Abstract
The usage of fixed dose combination (FDC) tablets of Lamivudine and Tenofovir Disoproxil Fumarate (TDF) is increasing due to increased incidences of HIV/Hepatitis B and HIV/TB co-infections. This is likely to increase the financial crisis due to limited resources for funding procurement of ready-made products from the pharmaceuticals manufacturing leading countries. Therefore, production of local oral tablets containing Lamivudine and TDF FDC is inevitable. Lamivudine 300 mg/TDF 300 mg tablets were developed and optimized by D-optimal mixture design and produced by direct compression technique. Twenty trial formulations with independent variables, including PVP-CL 1–12.00%, PVP-K30 1–10.00%, starch-1500 2.5–12.5% and Avicel-PH102 2–19.25% were prepared by direct compression technique. The formulations were assessed on assay, dissolution, friability, weight variation and disintegration time. It was found that assay ranged from 98.13–101.95% for Lamivudine, 98.25–102.84 for TDF, both were within the in-house assay specification of 95 to 105%. Dissolution at single point was above 80% for Lamivudine 93.96–100.55% and 95.85–103.15% for TDF, disintegration time was between 1.92–66.33 min and friability 0.06–12.56%. Out of twenty formulation trials, eight formulations had all parameters in proven acceptable range. On optimization, one formulation with independent variables, PVP-CL 5.67%, PVP-K30 1.00%, Starch-1500 5.76% was selected. The optimized formulation was comparable to the reference product on the market with similarity factor (f2) and difference factor (f1) within the acceptable range for both Lamivudine and TDF.
Keywords: Chemistry, Pharmaceutical chemistry
1. Introduction
Tuberculosis (TB) is a bacterial infectious disease caused by Mycobacterium tuberculosis. Whereas the Acquired Immunodeficiency Syndrome (AIDS) is an epidemic condition due to the viral manifestation of Human Immunodeficiency Virus (HIV). The HIV/TB co-infection burden is profoundly high in sub-saharan Africa and the concern for HIV/TB have been reported to grow in Asia [1, 2]. The levels of multi-drug resistant TB is reported to increase in Africa and other parts of the world [3, 4].
The two epidemics have been found to concurrently appear in patients due to the fact that HIV attacks the CD4 cells of the hosting human and reduces immunity of the host, whereas TB normally attacks an individual with the compromised immunity, therefore HIV provides an avenue for TB infection in the host, hence the appearance of the co-infection in the patient [5, 6]. Therefore, scientists are faced with the challenge to prevent TB and HIV simultaneously and concomitantly improve diagnosis and management of the co-infection [7, 8]. Hence a suitable FDC formulation devoid of nevirapine is required to be developed and made available for the TB and HIV co-infected individuals [9, 10, 11].
Lamivudine-300 mg/Tenofivir DF-300 mg FDC is among the recommended WHO regimens for management of TB and HIV co-infections. Highly Active Anti-Retroviral Therapy (HAART) regimen may comprise of one or two Nucleoside Reverse Transcriptase Inhibitors (NRTI), one Nucleotide Reverse Transcriptase Inhibitor (NtRTI) and one Non Nucleoside Reverse Transcriptase Inhibitor (NNRTI) or protease inhibitor. Regimens devoid of Nevirapine or any antiretroviral interacting with anti-TB drugs should be used in HIV/TB co-infected patients as Nevirapine interacts with Rifampicin (anti-TB drug). The regimens comprising of Lamivudine (3TC) and Tenofovir DF are also of choice in HIV/Hepatitis B co-infected patients as 3TC and TDF are effective against both HIV and Hepatitis B and therefore should be used in combination as part of first line treatment in Hepatitis B/HIV co-infection [12].
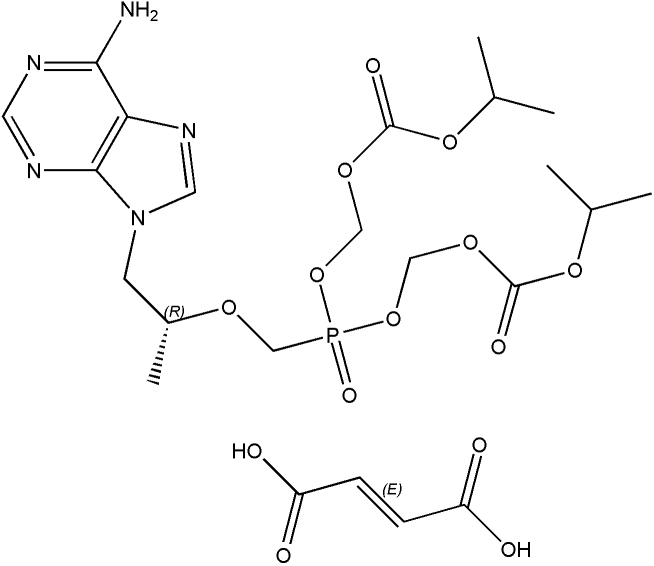
Lamivudine as described in Fig. 1 is a (2R, cis)-4-amino-1-(2-hydroxymethyl-1, 3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (−) enantiomer of a dideoxy analogue of cytidine. Also it has been referred to as (−) 2′, 3′-dideoxy, 3′-thiacytidine. Lamivudine (3TC) is a white to off-white crystalline solid with a solubility of approximately 70 mg/mL in water at 20 °C and has a molecular formula of C8H11N3O3S and a molecular weight of 229.3 g/mole [13].
Fig. 1.
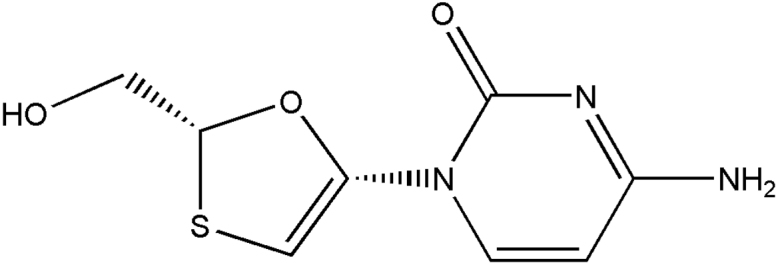
Chemical Structure of Lamivudine.
The drug substance, tenofovir is poorly absorbed from the small intestine due to its highly polarized form and hence reduced lipophilicity. The diester form of the drug Tenofovir disoproxil fumarate (TDF) is a salt of an oral prodrug of tenofovir with increased lipophilicity and hence improved oral bioavailability of the parent compound [14]. The chemical structure of tenofovir DF has been presented in Fig. 2 of this study.
Fig. 2.
Chemical Structure of Tenofovir Disoproxil fumarate.
2. Materials, instruments and method
2.1. Reagents and chemicals
Analytical grade solvents were used for analytical requirements. Methanol from Scharlab S.L, Sentmenat, Spain and Techno Pharmchem Bahadurggarh, Haryana, India. Whereas acetonitrile and glacial acetic acid were made from Scharlab S.L, Sentmenat, Spain. Ethyl acetate was made from Techno Pharmchem Bahadurggarh, Haryana, India and Fisher Scientific, Leicestershire, UK. Distilled water was in-house prepared at MUHAS Pharmaceutical Research and Development Laboratory (MUHAS Pharm R&D Lab), Tanzania by reverse osmosis using RO- Purification System equipment made from Millipore® France. Acetone, diaminoethane, ammonium hydroxide and triethyl amine were made from Fisher Scientific, Leicestershire, UK. Excipients included microcrystalline cellulose made from FMC BioPolymer, Philadelphia, sodium carboxymethyl cellulose and polyvinylpyrrolidone cross-linked (PVP CL) made from Associate Co. Ltd, Shenzhen, China and magnesium stearate was made from Shandong Liaocheng Ehua Medicine Co. Ltd, China. Lamivudine and tenofovir disoproxil fumarate were manufactured and supplied by Desano Chemical Pharmaceutical Co. LTD, Shanghai-China.
2.2. Instruments
The instruments that were used included a densitometer with TLC scanner 3 operated by Wincats (version 1.4.3) planar chromatograph software as data manager and integrator, Linomat 5, a semi automatic applicator with Hamilton syringe of 100 μL capacity for sample application and CAMAG developing tank single rectangular with internal dimensions (21.6 × 11.2 × 6) cm all were made from CAMAG, Muntez, Switzerland. The TLC (5 × 10) cm, HPTLC (10 × 10) and (20 × 10) cm plate pre coated with silica gel 60 F 254 were made from Merck, Darmstadt, Germany and a fluid bed drier that was made from GEA Niro pharma system, Bubendorf, Switzerland. A tablet press, EKO1 made in Germany and turbular mixer made in Switzerland.
2.3. Procedure
The study focused on galenical activities which included the formulation development and optimization by the aid of benchmarking experiments developed by design expert version 7.0 and version 10.0 software. The formulated batches were analyzed by using in-house validated HPLC and HPTLC analytical methods [15, 16], disintegration, friability and weight variation were done as according to pharmacopoeial recommendations.
3. Results and discussions
3.1. Formulation development
For the purpose of this formulation the particle size distribution for Lamivudine and Tenofovir Disoproxil Fumarate (TDF) was set at D90 > 250 μm to prevent the possibility of capping, picking and sticking of formulation during direct compression process. A complete list of all excipients used in the formulation and their rationale has been summarized in Table 1. The excipients were the common ones and most of them were utilized by the innovator. The percentage composition of each ingredient was proposed by the design expert software version 7.0 and presented in Table 2. Different formulation trials were randomized and presented in Table 3, the randomization was provided by the design expert software version 7.0. Lower and upper limits of pharmaceutical formulation excipients were proposed based on literature search and provided as design constraints in Table 4. Independent variables (PVP K30, PVP CL, Starch and Avicel PH 102) and dependent variables (friability and disintegration) were pointed out and presented in Table 5 due to the fact that for a direct compression a balance for having hard tablets with good friability and sufficient good disintegration is of great importance and poses a significant challenge for formulation development and optimization.
Table 1.
Proposed formulation included the following ingredients.
| SN | Ingredient | Function |
|---|---|---|
| 1 | Lamivudine | Active ingredient |
| 2 | Tenofovir Disoproxil Fumarate (TDF) | Active ingredient |
| 3 | Polyvinylpyrrolidone (PVP) K30 | Binder |
| 4 | Polyvinylpyrrolidone Cross Linked (PVP CL) | Disintegrant |
| 5 | Starch | Binder/disintegrant |
| 6 | Avicel PH 102 | Filler |
| 7 | Talc | Flowability enhancer, Glidant |
| 8 | Magnesium stearate | Lubricant |
Table 2.
Percentage composition of excipients in different formulation trials.
| Component 1 |
Component 2 |
Component 3 |
Component 4 |
Constant 1 |
Constant 2 |
|
|---|---|---|---|---|---|---|
| F | A:PVP CL % | B:PVP K30 % | C:Starch 1500 (%) | D:Avicel PH102 (%) | Talc (%) | MgSt (%) |
| 1 | 1.0 | 1.0 | 12.5 | 9.2 | 0.5 | 0.75 |
| 2 | 1.0 | 9.8 | 10.9 | 2.0 | 0.5 | 0.75 |
| 3 | 1.0 | 1.0 | 2.5 | 19.2 | 0.5 | 0.75 |
| 4 | 1.0 | 10.0 | 2.6 | 10.1 | 0.5 | 0.75 |
| 5 | 12.0 | 5.5 | 2.9 | 3.3 | 0.5 | 0.75 |
| 6 | 6.2 | 10.0 | 4.3 | 3.2 | 0.5 | 0.75 |
| 7 | 4.2 | 2.6 | 12.5 | 4.5 | 0.5 | 0.75 |
| 8 | 10.9 | 1.0 | 2.5 | 9.3 | 0.5 | 0.75 |
| 9 | 12.0 | 1.1 | 8.4 | 2.2 | 0.5 | 0.75 |
| 10 | 1.0 | 4.9 | 7.2 | 10.7 | 0.5 | 0.75 |
| 11 | 6.2 | 5.8 | 3.3 | 8.5 | 0.5 | 0.75 |
| 12 | 4.9 | 1.8 | 3.3 | 13.8 | 0.5 | 0.75 |
| 13 | 8.1 | 2.1 | 7.1 | 6.5 | 0.5 | 0.75 |
| 14 | 4.4 | 1.0 | 8.4 | 9.9 | 0.5 | 0.75 |
| 15 | 12.0 | 1.3 | 5.1 | 5.4 | 0.5 | 0.75 |
| 16 | 1.0 | 9.8 | 10.9 | 2.0 | 0.5 | 0.75 |
| 17 | 1.0 | 10.0 | 2.6 | 10.1 | 0.5 | 0.75 |
| 18 | 1.0 | 1.0 | 2.5 | 19.2 | 0.5 | 0.75 |
| 19 | 1.0 | 1.0 | 12.5 | 9.2 | 0.5 | 0.75 |
| 20 | 6.2 | 10.0 | 4.3 | 3.2 | 0.5 | 0.75 |
Table 3.
Formulation trials with their results.
| Formulation | Weight variation (% rsd) | Friability (%) | Disintegration (min) | Dissolution % at 30 min |
Assay % |
||
|---|---|---|---|---|---|---|---|
| L | T | L | T | ||||
| F-1 | 1.02 | 0.50 | 6.67 | 98.95 | 96.83 | 100.85 | 101.22 |
| F-2 | 1.55 | 0.06 | 40.83 | 93.96 | 95.85 | 98.23 | 99.27 |
| F-3 | 1.35 | 0.25 | 66.33 | 99.23 | 99.87 | 99.25 | 100.38 |
| F-4 | 1.71 | 0.12 | 38.42 | 98.50 | 102.84 | 98.88 | 99.85 |
| F-5 | 0.66 | 0.31 | 2.5 | 100.55 | 103.15 | 100.56 | 100.36 |
| F-6 | 2.27 | 0.25 | 10.32 | 99.58 | 101.20 | 100.35 | 99.50 |
| F-7 | 1.015 | 0.3 | 6.17 | 99.45 | 101.25 | 99.50 | 98.25 |
| F-8 | 2.55 | 0.24 | 8.5 | 100.16 | 99.86 | 99.58 | 100.16 |
| F-9 | 0.69 | 0.38 | 1.33 | 98.93 | 100.88 | 101.95 | 100.87 |
| F-10 | 0.82 | 0.37 | 20.87 | 99.97 | 98.99 | 98.50 | 99.87 |
| F-11 | 1.87 | 0.19 | 1.92 | 98.88 | 99.98 | 97.96 | 98.87 |
| F-12 | 1.18 | 0.29 | 8 | 97.75 | 98.89 | 98.13 | 98.55 |
| F-13 | 1.61 | 12.56 | 5.16 | 99.26 | 99.97 | 98.98 | 99.89 |
| F-14 | 1.71 | 0.19 | 4.85 | 99.98 | 100.35 | 99.87 | 101.74 |
| F-15 | 1.01 | 0.30 | 8.33 | 98.88 | 101.35 | 97.93 | 99.57 |
| F-16 | 1.50 | 0.30 | 42.5 | 98.88 | 98.98 | 99.96 | 98.87 |
| F-17 | 2.00 | 0.14 | 37.75 | 99.95 | 98.89 | 98.13 | 98.55 |
| F-18 | 1.03 | 0.18 | 38.42 | 98.26 | 97.97 | 99.98 | 99.99 |
| F-19 | 1.29 | 0.48 | 2.56 | 99.85 | 100.95 | 100.97 | 102.84 |
| F-20 | 2.04 | 0.25 | 10.2 | 99.89 | 102.35 | 100.83 | 99.87 |
Table 4.
Design constraints in formulation development and optimization of LT tablets.
| Low≤ | Constraint | ≤High |
|---|---|---|
| 1.00≤ | A:PVP CL | ≤12.00 |
| 1.00≤ | B:PVP K30 | ≤10.00 |
| 2.50≤ | C:Starch 1500 | ≤12.50 |
| 2.00≤ | D:Avicel PH102 | ≤19.25 |
| A + B + C + D | =23.75 |
Table 5.
Independent and dependent variables for formulation development and optimization.
| Component 1 |
Component 2 |
Component 3 |
Component 4 |
Response 1 |
Response 2 |
||
|---|---|---|---|---|---|---|---|
| Std | Run | A:PVP CL % | B:PVP K30 % | C:Starch 1500 (%) | D:Avicel PH102 (%) | Disintegration time (Min) | Friability (%) |
| 13 | 1 | 8.1 | 2.1 | 7.1 | 6.5 | 5.16 | 12.56 |
| 2 | 2 | 1.0 | 9.8 | 10.9 | 2.0 | 40.83 | 0.06 |
| 3 | 3 | 1.0 | 1.0 | 2.5 | 19.2 | 66.33 | 0.25 |
| 11 | 4 | 6.2 | 5.8 | 3.3 | 8.5 | 1.92 | 0.19 |
| 14 | 5 | 4.4 | 1.0 | 8.4 | 9.9 | 4.85 | 0.19 |
| 7 | 6 | 4.2 | 2.6 | 12.5 | 4.5 | 6.17 | 0.3 |
| 18 | 7 | 1.0 | 1.0 | 2.5 | 19.2 | 38.42 | 0.18 |
| 9 | 8 | 12.0 | 1.1 | 8.4 | 2.2 | 1.33 | 0.38 |
| 4 | 9 | 1.0 | 10.0 | 2.6 | 10.1 | 38.42 | 0.12 |
| 17 | 10 | 1.0 | 10.0 | 2.6 | 10.1 | 37.75 | 0.14 |
| 19 | 11 | 1.0 | 1.0 | 12.5 | 9.2 | 2.56 | 0.48 |
| 16 | 12 | 1.0 | 9.8 | 10.9 | 2.0 | 42.5 | 0.30 |
| 10 | 13 | 1.0 | 4.9 | 7.2 | 10.7 | 20.87 | 0.37 |
| 1 | 14 | 1.0 | 1.0 | 12.5 | 9.2 | 6.67 | 0.50 |
| 6 | 15 | 6.2 | 10.0 | 4.3 | 3.2 | 10.32 | 0.25 |
| 20 | 16 | 6.2 | 10.0 | 4.3 | 3.2 | 10.2 | 0.25 |
| 5 | 17 | 12.0 | 5.5 | 2.9 | 3.3 | 2.5 | 0.31 |
| 12 | 18 | 4.9 | 1.8 | 3.3 | 13.8 | 8.0 | 0.29 |
| 8 | 19 | 10.9 | 1.0 | 2.5 | 9.3 | 8.5 | 0.24 |
| 15 | 20 | 12.0 | 1.3 | 5.1 | 5.4 | 8.33 | 0.30 |
In order to obtain the best proportions of the ingredients the design expert version 7 software, D-Optimal design was used to generate and evaluate the trial batches. In this study the experimental range w/w% per tablet laid between 1% to 23.8% equivalents to 8 mg to 190.4 mg per tablet.
3.1.1. Friability
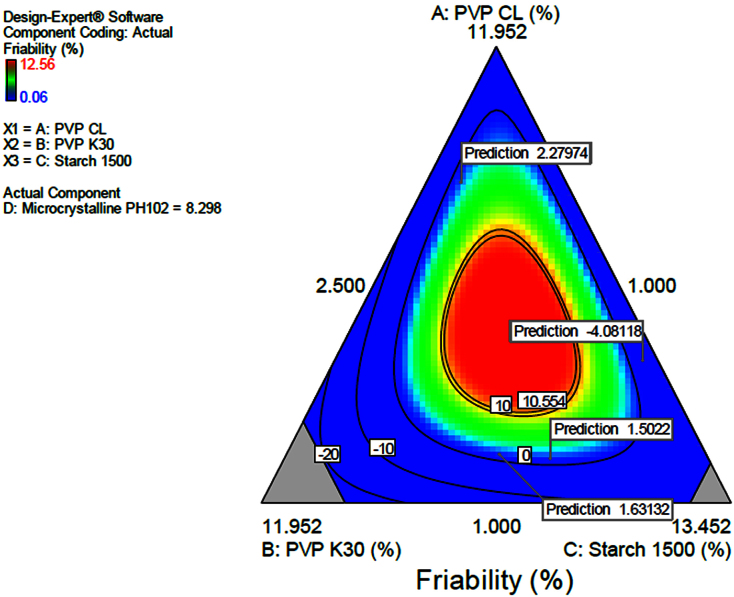
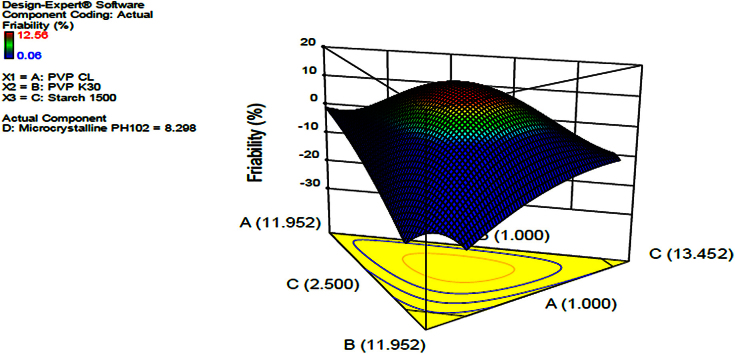
It was found that the higher the amount of microcrystalline cellulose the less friable the tablets become and increased disintegration time is noted, this could probably result from an increased hardness. Also stipulated that the increase in binder resulted in increased disintegration time and decreased friability. The contour diagram provided in Fig. 3 indicating the optimization of the formulation and the possible predicted values for friability from the model. The same was applied in Fig. 4 in which a 3-Dimension Figure showing the model with the possible predictions for friability in line with the levels of each component in the formulation was presented. The interrelationship between the components was generally summarized and given by the equation in Table 6. The provided equation in terms of actual components could be used to make predictions of the responses for the given level of each component. In this case, the levels of each component were to be specified in the original units for each component. The provided equation could not be used to determine the relative impact of each component due to the fact that the coefficients were scaled to accommodate the units of each component and the intercept was not at the centre of the design space. Friability was given by an equation in Table 6. The equation can be used to predict friability data.
Fig. 3.
Contour diagram indicating optimization of dependent variables and prediction of friability.
Fig. 4.
3D surface response diagram indicating the effect of independent variables, PVP CL, PVP K30 and starch on friability.
Table 6.
Equation for friability in terms of actual components.
| Final Equation in Terms of Actual Components: | |
|---|---|
| Friability | = |
| +1.47627 | * PVP CL |
| -22.66508 | * PVP K30 |
| -10.78748 | * Starch 1500 |
| -3.31625 | * Microcrystalline PH102 |
| -0.31144 | * PVP CL * PVP K30 |
| +0.17053 | * PVP CL * Starch 1500 |
| +0.22057 | * PVP CL * Microcrystalline PH102 |
| +2.65500 | * PVP K30 * Starch 1500 |
| +2.67523 | * PVP K30 * Microcrystalline PH102 |
| +1.47956 | * Starch 1500 * Microcrystalline PH102 |
| +0.52335 | * PVP CL * PVP K30 * Starch 1500 |
| -0.10127 | * PVP CL * PVP K30 * Microcrystalline PH102 |
| -0.090103 | * PVP CL * Starch 1500 * Microcrystalline PH102 |
| -0.35247 | * PVP K30 * Starch 1500 * Microcrystalline PH102 |
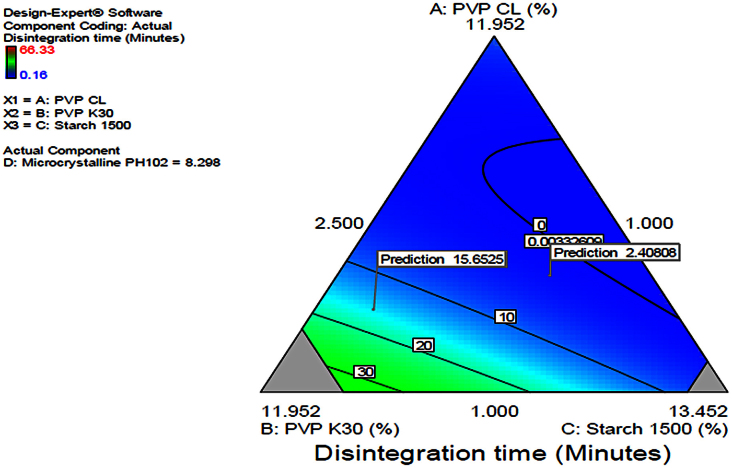
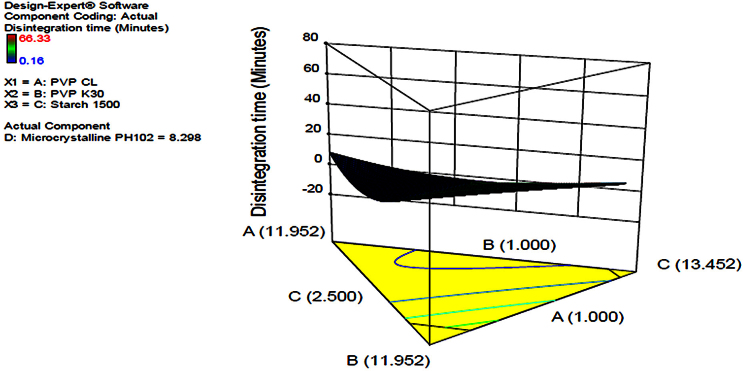
3.1.2. Disintegration time
Disintegration time was studied as it is potential for the quality of drug and prone to be affected as the formulator strives to achieve good hardness and good friability of the solid dosage form. In this case, the more the amount of super disintegrant, PVP CL, the reduced the disintegration time of the dosage form [17, 18]. This was in line with the theoretical knowledge that the super disintegrants in a pharmaceutical formulation are intended to enhance disintegration of the dosage form in an appropriate medium. The contour diagram provided in Fig. 5 indicating the optimization of the formulation and the possible predicted values for disintegration time from the model. The same was applied in Fig. 6 in which a 3-Dimension figure showing the model with the possible predictions for disintegration time in line with the levels of each component in the formulation was presented. The interrelationships between the components were generally summarized and given by the equation in Table 7. The equation in terms of actual components could be used to make predictions about the response for given levels of each component. In this case, the levels of each component were to be specified in the original units for each component. The provided equation could not be used to determine the relative impact of each component due to the fact that the coefficients were scaled to accommodate the units of each component and the intercept was not at the centre of the design space.
Fig. 5.
Contour diagram indicating optimization of the independent variables and prediction of disintegration time.
Fig. 6.
3D diagram indicating optimization of independent variables and prediction of disintegration time.
Table 7.
Equation for disintegration time in terms of actual components.
| Final Equation in Terms of Actual Components: | |
|---|---|
| Disintegration time | = |
| +3.85225 | * PVP CL |
| +4.88321 | * PVP K30 |
| +1.02884 | * Starch 1500 |
| +3.77274 | * Microcrystalline PH102 |
| -0.70695 | * PVP CL * PVP K30 |
| -0.31655 | * PVP CL * Starch 1500 |
| -0.49076 | * PVP CL * Microcrystalline PH102 |
| -0.033147 | * PVP K30 * Starch 1500 |
| -0.33317 | * PVP K30 * Microcrystalline PH102 |
| -0.32293 | * Starch 1500 * Microcrystalline PH102 |
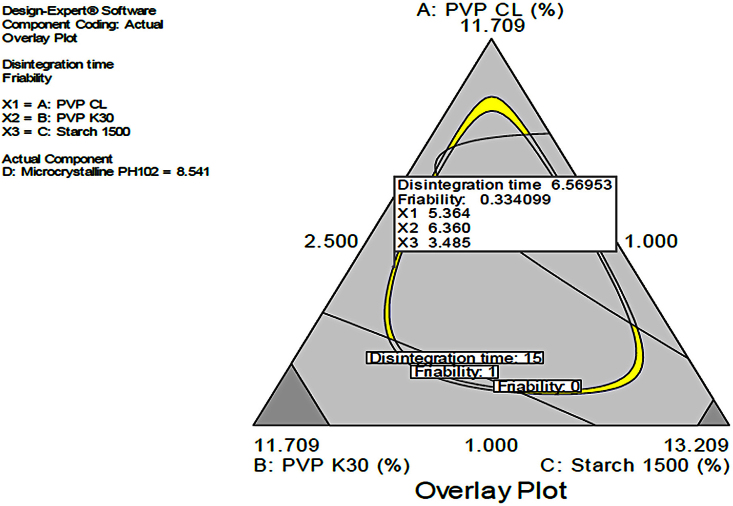
3.1.3. Combined disintegration time and friability
In this scenario the combined effects of PVP K30, Starch 1500 and PVP CL were studied to provide a design space with dependent variables within the acceptable range i.e Disintegration <15 min and Friability <1%. The yellow region in Fig. 7 indicated the acceptable design space. The design space was taken for further studies to generate predicted values for disintegration and friability as shown in Table 8. The design space was confirmed by testing the predicted values of friability and disintegration. It was generally inferred that the more the binder, PVP K30 the lower the friability and the higher the disintegration time if at allow the PVP CL is insignificantly altered in the formulation.
Fig. 7.
Effects of combined independent variables on simultaneous prediction of disintegration time and friability.
Table 8.
Predictions from the models and actual results.
| Predicted |
Actual |
|||||||
|---|---|---|---|---|---|---|---|---|
| S/N | PVP CL | PVP K30 | Starch 1500 | Avicel PH102 | Disintegration time (Min) | Friability (%) | Disintegration time (Min) | Friability (%) |
| 1* | 5.67 | 1.00 | 5.76 | 11.32 | 4.49 | 0.33 | 5.42 | 0.3 |
| 2 | 11.58 | 1.15 | 7.85 | 3.17 | 4.49 | 0.33 | 5.50 | 0.28 |
| 3 | 7.89 | 2.18 | 3.19 | 10.49 | 4.49 | 0.33 | 5.52 | 0.31 |
| 4 | 1.75 | 1.00 | 11.65 | 9.35 | 4.49 | 0.33 | 6.20 | 0.3 |
| 5 | 3.35 | 2.99 | 12.48 | 4.94 | 4.49 | 0.33 | 5.42 | 0.3 |
| 6 | 2.89 | 2.88 | 10.91 | 7.07 | 4.49 | 0.33 | 5.30 | 0.28 |
| 7 | 12.00 | 1.13 | 5.29 | 5.32 | 4.91 | 0.33 | 5.52 | 0.31 |
| 8 | 12.00 | 1.56 | 2.97 | 7.22 | 6.27 | 0.33 | 7.20 | 0.3 |
| 9 | 6.39 | 3.95 | 2.50 | 10.92 | 7.04 | 0.33 | 7.42 | 0.3 |
| 10 | 5.46 | 8.97 | 4.42 | 4.91 | 9.51 | 0.33 | 10.00 | 0.4 |
| 11 | 12.00 | 6.76 | 2.99 | 2.00 | 2.27 | 0.33 | 3.30 | 0.31 |
| 12 | 1.00 | 9.78 | 10.97 | 2.00 | 41.90 | 0.33 | 42.20 | 0.25 |
This batch was taken for further studies including comparative dissolution at three different pH.
3.1.4. Comparative dissolution
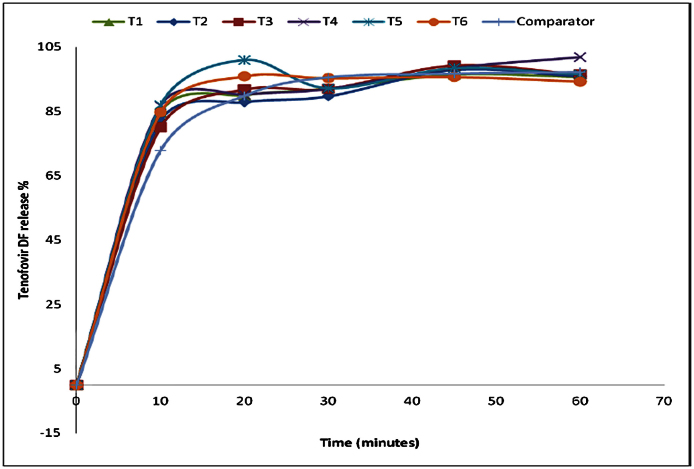
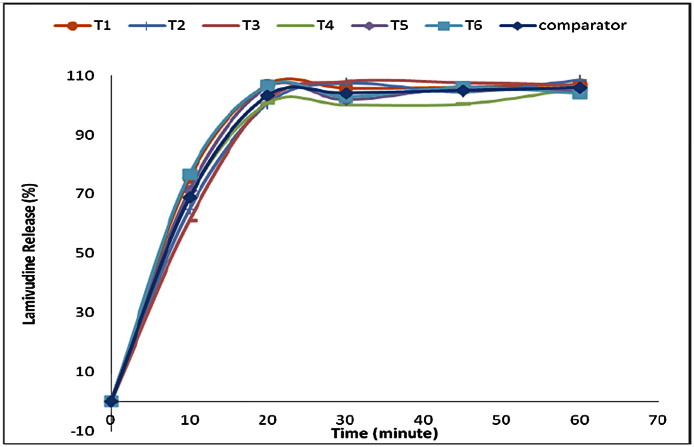
The selected formulation was compared to the reference product. In this case Lamivudine 300 mg/TDF 300 mg/Efavirenz 600 mg fixed dose combination tablets produced by Mylan Laboratories in India with batch no. 3035127, manufactured in Jan 2015 and expired in 2016 was used as a comparator product as it was the available product containing Lamividine and Tenofovir in a fixed combination available and registered by the local authority, Tanzania Food and Drug Authority (TFDA). The comparative dissolution study was conducted in three media named pH 1.2,4.5 and 6.8, at pH 6.8 phosphate buffer the dissolution profile indicating similar drug release for test and comparator product as in Fig. 8 for TDF and in Fig. 9 for lamivudine, analytical error was noted at time 30 min at which the TDF release dropped unexpectedly. At pH 4.5 acetate buffer with the dissolution profile indicating similar drug release for test and comparator product, whereas At pH 1.2 the dissolution profile indicated similar drug release for test and comparator products.
Fig. 8.
Comparison of Tenofovir DF release at pH 6.8 for Reference and test product.
Fig. 9.
Comparison of lamivudine release at pH 6.8 for reference and test product.
In reference to Table 9, the similarity factor (f2) was found to fall between 51 and 88 implying that the reference and test product were similar at the prescribed test conditions as since the acceptable similarity range of FDA is 50–100. The difference factor was between 1 and 7 where as the FDA acceptance criteria for the difference in the amount of drug dissolved falls between 0 and 15, hence acceptable. The developed LT formulation was found to be similar to the reference product on the market. In reference to Table 10, it was noted that the two products (reference and test product) had similar physical characteristics.
Table 9.
Similarity factor (f2) and difference factor (f1) consideration for comparison of reference product with test product.
| pH-1.2 |
pH-4.5 |
pH-6.8 |
||||
|---|---|---|---|---|---|---|
| L | T | L | T | L | T | |
| f1 | 3 | 1 | 7 | 4 | 6 | 4 |
| f2 | 69 | 88 | 51 | 63 | 54 | 60 |
Table 10.
Summary on comparison of other parameters excluding dissolution profile between the comparator product and test product.
| s/n | Parameters | Reference product | Test product |
|---|---|---|---|
| 1 | Appearance | White film coated tablet | White uncoated tablet |
| 2 | Assay | L-101.15%, T-99.06% | L-102.03%, T-100.03% |
| 3 | Disintegration time | 19 Min | 5.42 Min |
| 4 | Friability | 0.01% | 0.3% |
4. Conclusion
The Lamivudine and Tenofovir Disoproxil Fumarate FDC tablets formulation was developed by using D-optimal design through quality testing assessment involved disintegration, friability, assay and dissolution. The best formulation out of the twenty prepared formulations was selected.
The best selected formulation with PVP-CL 5.67%, PVP-K30 1.00%, Starch-1500 5.76% and Avicel PH102 was comparable to the market product in terms of assay between 95 to 105% (In house specification) for both Lamivudine and Tenofovir Disoproxil Fumarate (TDF), drug release from the formulated product was similar to the comparator product in three different media i.e pH 1.2 (0.1 N HCl), pH 4.5 (Acetate buffer) and pH 6.8 (Phosphate buffer). This formulation can be adopted by local pharmaceutical Industries and manufactured for public consumption.
4.1. Recommendations
Further formulation development trials could be conducted by varying ratios of binders and disintegrants which were not tried in this study. The undertaking of the proposed trials could lead into other optimized drug formulations and hence providing alternative formulations to the proposed formulation in this study.
In any case, this formulation is to be adopted for mass production, then should be scaled up to not less than 10% of the commercial batch size prior to production of the bio-batch which should be subjected to stability testing.
Declarations
Author contribution statement
Prosper Tibalinda: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Joseph Sempombe, Raphael Shedafa, Nelson Masota, Dickson Pius, Mary Temu: Analyzed and interpreted the data.
Eliangiringa Kaale: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This study was supported by SIDA.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Corbett E.L., Watt C.J., Walker N., Maher D., Williams B.G., Raviglione M.C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Trinh Q.M., Nguyen H.L., Nguyen V.N., Nguyen T.V.A., Sintchenko V., Marais B.J. Tuberculosis and HIV co-infection-focus on the Asia-Pacific region. Int. J. Infect. Dis. 2015:170–178. doi: 10.1016/j.ijid.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Klopper M., Warren R.M., Hayes C., van Pittius N.C.G., Streicher E.M., Miller B. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg. Infect. Dis. 2013;19(3):449–455. doi: 10.3201//EID1903.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mungrue K., Beharry A., Kalloo J., Mahabir S., Maraj T., Ramoutar R. Trends in HIV/TB coinfection in Trinidad and Tobago for the period 1998-2007. J. Int. Assoc. Physicians AIDS Care (Chic) 2009;8(3):170–175. doi: 10.1177/1545109709331471. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S.K., Mohan A., Kadhiravan T. HIV-TB co-infection: Epidemiology, diagnosis & management. Indian J. Med. Res. 2005:550–567. [PubMed] [Google Scholar]
- 6.Alexander P.E., De P. The emergence of extensively drug-resistant tuberculosis (TB): TB/HIV coinfection, multidrug-resistant TB and the resulting public health threat from extensively drug-resistant TB, globally and in Canada. Can. J. Infect. Dis. Med. Microbiol. 2007:289–291. doi: 10.1155/2007/986794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chintu C. Tuberculosis and human immunodeficiency virus co-infection in children: management challenges. Paediatr. Respir. Rev. 2007:142–147. doi: 10.1016/j.prrv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Shipton L.K., Wester C.W., Stock S., Ndwapi N., Gaolathe T., Thior I. Safety and efficacy of nevirapine- and efavirenz-based antiretroviral treatment in adults treated for TB-HIV co-infection in Botswana. Int. J. Tuberc. Lung Dis. 2009;13(3):360–366. [PMC free article] [PubMed] [Google Scholar]
- 9.Manosuthi W., Tantanathip P., Chimsuntorn S., Eampokarap B., Thongyen S., Nilkamhang S. Treatment outcomes of patients co-infected with HIV and tuberculosis who received a nevirapine-based antiretroviral regimen: A four-year prospective study. Int. J. Infect. Dis. 2010;14(11) doi: 10.1016/j.ijid.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Cohen K., Meintjes G. Management of individuals requiring antiretroviral therapy and TB treatment. Curr. Opin. HIV AIDS. 2010;5(1):61–69. doi: 10.1097/COH.0b013e3283339309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uttayamakul S., Likanonsakul S., Manosuthi W., Wichukchinda N., Kalambaheti T., Nakayama E.E. Effects of CYP2B6 G516T polymorphisms on plasma efavirenz and nevirapine levels when co-administered with rifampicin in HIV/TB co-infected Thai adults. AIDS Res. Ther. 2010;7:8. doi: 10.1186/1742-6405-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann C., Wyen C., Fätkenheuer G. Rapid improvement of liver function in a patient with HIV and hepatitis B coinfection treated with lamivudine and tenofovir. Infection. 2006;34(4):234–235. doi: 10.1007/s15010-006-4145-5. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V.R., Rupa Y., Chaitanya M. Estimation of lamivudine in bulk and formulation by titrimetric and uv-visible spectrophotometry. 2014;4(1):75–79. [Google Scholar]
- 14.Grim S.A., Romanelli F. Tenofovir disoproxil fumarate. Ann. Pharmacother. 2003;37(6):849–859. doi: 10.1345/aph.1C388. [DOI] [PubMed] [Google Scholar]
- 15.Nyamweru B., Kaale E., Mugoyela V., Chambuso M. Development and validation of an HPTLC-densitometric method for simultaneous analysis of lamivudine, tenofovir disoproxil fumarate, and efavirenz (LTE) in tablets. J. Planar Chromatogr. - Mod. TLC. 2013;26(3):226–231. [Google Scholar]
- 16.Joseph B. Development and validation of a method for the assay and dissolution of a fixed dose combination of lamivudine tenofovir and efavirenz tablet. 2011. A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Pharmacy in Quality Control and Quality Assurance of Muhimbili University of Health and Allied Sciences. [Google Scholar]
- 17.Bhowmik D., Chiranjib B., Yadav R., Kumar K. Emerging Trends of Disintegrants used in Formulation of Solid Dosage Form. Sch. Res. Libr. 2010;2(1):495–504. [Google Scholar]
- 18.Hari Har Prasad M., Duraivel S. Effect of different binders and super disintegrants on formulation of glimepiride immediate release tablets by wet granulation method. Int. J. Pharm. Clin. Res. 2012;4(4):44–47. [Google Scholar]