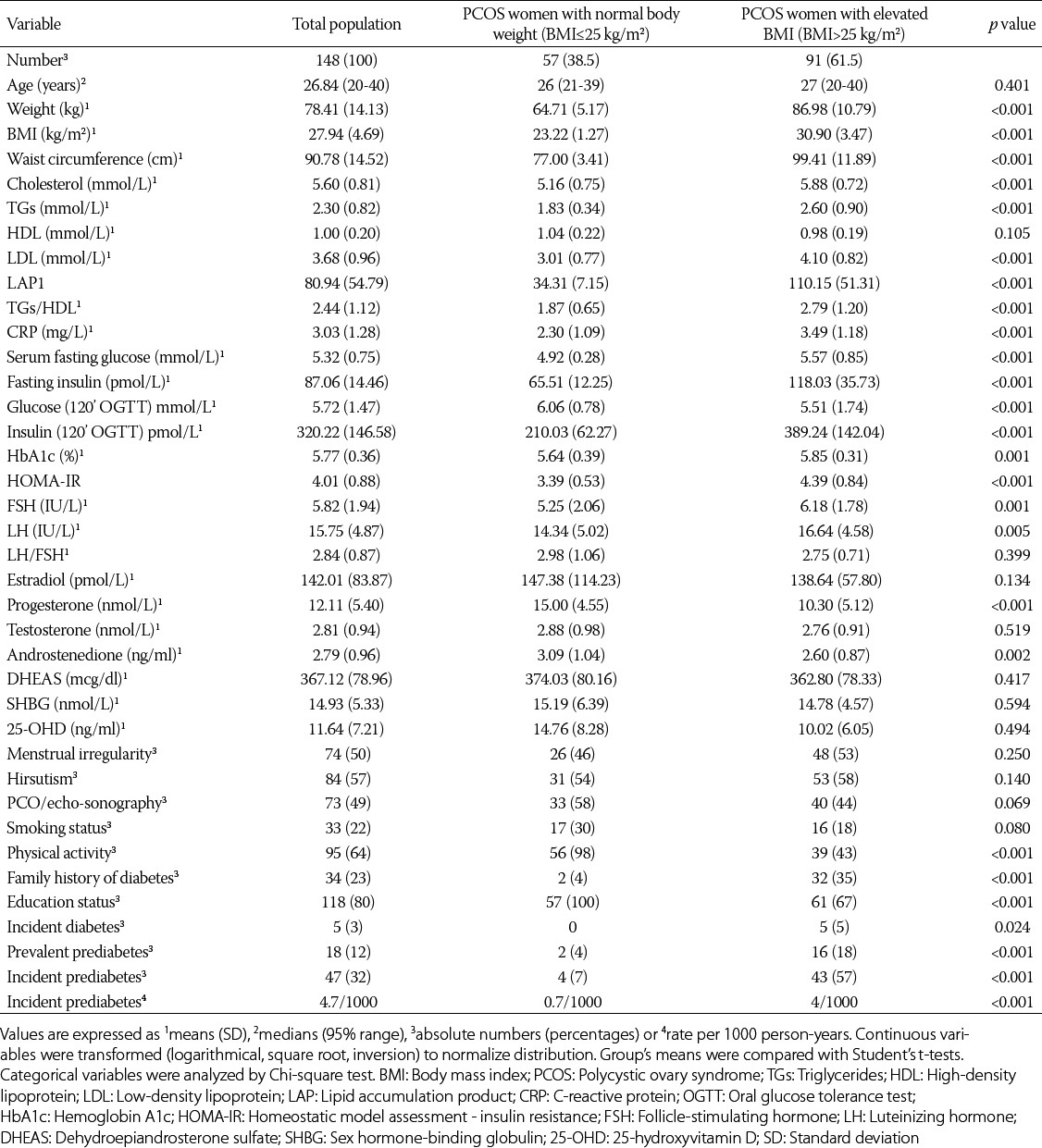
The characteristics of women with PCOS according to their BMI are presented in Supplemental Table 1. Out of 91 women with PCOS with elevated BMI, 16 (18%) had prediabetes at baseline, while out of 57 women with PCOS with normal body weight, only 2 (4%) had prediabetes at baseline. The incident prediabetes was more common in the PCOS patients with elevated BMI as compared to the participants with normal body weight (OR = 1.089, CI: 1.010; 1.174, p = 0.026). The positive family history of diabetes was more common in the PCOS patients with elevated BMI as compared to the participants with normal body weight. There were no differences in menstrual irregularity, echo-sonographic findings, and smoking status between the two groups of patients with PCOS. The women with normal body weight were more educated, and they were exercising more frequently than the women with elevated BMI. The levels of LDL, total cholesterol, LAP, HDL/TG ratio, CRP, TGs, HOMA-IR and glucose and insulin 2 hours after OGTT were significantly higher in the PCOS group with elevated BMI as compared to the women with PCOS with normal body weight.
SUPPLEMENTAL TABLE 1.
The clinical and laboratory characteristics of study population according to BMI
DISCUSSION
PCOS is the most common ovarian disorder related to the androgen excess in women, which explains the growing interest of endocrinologists to better understand the mechanisms of its development. In the last two decades, great efforts have been made to define PCOS [21]. Based on the well-known relationship of PCOS with IR, which is independent of obesity, a higher prevalence of prediabetes and T2DM in patients with PCOS is expected.
Our results indicate an association of prediabetes with high BMI, IR, markers of inflammation, LAP, and low serum 25-OHD concentration. IR appears to be more relevant than the other predictors of prediabetes risk in this study.
We showed a high prevalence of prediabetes (18%) in the women with PCOS with elevated BMI. Similarly, Legro et al. [22] demonstrated a significantly higher prevalence of both IGT (30%) and T2DM (4%) in obese women with PCOS as compared with control group of patients without PCOS (15.7% IGT, 0 T2DM). Furthermore, Ehrmann et al. [23] demonstrated a similar prevalence of impaired IR among women with PCOS (35% IGT, 10% T2DM).
Previous studies also suggested that the conversion from IGT to T2DM is accelerated in PCOS patients [24]. Accordingly, here, we demonstrated the conversion of prediabetes to diabetes in 3% of the participants during the follow-up period of 3 years. Furthermore, a similar study performed in women with PCOS and IGT reported that the conversion rates of prediabetes to diabetes ranged from 6% over 3 years to 13.4% over the period of 8 years in older women [24].
Interestingly, women who are obese, especially those with PCOS, have been recognized as specific high-risk subgroups for further development of prediabetes, T2DM, and potentially CVD [25]. Similarly, our results also showed that the incident prediabetes was more common in the PCOS patients with elevated BMI as compared to the participants with normal body weight. Women with PCOS with a positive family history of both T2DM and PCOS had an adverse metabolic and endocrine profile, including a linear increase in the risk of obesity, central fat accumulation, metabolic syndrome, prediabetes, and low HDL levels [26]. In accordance with these studies, our results showed that the positive family history of diabetes was more common in the PCOS patients with elevated BMI as compared to the participants with normal body weight. Likewise, the HOMA-IR, HbA1c, CRP, LAP, and 25-OHD levels were significantly associated with obesity. Furthermore, obesity significantly increased the incidence of prediabetes in the women with PCOS.
Smith-Marsh reported that the lifestyle improvement, including weight loss and increased physical activity, was effective in reducing the conversion of IGT to T2DM by 58% [27]. According to our results, a significantly higher number of non-diabetic women with PCOS were physically active as compared to the lower number of the PCOS patients who developed T2DM.
Adolescents with PCOS in South China had more metabolic abnormalities such as prediabetes, IR, hyperinsulinemia, dyslipidemia, and metabolic syndrome than their age- and BMI-matched non-PCOS counterparts [28]. Our study showed that the levels of LDL, total cholesterol, LAP, HDL/TG ratio, CRP, TGs, HOMA-IR, as well as glucose and insulin levels at 2 hours after OGTT were significantly higher in the PCOS group with elevated BMI as compared to the PCOS group with normal body weight.
Furthermore, our data showed that the PCOS patients with incident prediabetes had similar frequencies of menstrual irregularity, hirsutism, echo-sonographic finding, smoking status, and educational status as the PCOS participants without incident prediabetes. However, the women with PCOS with incident prediabetes had significantly higher levels of fasting glucose, fasting insulin, HOMA-IR, HbA1c, LAP, BMI, and DHEA-S, as well as lower levels of 25-OHD than the women with PCOS without incident prediabetes. Many studies showed similar results [3,29,30].
One-third of T2DM cases can be associated with elevated serum CRP. These findings substantiate a role of CRP as a possible candidate biomarker for early T2DM risk detection [31]. This was in line with our results, which also demonstrated a significant association between the CRP levels and prediabetes incidence. Interestingly, our data showed a significant association of CRP levels with the waist circumference, and the levels of fasting insulin, total cholesterol, LDL, LAP, HbA1c and fasting glucose in the women with PCOS. This is in line with the fact that women with PCOS often suffer from various metabolic disturbances. One of the emerging cardiovascular risk factors is LAP. A recent study performed by Wehr et al. [32] found that women with PCOS had significantly higher LAP levels than control group in age-adjusted analyses. Furthermore, they reported that OR for IGT in women with PCOS in the highest LAP quartile was significantly different compared to women with PCOS in the lowest LAP [32]. Our results showed that after adjusting for confounding, LAP was significantly associated with the incident prediabetes According to Wiltgen et al., LAP levels were better predictor than BMI for identifying adults at cardiovascular risk [33]. Furthermore, the cross-sectional study performed by Roa Barrios et al. [34] demonstrated that women with PCOS showed significantly higher values of the TG/HDL-cholesterol (HDL-C) ratio than women without PCOS, which is closely related to waist circumference and IR. Thus, the TG/HDL-C ratio could be considered as a useful and practical method to identify an increased risk of CVD in patients with PCOS. Here, we also found a significant relationship of LAP with BMI, waist circumference, HbA1c, total cholesterol, TGs, HDL, LDL, and TGs/HDL ratio.
The results of a recent study performed in U.S. adults suggested that lower serum 25-OHD levels are associated with prediabetes development [35,36]. Recently, it was also demonstrated that 25-OHD deficiency may play a role in exacerbating PCOS and that there may be a place for 25-OHD supplementation in the management of this syndrome [37]. Furthermore, Li et al. [38] demonstrated correlations of 25-OHD status with insulin sensitivity, HDL-C, and CRP in PCOS patients, which support the increasing evidence that 25-OHD deficiency is associated with multiple metabolic risk factors in women with PCOS. Our results also demonstrated a significant association between the 25-OHD levels and prediabetes incidence in the women with PCOS, where the 25-OHD levels were inversely associated with the development of prediabetes, suggesting a potential benefit of 25-OHD supplementation in these patients.
Cardiovascular risk is very often associated with PCOS and with its metabolic disorders [3-5,39]. Our data showed a high risk of developing CVD in the women with PCOS associated with prediabetes incidence.
This study has some limitations. We were not able to evaluate vascular endothelial function in the women with PCOS. If available, this analysis would potentially lead to better understanding of CVD development in PCOS patients. Furthermore, additional studies are necessary to confirm the potential benefits of 25-OHD supplementation in PCOS patients. Importantly, these strategies may potentially prevent the development of prediabetes and its progression into an overt diabetes, as well as hinder the development of cardiovascular complications in women with PCOS.
This study has a number of strengths including the longitudinal design. The prospective nature of the study ensured that all metabolic measurements were assessed blind to the eventual later prediabetes diagnosis. Furthermore, we were able to analyze a large number of covariates.
CONCLUSION
To the best of our knowledge, this follow-up study is one of the rare studies in which prediabetes incidence and cardiovascular risk were estimated in women with PCOS using a comprehensive set of hormonal, metabolic, and inflammation parameters and anthropometric measures.
Our results demonstrated a high incidence of prediabetes in women with PCOS and its association with high BMI, IR, markers of inflammation, LAP, and low serum 25-OHD concentration. IR appears to be more relevant than the other predictors of prediabetes risk in this study. The risk of developing CVD according to prediabetes incidence in the women with PCOS was high. Women with PCOS are considered a high-risk population for prediabetes.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Nitsche K, Ehrmann DA. Obstructive sleep apnea and metabolic dysfunction in polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2010;24(5):717–30. doi: 10.1016/j.beem.2010.08.001. http://dx.doi.org/10.1016/j.beem.2010.08.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–9. doi: 10.1210/jc.2003-032046. http://dx.doi.org/10.1210/jc.2003-032046 . [DOI] [PubMed] [Google Scholar]
- [3].Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, Type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–63. doi: 10.1093/humupd/dmq001. http://dx.doi.org/10.1093/humupd/dmq001 . [DOI] [PubMed] [Google Scholar]
- [4].Cho LW, Randeva HS, Atkin SL. Cardiometabolic aspects of polycystic ovarian syndrome. Vasc Health Risk Manag. 2007;3(1):55–63. [PMC free article] [PubMed] [Google Scholar]
- [5].Cussons AJ, Stuckey BG, Watts GF. Metabolic syndrome and cardiometabolic risk in PCOS. Curr Diab Rep. 2007;7(1):66–73. doi: 10.1007/s11892-007-0012-8. http://dx.doi.org/10.1007/s11892-007-0012-8 . [DOI] [PubMed] [Google Scholar]
- [6].Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. 2010;95(5):2038–49. doi: 10.1210/jc.2009-2724. http://dx.doi.org/10.1210/jc.2009-2724 . [DOI] [PubMed] [Google Scholar]
- [7].Tomlinson J, Millward A, Stenhouse E, Pinkney J. Type 2 diabetes and cardiovascular disease in polycystic ovary syndrome: What are the risks and can they be reduced? Diabet Med. 2010;27(5):498–515. doi: 10.1111/j.1464-5491.2010.02994.x. http://dx.doi.org/10.1111/j.1464-5491.2010.02994.x . [DOI] [PubMed] [Google Scholar]
- [8].Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56(3):872–8. doi: 10.2337/db06-0922. http://dx.doi.org/10.2337/db06-0922 . [DOI] [PubMed] [Google Scholar]
- [9].Keskin Kurt R, Okyay AG, Hakverdi AU, Gungoren A, Dolapcioglu KS, Karateke A, et al. The effect of obesity on inflammatory markers in patients with PCOS: A BMI-matched case-control study. Arch Gynecol Obstet. 2014;290(2):315–9. doi: 10.1007/s00404-014-3199-3. http://dx.doi.org/10.1007/s00404-014-3199-3 . [DOI] [PubMed] [Google Scholar]
- [10].Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–7. doi: 10.1093/humrep/deh098. http://dx.doi.org/10.1093/humrep/deh098 . [DOI] [PubMed] [Google Scholar]
- [11].Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, Trakatelli M, et al. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol. 2011;164(1):5–15. doi: 10.1111/j.1365-2133.2010.10011.x. http://dx.doi.org/10.1111/j.1365-2133.2010.10011.x . [DOI] [PubMed] [Google Scholar]
- [12].Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. http://dx.doi.org/10.1210/jcem-21-11-1440 . [DOI] [PubMed] [Google Scholar]
- [13].Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–219. doi: 10.1093/eurheartj/eht151. http://dx.doi.org/10.1093/eurheartj/eht151 . [DOI] [PubMed] [Google Scholar]
- [14].International Diabetes Federation. The IDF consensus worldwide definition of metabolic syndrome. Brussels: International Diabetes Federation; 2006. [Google Scholar]
- [15].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. http://dx.doi.org/10.1007/BF00280883 . [DOI] [PubMed] [Google Scholar]
- [16].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- [17].Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. American association of clinical endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. http://dx.doi.org/10.4158/EP.17.S2.1 . [DOI] [PubMed] [Google Scholar]
- [18].American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. http://dx.doi.org/10.2337/dc13-S011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: When do the risks of diabetes begin? A consensus statement from the American college of endocrinology and the American association of clinical endocrinologists. Endocr Pract. 2008;14(7):933–46. doi: 10.4158/EP.14.7.933. http://dx.doi.org/10.4158/EP.14.7.933 . [DOI] [PubMed] [Google Scholar]
- [20].Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc Disord. 2005;5(1):26. doi: 10.1186/1471-2261-5-26. http://dx.doi.org/10.1186/1471-2261-5-26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: A position statement from the European society of endocrinology. Eur J Endocrinol. 2014;171(4):P1–29. doi: 10.1530/EJE-14-0253. http://dx.doi.org/10.1530/EJE-14-0253 . [DOI] [PubMed] [Google Scholar]
- [22].Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for Type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84(1):165–9. doi: 10.1210/jcem.84.1.5393. http://dx.doi.org/10.1097/00006254-199906000-00019 . [DOI] [PubMed] [Google Scholar]
- [23].Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141–6. doi: 10.2337/diacare.22.1.141. http://dx.doi.org/10.2337/diacare.22.1.141 . [DOI] [PubMed] [Google Scholar]
- [24].Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: A controlled study. J Clin Endocrinol Metab. 2005;90(6):3236–42. doi: 10.1210/jc.2004-1843. http://dx.doi.org/10.1210/jc.2004-1843 . [DOI] [PubMed] [Google Scholar]
- [25].Rachon D, Teede H. Ovarian function and obesity - interrelationship, impact on women’s reproductive lifespan and treatment options. Mol Cell Endocrinol. 2010;316(2):172–9. doi: 10.1016/j.mce.2009.09.026. http://dx.doi.org/10.1016/j.mce.2009.09.026 . [DOI] [PubMed] [Google Scholar]
- [26].Lerchbaum E, Schwetz V, Giuliani A, Obermayer-Pietsch B. Influence of a positive family history of both Type 2 diabetes and PCOS on metabolic and endocrine parameters in a large cohort of PCOS women. Eur J Endocrinol. 2014;170(5):727–39. doi: 10.1530/EJE-13-1035. http://dx.doi.org/10.1530/EJE-13-1035 . [DOI] [PubMed] [Google Scholar]
- [27].Smith-Marsh D. Pharmacological strategies for preventing Type 2 diabetes in patients with impaired glucose tolerance. Drugs Today (Barc) 2013;49(8):499–507. doi: 10.1358/dot.2013.49.8.2002839. http://dx.doi.org/10.1358/dot.2013.49.8.2002839 . [DOI] [PubMed] [Google Scholar]
- [28].Huang J, Ni R, Chen X, Huang L, Mo Y, Yang D. Metabolic abnormalities in adolescents with polycystic ovary syndrome in South China. Reprod Biol Endocrinol. 2010;8:142. doi: 10.1186/1477-7827-8-142. http://dx.doi.org/10.1186/1477-7827-8-142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: Purposes and pitfalls. Obstet Gynecol Surv. 2004;59(2):141–54. doi: 10.1097/01.OGX.0000109523.25076.E2. http://dx.doi.org/10.1097/01.OGX.0000109523.25076.E2 . [DOI] [PubMed] [Google Scholar]
- [30].Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324–32. doi: 10.1016/j.molmed.2006.05.006. http://dx.doi.org/10.1016/j.molmed.2006.05.006 . [DOI] [PubMed] [Google Scholar]
- [31].Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohemy A, et al. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;3:173–86. doi: 10.2147/dmsott.s9089. http://dx.doi.org/10.2147/DMSO.S9089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wehr E, Gruber HJ, Giuliani A, Möller R, Pieber TR, Obermayer-Pietsch B. The lipid accumulation product is associated with impaired glucose tolerance in PCOS women. J Clin Endocrinol Metab. 2011;96(6):E986–90. doi: 10.1210/jc.2011-0031. http://dx.doi.org/10.1210/jc.2011-0031 . [DOI] [PubMed] [Google Scholar]
- [33].Wiltgen D, Benedetto IG, Mastella LS, Spritzer PM. Lipid accumulation product index: A reliable marker of cardiovascular risk in polycystic ovary syndrome. Hum Reprod. 2009;24(7):1726–31. doi: 10.1093/humrep/dep072. http://dx.doi.org/10.1093/humrep/dep072 . [DOI] [PubMed] [Google Scholar]
- [34].Roa Barrios M, Arata-Bellabarba G, Valeri L, Velázquez-Maldonado E. Relationship between the triglyceride/high-density lipoprotein-cholesterol ratio, insulin resistance index and cardiometabolic risk factors in women with polycystic ovary syndrome. [Article in Spanish] Endocrinol Nutr. 2009;56(2):59–65. doi: 10.1016/S1575-0922(09)70553-4. http://dx.doi.org/10.1016/S1575-0922(09)70553-4 . [DOI] [PubMed] [Google Scholar]
- [35].de las Heras J, Rajakumar K, Lee S, Bacha F, Holick MF, Arslanian SA. 25-hydroxyvitamin D in obese youth across the spectrum of glucose tolerance from normal to prediabetes to Type 2 diabetes. Diabetes Care. 2013;36(7):2048–53. doi: 10.2337/dc12-1288. http://dx.doi.org/10.2337/dc12-1288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes. Diabetes Care. 2011;34(5):1114–9. doi: 10.2337/dc10-1203. http://dx.doi.org/10.2337/dc10-1203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thomson RL, Spedding S, Buckley JD. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;77(3):343–50. doi: 10.1111/j.1365-2265.2012.04434.x. http://dx.doi.org/10.1111/j.1365-2265.2012.04434.x . [DOI] [PubMed] [Google Scholar]
- [38].Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism. 2011;60(10):1475–81. doi: 10.1016/j.metabol.2011.03.002. http://dx.doi.org/10.1016/j.metabol.2011.03.002 . [DOI] [PubMed] [Google Scholar]
- [39].Carmina E, Campagna AM, Lobo RA. Emergence of ovulatory cycles with aging in women with polycystic ovary syndrome (PCOS) alters the trajectory of cardiovascular and metabolic risk factors. Hum Reprod. 2013;28(8):2245–52. doi: 10.1093/humrep/det119. http://dx.doi.org/10.1093/humrep/det119 . [DOI] [PubMed] [Google Scholar]


