Abstract
The aim of this study was to investigate the responses of atherosclerosis plaque biomarkers to purslane seed consumption and aerobic training in women with T2D. 196 women with T2D were assigned into; (1) placebo (PL), (2) aerobic training+placebo (AT + PL), 3) purslane seeds (PS), aerobic training+purslane seeds (AT + PS). The training program and purslane seeds consumption (2.5 g lunch and 5 g dinner) were carried out for 16 weeks. The components of purslane seed were identified and quantified by GC–MS. Blood samples were withdrawn via venipuncture to examine blood glucose, low-density lipoprotein (LDL), high-density lipoprotein (HDL), cholesterol, triglycerides (TG), creatinine, urea, uric acid, NF-κB, GLP1, GLP1R, TIMP-1, MMP2, MMP9, CRP, CST3, and CTSS expressions. Blood glucose, LDL, cholesterol, TG, creatinine, urea, and uric acid levels in the (P), (AT), and (AT + PS) groups were significantly decreased compared to the pre-experimental levels or the placebo group, while HDL, significantly increased. Furthermore, the protein and mRNA levels of NF-κB, TIMP-1, MMP2 &9, CRP, CST3, and CTSS in the (P), (AT), (AT + PS) significantly decreased compared to pre-experimental or the placebo group, while level of GLP1 and GLP1-R increased drastically. Findings suggest that purslane seed consumption alongside exercising could improve atherosclerosis plaque biomarkers through synergistically mechanisms in T2D.
Diabetes mellitus (DM) is a metabolic disease with 8% worldwide prevalence in 2011 whereby it’s prevalence estimated to reach 10% by 20301. DM is characterized by hyperglycemia, glucose intolerance, abnormal lipid and protein metabolisms along with specific long–term complications affecting several major organs, including heart, blood vessels, nerves, eyes, and kidneys. Diabetic complications, such as cardiac dysfunction, atherosclerosis, neuropathy, retinopathy, and nephropathy2,3 are linked to defective insulin secretion, insulin resistance, or both4,5. Patients with type 2 diabetes (T2D) are at higher risk of complications arising from macro vascular, peripheral vascular and coronary heart disease, stroke, and insulin resistance associated with atherosclerosis6.
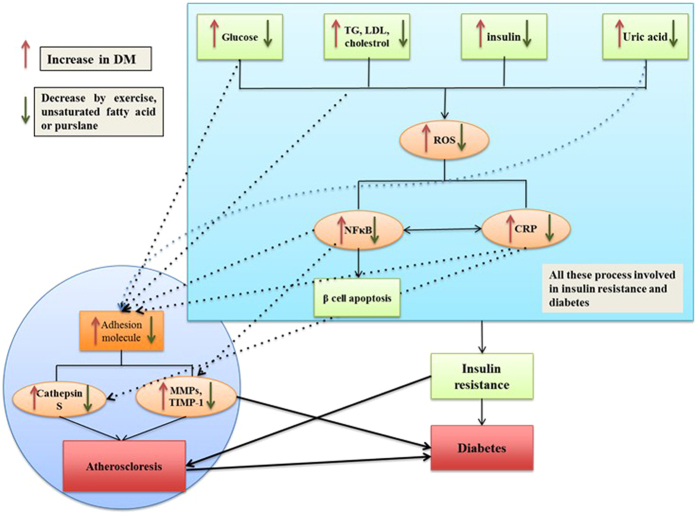
Atherosclerosis is a chronic inflammatory disease of blood vessels and it is characterized by formation of atherosclerotic plaques in arteries including calcified regions, necrotic cores, inflamed smooth muscle cells, accumulated modified lipids, endothelial cells, leukocytes, and foam cells7. Atherosclerosis causes cardiovascular diseases (CVD) in the components of the vascular, immune systems are involved8. Atherosclerosis diagnosis comprises of the identification of biomarkers linked to the development of this disease. Among the markers, nuclear factors kappa beta (NF-κB) and C-reactive protein (CRP) are the master key and prototypic markers respectively9,10. Activation of NF-κB is a key event in the pathobiology of diabetes and leads to apoptosis of beta cells. It is required for the transcription of inflammatory molecules containing adhesion molecule, cytokine and chemokines11. Matrix metalloproteinases (MMP) and cysteine proteases degrade extracellular matrix (ECM) proteins which are essential for vascular remodeling thus contributing to CVD12,13,14. High levels of glucose, LDL, VLDL, and uric acid activate CRP and NF-κB pathways which increase the expression of MMP2, MMP9 and CTSS (Fig. 1)15,16,17,18. In addition, glucagon like peptide −1 (GLP-1) is an insulinotropic agent which improves beta cell functions19 and protects heart muscle cells against adverse cardiac remodeling20.
Figure 1. Schematic illustration of some of the key pathological pathways that are involved in both type 2 diabetes and atherosclerosis.
Hyperglycemia, hyperlipidemia, hyperinsulinia, and hyperuricimia increase ROS generation through oxidative stress. ROS activates a number of stress sensitive kinase which mediates insulin resistance via activation of NF-κB and CRP. On the other hand, activation of NF-κB and CRP affect the structure of extracellular matrix and increase the activity of MMP and cathepsin S which are involved in atherosclerosis and diabetes. Exercise and purslane intake improve the regulation of biomarkers involved in the activation of these pathways. TG; triglycerides, LDL; Low-density lipoprotein, ROS; reactive oxygen species, NF-κB; nuclear factor kappa-light-chain-enhancer of activated B cells, CRP; C-reactive protein, MMPs; Matrix metalloproteinase, TIMP-1; metallopeptidase inhibitor 1.
Maintaining a healthy life style with regular physical activity has shown to reduce complications resulting from T2D21. In recent years, attention has been given to alternative medicines such as herbal remedies for treating and preventing plenty different diseases such as T2D22,23. Portulaca oleracea commonly known as purslane, is an herb from the Portulacaceae family with anti-diabetic properties5 and has been used for therapeutic purposes. It is listed by in the World Health Organization as one of the most used medicinal plants termed as ‘Global Panacea’24. P. oleracea is an excellent source of antioxidants such as vitamins A, C, E and β-carotene25,26. Several studies have indicated purslane extract as a factor in lowering blood sugar, triglycerides, total cholesterol, high-density-lipoprotein (HDL), low-density-lipoprotein (LDL) and body weight in diabetic rat and mice27,28. Moreover, consumption of purslane seeds alongside 8 weeks of resistance training by women with T2D, improved indicators associated with liver damage29, proxidant and antioxidant balance30 and blood pressure31. It also prevented exercise-induced oxidative stress30.
Physical activities such as resistance training promote an increase in lean muscle mass, muscle strength, the basal metabolic rate and sensitivity to insulin in diabetic individuals32. Regular exercising affects the regulation of enzymatic antioxidants such as catalase, super oxide dismutase and non-enzymatic antioxidants including vitamin E and C. Regular exercise is linked to CRP reduction, lipid profile regulation, increase of nitric oxide synthase, improvement in insulin sensitivity, and preservation of beta cell mass. Thus regular exercise leads to the adaptation in antioxidant capacity, protecting cells against harmful effects of oxidative stress33.
In this study our aim was to investigate the responses of atherosclerosis plaque biomarkers to purslane seed consumption (as non-medical intervention) along with aerobic training in women with T2D within a 16 week time period. We evaluated the lipid profile as well as traditional biomarkers and other biomarkers related with tissue inflammation and inflammatory response in diabetes patients including MMP 2&9, CRP, cystain C, creatinine, uric acid.
Patients and Methods
Subject recruitment
The study involved 196 women with type 2 diabetes whose fasting blood glucose levels were greater than 200 mg/dL (not taking metformin) and were diagnosed with the illness for an average of 8 years. The patients were on metformin with the dose of 500 mg/day and were registered with Tehran Hospital (Iran). Characteristics and demographics of the patients are presented in Table 1. The average age and BMI of these women were 52.08 ± 3.45 years and 29.5 ± 6.5 kg/m2 respectively. Eligible participants lacked any form of complications arising from acute and chronic diabetes and did not exercise regularly. The participants did not have any history of other diseases such as chronic cardiovascular and inflammatory diseases, diabetic ulcers, and hepatitis. Patients consuming vitamins and supplements or those who smoked were excluded from the study. Patients were well informed about the study prior to the experiment and written consent was obtained from them. A double-blind study method was applied and the participants were randomly assigned into 4 groups of 8; (1) placebo (PL), (2) aerobic training + placebo (AT + PL), (3) purslane seeds (PS), and (4) aerobic training + purslane seeds (AT + PS). All procedures involving experiments were carried out in strict accordance of the United States Institute of Research guidelines and approved by the Medical Centre Board of Tehran Hospital with Medical Ethics Number 4382.30.
Table 1. Characteristics and demographics of the diabetics’ subjects.
| Placebo (PL) Mean ± SEM | Aerobic Training + Placebo (AT + PL) Mean ± SEM | Purslane Seeds (PS) Mean ± SEM | Aerobic Training + Purslane Seeds (AT + PS) Mean ± SEM | |
|---|---|---|---|---|
| Age, year | 50.17 ± 5.34 | 58.83 ± 6.79 | 52.33 ± 4.08 | 61.17 ± 4.88 |
| Height, cm | 160.67 ± 6.44 | 162.50 ± 6.53 | 159.17 ± 6.65 | 154.50 ± 10.60 |
| Weight, cm | 75.67 ± 9.44 | 79.50 ± 8.96 | 73.50 ± 6.65 | 70.83 ± 7.88 |
| BMI, Kg/m2 | 29.8 ± 6.4 | 29.5 ± 7.2 | 29.0 ± 5.0 | 29.9 ± 7.3 |
Plant sample preparation
Purslane seeds were purchased from a grocery shop in Tehran (Iran). The seeds were washed and air dried at room temperature for seven days. The dry seeds were powdered and packaged in capsules (5 g each). Portulaca oleracea was identified and verified by a botanist and deposited at Herbarium of Department of Biochemistry, (voucher specimen no. 15–04979). The consumer groups (AT + PS, PS) consumed 2.5 g of purslane seeds with lunch and 5 g with dinner daily for 16 weeks. The placebo group similarly received placebo pills (the flavored maltodextrin).
Sample preparation and identification of compounds by Gas Chromatography/Mass Spectroscopy (GC/MS/MS)
Dried powdered (100 g) purslane seed was added with 100 ml of methanol and left at room temperature overnight. The eluate was filtered through Whatman filter paper and shade dried to remove the solvent. The extract was then weighed (0.563 g) and kept in −20 °C for further analysis. The extract was used in Gas Chromatography. The detail of Chromatography is available in supplementary file.
Aerobic training Guideline
Patients in groups AT + PL and AT + PS performed their training under a trainer’s supervision for 16 weeks in a gym. The trainer is blinded to the subject’s treatment group. Jogging was considered for this study as a moderate-intensity aerobic activity. Progressive training was performed for a minimum of 60 min per session for three days a week at 50 to 70% of maximum heart rate (MHR) during the 16 weeks of experiment. Written informed consent was obtained from all patients before any study-related procedures were performed. Patients were familiarized with the protocol and a heart rate strap was applied on them to monitor their heart rate within the first two sessions. The training included a 15 min warm-up through walking with light static and dynamic stretching and cooling down with stretching in standing and lying position in the final 15 min. All movements were performed with medium tempo and separate movement for arms, legs and trunk. The main training time increased 40 to 45 min and heart rate has reached 70% MHR. At the end of 16 weeks participants were able to perform muscle movements with greater coordination.
Blood Sample Collection
Blood samples were collected at the certain time of the trainings through the elbow antecubital vein of all patients, 24 hours before and after the 16 weeks training and purslane consumption for measurement of blood glucose, LDL, HDL, cholesterol, TG, creatinine, urea, uric acid, NF-κB, GLP1, GLP1R, TIMP-1, MMP2, MMP9, CRP, CST3, and CTSS mRNA and protein expressions.
Measurement of Serum parameters
Blood samples were collected in serum separating tubes (SST) and allowed to clot at room temperature for 30 min. The blood clot was then centrifuge at 3000 g for 15 min. Aliquots of the serum samples were stored at −20 °C for further use. Fasting serum glucose and total cholesterol levels were determined using the glucose oxidase method34 with a digital spectrophotometer (Spectronic, US). LDL level was calculated using the Friedewald equation35, and to measure HDL level, diagnosis kits were used following the photometric method. Serum triglycerides was measured by using the Fossati and Prencipe method36. Based on manufacturer’s instructions of Fortress Diagnostics Limited, creatinine, urea, and uric acid were examined by alkaline picrate method, urease-hypochlorite and uricase-peroxidase methods respectively.
Enzyme-linked immunosorbent assay (ELISA) was performed by using commercial kits (CUSABIO - USA) for NF-κB, GLP-1, GLP1R, TIMP-1, MMP2, MMP9, CRP, CST3, and CTSS. The ELISA kits detail information are available in supplementary file.
RNA purification and mRNA Expression Analysis by Real Time PCR (qPCR)
QIAamp RNA Blood Mini Kit (Qiagen, Germany) was used to isolate total cellular RNA from fresh whole blood. The concentration and purification of isolated RNA were evaluated by 260/280 UV absorption ratios (Gene Quant 1300, UK). Specific amplification fragments of DNA/RNA, Two-step Real time qPCR (quantitative Polymerase Chain Reaction) technique was used to calculate gene expression during the PCR amplification process with application of TaqMan reagent. This method was able to detect small differences between samples compared to other methods37. All reagents including probes and primers were obtained from Applied Biosystems, USA. TaqMan probe (known as fluorogenic 5′ nuclease) was chosen to perform qPCR. This probe has a sensitivity of 100% and a specificity of 96.67%17 and is capable of detecting as few as 50 copies of RNA/ml and as low as 5–10 molecules18. The same company designed specific primers for specific targets. The primers detail information are available in supplementary file.
All experiments were conducted in 3 biological replicates in a Step One Plus real time PCR machine (Applied Biosystems, USA). The Real time PCR program includes reverse transcription, at 48 °C for 15 min, activation of ampli Taq gold DNA polymerase at 95 °C for 10 min, denaturation at 95 °C for 15 sec and annealing at 60 °C for 1 min. Denaturation and annealing steps were performed for 40 cycles. The fold changes of each target per average of ACTB were calculated and considered as mRNA expression levels of the target gene. Data was analyzed according to Comparative Ct (2−ΔΔCt) method, where amplification of the target and the reference genes were measured in the sample and reference.
Statistical Analysis
Levene’s equality of variable assumption was applied and the results revealed no significant differences among the observations. All data were presented as mean ± SEM. Hormone levels of each subject were analyzed by descriptive statistics. Two-way analysis of variance with repeated measure (ANOVA) was used to compare changes between groups. A Bonferroni post-hoc test was used to check for significant differences between training alone vs. purslane seeds alone vs. aerobic + purslane seeds combined. For variables with normal distribution, Pearson correlation coefficient and for non-normal distributed variable, Spearman correlation coefficient were applied. SPSS 18.0 was used in this study and p < 0.05 was considered as statistically significant.
Results
Gas Chromatography/Mass Spectroscopy of Purslane Seed Extract
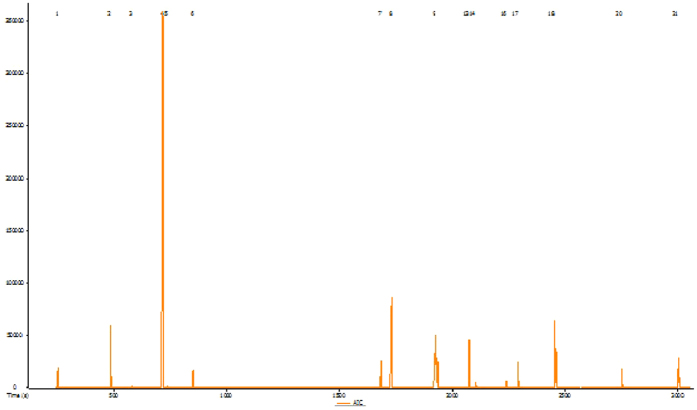
The components of Purslane seed were identified and quantified by GC–MS. A total of 21 known compounds are presented in Table 2. Among these compounds, 27% are unsaturated fatty acid (linoleic and palmitoleic acid); 11.18% are phytosterols (stiotestrol), and 7.9% are saturated fatty acids (palmitic acid). Additionally, smaller amounts of unknown substances were also detected (Fig. 2).
Table 2. Composition of total components contents of purslane seeds.
| Peak # | Name | R.T. (s) | Area | Relative Amount (RA) | Mass |
|---|---|---|---|---|---|
| 1 | 2-Propanol, 1-[(1-methylethyl) amino]-3-[2-(2-propenyl) phenoxy]-(Alprenolol, or alfeprol, alpheprol) | 252.15 | 311449 | 0.04 | 249 |
| 2 | 4-Methylpiperidine-1-carboxylic acid, phenyl ester (acid amin3) | 485.35 | 6340394 | 0.81 | 219 |
| 3 | N-(1-Methoxycarbonyl-1-methylethyl)-4-methyl-2-aza-1,3-dioxane | 582.25 | 4477291 | 0.57 | 203 |
| 4 | Tetrahydropyran-4-ol, 2,2-dimethyl-4-[[(thiophen-2-ylmethyl) amino]methyl]- | 717.9 | 129487357 | 16.61 | 255 |
| 5 | 1,3-Oxathiolane, 2-[[(2-chloroethyl) thio]methyl]-2-methyl- | 736.3 | 388091 | 0.05 | 212 |
| 6 | Benzoic acid, 2-(2-isopropyl-5-methylphenoxymethyl)- | 853 | 6451312 | 0.83 | 284 |
| 7 | 29.82 Methyl dodecanoate | 1684.55 | 2410439 | 0.31 | 214 |
| 8 | n-Hexadecanoic acid (Palmitic acid) | 1731.6 | 61905629 | 7.94 | 256 |
| 9 | Decanamide, N-(2-hydroxyethyl)- | 1925.3 | 52686347 | 6.76 | 215 |
| 10 | 9,12-Octadecadienoic acid (Z,Z)- Linoleic acid (LA) | 1927.4 | 120792530 | 15.49 | 280 |
| 11 | 9-Hexadecenoic acid-(Palmitoleic acid) | 1933.25 | 93736575 | 12.02 | 254 |
| 12 | 9,12,15-Octadecatrien-1-ol, (Z,Z,Z)- | 1934.05 | 24934192 | 3.20 | 264 |
| 13 | S-[2-[N,N-Dimethylamino]ethyl]N,N-dimethylcarbamoyl thiocarbohydroximate | 2074.5 | 4196678 | 0.54 | 219 |
| 14 | Palmidrol | 2103.5 | 10489224 | 1.35 | 299 |
| 15 | 4-(7-Methoxy-7-methyloxepan-2-ylidene) butan-2-one | 2110.3 | 5920620 | 0.76 | 212 |
| 16 | 6-Methoxy-2-methyl-quinoline-3-carboxylic acid 2-dimethylamino-ethyl ester | 2240.95 | 16583058 | 2.13 | 288 |
| 17 | Glycerol 1-palmitate | 2292.1 | 24680340 | 3.17 | 330 |
| 18 | Z-5,17-Octadecadien-1-ol acetate | 2453.7 | 79862448 | 10.24 | 308 |
| 19 | 9,12,15-Octadecatrien-1-ol, (Z,Z,Z)- | 2460.85 | 29437987 | 3.78 | 264 |
| 20 | 3H-Imidazo[4,5-b]pyridine, 2-(2-ethylhexylsulfanyl)- | 2753 | 17494020 | 2.24 | 263 |
| 21 | beta-Sitosterol | 3004.8 | 87157789 | 11.18 | 414 |
| Total | 779,743,770 | 100 |
Figure 2. Chromatographic profile of purslane seed methanolic extract, the 21 known and unknown peaks components are identified.
Serum Biochemical Parameters Analysis
The effects of purslane seed consumption and aerobic on the serum biochemicals of diabetic women are presented in Table 3. Blood glucose, LDL, Cholesterol, and TG concentration in the (PS), (AT + PL), (AT + PS) groups were significantly decreased after 16 weeks as compared to pre-experimental levels or the (PL) (p < 0.05). No significant differences observed between (PS) and (AT + PL) (p > 0.05), whereas significant interactions were detected between (PS) and (AT + PL) compared to (AT + PS) (p < 0.05).
Table 3. Changes in the serum variables of diabetic subjects between 4 groups pre and post 16 weeks experiments.
| Variable | Stage | Placebo (PL) Mean ± SEM | Aerobic Training + Placebo (AT + PL) Mean ± SEM | Purslane Seeds (PS) Mean ± SEM | Aerobic Training + Purslane Seeds (AT + PS) Mean ± SEM |
|---|---|---|---|---|---|
| Glucose, mg/dL | Pre Post | 168.2 ± 18.1 | 164.8 ± 17.5 | 167.4 ± 14.1 | 167.6 ± 20.6 |
| 173.1 ± 19.7 | 147.3 ± 18.3*,† | 152.1 ± 11.9*,† | 140.1 ± 15.2*,†,#,Ω | ||
| LDL-C, mg/dL | Pre Post | 83.3 ± 8.6 | 87.1 ± 12.4 | 84.1 ± 14.2 | 86.3 ± 11.7 |
| 82.1 ± 19.7 | 75.5 ± 17.2*,† | 79.9 ± 12.6*,† | 72.8 ± 19.7*,†,#,Ω | ||
| HDL-C, mg/dL | Pre Post | 35.8 ± 4.9 | 36.7 ± 8.3 | 35.3 ± 7.2 | 34.9 ± 3.7 |
| 33.6 ± 9.1 | 39.8 ± 10.3*,† | 39.2 ± 6.9*,† | 46.7 ± 7.4*,†,#,Ω | ||
| Cholesterol, mg/dL | Pre Post | 197.2 ± 22.4 | 198.5 ± 26.1 | 196.9 ± 22.6 | 198.7 ± 24.8 |
| 209.2 ± 20.1 | 169.7 ± 14.8*,† | 172.3 ± 19.3*,† | 155.2 ± 16.4*,†,#,Ω | ||
| TG, mg/dL | Pre Post | 183.1 ± 26.3 | 182.8 ± 21.7 | 183.6 ± 27.2 | 183.1 ± 23.8 |
| 182.9 ± 24.7 | 152.7 ± 29.2*,† | 159.3 ± 22.0*,† | 142.2 ± 21.5*,†,#,Ω | ||
| Creatinine, mg/dL | Pre Post | 1.43 ± 0.04 | 1.41 ± 0.05 | 1.40 ± 0.05 | 1.41 ± 0.03 |
| 1.43 ± 0.02 | 0.81 ± 0.06*,† | 0.88 ± 0.06*,† | 0.42 ± 0.07*,†,#,Ω | ||
| Urea, mg/dL | Pre Post | 19.31 ± 1.19 | 18.99 ± 1.04 | 18.86 ± 0.82 | 19.01 ± 0.63 |
| 19.31 ± 1.11 | 14.21 ± 0.98*,† | 14.42 ± 0.07*,† | 10.62 ± 0.98*,†,#,Ω | ||
| Uric Acid mg/dL | Pre Post | 4.65 ± 0.15 | 4.82 ± 0.18 | 4.59 ± 0.16 | 4.71 ± 0.15 |
| 4.66 ± 0.42 | 3.38 ± 0.21*,† | 3.11 ± 0.14*,† | 2.39 ± 0.23*,†,#,Ω |
*Denote significant differences from pre-test.
†Denote significant differences from Placebo group.
#Denote significant differences from Aerobic Training+ Placebo.
ΩDenote significant differences from Purslane Seeds group.
p < 0.05 as compared to pre-test, Placebo, Aerobic Training+ Placebo, and Purslane Seeds groups.
However, the HDL levels significantly increased in all (PS), (AT + PL), (AT + PS) groups as compared to pre-experimental levels or the (PL) (p < 0.05). No significant differences observed between (PS) and (AT + PL) (p > 0.05), whereas significant distinction were detected in (PS) and (AT + PL) compared to (AT + PS) (p < 0.05). The changes were more pronounced in (AT + PS) group.
Furthermore, Creatinine, Urea, and Uric Acid in the (PS), (AT + PL), (AT + PS) groups were significantly decreased after 16 weeks as compared to the pre experimental levels or the (PL) (p < 0.05). No significant differences observed between (PS) and (AT + PL) (p > 0.05), while significant distinction were detected in (PS) and (AT + PL) compared to (AT + PS) (p < 0.05). The reductions in levels were more pronounced in (AT + PS) group.
Atherosclerosis Biomarkers
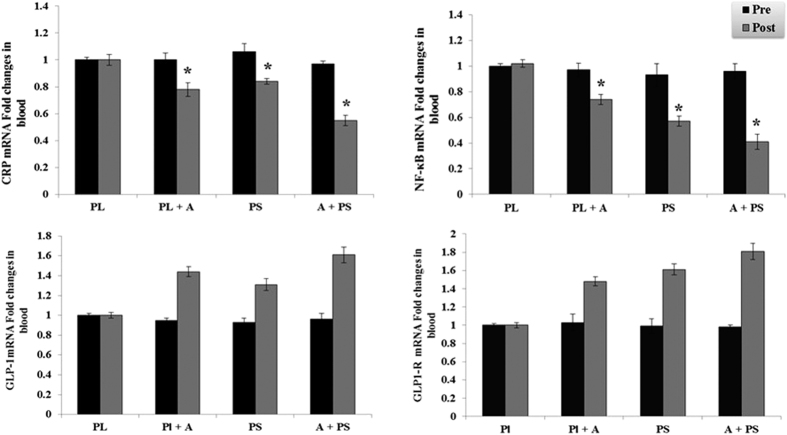
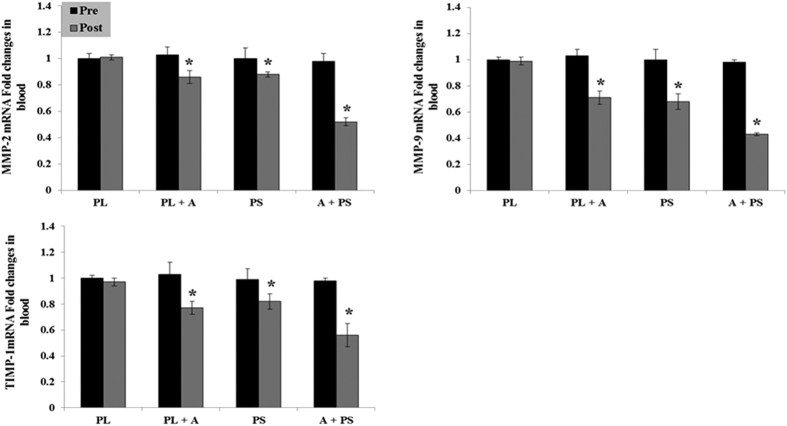
The effects of purslane seed consumption and aerobic on the serum protein and mRNA biomarkers changes in diabetic women are presented in Table 4 and Figs 3, 4 and 5. The protein and mRNA concentration levels of NF-κB, TIMP-1, MMP 2 & 9, CRP, CST3, and CTSS in the (PS), (AT + PL), (AT + PS) had significantly decreased after 16 weeks as compared to the pre experimental levels or the (PL) (p < 0.05). No significant differences observed between (PS) and (AT + PL) in the protein and mRNA concentration levels of NF-κB, MMP2 &9, CRP, CST3, and CTSS (p > 0.05), while significant distinction were detected in (PS) and (AT + PL) compared to (AT + PS) (p < 0.05). In addition, a significant difference observed between (PS) and (AT + PL) in the protein and mRNA concentration levels of TIMP-1, as well as significant distinction in (PS) and (AT + PL) compared to (AT + PS) (p < 0.05).
Table 4. Changes in the Serum biomarkers concentration diabetic subjects between 4 groups pre and post 16 weeks experiments.
| Variable | Stage | Placebo (PL) Mean ± SEM | Aerobic Training+ Placebo (AT + PL) Mean ± SEM | Purslane Seeds (PS) Mean ± SEM | Aerobic Training+Purslane Seeds (AT + PS) Mean ± SEM |
|---|---|---|---|---|---|
| NF-κB1, ng/mL | Pre Post | 8.74 ± 0.7 | 8.87 ± 0.5 | 8.52 ± 0.5 | 8.79 ± 0.6 |
| 8.69 ± 0.2 | 6.7 ± 0.3$,β | 6.5 ± 0.4$,β | 5.3 ± 0.4$,β,€,£ | ||
| GLP1, ng/mL | Pre Post | 5.44 ± 0.3 | 4.38 ± 0.6 | 3.82 ± 0.3 | 3.43 ± 0.3 |
| 5.55 ± 0.4 | 5.78 ± 0.3$,β | 4.93 ± 0.6$,β,€ | 5.77 ± 0.7$,β,€,£ | ||
| GLP1-R, ng/mL | Pre Post | 0.56 ± 0.012 | 0.52 ± 0.016 | 0.48 ± 0.017 | 0.49 ± 0.011 |
| 0.59 ± 0.018 | 0.69 ± 0.013$,β | 0.67 ± 0.015$,β | 0.71 ± 0.014$,β,€,£ | ||
| TIMP-1, ng/mL | Pre Post | 10.72 ± 0.56 | 10.47 ± 0.47 | 10.22 ± 0.68 | 10.86 ± 0.87 |
| 10.69 ± 0.6 | 6.98 ± 0.95$,†,β | 7.84 ± 0.44$,β,€ | 5.12 ± 0.65$,β,€,£ | ||
| MMP2, ng/mL | Pre Post | 0.88 ± 0.02 | 0.87 ± 0.03 | 0.88 ± 0.02 | 0.86 ± 0.02 |
| 0.87 ± 0.03 | 0.70 ± 0.04$,†,β | 0.67 ± 0.03$,β | 0.55 ± 0.04$,β,€,£ | ||
| MMP9, ng/mL | Pre Post | 1.62 ± 0.05 | 1.59 ± 0.07 | 1.58 ± 0.08 | 1.60 ± 0.07 |
| 1.61 ± 0.04 | 1.08 ± 0.22$,†,β | 1.05 ± 0.15$,β | 0.55 ± 0.19$,β,€,£ | ||
| CRP, mg/mL | Pre Post | 8.72 ± 0.4 | 7.91 ± 0.6 | 7.85 ± 0.6 | 8.56 ± 0.7 |
| 8.83 ± 0.5 | 6.1 ± 0.3$,β | 6.2 ± 0.1$,β | 5.1 ± 0.3$,β,€,£ | ||
| CST3, ng/mL | Pre Post | 35.13 ± 4.32 | 35.33 ± 3.52 | 34.71 ± 4.61 | 34.51 ± 3.22 |
| 35.75 ± 3.25 | 26.25 ± 3.95$,β | 25.98 ± 2.44$,β | 20.09 ± 3.65$,β,€,£ | ||
| CTSS, ng/mL | Pre Post | 0.77 ± 0.02 | 0.76 ± 0.03 | 0.79 ± 0.06 | 0.79 ± 0.03 |
| 0.77 ± 0.02 | 0.64 ± 0.05$,β | 0.61 ± 0.02$,β | 0.29 ± 0.02$,β,€,£ |
$Denote significant differences from pre-test.
βDenote significant differences from Placebo group.
€Denote significant differences from Aerobic Training+ Placebo.
£Denote significant differences from Purslane Seeds group.
p < 0.05 as compared to pre-test, Placebo, Aerobic Training+ Placebo, and Purslane Seeds groups.
Figure 3. Blood CRP, NF-κB, GPL-1, and GPL1-R, mRNA expression and activity at pre or post treatment in diabetic type 2 women. Data were expressed as mean ± SEM.
PL; Placebo, PL + A; Placebo + Aerobic, PS; Purslane Seed, and A + PS; Aerobic + Purslane Seed. *p < 0.05 as compared to Placebo group.
Figure 4. Blood MMP-2, MMP-9, and TMP-1 mRNA expression and activity at pre or post treatment in diabetic type 2 women. Data were expressed as mean ± SEM.
PL; Placebo, PL + A; Placebo + Aerobic, PS; Purslane Seed, and A + PS; Aerobic + Purslane Seed. *p < 0.05 as compared to Placebo group.
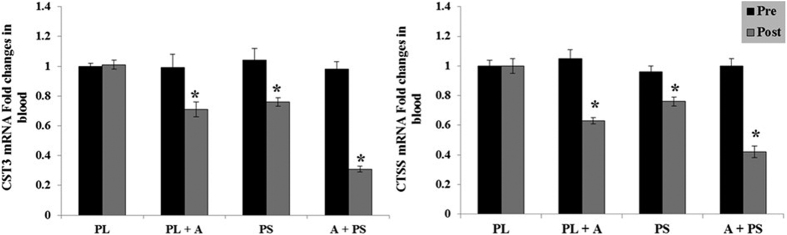
Figure 5. Blood CST3 and CTSS mRNA expression and activity at pre or post treatment in diabetic type 2 women.
Data were expressed as mean ± SEM. PL; Placebo, PL + A; Placebo + Aerobic, PS; Purslane Seed, and A + PS; Aerobic + Purslane Seed. *p < 0.05 as compared to Placebo group.
Furthermore, GLP1 and GLP1-R had profoundly increased in the (PS), (AT + PL), (AT + PS) as compared to the pre experimental levels or the (PL) (p < 0.05). No significant difference observed between (PS) and (AT + PL) in the protein and mRNA concentration levels of GLP1-R (p > 0.05), while a significant difference observed between (PS) and (AT + PL) in the protein and mRNA concentration levels of GLP1 (p < 0.05). Moreover, significant distinction were detected in the protein and mRNA concentration levels of GLP1 and GLP1-R in (PS) and (AT + PL) compared to (AT + PS) (p < 0.05). The changes were more pronounced in (AT + PS) group.
Discussion
The control and treatment of diabetes and its complications predominantly depends on chemical or biochemical agents. However, total recovery from diabetes has never been reported38,39. The data from the current study showed that 16 weeks of aerobic training or purslane seed consumption ere effective in reduction of markers of inflammations such as NF-κB, CRP, CST3, CTSS, MMP 2 & 9 and TIMP-1 in diabetic patients. In addition, glucose, TG, LDL, urea, uric acid and creatinine levels in all treated groups were significantly reduced. Furthermore, there was an increase in the levels of HDL, GLP-1 and GLP-1R. The improvement was more remarkable in women who received training and supplementation simultaneously. The changes in the level of biomarkers were further evaluated by the expression of mRNA from blood samples of the same subjects. Our results show that the mRNA levels of NF-κB, CRP, CST3, CTSS, MMP 2, 9 and TIMP-1 increased after intervention. GC-MS analyses results indicated the presence of 21 compounds, of which 27% are poly unsaturated fatty acid (linoleic and palmetoleic acid) and 11.18% are phytosterols (stiotestrol). The observed effects of purslane seeds may be due to the presence of these compounds.
The findings suggest that glucose levels had decreased in all groups. However the decrease was more evident in the group consuming purslane seed and exercising simultaneously. Purslane seed consumption has been shown previously to impact glucose levels27. High levels of glucose in diabetes appears to be an initiating factor for the eventual cascade of biomarkers involved in inflammation and coronary heart diseases. Improvements in lipid profile were observed in the subjects after 16 weeks of aerobic training or purslane seed consumption or both (Table 3). The reduction of lipid profile in the aerobic group could be due to the decrease of free fatty acids, which negatively affect insulin resistance and excess lipid availability40,41,42,43,44,45. Hence, aerobic training is beneficial for patients with T2D40,42,46. The results obtained from our study also highlights the positive effects of purslane seed consumption on TG, LDL, cholesterol and HDL levels, which is consistent with earlier reports in T2D of Yemeni and Iranian patients5,31.
Levels of blood urea, uric acid and creatinine (Table 3) were reduced in all treated groups. In T2D overweight patients, uric acid and urea serve as biomarkers for impaired physical performances and are associated with blood glucose levels47. Purslane consumption in T2D mice48 and T2D women has also been shown to reduce levels of creatinine, uric acid and urea49. Therefore, the reduction of blood nitrogen content seen in this study, may be lined to reduction in glucose levels (Table 3) following purslane seed consumption or aerobic training. In a study reported by Sousa50, twelve weeks of resistance training improved uric acid levels in T2D in Brazilian patients50,51,52,53. However, six months of aerobic training did not produce remarkable effects on creatinine and urea in untrained aged healthy women54.
The current study highlighted the beneficial effects of purslane seed consumption in alleviation of diabetes parameters. The beneficial effect may due to the presence of unsaturated fatty acids and beta-sistosterol as identified via GC-MS analysis (Table 2 and Fig. 2). Previous reports have suggested that, unsaturated fatty acids are responsible in reducing levels of LDL and cholesterol synthesis55, enhancing insulin function56 and improving glucose tolerance and lipid profile57,58. Another active substance in purslane seed is beta-sitosterol which is a phytosterol. Studies have found that beta-sitosterol has cholesterol59 and LDL60 lowering effects, increases the expression of VEGF (vascular endothelial growth factor) and FLK-1 (Fetal Liver Kinase 1) of VEGF receptors while modulating inflammation and regulation the immune systems. The effectiveness of purslane seed consumption is in lowering cholesterol levels explained by the synergistic effect of both phytosterols and unsaturated fatty acids61.
Hyperglycemia, hyperlipidemia, hyperinsulinemia17,18,53 and hyperuricemia62,63,64 of diabetics are common symptoms which enhance production of pro inflammatory markers such as CRP, IL6, TNFα, NF-κB17,18,53,62,64, reactive oxygen species (ROS) and reduce anti-inflammatory cytokine and adiponectin, which are involved in insulin resistance16,65 and diabetes66. Reduction in the level of NF-κB and CRP in the inflammatory cascade was observed in treated groups of diabetics’ subjects. Our experiments showed that purslane seed consumption resulted in reduction of CRP and NF-κB (Table 4 and Fig. 3). This may be the result of inhibitory effects of unsaturated fatty acids on NF-κB reported in diabetic patients67. The inhibitory effect of unsaturated fatty acids such as omega 3 on CRP68 in diabetic patients was reported even though the changes in CRP level were not significant. However, the significant reduction of CRP in the present study may indicate synergistic effect of various compounds found in purslane seed. Reduction in the level of CRP following resistance or strength training has been reported in patients with kidney disease45,69, older adults with T2D21 and diabetic men70 and in improving insulin sensitivity21. Various types of exercise may affect levels of NFκB differently. For instance, acute exercise did not change the level of NFκB in T2D patients53, but acute treadmill running raised the level of NFκB activity in rat skeletal muscles71. Therefore, reduction in levels of NFκB may be linked to CRP level reduction following aerobic exercise (Table 4) by impact on different synergistically pathways52,53,72. The expression of NFκB and CRP are interconnected whereby CRP enhances NFκB levels9, thus aerobic training and/or purslane seed consumption may directly or indirectly reduce the levels of NFκB via reducing the CRP.
The reduction of these two biomarkers is associated with the levels of inflammation and ROS in addition to regulation of ECM9,18. Matrix metalloproteinase (MMPs) and cathepsin S (CTSS) are proteases that degrade at least one component ECM and contribute for tissue remodeling and inflammation. Higher level of CRP and IL-6 are associated with higher level of CTSS17 and higher levels of NFκB enhance MMPs level16,73. In pathological conditions such as diabetes, the balance between the protease enzymes (MMPs and CTSS) and their inhibitors (metallopeptidase inhibitor 1; TIMP-1 and CST3) are dysregulated74. The concentrations of MMP-2, 9 and TIMP-1 has been shown higher in T2D patients as compared to non-diabetic subjects75,76 but in the current study, their levels were significantly reduced in all treated groups following different interventions (Table 4 and Fig. 4). Positive effects of exercise improve the balance between pro- and anti-inflammatory and oxidative stress markers which could improve nitric oxide bioavailability and alter MMPs and/or tissue inhibitors of MMPs (TIMP) activity. Chronic exercise training, that acts as a mechanical stressor for the arteries, could exert its effects by altering MMP/TIMPs activity directly and/or indirectly via the anti-inflammatory and oxidative stress responses of exercise77. The finding were concurrent with those of Kim et al.18 which states that the level of MMP-2 was reduced after low intensity exercise training in diabetic mice78. The suppressive effect of purslane seed on MMP and TIMP activity may be caused by the presence of the unsaturated fatty acids in the seeds. Similar outcomes were observed in patients with multiple sclerosis79 and in pregnant rats80.
CTSS is another important regulator of inflammation14 that plays a role in pathological conditions such as diabetes17,81. This regulator and its inhibitor CST3 are higher in diabetic patients as compared to healthy subjects82. Nonetheless the current interventions applied in this study reduced the protein and mRNA of both CTSS and CST3 (Table 4 and Fig. 5). This reduction following exercise may changes energy balance in adipose tissues83. Moreover, CTSS is positively correlated with weight loss83,84 and TG level in obese subjects85. Reduction in CTSS and LDL was also observed in diet induced weight loss in non-obese men and women86. This further supports the hypothesis that CTSS contributes to cardiovascular risks in obesity83. The reduction of CTSS and CST3 was also observed in the group consuming purslane seed alone which could be due to the presence of unsaturated fatty acids and beta-sistosterol found in the seeds. However, the mechanism of for their reduction is unknown.
This study also evaluated the effect of intervention on biomarkers for insulin and beta cell activity. The levels of GLP-1 and GLP-1R were measured. GLP-1 is an insulinotropic in T2D which improves insulin secretion via it’s receptor and by stimulation of glucose dependent insulin secretion19. It also has protective effect against degradation of ECM20 and inhibits apoptosis of beta cells87. In addition GLP-1 mimetics are potential drugs to treat T2D88. The present findings, showed improving effects of interventions on GLP-1 and its receptor levels (Table 4 and Fig. 3). This is possibly linked to effects of purslane and aerobic training on beta cells mediated by the AMPK activated pathway88,89. Exercise and physical activity induce the AMPK pathway and inhibit energy consuming pathways such as fatty acid and cholesterol synthesis and stimulate the ATP catabolic pathway90. Since AMPK signaling pathway is among the crucial pathways involved in inflammation, following activation of this pathway, inhibition of NFκB and its downstream signaling pathway occurs91.
Thus the reduction in the inflammatory state of diabetes may be an important factor leading to improved insulin sensitivity and better metabolic control21. Increased physical activity and weight loss are associated with lower CRP and reduction in concentration of other markers of inflammation33,92. Therefore, both intervention alone or in combination might act through AMPK pathway and inhibit the activation of downstream inflammation through inhibition of CRP and NFκB. Among different insulin signaling pathways, available treatments for diabetes usually trigger the AMPK pathway which is insulin independent93 and metformin activated that results in the inhibition of NF-kB activity in endothelial cells94,95. In this study, the level of NF-kB as a master inflammation key was reduced with purslane intake and/or aerobic training which could be due to activation of the AMPK pathway. Previous reports have indicated that aerobic exercise52,53 and the uptake of unsaturated fatty acids activate the AMP and AMPK pathways96.
Conclusion
The data obtained from this study indicated that 16 weeks of aerobic training or/and purslane seed consumption ere effective in regulation of diabetic parameters and biomarkers associated with atherosclerosis in women with T2D. This may be due to the synergistic effect of aerobic training and unsaturated fatty acids found in purslane seed which activate the AMP and AMPK pathways. This may result in the regulation of biomarkers involved in cardiovascular complications of T2D. The parallel effects of purslane intake and aerobic training in their simultaneous role were more prominent which could be a strong therapeutic effective factor on diabetic patients with CVD. Thus, with the benefits of this combination on reducing health risk factors, diabetic patients are advised to exploit this arrangement of alternative to control and manage their disease. However, further investigations are warranted, and such research should focus on identifying the specific properties of purslane seed, proper dose, role of various active compounds, as well as the possibilities for synergistic interactions not only between the various components of this seed, but also between various alternatives in treating and reducing symptoms of these diseases.
Additional Information
How to cite this article: Dehghan, F. et al. Purslane (Portulaca oleracea) Seed Consumption And Aerobic Training Improves Biomarkers Associated with Atherosclerosis in Women with Type 2 Diabetes (T2D). Sci. Rep. 6, 37819; doi: 10.1038/srep37819 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors wish to sincerely thank University of Malaya for High Impact Research grant UM.C/HIR/MOHE/MED/11 and research grant BKP065-2015 for supporting this research.
Footnotes
Author Contributions Conceived and designed the study: F.D., K.G., MA.A., and A.Y. Performed the experiments: F.D., S.H. Analyzed the data: F.D., M.A and MA.A. Contributed reagents/materials/analysis tools: F.D., A.Y., MA.A., P.F., and S.M. Wrote the manuscript: F.D., R.S., K.G., M.A., A.Y., and MA.A.
References
- Shamima A., Rahman M., Krull S. A. & Sultana P. Prevalence of diabetes and prediabetes and their risk factors among Bangladeshi adults: a nationwide survey. Bulletin of the World Health Organization 92, 204–213A (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini G. P., Iori E., Marescotti M. C., de Kreutzenberg S. V. & Avogaro A. Insulin-induced glucose control improves HDL cholesterol levels but not reverse cholesterol transport in type 2 diabetic patients. Atherosclerosis 235, 415–417 (2014). [DOI] [PubMed] [Google Scholar]
- Patiño M. N. et al. Caries, periodontal disease and tooth loss in patients with diabetes mellitus types 1 and 2. Acta odontologica latinoamericana: AOL 21, 127–133 (2007). [PubMed] [Google Scholar]
- Perez-Gallardo R. V. et al. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. J Bioenerg Biomembr 46, 511–518 (2014). [DOI] [PubMed] [Google Scholar]
- El-Sayed M. I. Effects of Portulaca oleracea L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy. J Ethnopharmacol 137, 643–651 (2011). [DOI] [PubMed] [Google Scholar]
- Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care 33, 442–449 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E. & Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annual review of immunology 27, 165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watala C. & Winocour P. The relationship of chemical modification of membrane proteins and plasma lipoproteins to reduced membrane fluidity of erythrocytes from diabetic subjects. Clinical Chemistry and Laboratory Medicine 30, 513–520 (1992). [DOI] [PubMed] [Google Scholar]
- Chang J. W. et al. C-reactive protein induces NF-kappaB activation through intracellular calcium and ROS in human mesangial cells. Nephron Exp Nephrol 101, e165–172 (2005). [DOI] [PubMed] [Google Scholar]
- Jialal I. & Devaraj S. The Role of C-Reactive Protein Activation of Nuclear Factor Kappa-B in the Pathogenesis of Unstable Angina*. Journal of the American College of Cardiology 49, 195–197 (2007). [DOI] [PubMed] [Google Scholar]
- Patel S. & Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep 61, 595–603 (2009). [DOI] [PubMed] [Google Scholar]
- Song W. & Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: relation to vessel size. Cardiovasc Diabetol 5, 3 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. W. et al. Role for Cysteine Protease Cathepsins in Heart Disease: Focus on Biology and Mechanisms With Clinical Implication. Circulation 125, 1551–1562 (2012). [DOI] [PubMed] [Google Scholar]
- Jobs E. et al. Serum cathepsin S is associated with decreased insulin sensitivity and the development of type 2 diabetes in a community-based cohort of elderly men. Diabetes Care 36, 163–165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru R. A., Zhong Q. & Santos J. M. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert Opin Investig Drugs 21, 797–805 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O. et al. Potential Role of Nuclear Factor κB in Diabetic Cardiomyopathy. Mediators of Inflammation 2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobs E. et al. Serum cathepsin S is associated with serum C-reactive protein and interleukin-6 independently of obesity in elderly men. J Clin Endocrinol Metab 95, 4460–4464 (2010). [DOI] [PubMed] [Google Scholar]
- Green C. J., Pedersen M., Pedersen B. K. & Scheele C. Elevated NF-κB Activation Is Conserved in Human Myocytes Cultured From Obese Type 2 Diabetic Patients and Attenuated by AMP-Activated Protein Kinase. Diabetes 60, 2810–2819 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. & Egan J. M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60, 470–512 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M., Robinson E., McDermott B. J. & Grieve D. J. Glucagon-like peptide-1 protects against cardiac dysfunction and extracellular matrix remodelling IN experimental diabetes. Heart 98, A1 (2012). [Google Scholar]
- Brooks N. et al. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci 4, 19–27 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan F. et al. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci Rep 6, 25139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbari A. et al. In vivo and in vitro evaluation of the effects of Urtica dioica and swimming activity on diabetic factors and pancreatic beta cells. BMC Complement Altern Med 16, 101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker N. & Debnath S. Impact of dehydration of purslane on retention of bioactive molecules and antioxidant activity. J Food Sci Technol 52, 6631–6638 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. et al. Fatty acids and beta-carotene in australian purslane (Portulaca oleracea) varieties. J Chromatogr A 893, 207–213 (2000). [DOI] [PubMed] [Google Scholar]
- Uddin M. K. et al. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. ScientificWorldJournal 2014, 951019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong F. et al. Hypoglycemic effects of crude polysaccharide from Purslane. International Journal of Molecular Sciences 10, 880–888 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdalla H. M. Jr. Purslane extract effects on obesity-induced diabetic rats fed a high-fat diet. Malays J Nutr 16, 419–429 (2010). [PubMed] [Google Scholar]
- Salehi A. & Farzanegi P. Effect of 8 weeks of resistance training with and without portulacalo seeds on some of liver injury markers in women with diabetes type 2. URMIA MEDICAL JOURNAL 25, 968–978 (2015). [Google Scholar]
- Fakoory Jouybari M., Farzanegi P. & A. B. The effect of 8-week aerobic exercise with purslane supplementation consumption on peroxidant and antioxidants indicators in women with type 2 diabetes. J Shahid Sadoughi Univ Med Sci 22, 928–939 (2014). [Google Scholar]
- Esmaillzadeh A., Zakizadeh E., Faghihimani E., Gohari M. & Jazayeri S. The effect of purslane seeds on glycemic status and lipid profiles of persons with type 2 diabetes: A randomized controlled cross-over clinical trial. J Res Med Sci 20, 47–53 (2015). [PMC free article] [PubMed] [Google Scholar]
- Ciolac E. G. & Physical G. G. exercise and metabolic syndrome. Rev Bras Med Esporte 10, 319–324 (2004). [Google Scholar]
- de Lemos E. T., Oliveira J., Pinheiro J. P. & Reis F. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid Med Cell Longev 2012, 741545 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabo B. E. & Terkildsen T. On the enzymatic determination of blood glucose. Scandinavian journal of clinical and laboratory investigation 12, 402–407 (1960). [DOI] [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I. & Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry 18, 499–502 (1972). [PubMed] [Google Scholar]
- Fossati P. & Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical chemistry 28, 2077–2080 (1982). [PubMed] [Google Scholar]
- Wong M. L. & Medrano J. F. Real-time PCR for mRNA quantitation. Biotechniques 39, 75 (2005). [DOI] [PubMed] [Google Scholar]
- Li W., Zheng H., Bukuru J. & De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. Journal of Ethnopharmacology 92, 1–21 (2004). [DOI] [PubMed] [Google Scholar]
- Ivorra M., Paya M. & Villar A. A review of natural products and plants as potential antidiabetic drugs. Journal of Ethnopharmacology 27, 243–275 (1989). [DOI] [PubMed] [Google Scholar]
- Chien K.-L., Chen M.-F., Hsu H.-C., Su T.-C. & Lee Y.-T. Sports activity and risk of type 2 diabetes in Chinese. Diabetes Research and Clinical Practice 84, 311–318 (2009). [DOI] [PubMed] [Google Scholar]
- Gordon L. A. et al. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Altern Med 8, 21 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashino Y., Jackson J. L., Fukumori N., Nakamura F. & Fukuhara S. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Diabetes Research and Clinical Practice 98, 349–360 (2012). [DOI] [PubMed] [Google Scholar]
- Miyatake N. et al. Daily walking reduces visceral adipose tissue areas and improves insulin resistance in Japanese obese subjects. Diabetes Res Clin Pract 58, 101–107 (2002). [DOI] [PubMed] [Google Scholar]
- Ross R. et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 133, 92–103 (2000). [DOI] [PubMed] [Google Scholar]
- Saremi A., Mosleh abadi M. & Parastesh M. ffects of Twelve-week Strength Training on Serum Chemerin, TNF-α and CRP Level in Subjects with the Metabolic Syndrome. Iranian Journal of Endocrinology and Metabolism 12, 536–543 (2011). [Google Scholar]
- Davey Smith G. et al. Incidence of type 2 diabetes in the randomized multiple risk factor intervention trial. Ann Intern Med 142, 313–322 (2005). [DOI] [PubMed] [Google Scholar]
- Bo S., Cavallo-Perin P., Gentile L., Repetti E. & Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest 31, 318–321 (2001). [DOI] [PubMed] [Google Scholar]
- Lee A. S. et al. An aqueous extract of Portulaca oleracea ameliorates diabetic nephropathy through suppression of renal fibrosis and inflammation in diabetic db/db mice. Am J Chin Med 40, 495–510 (2012). [DOI] [PubMed] [Google Scholar]
- Farzanegi P., Pour Amin Z. & Habibian M. Changes of Liver Trans-Aminases after a Period of Selected Aerobic Training in Postmenopausal Women. Medical Labrotoary Journal 8, 22–28 (2014). [Google Scholar]
- Sousa M. S. et al. Resistance Training in Type 2 Diabetic Patients Improves Uric Acid levels. J Hum Kinet 43, 17–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine S. C. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc 38, 1950–1957 (2006). [DOI] [PubMed] [Google Scholar]
- Sriwijitkamol A. et al. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes 55, 760–767 (2006). [DOI] [PubMed] [Google Scholar]
- Tantiwong P. et al. NF-kappaB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab 299, E794–801 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijeh N. & Farahati S. The Effect of Six Months of Aerobic training on Renal Function Markers in Untrained Middle-Aged Women. Int J Sports Studies 3, 218–224 (2013). [Google Scholar]
- Nestel P. J. Fish oil and cardiovascular disease: lipids and arterial function. The American Journal of Clinical Nutrition 71, 228S–231S (2000). [DOI] [PubMed] [Google Scholar]
- Oh J. Y. Serum cystatin C as a biomarker for predicting coronary artery disease in diabetes. Korean Diabetes J 34, 84–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. et al. Chronic dietary n-3 PUFA intervention improves dyslipidaemia and subsequent cardiovascular complications in the JCR:LA-cp rat model of the metabolic syndrome. British Journal of Nutrition 105, 1572–1582 (2011). [DOI] [PubMed] [Google Scholar]
- Samimi M., Jamilian M., Asemi Z. & Esmaillzadeh A. Effects of omega-3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Clin Nutr 34, 388–393 (2015). [DOI] [PubMed] [Google Scholar]
- Ostlund R. E. Jr. Phytosterols and cholesterol metabolism. Curr Opin Lipidol 15, 37–41 (2004). [DOI] [PubMed] [Google Scholar]
- Abumweis S. S., Barake R. & Jones P. J. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr Res 52 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef M. A. & Garg M. L. The lipid-lowering effects of phytosterols and (n-3) polyunsaturated fatty acids are synergistic and complementary in hyperlipidemic men and women. J Nutr 138, 1086–1090 (2008). [DOI] [PubMed] [Google Scholar]
- Lyngdoh T. et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One 6, e19901 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M. G., Kullberg B. J., Blok W. L., Netea R. T. & van der Meer J. W. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood 89, 577–582 (1997). [PubMed] [Google Scholar]
- Zapolski T. et al. Uric acid as a link between renal dysfunction and both pro-inflammatory and prothrombotic state in patients with metabolic syndrome and coronary artery disease. Kardiol Pol 69, 319–326 (2011). [PubMed] [Google Scholar]
- Cheung B. M. & Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep 14, 160–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbidi S. & Laher I. Exercise Induced Adipokine Changes and the Metabolic Syndrome. Journal of Diabetes Research 2014, 16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellenger J. et al. High Pancreatic n-3 Fatty Acids Prevent STZ-Induced Diabetes in Fat-1 Mice: Inflammatory Pathway Inhibition. Diabetes 60, 1090–1099 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekshahi Moghadam A. et al. Efficacy of omega-3 fatty acid supplementation on serum levels of tumour necrosis factor-alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singapore Med J 53, 615–619 (2012). [PubMed] [Google Scholar]
- Castaneda C. et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 43, 607–616 (2004). [DOI] [PubMed] [Google Scholar]
- Roberts C. K., Won D., Pruthi S., Lin S. S. & Barnard R. J. Effect of a diet and exercise intervention on oxidative stress, inflammation and monocyte adhesion in diabetic men. Diabetes Res Clin Pract 73, 249–259 (2006). [DOI] [PubMed] [Google Scholar]
- Ho R. C. et al. Regulation of IkappaB kinase and NF-kappaB in contracting adult rat skeletal muscle. Am J Physiol Cell Physiol 289, C794–801 (2005). [DOI] [PubMed] [Google Scholar]
- Durham W. J. et al. Fatiguing exercise reduces DNA binding activity of NF-kappaB in skeletal muscle nuclei. J Appl Physiol (1985) 97, 1740–1745 (2004). [DOI] [PubMed] [Google Scholar]
- Newby A. C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 28, 2108–2114 (2008). [DOI] [PubMed] [Google Scholar]
- Lewandowski K. C., Banach E., Bienkiewicz M. & Lewinski A. Matrix metalloproteinases in type 2 diabetes and non-diabetic controls: effects of short-term and chronic hyperglycaemia. Arch Med Sci 7, 294–303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa G. et al. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab 33, 129–134 (2007). [DOI] [PubMed] [Google Scholar]
- Signorelli S. S. et al. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc Med 10, 1–6 (2005). [DOI] [PubMed] [Google Scholar]
- Donley D. A. et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985) 116, 1396–1404 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S. et al. Effect of exercise training of different intensities on anti-inflammatory reaction in streptozotocin-induced diabetic rats. Biol Sport 31, 73–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto L., Marracci G., Bumgarner L. & Yadav V. The Effects of Omega-3 Fatty Acids on Matrix Metalloproteinase-9 Production and Cell Migration in Human Immune Cells: Implications for Multiple Sclerosis. Autoimmune Diseases 2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. A. et al. Effects of conjugated linoleic acids and docosahexaenoic acid on rat liver and reproductive tissue fatty acids, prostaglandins and matrix metalloproteinase production. Prostaglandins Leukot Essent Fatty Acids 65, 23–29 (2001). [DOI] [PubMed] [Google Scholar]
- Gupta S., Singh R. K., Dastidar S. & Ray A. Cysteine cathepsin S as an immunomodulatory target: present and future trends. Expert Opin Ther Targets 12, 291–299 (2008). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis 186, 411–419 (2006). [DOI] [PubMed] [Google Scholar]
- Naour N. et al. Cathepsins in human obesity: changes in energy balance predominantly affect cathepsin s in adipose tissue and in circulation. J Clin Endocrinol Metab 95, 1861–1868 (2010). [DOI] [PubMed] [Google Scholar]
- Taleb S. et al. Weight loss reduces adipose tissue cathepsin S and its circulating levels in morbidly obese women. J Clin Endocrinol Metab 91, 1042–1047 (2006). [DOI] [PubMed] [Google Scholar]
- Taleb S. et al. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. FASEB J 19, 1540–1542 (2005). [DOI] [PubMed] [Google Scholar]
- Jobs E. et al. Influence of a prudent diet on circulating cathepsin S in humans. Nutr J 13, 84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farilla L. et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143, 4397–4408 (2002). [DOI] [PubMed] [Google Scholar]
- Coughlan K. A., Valentine R. J., Ruderman N. B. & Saha A. K. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7, 241–253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati-Baroni G. et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 31, 1285–1297 (2011). [DOI] [PubMed] [Google Scholar]
- Viollet B. et al. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front Biosci (Landmark Ed) 14, 3380–3400 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Hyttinen J. M. & Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 89, 667–676 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M. G., McFarlin B. K. & Markofski M. M. The Anti-Inflammatory Actions of Exercise Training. Am J Lifestyle Med 1, 220–235 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze S. M., Hemmings B. A., Niessen M. & Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev Mol Med 14, e1 (2012). [DOI] [PubMed] [Google Scholar]
- Katerelos M. et al. 5-aminoimidazole-4-carboxamide ribonucleoside and AMP-activated protein kinase inhibit signalling through NF-kappaB. Immunol Cell Biol 88, 754–760 (2010). [DOI] [PubMed] [Google Scholar]
- Huang N. L. et al. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol 134, 169–175 (2009). [DOI] [PubMed] [Google Scholar]
- Higuchi T., Shirai N., Saito M., Suzuki H. & Kagawa Y. Levels of plasma insulin, leptin and adiponectin, and activities of key enzymes in carbohydrate metabolism in skeletal muscle and liver in fasted ICR mice fed dietary n-3 polyunsaturated fatty acids. The Journal of Nutritional Biochemistry 19, 577–586 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.