Abstract
TRPA1 is a Ca2+-permeable cation channel that is activated by painful low temperatures (˂17 °C), irritating chemicals, reactive metabolites and mediators of inflammation. In the bladder TRPA1 is predominantly expressed in sensory afferent nerve endings, where it mediates sensory transduction. The contractile effect of its activation on detrusor smooth muscle (DSM) is explained by the release from sensory afferents of inflammatory factors – tachykinins and prostaglandins, which cause smooth muscle cell contraction. Diabetes is a systemic disease, with common complications being diabetic cystopathies and urinary incontinence. However, data on how diabetes affects bladder contractility associated with TRPA1 activation are not available. In this study, by using a rat model with streptozotocin-induced type I diabetes, contractility measurements of DSM strips in response to TRPA1-activating and modulating pharmacological agents and assessment of TRPA1 mRNA expression in bladder-innervating dorsal root ganglia, we have shown that diabetes enhances the TRPA1-dependent mechanism involved in bladder DSM contractility. This is not due to changes in TRPA1 expression, but mainly due to the general inflammatory reaction caused by diabetes. The latter leads to an increase in cyclooxygenase-2-dependent prostaglandin synthesis through the mechanisms associated with substance P activity. This results in the enhanced functional coupling between the tachykinin and prostanoid systems, and the concomitant increase of their impact on DSM contractility in response to TRPA1 activation.
Keywords: diabetes, bladder detrusor smooth muscle, contraction, TRPA1, TRPV1, prostaglandins, tachykinins
Introduction
TRPA1 is a Ca2+-permeable cation channel that is activated by painful low temperatures (˂17 °C), irritating chemicals, reactive metabolites and mediators of inflammation (1). In lower urinary tract tissues, TRPA1-channel protein expression was first discovered in nociceptive nerve endings that penetrate the urothelium and which innervate the trygone of the mouse bladder (2). Further studies have shown that in the bladder, TRPA1-channels are predominantly expressed in sensory afferent nerve endings, whilst their expression and sensory function in the epithelial cells is species-specific, with a virtual absence of TRPA1 expression in the detrusor smooth muscle (DSM) cells (3, 4). In addition to being present in nerve endings, TRPA1-channels are in significant numbers in the mucosa of the human bladder where their expression may increase even further in pathologies associated with bladder outlet obstruction (5).
TRPA1-channels of either sensory nerve endings or bladder epithelium are involved in sensory transduction, and under certain conditions their activation can cause bladder hyperactivity. Agonists of TRPA1-channels are known to induce concentration-dependent contraction of isolated muscle strips of the rat bladder via stimulation of TRPA1-expressing sensory fibers (6). This effect is attributed to the release from afferent nerve endings in response to TRPA1 activation of tachykinins and prostaglandins, which can cause contraction of DSM cells (6). Intravesical instillation of TRPA1-channel agonists into the rat bladder causes hyperreflexia involving C-fiber afferent nerve pathways (7).
Diabetes is a systemic disease characterized by the impairment of both the peripheral and autonomic nervous systems, termed diabetic neuropathy, which in turn affects both the perception of sensory information, and the regulation of many organs (8). In particular, the negative impact of diabetes on the functioning of the urinary system is manifested by diabetic cystopathies and urinary incontinence (9, 10). It has been shown that in diabetes, TRPA1-channels expressed in nociceptive afferent nerve endings are involved in the development and maintenance of polymodal peripheral hypersensitivity due to channel sensitization by reactive compounds (4 hydroxynonenal and methylglyoxal) formed in diabetes (11). Furthermore, activation of the TRPA1 channel is positively modulated by calcium (12,13,14). Since both basal intracellular calcium concentration and voltage-gated calcium influx are increased in sensory neurons in diabetes (15, 16), this may also influence TRPA1-dependent processes. However, data on how diabetes affects the contractility of the bladder associated with TRPA1 activation are still lacking.
The aim of this study was to investigate the mechanisms of TRPA1-channels involvement in the activation of bladder DSM contraction of normal rats and rats with experimental diabetes. Our data show that diabetes affects TRPA1-dependent mechanisms of DSM contractility mainly via an overall inflammatory reaction associated with diabetes, which leads to an enhanced functional coupling between the tachykinin and prostanoid systems.
Materials and Methods
Induction of diabetes and preparation of DSM strips
Animal protocols used in the study complied with EU Directive 2010/63/EU for animal experiments. All efforts were made to minimize animal suffering. The procedures of diabetes induction, DSM strips preparation and contraction measurements did not differ from those described elsewhere (17, 18). Briefly, type I diabetes was induced in Wistar male rats weighing 200–250 g by a single intraperitoneal injection of 42 mg/kg of streptozotocin (STZ) diluted in 100 mM acetic buffer at pH 4.5. Age-mates injected with a similar volume of buffer only served as controls. Animals whose blood glucose level after 3 days of STZ injection was 20 mmole/l or higher were assigned to the diabetic group. Animals from this group were used in experiments after 8 weeks of diabetes induction. This period was selected based on previous studies showing that diabetic cystopathy in the rat model of STZ-induced diabetes undergoes time-dependent changes with the strongest alterations in detrusor contractility taking place between the 6th and 9th weeks of diabetes induction (10). Measurement of blood glucose level in animals from the diabetic group just before the experiments gave values of 25–33 mmole/l.
Animals were anesthetized by brief carbon dioxide exposure and sacrificed by decapitation. The intact urinary bladder was removed and placed in warmed (37 °C), oxygenated (95% O2 and 5% CO2) Krebs solution (in mM): 120.4 NaCl, 5.9 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 1.8 CaCl2, 15.5 NaHCO3, 11.5 glucose (pH 7.4). The bladder was cut ventrally from base to dome, cleaned of connective tissue and urothelium, and four longitudinal strips (diameter ∼2 mm, length ∼7 mm) were then excised from its walls.
Tensiometric measurements of contraction
For the recording of contraction each strip was placed in an organ bath continuously superfused with experimental Krebs solutions at a constant flow rate (1 ml/min) and temperature (36 °C). One end of the strip was fixed while the other end was attached by means of a ligature to the lever of the capacitative force sensor with a baseline load of 3 mN (18). Electric field stimulation (EFS) with a 10-second-long train of pulses (pulse duration 0.5 ms, amplitude 100 V, frequency 10 Hz) was applied via Ag/AgCl electrodes every 3 min which was sufficient for complete restoration of basal tone.
Atropine (ATR, 1 µM) and phentolamine (PHE, 1 µM) were added to the experimental Krebs solutions to inhibit the cholinergic component of the EFS-evoked contractions and to prevent possible impact of diabetes-associated changes in α1-adrenoreceptors expression or sensitivity (19). All compounds used in the study were added directly to the experimental solutions in the required concentrations and applied by gravitational flow (1 ml/min) via a multi-positional tap. Complete solution exchange in the organ bath was achieved in less than 1 min. The recording of contractile activity was made on a pen recorder and in parallel via an A/D converter to the computer.
The following reagents (all from Sigma-Aldrich) were used in the study: allyl isothiocyanate (AITC, TRPA1 agonist), capsaicin (CAP, TRPV1 agonist), HC-030031 (TRPA1 blocker), AMG 9810 (TRPV1 blocker), substance P (SP), indomethacin (INDO, non-selective cyclooxygenase COX-1 and COX-2 inhibitor), nimesulide (NIM, selective COX-2 inhibitor), aristolochic acid (phospholipase A2 inhibitor), prostaglandins F2α (PGF2α) and E2 (PGE2).
Quantitative RT-PCR (qRT-PCR)
Analysis by qRT-PCR of the expression of TRPA1 mRNA was performed on lumbosacral (L1-L2, L6-S1) dorsal root ganglia (DRG) that innervate the bladder (20) from control animals and animals on various stages of STZ-induced diabetes. DRGs were cleaned of connective tissue, homogenized, and total RNA was extracted using RNAzol®RT (Molecular Research Center, Inc.). cDNA was synthesized with RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo scientific, Inc.) in a mixture of 600 ng of total RNA, Oligo(dT)18 primer (Thermo Scientific, Inc.) and RiboLock ribonuclease inhibitor (Thermo Scientific, Inc.). The extracted cDNA was used for real-time quantitative PCR in a mixture of 2 µl cDNA, Maxima™ SYBR Green qPCR Master Mix (Thermo Scientific, Inc.) 10 nM ROX solution (Thermo Scientific, Inc.) and 5 µM of each primer.
Primers were designed using NCBI/Primer-BLAST and tested with FastPCR 4.0.13 (PCR Team) software for the "Primer quality" parameter to exceed 70. Their sequences were as follows: TRPA1: 5′-TCCTGTGAAGCGCTGAATGT-3′ (F), 5′-ACAGCTATGTGAAGGGGTGC-3′ (R). Beta-actin (Actb): CTGTGTGGATTGGTGGCTCT (F), GCTCAGTAACAGTCCGCCTA (R). Quantitative PCR (qPCR) was carried out on 7500 Fast Real-Time PCR System (Applied Biosystems) according to the program: 50 cycles of 95 °C for 15 s and 60 °C for 60 s followed by a melting curve from 60 °C to 95 °C with 1% steps. Analysis of the results was performed with 7500 Software v2.0.5 (Applied Biosystems). The data on TRPA1 expression was normalized to beta-actin expression and averaged for each group of animals.
Data analysis and statistics
For the same type of experiment the amplitude of contractile response following administering of certain intervention were normalized to the amplitude of contraction before the intervention and then averaged. The results were expressed as the mean ± s.e.m. with the number of studied strips indicated by "n". Each experiment was performed on 8–10 strips from at least 3 animals (N). Statistical comparison of control responses and responses under the influence of certain compounds was made using an unpaired t-test with P<0.05 being considered significant. Differences in TRPA1 mRNA expression were analyzed using ANOVA.
Results
Influence of AITC on the contraction of control DSM
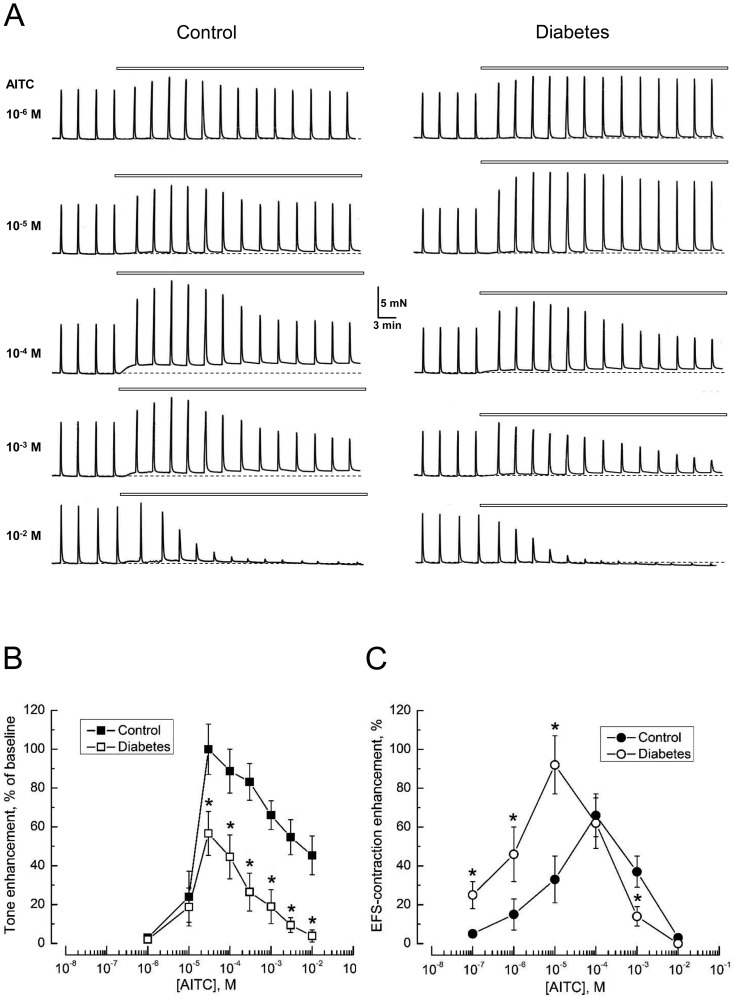
Exposure of bladder DSM strips from control rats to the TRPA1-channel agonist, AITC, caused two effects: 1) quasi-stationary increase of basal tone and 2) transient increase in the amplitude of EFS-contractions, with both effects demonstrating a biphasic dependence on AITC concentration (Fig. 1). Using drug concentrations ranging from 10−7 – 3 × 10−5 M, there was first of all an enhancement of the amplitude of EFS-contraction on the background of a largely stable tone, with the latter starting to pick up only from 10−6 M (Figs. 1A–C). Increasing AITC concentrations above 3 × 10−5 M led to a decrease in the relative enhancement of EFS-contractions amplitude as well as of basal tone (Figs. 1A–C).
Fig. 1.
The TRPA1 channel activator, allyl isothiocyanate (AITC), enhances the basal tone and increases the amplitude of electric field stimulation-evoked contractions (EFS-contractions) of detrusor smooth muscle (DSM) strips of the rat bladder. A: Representative recordings of the contractions of DSM strips from control rats (left) and rats with STZ-induced diabetes (right) in response to the application of different concentrations of AITC (specified on the left). In the recordings presented in this and subsequent figures, the dotted line indicates the initial basal tone, and the horizontal bars above the recordings correspond to the period of application of the compound; EFS-contractions in the recordings are seen as the sharp upward spikes. B, C: Concentration-dependencies for the relative enhancement of basal tone (B) and the amplitude of EFS-contractions (C) in response to AITC in control (filled symbols) and diabetic (open symbols) DSM strips; points – mean ± s.e.m, n=8–10 strips for each point; "*" P<0.05 in diabetes compared to respective controls.
It should be noted that the transient increase in the amplitude of the EFS-contractions in response to AITC concentrations below 10−3 M was followed by the amplitude returning close to its pre-drug value, and it was only at concentrations higher than 10−3 M that the transient increase changed to partial inhibition, which at 10−2 M caused almost complete blockade (Fig. 1A).
Thus, despite these concentration-dependencies of the AITC effects on basal tone and EFS-contractions had a similar biphasic shape, they demonstrated a shift with respect to the drug concentration. Because application of EFS induces depolarization of the nerve endings, we suggest that the voltage-gated calcium entry that accompanies such depolarization promotes TRPA1 activation at lower concentrations of agonist (12,13,14).
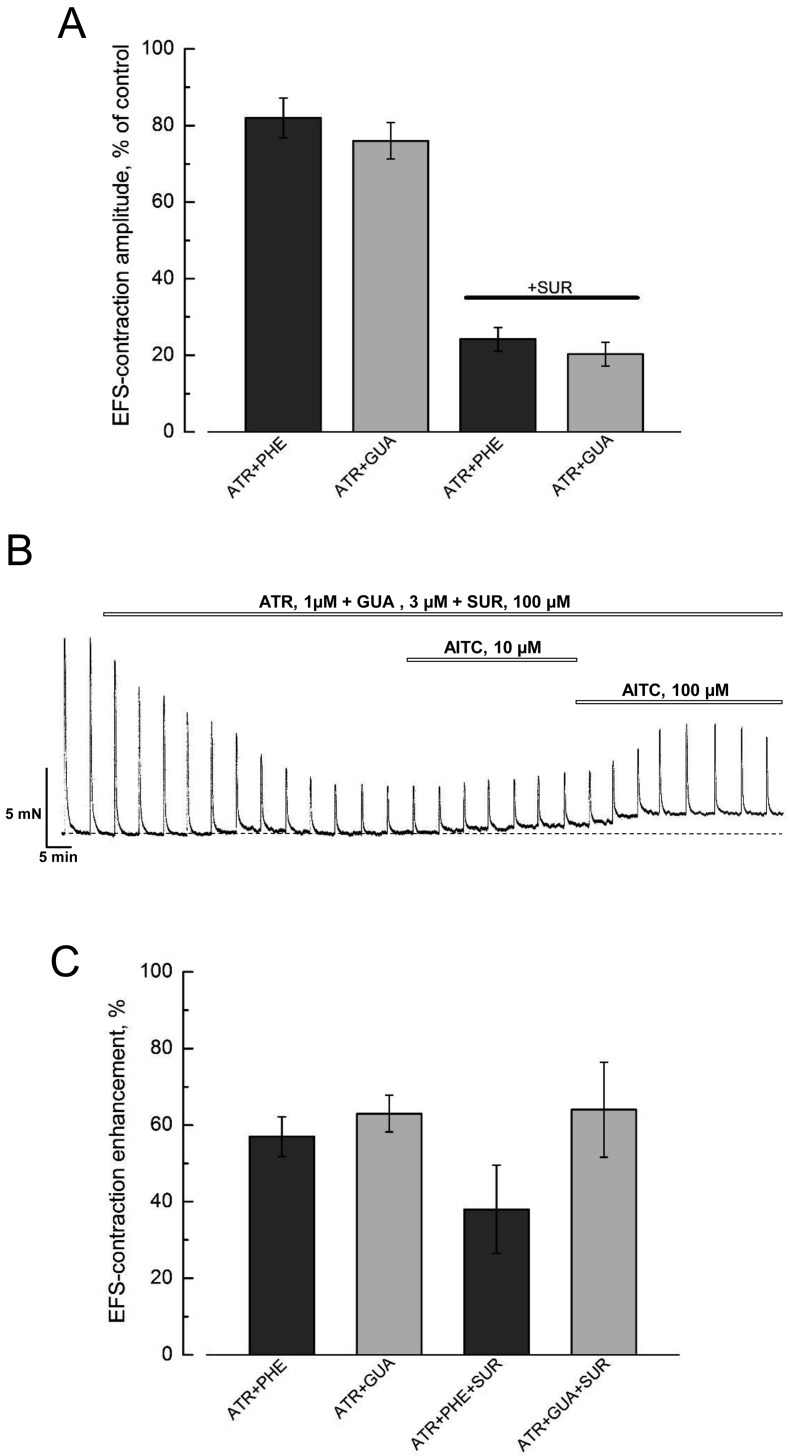
All our experimental solutions contained atropine (1 µM) and phentolamine (1 µM) to suppress m-cholinergic and α1-adrenergic neurotransmission. To verify to what extent the presence of these blockers establishes conditions for generation of the so called non-adrenergic, non-cholinergic (NANC) contractions in DSM we have performed control experiments using an inhibitor of catecholamine release from sympathetic nerves, guanethidine (GUA, 3 µM), instead of phentolamine. Fig. 2A shows that irrespective of whether phentolamine or guanethidine was used in combination with atropine the amplitude of EFS-contractions was inhibited to basically the same level of 80% of its control value. Further addition of the purinergic antagonist, suramin (SUR, 100 µM), to both cocktails of blockers reduced the amplitude of EFS-contractions to about 20% (Figs. 2A and B), indicating that a purinergic component is dominant in the EFS-contractions. Moreover, application of AITC in the presence of any combination of blockers produced similar relative potentiation of EFS-contractions amplitude (Figs. 2B and C) and tone enhancement. From these experiments we have concluded that: 1) the combination of atropine and phentolamine, which we used throughout all our experiments, was sufficient for establishing NANC conditions and 2) the action of AITC is not dependent on the cocktails of blockers used to inhibit the major components of efferent neurotransmission.
Fig. 2.
Effects of AITC on DSM contractility are not influenced by the inhibitors of efferent neurotransmission. A: The bar graph (mean ± s.e.m., n=6–8) shows no significant difference in the residual amplitude of EFS-contractions of DSM strips from control rats in the presence of atropine (1 µM) plus phentolamine (1 µM) (ATR+PHE, dark grey columns) and atropine (1 µM) plus guanethidine (3 µM) (ATR+GUA, dark grey columns) without or with 100 µM suramine (SUR, horizontal bar). B: A representative recording of the contractions of DSM strips from control rats during application of atropine (ATR, 1 µM) plus guanethidine (GUA, 3 µM) plus (SUR, 100 µM) followed by consecutive applications of 10 µM and 100 µM of AITC; note the strong inhibition of the amplitude of the EFS-contractions as a result of ATR+GUA+SUR and its transient potentiation by AITC accompanied by tone enhancement. C: The bar graph (mean ± s.e.m., n=6–8), shows the similarity of relative potentiation of the amplitude of the EFS-contractions in response to AITC (100 µM) applied in the presence of atropine (1 µM) plus phentolamine (1 µM) (ATR+PHE), atropine (1 µM) plus guanethidine (3 µM) (ATR+GUA) without or with 100 µM suramine (ATR+PHE+SUR and ATR+GUA+SUR).
Influence of AITC on the contraction of diabetic DSM
AITC application to DSM strips from the bladders of diabetic rats caused similar effects to the controls, but with differences in quantitative characteristics. In particular, tone enhancement in diabetic DSM in response to the same AITC concentrations was smaller than in controls, whereas the increase of EFS-contractions' amplitude, on the contrary, was higher (Fig. 1).
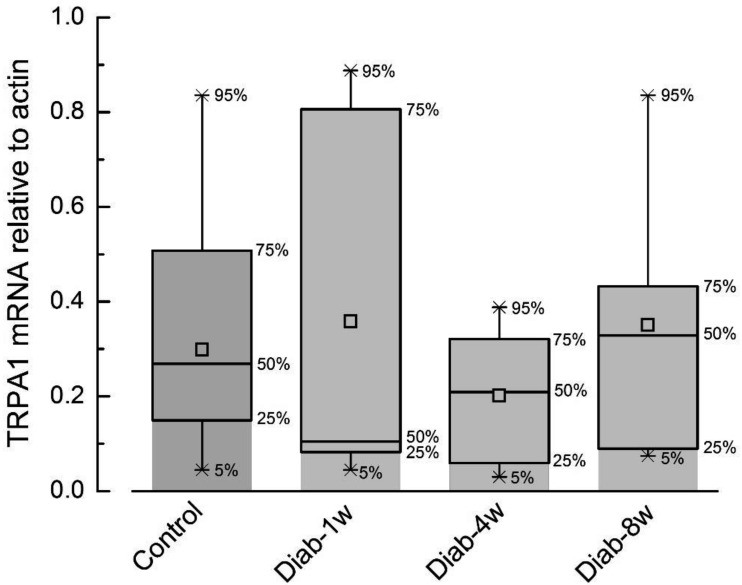
To verify whether or not these differences were related to the impact of diabetes on TRPA1-channel expression, we compared TRPA1 mRNA levels in bladder-innervating lumbosacral, L1-L2, L6-S1, DRGs (20) in control rats and rats at various periods of STZ-induced diabetes by means of qRT-PCR. The results of this analysis (Fig. 3) have shown that TRPA1 mRNA levels during the 8-week-period of diabetes does not undergo statistically significant change compared to the control. Thus, the changes in TRPA1-dependent contractility during diabetes are most likely to be determined by the peculiarities in TRPA1 regulation and/or coupling of its activation to DSM contraction.
Fig. 3.
STZ-induced type I diabetes does not significantly change the expression of TRPA1 mRNA in the L1-L2, L6-S1 dorsal root ganglia (DRG) that innervate the rat bladder. The graph presents a box plot of the expression of TRPA1 mRNA (relative to actin) in DRGs of control rats (control, N=16) and rats at 1st (N=7), 4th (N=10) and 8th (N=6) weeks of diabetes (diab-1w, -4w and -8w, respectively). The upper and lower values of the error bars correspond to 95% and 5% of the data range, while the upper and lower limits of the boxes correspond to 75% and 25% of the data range, the middle line – median value, and the square in the middle denotes the average value. ANOVA test P>0.05.
Interaction of TRPA1 with prostanoid system
The literature indicates that AITC-sensitive TRPA1 channels are often localized on the same nerve endings of urinary bladder-innervating sensory neurons as the CAP-sensitive TRPV1 channels (6, 7). Such co-localization even leads to a functional interaction and cross-regulation of both channels (6, 21).
Moreover, the mechanisms of TRPA1 and TRPV1 agonists action on bladder contractions are also similar. They involve the release from TRPA1- and TRPV1-expressing sensory afferents of neurotransmitters which have a contractile action on DSM: prostaglandins and substance P (SP) with TRPA1 activation (6), and SP and calcitonin gene-related peptide (CGRP) with TRPV1 activation (22,23,24). The presence of a SP component in the EFS-contractions upon CAP-mediated TRPV1 stimulation was demonstrated by using tachykinin receptor antagonists (23). Since prostaglandins are able to sensitize TRPV1-channels (25), the prostanoid system may be the factor underlying the functional coupling between the TRPA1- and TRPV1-dependent contractile mechanisms.
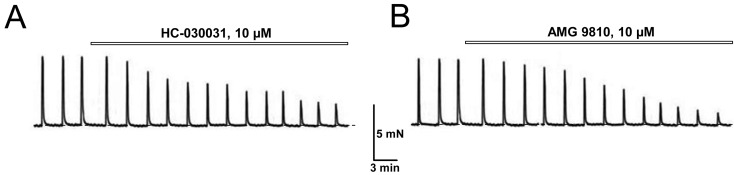
The presence of TRPA1- and TRPV1-dependent components in EFS-induced contraction even under basal conditions was confirmed by using both the selective TRPA1 blocker HC-030031 and the selective TRPV1 blocker AMG 9810. Exposure of DSM strips from control rats to either HC-030031 (10 µM) or AMG 9810 (10 µM) caused suppression of EFS-contractions amplitude (Fig. 4), with steady-state levels of 58% ± 6% (n=6) and 76 ± 8% (n=6) for HC-030031 and AMG 9810, respectively. Since both TRPA1 and TRPV1, in addition to the sensitivity to their specific physical and chemical agonists are also characterized by voltage-dependent activation (14, 26), this indicates that the EFS-evoked depolarization of TRPA1- and TRPV1-expressing sensory afferents is by itself sufficient to activate these channels and cause concomitant release of transmitters with contractile action on DSM.
Fig. 4.
EFS-contractions of control DSM contain TRPA1- and TRPV1-dependent components. A, B: Representative recordings of the contractions of DSM strips from control rats in response to application of specific blockers: (A) of TRPA1 by HC-030031 and (B) of TRPV1 by AMG 9810 (B); note that both blockers inhibit the amplitude of EFS-activated NANC contractions; explanations and designations are the same as in Fig. 1A.
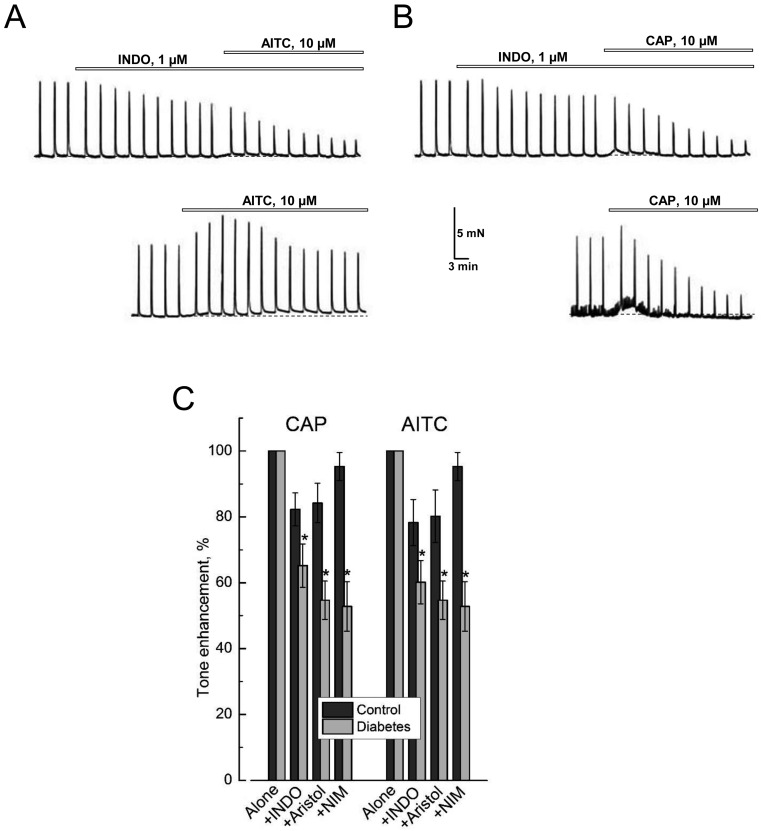
Evidence in favor of the fact that prostaglandins are among these transmitters was obtained by using indomethacin (INDO), a nonselective inhibitor of COX-1 and COX-2 enzymes (COX-1/2) which catalyze the synthesis of prostaglandins from arachidonic acid. Indeed, as shown in Fig. 5A, B, INDO (1 µM) per se inhibited the amplitude of control DSM EFS-contractions, although the extent of inhibition (i.e., 17.5 ± 3.9%, n=7) was smaller compared to either HC-030031 or AMG 9810. Moreover, TRPA1 and TRPV1 agonists, AITC and CAP, applied on top of INDO were no longer able to produce transient enhancement of the amplitude of the EFS-contractions (Figs. 5A and B), whilst enhancement of basal tone in response to them was reduced by about 20% compared to the case when they were applied alone (Fig. 5C).
Fig. 5.
Activation of TRPA1 and TRPV1 is associated with the presence of a prostaglandin-dependent component in DSM contractions, which increases in experimental diabetes. A, B: Representative recordings of the contractions of DSM strips from control rats in response to the application in (A) of the specific TRPA1 agonist, AITC and in (B) of the TRPV1 agonist, capsaicin (CAP), in the presence of the non-specific COX-1/2 inhibitor, indomethacin (INDO, top recordings) and in its absence (lower recordings); note that in the presence of INDO, the contractile responses to AITC and CAP in the form of basal tone enhancement and transient stimulation EFS-contractions become reduced; explanation and symbols are the same as in Fig. 1A. C: Quantification (mean ± s.e.m., n=8–10) of basal tone enhancement of control (dark gray columns) and diabetic (light gray column) DSM strips in response to the application of CAP or AITC in the presence of INDO (+INDO, 1 µM), aristolochic acid (+Aristol, 10 µM) or nimesulide (+NIM, 10 µM) relative to the applications of CAP or AITC alone; "*" P<0.05 in diabetes compared to respective controls; additional explanation in the text.
The substrate of COX-1/2 in the synthesis of prostaglandins is arachidonic acid, which in turn is a product of catalytic activity of phospholipase A2 (PLA2) (27). In control DSM strips, blockade of PLA2 by aristolochic acid (Aristol, 10 µM) produced the same ∼20% reduction of basal tone enhancement in response to AITC or CAP, as did COX-1/2 blockade by INDO (Fig. 5C).
However, in contrast to INDO and aristolochic acid, the use of selective COX-2 inhibitor, nimesulide (NIM, 10 µM), only led to a ∼5% decrease in AITC- or CAP-evoked basal tone enhancement of control DSM strips (see Fig. 5C), consistent with the notion that under non-pathologic conditions it is the activity of COX-1 that provides the most synthesis of prostaglandins (27, 28), which are involved in the activation of DSM contractile response.
In diabetic DSM strips we have observed statistically significant enhancement of inhibitory influence of the blockers of the PLA2/COX-1/2 pathway of prostaglandin synthesis on AITC- and CAP-evoked contractions (Fig. 5C). This was especially evident for the action of the COX-2 blocker, nimesulide, for which the inhibitory effect on these contractions increased from ∼5% in the control DSM to ∼45% in diabetic ones (Fig. 5C). Thus, under diabetic conditions synthesis of prostaglandins, which are involved in TRPA1- and TRPV1-dependent DSM contractions, obviously increases, and this increase largely occurs due to inducible COX-2, which expression is known to be stimulated in pathological processes, especially accompanied by inflammation (28).
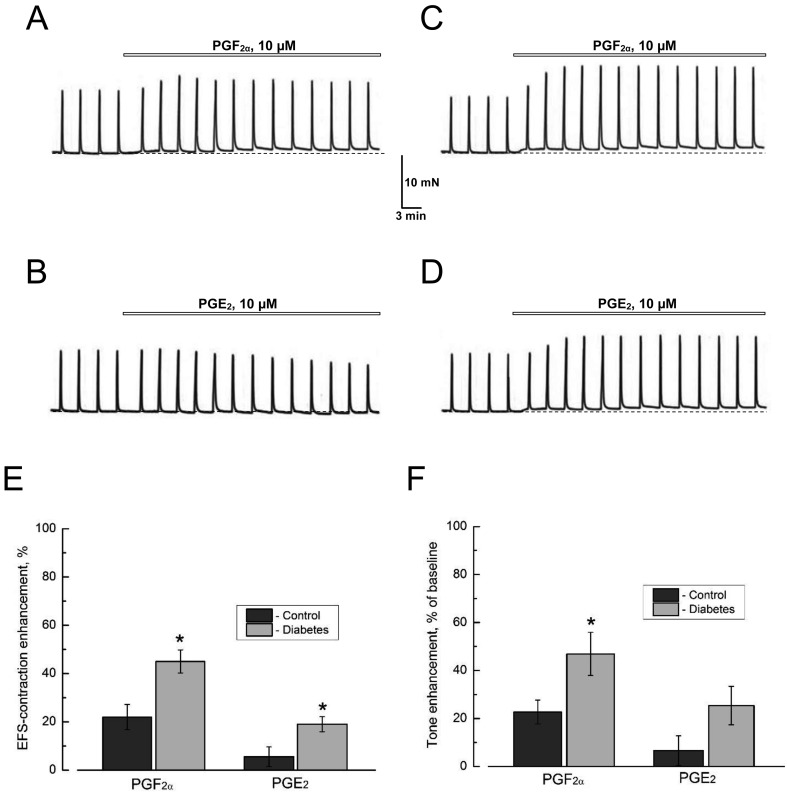
An additional strong argument in favor of the involvement of prostaglandins release in the contractile effects of AITC in urinary bladder, came from experiments on direct exogenous application of prostaglandin F2α (PGF2α,) and E2 (PGE2). These experiments have shown that either PGF2α (10 µM) or PGE2 (10 µM) per se largely mimic the actions of AITC on both basal tone and on the amplitude of the EFS-contractions (Figs 6A and B). The effects of PGF2α and PGE2 were generally greater in diabetic vs. control DSM (Figs. 6A–F), suggesting that diabetes, not only stimulates the release of prostaglandins, but also increases the sensitivity to them in DSM, probably due to increased expression of respective receptors and/or modification of intracellular signaling pathways coupled to them.
Fig. 6.
Prostaglandins partially reproduce the effects of TRPA1 activation on DSM contractions. A, B: Representative recordings of the contractions of DSM strips from control rats in response to the application of prostaglandins (A) F2α (PGF2α,) and (B) E2 (PGE2). C, D: As in panels A and B, respectively, but for DSM strips from diabetic animals. E, F: Quantification of the actions of PGF2α and PGE2 (both at 10 µM) on the amplitude of the EFS-contractions (E) and of the tone (F) in control (dark grey columns) and diabetic (light grey columns) DSM strips (mean ± s.e.m., n=6 in both cases); "*" P<0.05 in diabetes compared with respective controls. Other explanations and designations are the same as in Fig. 1A; note that in diabetic DSM, the contractile effects of the prostaglandins are amplified.
Interaction of TRPA1 with tachykinin system
It is known that the activation of both TRPA1 and TRPV1 also leads to the release of substance P (SP) from sensory afferents to the surrounding milieu. SP is a neuropeptide from the tachykinin family with a contractile action on bladder DSM (6, 23), and an important factor in neurogenic inflammation (29). However, the mechanisms of SP modulatory action on DSM contractility associated with TRPA1 and TRPV1 activation have not been sufficiently studied, especially in diabetes.
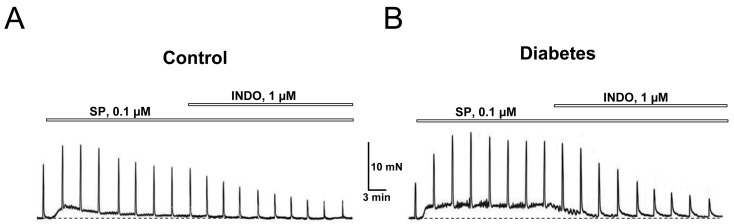
Exposure of DSM strips from control animals to SP (0.1 µM) per se caused transient enhancement of basal tone and an increase in the amplitude of EFS-evoked contractions (Fig. 7A). DSM strips from diabetic animals responded to such exposure in a similar manner except the enhancement of basal tone was not transient, but rather long-lasting and the increase of the amplitude of the EFS-contractions was more pronounced (Fig. 7B). Thus, the SP action on normal and diabetic DSM was similar to that of AITC, indicating that the contractile effects of TRPA1 activation are at least in part mediated via the tachykinin system.
Fig. 7.
Functional coupling between tachykinin and prostanoid systems in the activation of bladder DSM contractions. A, B: Representative recordings the contractions of control (A) and diabetic (B) DSM strips showing partial inhibition by INDO of the stimulating effect of substance P (SP) on the DSM contractions; explanations and designations are the same as in Fig. 1A; note that in diabetic DSM, SP evokes quasi stationary tone enhancement vs. a transient increase in tone in the control DSM, and stronger potentiation of the EFS-contractions that are removed by INDO.
It is known that substance P, as a proinflammatory factor, can affect the prostanoid system by stimulating PGE2 production through the induction of COX-2 expression (30). One possible mechanism for this includes activation by SP of neurokinin-1 receptors (NK-1R) with the subsequent involvement of JAK2-kinase and the STAT3/5 transcription factor signaling pathway (30), although the role of other signaling mechanisms is also possible (31). In support of such a possibility, INDO (1 µM) partially inhibited the contractile effect of SP, and this effect was stronger in DSM strips from diabetic animals (Figs. 7A and B). In this regard, we hypothesized that both tachykinin and prostanoid systems can also interact during TRPA1 and TRPV1 activation.
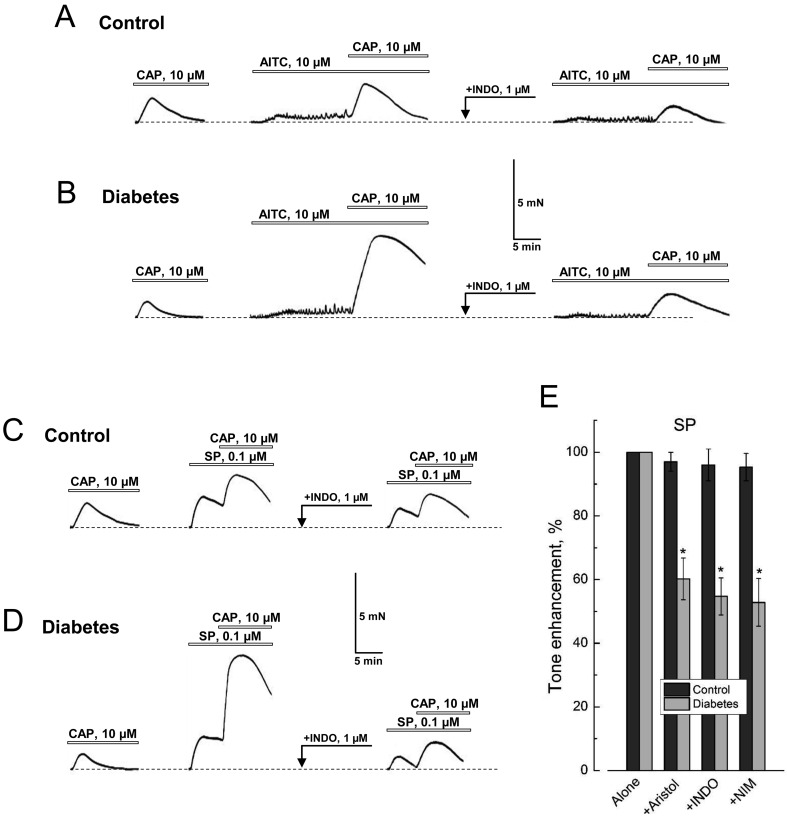
Fig. 8A shows that application of CAP (10 µM) in the presence of AITC (10 µM) in normal DSM causes a 1.6-times greater contractile response compared to that of CAP alone. However, if DSM strips were pre-exposed to the nonselective COX-1/2 inhibitor, INDO (10 µM), prior to consecutive applications of AITC and CAP, the facilitation of the CAP responses becomes lost (Fig. 8A). Moreover, in diabetic DSM these effects become more prominent. As a result, CAP in the presence of AITC could evoke contractions with not just a 1.6-fold, but with a 5.5-fold greater amplitude compared to that with CAP alone, which, however, could still be eliminated by INDO (Fig. 8B).
Fig. 8.
DSM contractions in response to co-activation of TRPA1 and TRPV1 involve interactions of tachykinin and prostanoid systems. A, B: Representative recordings of DSM strips contraction from control (A) and diabetic (B) animals in response to the application of CAP alone (left recording), of CAP in the presence of AITC (middle recording) and CAP in the presence of both INDO (application is marked by arrow) and AITC (right recording). C, D: Same as in A and B, respectively, but for applications of CAP alone (left recording), CAP in the presence of SP (middle recording) and CAP in the presence of INDO (application is marked by arrow) and SP (right recording); other explanations and designations are the same as in Fig. 1A. Note that augmentation of contraction in response to CAP applied in the presence of AITC or SP is much larger in diabetic vs. control DSM, and in both cases it can be removed by INDO. D: The bar graph (mean ± s.e.m., n=8–10), shows changes in the amplitude of basal tone enhancement of control (dark gray columns) and diabetic (light gray column) DSM strips in response to the application of SP in the presence of INDO (+INDO, 1 µM), aristolochic acid (+Aristol, 10 µM) or nimesulide (+NIM, 10 µM) relative to the applications of SP alone; "*" P<0.05 in diabetes compared to respective controls; additional explanation in the text.
In our opinion, these experiments indicate that SP, which is released in response to AITC, stimulates PGE2 production in nerve endings via an autocrine process and probably also in smooth muscle cells via a paracrine process. PGE2 in turn sensitizes TRPV1 to its agonist, CAP, so that the latter, when applied in the presence of AITC is able to cause a larger INDO-sensitive, PGE2-dependent contraction component. Enhancement of this effect in diabetes is apparently explained by a higher expression of inducible COX-2, as mentioned above (see. Fig. 5B).
Confirmation of this hypothesis was obtained in experiments conducted according to a similar protocol, but by using directly SP (0.1 µM) instead of AITC. These experiments have shown that contraction of control DSM in response to CAP applied in the presence of SP, was 2.2-fold greater than without SP, and that this increase could be removed by INDO treatment (Fig. 8C). In diabetic DSM, the enhancement of contraction in response to CAP increased by 7-fold in the presence of SP, and was almost entirely removed by INDO (Fig. 8D).
Finally, the contractile responses of diabetic, but not control DSM, to direct application of SP, appeared to be almost equally sensitive to all three pharmacological agents that block the production of prostaglandins, indomethacin, nimesulide and aristolochic acid, all of which inhibited these responses by 55–60% (Fig. 8E). This suggests that in diabetes, even SP-activated contractions contain a prostaglandin-dependent component, and that this component is largely due to inducible COX-2.
Discussion
The data presented in this study allow us to draw the following main conclusions: 1) diabetes affects both TRPA1- and TRPV1-dependent mechanisms of the contraction of bladder DSM, 2) the effects of TRPA1 and TRPV1 agonists on DSM contractions are realized via prostanoid and tachykinin systems, 3) the general pro-inflammatory reaction in diabetes enhances the interaction between prostanoid and tachykinin systems in TRPA1- and TRPV1-dependent DSM contractions.
The main purpose of the current study was to compare the effects of TRPA1 activation on bladder DSM contractility in normal and diabetic rats (with STZ-induced type I diabetes). However, during the progress of this study we observed interesting interactions between the TRPA1- and TRPV1-dependent mechanisms in DSM contractility which prompted us to broaden the initial scope of the research to include TRPV1 as well.
The concentration-dependence of the TRPA1 agonist, AITC, action on DSM contractions was biphasic: with increasing concentration in the range 10−7–10−5 M, AITC produced a progressively bigger stimulating effect, whilst at concentrations above 10−5 M its stimulatory effect gradually decreased. Such biphasic action most likely reflects the predominance of TRPA1 channel desensitization at high AITC concentrations (21, 32). On the other hand, the fact that the concentration threshold for stimulation of EFS-contractions appeared to be somewhat lower than that for the enhancement of basal tone can be explained by the synergy among voltage-dependent, Ca2+-dependent and agonist-dependent modes of TRPA1 channel activation (12,13,14) that may take place during EFS-evoked depolarization of sensory afferents.
In the diabetic animals, the biphasic concentration dependence of AITC actions on DSM contractions remained intact. Nevertheless, the relative stimulation of EFS-contractions by AITC in diabetic DSM appeared to be larger than in the controls, while enhancement of basal tone was smaller. In our opinion, the increase of the stimulatory influence of AITC on EFS-contractions of diabetic DSM can be explained if one considers that voltage-gated Ca2+ entry as well as basal concentration of intracellular calcium in sensory neurons become upregulated in diabetes (15, 16), thereby contributing to Ca2+-dependent mechanism of TRPA1 activation.
Overall, the effect of TRPA1 activation on bladder contractility is attributed to the stimulation of TRPA1-expressing sensory nerve fibers causing them to release both SP and PGE2, each of which is capable of activating contraction via tachykinin and prostaglandin receptors on the surface of DSM cells (6). At the same time, tachykinins and prostaglandins are known to be factors involved in neurogenic inflammation that may enhance pro-inflammatory reaction by further sensitizing nerve endings via autocrine and/or paracrine processes (33). The activation of yet another TRP channel family member, heat- and capsaicin-sensitive TRPV1, is coupled to DSM contraction in a manner similar to that of TRPA1 (6, 23). Moreover, both TRPA1 and TRPV1 are co-localized on the same nerve endings and are capable of interacting with each other owing to largely overlapping mechanisms of activation, desensitization and regulation (6, 21).
Our data show that diabetes leads to changes in the TRPA1- and TRPV1-dependent DSM contractility, and that these changes most likely result from general pro-inflammatory reaction associated with diabetes. Indeed, under the experimental diabetes we observed an increase in COX-1/2-dependent component in AITC- and CAP-activated contractions, and this increase was almost entirely due to the contribution of nimesulide-sensitive, inducible COX-2, the expression of which is stimulated in pathological processes accompanied by inflammation (28), including diabetes (34). The fact that the contractile action of AITC could be largely reproduced by direct application of PGF2α or PGE2 agrees well with the cause-effect relationship between activation of TRPA1 in the nerve endings and the release of prostaglandins. On the other hand, the more potent contractile effect of exogenous PGF2α or PGE2 in diabetic vs. control DSM indicates that sensitivity of DSM cells to prostaglandins in diabetes becomes higher due to either increased expression of prostaglandin receptors or more effective intracellular signaling through them leading to contraction. For example, increased expression of EP1 and EP2 prostaglandin receptors was documented in the kidneys of type I STZ-induced diabetic mice (35).
The contractile action of AITC could be partially reproduced not only by prostaglandins, but by application of exogenous SP as well. Moreover, we discovered that diabetes promotes the interaction between the tachykinin and prostanoid systems. If in control DSM the contraction caused by SP was insensitive to the blockers of prostaglandin synthesis, then in diabetic DSM such sensitivity became obvious, and specific to the inducible COX-2. Since it is known that SP, as a pro-inflammatory factor, can stimulate the expression of COX-2 (30, 31) and, consequently, the release of prostaglandins, we hypothesize that in diabetes the signaling pathways involved such stimulation, particularly NK-1R/JAK2/STAT3/5 (30), may experience upregulation.
Increased functional coupling between tachykinin and prostanoid systems in diabetes are also well illustrated by the contractions in responses to combined applications of AITC, SP, CAP, and their sensitivity to COX blockers. Indeed, our data show that CAP applied in the presence of AITC or SP is able to cause stronger contraction compared to the event when it is applied alone, and that such facilitating effect of AITC or SP on CAP contractions significantly increases in diabetes. Removal of AITC- or SP-induced facilitation of responses to CAP in control and diabetic DSM by indomethacin agrees well with the involvement of prostaglandins in both cases, which are known to sensitize TRPV1-channels (25). Thus, CAP-activated TRPV1-mediated DSM contractions in the presence of AITC or SP serve as a good indicator for the release of prostaglandins by sensory nerve endings, which in diabetes is significantly increased.
Thus, the contractility of bladder DSM associated with TRPA1 activation becomes generally up-regulated in diabetes, and this up-regulation mostly occurs due to an overall inflammatory reaction accompanying diabetes. This reaction results in enhanced prostaglandin synthesis and facilitated functional interaction between tachykinin and prostanoid systems, each of which is involved in mediation of the contractile effects of TRPA1 channel activation. To reduce TRPA1- and TRPV1-dependent bladder overactivity in diabetes, pharmacological inhibition of prostanoid system would seem to be a promising therapeutic tool.
It should be noted, however, that the increase of TRPA1-dependent DSM contractility in diabetes was not straightforward by far. In particular, it was most evident on EFS-contractions, whereas the enhancement of basal tone in response to AITC in diabetic DSM was even smaller compared to that in control (see. Fig. 1). A similar situation has been observed by ourselves and others for the TRPV1-dependent DSM contractility in experimental diabetes (17, 36, 37). One explanation for this may be that diabetes also influences the expression and/or activity of endopeptidases involved in termination of the action of peptide neurotransmitters, including tachykinins, via their proteolytic degradation (17). Therefore, it seems that the end result of the impact of diabetes on TRPA1-dependent bladder contractility may depend from multiple factors including the effectiveness of the coupling of the prostanoid and tachykinin systems, the degradation of neurotransmitters, as well as direct TRPA1 regulation by intracellular calcium, inflammatory factors and reactive compounds that are formed during diabetes.
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Acknowledgments
Supported by the National Academy of Sciences of Ukraine.
References
- 1.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012; 464(5): 425–58. doi: 10.1007/s00424-012-1158-z [DOI] [PubMed] [Google Scholar]
- 2.Nagata K, Duggan A, Kumar G, García-Añoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005; 25(16): 4052–61. doi: 10.1523/JNEUROSCI.0013-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du S, Araki I, Yoshiyama M, Nomura T, Takeda M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology. 2007; 70(4): 826–31. doi: 10.1016/j.urology.2007.06.1110 [DOI] [PubMed] [Google Scholar]
- 4.Skryma R, Prevarskaya N, Gkika D, Shuba Y. From urgency to frequency: facts and controversies of TRPs in the lower urinary tract. Nat Rev Urol. 2011; 8(11): 617–30. doi: 10.1038/nrurol.2011.142 [DOI] [PubMed] [Google Scholar]
- 5.Du S, Araki I, Kobayashi H, Zakoji H, Sawada N, Takeda M. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology. 2008; 72(2): 450–5. doi: 10.1016/j.urology.2007.11.127 [DOI] [PubMed] [Google Scholar]
- 6.Andrade EL, Ferreira J, André E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol. 2006; 72(1): 104–14. doi: 10.1016/j.bcp.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 7.Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Högestätt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008; 53(2): 391–9. doi: 10.1016/j.eururo.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 8.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003; 23(4): 365–72. doi: 10.1055/s-2004-817720 [DOI] [PubMed] [Google Scholar]
- 9.Brown JS, Wessells H, Chancellor MB, Howards SS, Stamm WE, Stapleton AE, Steers WD, Van Den Eeden SK, McVary KT. Urologic complications of diabetes. Diabetes Care. 2005; 28(1): 177–85. doi: 10.2337/diacare.28.1.177 [DOI] [PubMed] [Google Scholar]
- 10.Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009; 182(6 Suppl): S18–26. doi: 10.1016/j.juro.2009.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, Viisanen H, Akerman KE, Lindstedt K, Pertovaara A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res. 2012; 65(1): 149–58. doi: 10.1016/j.phrs.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 12.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007; 282(18): 13180–9. doi: 10.1074/jbc.M607849200 [DOI] [PubMed] [Google Scholar]
- 13.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci. 2007; 10(3): 277–9. doi: 10.1038/nn1843 [DOI] [PubMed] [Google Scholar]
- 14.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009; 106(4): 1273–8. doi: 10.1073/pnas.0808487106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N. Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Arch. 2004; 448(4): 395–401. doi: 10.1007/s00424-004-1263-8 [DOI] [PubMed] [Google Scholar]
- 16.Cao XH, Byun HS, Chen SR, Pan HL. Diabetic neuropathy enhances voltage-activated Ca2+ channel activity and its control by M4 muscarinic receptors in primary sensory neurons. J Neurochem. 2011; 119(3): 594–603. doi: 10.1111/j.1471-4159.2011.07456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philyppov IB, Paduraru ON, Andreev YA, Grishin EV, Shuba YM. Modulation of TRPV1-dependent contractility of normal and diabetic bladder smooth muscle by analgesic toxins from sea anemone Heteractis crispa. Life Sci. 2012; 91(19-20): 912–20. doi: 10.1016/j.lfs.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 18.Vladimirova IA, Lankin YN, Philyppov IB, Sushiy LF, Shuba YM. Frequency dependence of excitation-contraction of multicellular smooth muscle preparations: the relevance to bipolar electrosurgery. J Surg Res. 2014; 186(1): 119–25. doi: 10.1016/j.jss.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 19.Gonulalan U, Kosan M, Hafez G, Arıoglu E, Akdemir O, Ozturk B, Gur S, Cetinkaya M. The effect of diabetes mellitus on α1-adrenergic receptor subtypes in the bladder of rats. Urology. 2012. Oct; 80(4): 951. e9–16. [DOI] [PubMed]
- 20.Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992; 319(4): 615–23. doi: 10.1002/cne.903190411 [DOI] [PubMed] [Google Scholar]
- 21.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007; 583(Pt 1): 175–93. doi: 10.1113/jphysiol.2007.133231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggi CA. The dual function of capsaicin-sensitive sensory nerves in the bladder and urethra. Ciba Found Symp. 1990; 151: 77–83 discussion 83–90. [DOI] [PubMed] [Google Scholar]
- 23.Meini S, Maggi CA. Evidence for a capsaicin-sensitive, tachykinin-mediated, component in the NANC contraction of the rat urinary bladder to nerve stimulation. Br J Pharmacol. 1994; 112(4): 1123–31. doi: 10.1111/j.1476-5381.1994.tb13200.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitoh C, Kitada C, Uchida W, Chancellor MB, de Groat WC, Yoshimura N. The differential contractile responses to capsaicin and anandamide in muscle strips isolated from the rat urinary bladder. Eur J Pharmacol. 2007; 570(1-3): 182–7. doi: 10.1016/j.ejphar.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005; 1: 3. doi: 10.1186/1744-8069-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004; 430(7001): 748–54. doi: 10.1038/nature02732 [DOI] [PubMed] [Google Scholar]
- 27.Meyer MC, Rastogi P, Beckett CS, McHowat J. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des. 2005; 11(10): 1301–12. doi: 10.2174/1381612053507521 [DOI] [PubMed] [Google Scholar]
- 28.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002; 68-69: 165–75. doi: 10.1016/S0090-6980(02)00029-1 [DOI] [PubMed] [Google Scholar]
- 29.O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004; 201(2): 167–80. doi: 10.1002/jcp.20061 [DOI] [PubMed] [Google Scholar]
- 30.Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol. 2006; 176(8): 5050–9. doi: 10.4049/jimmunol.176.8.5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sio SW, Ang SF, Lu J, Moochhala S, Bhatia M. Substance P upregulates cyclooxygenase-2 and prostaglandin E metabolite by activating ERK1/2 and NF-kappaB in a mouse model of burn-induced remote acute lung injury. J Immunol. 2010; 185(10): 6265–76. doi: 10.4049/jimmunol.1001739 [DOI] [PubMed] [Google Scholar]
- 32.Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008; 457(1): 77–89. doi: 10.1007/s00424-008-0493-6 [DOI] [PubMed] [Google Scholar]
- 33.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002; 302(3): 839–45. doi: 10.1124/jpet.102.032797 [DOI] [PubMed] [Google Scholar]
- 34.Shanmugam N, Gaw Gonzalo IT, Natarajan R. Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes. 2004; 53(3): 795–802. doi: 10.2337/diabetes.53.3.795 [DOI] [PubMed] [Google Scholar]
- 35.Nasrallah R, Xiong H, Hébert RL. Renal prostaglandin E2 receptor (EP) expression profile is altered in streptozotocin and B6-Ins2Akita type I diabetic mice. Am J Physiol Renal Physiol. 2007; 292(1): F278–84. doi: 10.1152/ajprenal.00089.2006 [DOI] [PubMed] [Google Scholar]
- 36.Pinna C, Bolego C, Puglisi L. Effect of substance P and capsaicin on urinary bladder of diabetic rats and the role of the epithelium. Eur J Pharmacol. 1994; 271(1): 151–8. doi: 10.1016/0014-2999(94)90275-5 [DOI] [PubMed] [Google Scholar]
- 37.Benkó R, Lázár Z, Pórszász R, Somogyi GT, Barthó L. Effect of experimental diabetes on cholinergic, purinergic and peptidergic motor responses of the isolated rat bladder to electrical field stimulation or capsaicin. Eur J Pharmacol. 2003; 478(1): 73–80. doi: 10.1016/j.ejphar.2003.08.035 [DOI] [PubMed] [Google Scholar]