Significance
Sodium appetite is an important instinctive behavior with high survival value. Although a role of opioid signaling in salt appetite has been identified in rats, the exact contribution made by different opioid receptor subtypes within specific brain regions is not fully characterized. Here, we report that mu-opioid receptor (MOR) signaling is intrinsically responsible for opioid-dependent sodium appetite rendered by sodium deficiency in mice. Furthermore, we identified that during gratification of sodium appetite, the central amygdala is activated, and endogenous MOR signaling within this region promotes sodium intake in sodium-depleted mice. Accordingly, we reveal a key nucleus within the endogenous opioid circuit that is likely to be conserved across mammals and important in the control of dietary sodium consumption.
Keywords: sodium appetite, mu-opioid receptor, central amygdala
Abstract
Due to the importance of dietary sodium and its paucity within many inland environments, terrestrial animals have evolved an instinctive sodium appetite that is commensurate with sodium deficiency. Despite a well-established role for central opioid signaling in sodium appetite, the endogenous influence of specific opioid receptor subtypes within distinct brain regions remains to be elucidated. Using selective pharmacological antagonists of opioid receptor subtypes, we reveal that endogenous mu-opioid receptor (MOR) signaling strongly drives sodium appetite in sodium-depleted mice, whereas a role for kappa (KOR) and delta (DOR) opioid receptor signaling was not detected, at least in sodium-depleted mice. Fos immunohistochemistry revealed discrete regions of the mouse brain displaying an increased number of activated neurons during sodium gratification: the rostral portion of the nucleus of the solitary tract (rNTS), the lateral parabrachial nucleus (LPB), and the central amygdala (CeA). The CeA was subsequently targeted with bilateral infusions of the MOR antagonist naloxonazine, which significantly reduced sodium appetite in mice. The CeA is therefore identified as a key node in the circuit that contributes to sodium appetite. Moreover, endogenous opioids, acting via MOR, within the CeA promote this form of appetitive behavior.
Sodium is an essential dietary component and is required for a range of general physiological and cellular functions and specialized roles including blood volume/blood pressure regulation and nerve conduction. However, environmental sources of sodium are often scarce. Although rainwater derived from marine aerosols initially contains sodium, most of this is lost by 100–200 km from the sea coast. In the absence of geological sources, the soil and plants within a large portion of the earth’s continental interior, including jungle, mountain, and desert habitats, are sodium deficient. Consequently, there has been a powerful evolutionary selection pressure underlying the emergence of a specific neural organization subserving sodium appetite (1). It is an exemplar of a neural organization subserving instinctive behavior and is perhaps most conspicuous in ruminants.
A core feature of sodium appetite in terrestrial animals is that sodium depletion induces neuronal changes such that dietary sodium (e.g., food with high NaCl content) becomes highly wanted or “craved” and is more hedonically palatable when consumed (2). Consequently, substances with sodium concentrations that are so high they are usually avoided when an animal is sodium-replete are nonetheless appetitively consumed in large quantities when an animal is sodium-depleted. This phenomenon has been reported in sodium-depleted humans [for example, with Addison’s disease (3)] and is a feature of most long-established empirical sodium depletion–gratification methods that have quantified salt appetite in laboratory sheep (4), rats (5), and mice (6). In rodent models, sodium depletion is readily achieved by removing all sources of dietary sodium in combination with infusions of a diuretic such as furosemide. Upon reintroduction of a concentrated sodium source that is usually aversive (such as 0.3 M NaCl), rodents will consume a relatively large volume over a short time frame. Notably, this behavior ceases sometime before renormalization of plasma osmolarity, and we therefore operationally define this rapid consummatory act as gratification of a sodium appetite. Measurement of the amount consumed enables quantification of such a gratification response. Therefore, the magnitude of sodium gratification is subject to the influence of multiple factors, such as changes in the palatability of the sodium tastant and the incentive salience or motivation to consume sodium (1, 2).
A role for opioids in sodium appetite is well established, in line with a broader role that opioids play in food palatability and ingestive behaviors (7). Early studies demonstrated that systemic infusion of naltrexone, a nonselective opioid receptor antagonist, reduced the amount of 3% (0.5 M) NaCl solution consumed by sodium-depleted rats (8). However, multiple endogenous opioids exist (such as enkephalins, dynorphins, and endorphins/endomorphins), which despite considerable promiscuity bind preferentially to delta (DOR), kappa (KOR), and mu-opioid receptors (MORs), respectively (9). In rats, selective pharmacological antagonism of MOR (10), DOR (11), or KOR (12) signaling can reduce sodium appetite. However, each opioid receptor subtype is broadly expressed throughout a multitude of brain regions (13, 14), and therefore characterizing the precise opioidergic circuitries that contribute to sodium appetite is an important and ongoing focus of research. Studies in which exogenous opioid receptor agonists are infused into local regions of rat brain have proven informative and, for example, have demonstrated that increased MOR signaling within both the nucleus accumbens and ventral pallidum enhances the hedonic palatability of NaCl (15). However, experiments such as these fail to determine whether such mechanisms act endogenously and are physiologically relevant during sodium deficiency. To address this, an alternative approach has been to locally infuse opioid receptor antagonists, which have revealed a role of endogenous MOR signaling in the lateral parabrachial nucleus (LPB), a region known to relay gustatory signals related to sodium consumption from the rostral nucleus of the solitary tract (rNTS) (16). Further research to elucidate such endogenous nodes is important. However, conducting longitudinal studies of salt appetite in rats is confounded by the tendency of this species to sensitize to multiple rounds of sodium deprivation/gratification (17). In contrast, mice exhibit stable levels of salt gratification over multiple trials and represent an important experimental model in which the role of opioids in sodium appetite remains relatively underexplored (18).
Here, we reveal that MORs are predominantly responsible for driving opioid-mediated sodium appetite in sodium-depleted mice. Analysis of Fos immunoreactivity revealed that three regions within the mouse brain are most prominently activated following gratification of sodium appetite: the central amygdala (CeA), rNTS, and LPB. Local infusion of a selective MOR antagonist (naloxonazine) within the CeA significantly reduced salt appetite, identifying CeA MOR signaling as an endogenous instinctive component of sodium appetite in mice. These findings align well with the established role of CeA in sodium appetite (19) and motivated consummatory behavior (20) and may have wider implications for understanding the neuronal mechanisms underlying the overconsumption of dietary sodium, which is associated with hypertension, obesity, and other diseases with significant health burden (21).
Results
MOR Signaling Is Primarily Responsible for Endogenous Opioid-Driven Sodium Intake in Sodium-Depleted Mice.
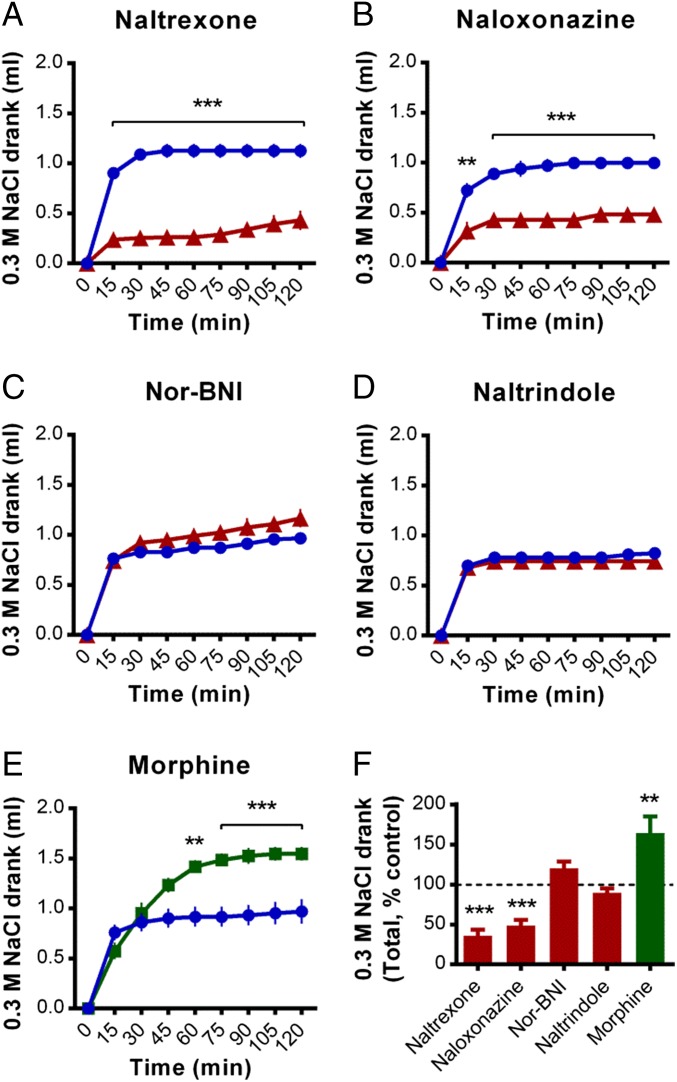
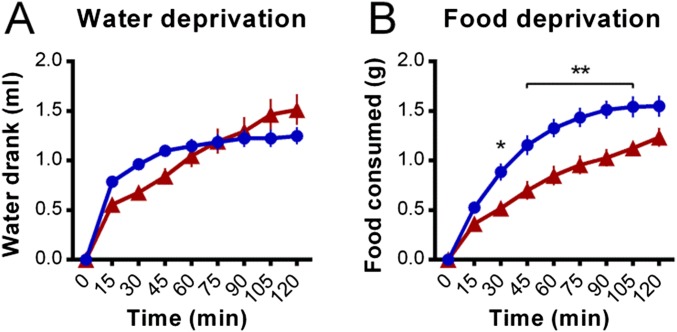
The nonselective opioid receptor antagonist naltrexone reduced the cumulative amount of sodium consumed by sodium-depleted mice [main effect of treatment, F(1, 14) = 75.3, P < 0.001; Fig. 1 A and F for normalized 120-min totals]. Naltrexone had no effect on water consumption following 24 h of water deprivation [main effect of treatment, F(1, 13) = 0.1, P = 0.078; Fig. S1A], however reduced food consumption following 24 h food deprivation was observed [main effect of treatment, F(1, 12) = 13.0, P = 0.004; Fig. S1B]. The ability of naltrexone to reduce sodium intake is mostly conferred via antagonism of MORs, as naloxonazine (MOR antagonist) treatment reduced sodium intake to a similar extent [main effect of treatment, F(1, 7) = 38.7, P < 0.001; Fig. 1B], whereas neither nor-BNI [KOR antagonist; main effect of treatment, F(1, 14) = 2.6, P = 0.131; Fig. 1C] nor naltrindole [DOR antagonist; main effect of treatment, F(1, 14) = 0.5, P = 0.502; Fig. 1D] altered sodium intake. In line with an ability of MORs to modulate this behavior in either direction, acute morphine (predominantly a MOR partial agonist) significantly increased sodium consumption [main effect of treatment, F(1, 12) = 11.5, P = 0.005; Fig. 1E].
Fig. 1.
Systemic infusion of the MOR antagonist naloxonazine reduced sodium gratification in sodium-depleted mice. (A) The i.p. preinfusion (30 min before) of the nonselective opioid receptor antagonist naltrexone (1 mg/kg, n = 8, red line) significantly reduced the cumulative amount of 0.3 M NaCl solution drank (introduced at time = 0) by sodium-depleted mice, relative to vehicle controls (n = 8; vehicle controls are represented by a blue line in A–E). (B) A similar reduction in sodium gratification was achieved following administration of the MOR antagonist naloxonazine (5 mg/kg, n = 5, red line; vehicle, n = 4). In contrast, sodium gratification was not altered by the KOR antagonist nor-BNI (10 mg/kg, n = 8, red line; vehicle, n = 8) (C) or the DOR antagonist naltrindole (5 mg/kg, n = 8, red line; vehicle, n = 8) (D). (E) Infusion of the MOR agonist morphine (1.5 mg/kg, n = 7, green line; vehicle, n = 7) significantly increased sodium gratification. (F) Total amounts of 0.3 M NaCl solution drank during the 120-min measurement period following each treatment, normalized as a percentage of each corresponding vehicle control group (which are assigned 100%). Data are expressed as mean ± SEM. Two-way repeated-measures ANOVA, post hoc tests between treatments within each time bin as indicated (A–E), and main effects of treatment (F): **P < 0.01, ***P < 0.001.
Fig. S1.
Effects of naltrexone on consummatory behavior in water- and food-deprived mice. (A) The i.p. preinfusion (30 min before) of the nonselective opioid receptor antagonist naltrexone (1 mg/kg, n = 8; naltrexone represented by a red line for A and B) did not alter the cumulative amount of water drank (introduced at time = 0) by water-deprived mice relative to vehicle controls (n = 7; vehicle controls represented by a blue line for A and B). (B) However, naltrexone treatment (n = 7; vehicle, n = 7) reduced the cumulative amount of food consumed by food-deprived mice. Data are expressed as mean ± SEM. Two-way repeated-measures ANOVA, post hoc tests between treatments within each time bin as indicated: *P < 0.05, **P < 0.01.
Sodium Gratification Activates Neurons Within the CeA, rNTS, and LPB.
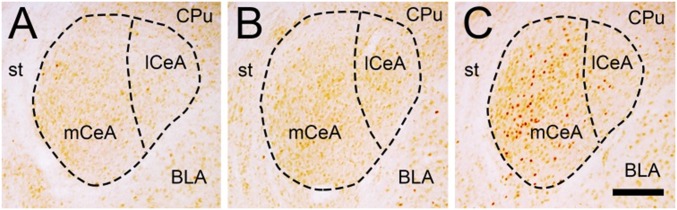
MORs are widely distributed throughout the mouse brain. To determine which candidate regions are involved in the sodium gratification response, brains were collected from sodium-replete, sodium-depleted, and sodium-gratified mice and assessed for Fos immunoreactivity (Table 1). The CeA displayed a significantly increased number of Fos-positive neurons following sodium gratification, relative to both sodium-replete (P < 0.05) and sodium-depleted mice (P < 0.05). Within the brainstem, similar increases in Fos immunoreactivity following sodium gratification were observed within the rNTS (P < 0.01 versus sodium-replete, P < 0.01 versus sodium-depleted) and LPB (P < 0.01 versus sodium-replete, P < 0.05 versus sodium-depleted).
Table 1.
Number of Fos-positive cells within key brain regions following sodium depletion and gratification
| Brain region | Sodium replete | Sodium depleted | Sodium gratified |
| Nucleus accumbens, core | 3.8 ± 1.8 | 5.3 ± 1.1 | 8.2 ± 2.5 |
| Nucleus accumbens, shell | 15.4 ± 6.9 | 25.5 ± 6.8 | 27.4 ± 4.1 |
| Ventral pallidum | 0.8 ± 0.4 | 0.8 ± 0.5 | 2.0 ± 1.3 |
| Lateral preoptic area | 16.4 ± 4.7 | 32.6 ± 8.6 | 29.0 ± 7.5 |
| Median preoptic nucleus | 21.1 ± 4.2 | 29.3 ± 6.4 | 28.2 ± 8.3 |
| Vascular organ of the lamina terminalis | 0.4 ± 0.3 | 1.8 ± 0.6 | 1.7 ± 0.5 |
| Subfornical organ | 0.5 ± 0.2 | 9.3 ± 1.1^^^ | 7.3 ± 0.6*** |
| Arcuate nucleus | 54.0 ± 13.6 | 41.3 ± 8.4 | 75.8 ± 27.7 |
| Paraventricular hypothalamic nucleus | 77.3 ± 13.2 | 66.8 ± 10.7 | 85.2 ± 14.4 |
| Posterior hypothalamic area | 323 ± 31.0 | 272.3 ± 57.7 | 303.8 ± 35.5 |
| Lateral hypothalamic area | 39.0 ± 8.5 | 43.5 ± 7.5 | 58.2 ± 13.5 |
| Supramammillary nucleus | 61.7 ± 9.2 | 70.8 ± 8.9 | 71.4 ± 10.6 |
| Paraventricular thalamic nucleus, rostral | 201.7 ± 38.2 | 207.6 ± 47.7 | 170.3 ± 43.6 |
| Paraventricular thalamic nucleus, caudal | 88.8 ± 13.4 | 142.2 ± 20.8 | 150.3 ± 19.2 |
| Bed nucleus of the stria terminalis, dorsal | 9.6 ± 5.9 | 9.2 ± 2.5 | 18.2 ± 5.7 |
| Bed nucleus of the stria terminalis, ventral | 1.6 ± 0.9 | 4.8 ± 1.7 | 3.0 ± 1.3 |
| Central nucleus of the amygdala | 14.3 ± 6.0 | 17.8 ± 7.9 | 56.3 ± 14.8*# |
| Basolateral nucleus of the amygdala | 22.8 ± 9.9 | 20.2 ± 7.2 | 29.2 ± 7.6 |
| Prelimbic cortex | 6.0 ± 2.9 | 8.8 ± 4.8 | 13.4 ± 4.9 |
| Infralimbic cortex | 3.2 ± 1.5 | 10.5 ± 3.5 | 9.4 ± 3.4 |
| Nucleus of the solitary tract, rostral | 3.0 ± 1.6 | 5.5 ± 1.9 | 35.5 ± 11.8**## |
| Lateral parabrachial nucleus | 14.9 ± 2.3 | 22.5 ± 6.2 | 50.3 ± 8.0**# |
| Medial parabrachial nucleus | 2.0 ± 0.6 | 2.3 ± 0.5 | 5.6 ± 1.4 |
| Kolliker–Fuse nucleus | 5.8 ± 2.8 | 6.6 ± 2.3 | 4.1 ± 1.2 |
Brain sections from mice that were culled either sodium replete, following sodium depletion, or 90 min after sodium gratification were subjected to immunohistochemistry to visualize Fos-positive (“activated”) neurons. For each midline and bilateral brain region, the number of Fos-positive cells within one or two 40-µm thick representative coronal sections (location-matched across all subjects) was automatically and blindly counted using ImageJ (n = 4–6 mice per group). Data are expressed as mean ± SEM. One-way ANOVA: sodium gratified versus sodium replete, *P < 0.05, **P < 0.01, ***P < 0.001; sodium gratified versus sodium depleted, #P < 0.05, ##P < 0.01; sodium depleted versus sodium replete, ^^^P < 0.001.
MOR Antagonism Within the CeA Reduces Sodium Intake in Sodium-Depleted Mice.
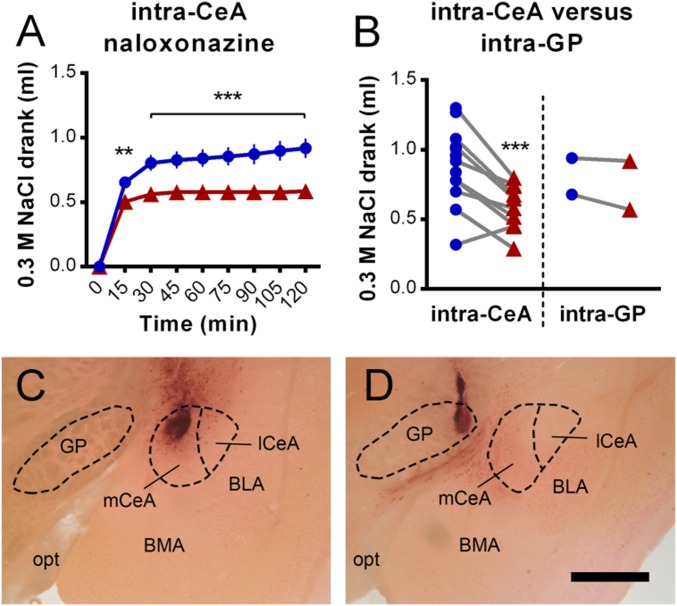
Due to the increased Fos expression within the CeA following sodium gratification (enriched within the medial CeA, mCeA; Fig. 2), bilateral guide cannulae were surgically implanted in mice to target the CeA (centered within the mCeA) or the adjacent region directly medial as a control. Intra-CeA infusion of naloxonazine significantly reduced sodium intake in sodium-depleted mice compared with vehicle controls [main effect of treatment, F(1, 23) = 10.3, P = 0.004; Fig. 3A]. This effect was not due to generalized behavioral inhibition or sedation, as intra-CeA naloxonazine treatment did not alter locomotor activity [main effect of treatment, F(1, 15) = 1.2, P = 0.292; Fig. S2]. Furthermore, the ability of naloxonazine to reduce sodium intake was not due to antagonism of MORs within the nearby globus pallidus (GP), as data from mice that received injections centered within this region did not display reduced sodium consumption (Fig. 3 B–D).
Fig. 2.
Sodium gratification increased the number of Fos-positive neurons within the CeA, most prominently within the medial subdivision (mCeA). Micrographs of coronal sections through the CeA (bregma –1.4 mm) from representative sodium-replete (A), sodium-depleted (B), and sodium-gratified mice (C) that have been subjected to Fos immunohistochemistry. BLA, basolateral amygdala; CPu, caudate putamen; lCeA, lateral subdivision of the CeA; st, stria terminalis. (Scale bar, 200 µm.)
Fig. 3.
Intra-CeA infusion of the MOR antagonist naloxonazine reduced sodium gratification in sodium-depleted mice. (A) Bilateral intra-CeA preinfusion (30 min before) of naloxonazine (250 ng in 250 nL, n = 11, red line) significantly reduced the cumulative amount of 0.3 M NaCl solution drank (introduced at time = 0) by sodium-depleted mice, relative to vehicle controls (n = 14, blue line). (B) Total amounts of 0.3 M NaCl solution drank during the 120-min measurement period following each treatment (naloxonazine, red; vehicle, blue) by individual mice, which received infusions into either the CeA or the adjacent GP. At the conclusion of studies, brains were sectioned and counterstained for histological target validation. Coronal sections (–1.4 mm posterior from bregma) from representative mice that received infusions either targeting the CeA (C) or the adjacent GP (D). For CeA infusions, although the center of the medial division of the CeA (mCeA) was targeted, the entire CeA was likely perfused with naloxonazine and vehicle. BLA, basolateral amygdala; BMA, basomedial amygdala; lCeA, lateral subdivision of the CeA; opt, optic tract. (Scale bar, 500 µm.) Data are expressed as mean ± SEM. Two-way repeated-measures ANOVA, post hoc tests between treatments within each time bin as indicated (A), and main effect of treatment (B): **P < 0.01, ***P < 0.001.
Fig. S2.
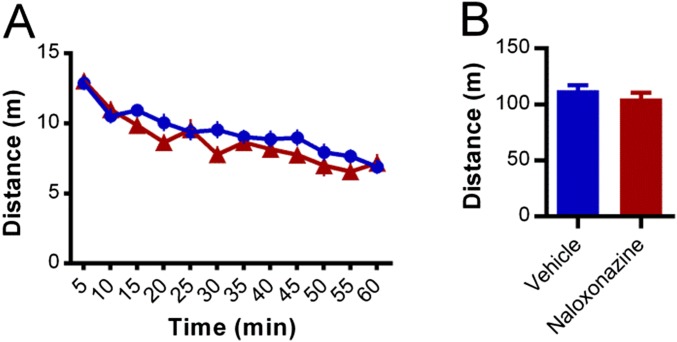
Intra-CeA infusion of naloxonazine did not alter locomotor activity. Bilateral intra-CeA preinfusion (30 min before) of naloxonazine (250 ng in 250 nL, n = 9, red line) did not alter the time course (5 min time bins) of distance traveled by mice placed in automated locomotor cells (A) or the total distance traveled during the 60-min session (B) relative to vehicle controls (n = 8, blue line). Data are expressed as mean ± SEM.
Discussion
Here we report that intra-CeA infusion of a selective MOR antagonist reduced sodium intake in sodium-depleted mice. Following early observations that bilateral CeA lesion abolishes sodium appetite in rats (22), the CeA is now considered one of several pivotal brain nuclei required for sodium appetite. The present study extends this knowledge, showing that endogenous MOR signaling within the CeA is a physiological mechanism to increase sodium intake when sodium deplete. Moreover, our finding that DOR and KOR signaling does not significantly contribute to sodium appetite in sodium-deplete mice is in line with previous studies where sodium-depleted beta-endorphin knockout mice consumed ∼50% less 2% (wt/vol) NaCl than wild-type controls (23) and suggests that this CeA MOR-dependent mechanism may well be more highly conserved than mechanisms involving DOR (11) and KOR (12) signaling that have been identified in rats. Intra-CeA infusion of the exogenous MOR agonist DAMGO [(D-Ala2, N-MePhe4, Gly-ol)-enkephalin] increases sodium appetite in rats (24, 25); however, until now the endogenous physiological relevance of this mechanism was unknown. In conjunction with the current data, we suggest that CeA MOR signaling is a mechanism to regulate sodium appetite in rodents.
Pharmacological enhancement of MOR signaling within the nucleus accumbens shell and ventral pallidum increases the hedonic palatability of sodium in rats (7, 15), which can be accurately measured using taste reactivity tests that assess characteristic hedonic and aversive orofacial gestures (26). In the present studies, although local CeA infusion of the MOR antagonist naloxonazine significantly reduced sodium consumption (by approximately one-third), systemic naloxonazine infusion reduced sodium intake to an even greater extent (approximately one half). This observation is therefore likely due to an ability of systemic naloxonazine administration to additionally reduce MOR signaling within the nucleus accumbens shell and ventral pallidum, and possibly other key areas, which decreases sodium palatability or otherwise impairs sodium appetite. In contrast to the role of endogenous MOR signaling in increasing hedonic palatability in extra-CeA sites, the work of Berridge and others has demonstrated that pharmacological MOR activation within the CeA of rats increases the incentive salience of various goals or rewards such as sucrose (how much sucrose is “wanted”), in response to cues (27–29). However, CeA MOR activation does not increase the palatability and hedonic reward value of sugar when it is tasted (how much the sugar is “liked”) in slightly food-restricted rats (20). In light of these findings, it seems probable that endogenous MOR signaling within the CeA of sodium-depleted mice increases the incentive salience of sodium (“wanting”) in response to cues that indicate sodium availability (such as reintroduction of the sodium solution and initial detection of the sodium tastant), rather than by increasing the hedonic palatability (“liking”) of sodium when consumed. This mechanism is in agreement with our finding that the CeA was activated (displayed an increased number of Fos-positive neurons) following sodium gratification but not during sodium depletion when the sodium-related cues were absent. However, as the mice used in these Fos studies consumed large volumes of 0.3 M NaCl solution during the 10 min in which access was provided, it is also possible that the observed increase in CeA Fos may be due to indirect stimulation from the mouth and gastrointestinal tract (via the NTS and LPB) to signal the cessation of sodium consumption (30). A similar pattern of Fos immunoreactivity has been reported in rats, where sodium gratification also appeared to approximately double the number of CeA Fos-positive neurons compared with sodium-deficient rats that were killed 24 h after their last furosemide injection (a time point comparable to the present studies in mice) (31). In contrast to the present study, however, sodium-depleted rats display more Fos immunoreactivity throughout the extended amygdala than sodium-replete controls, including in the central subdivision of the lateral part of the CeA (CeLCn) (19). Although this finding may suggest a physiological difference between species, the expression and detection of Fos immunoreactivity can be highly sensitive to subtle variations in technical approach. For example, a previous mouse study that used somewhat similar protocols reported that sodium depletion significantly increased Fos immunoreactivity within the median preoptic area, vascular organ of the lamina terminalis, and subfornical organ (which together form the lamina terminalis) (32). In contrast, our experimental protocols detected a statistically significant increase only in the subfornical organ.
Dynamic and fast-changing incentive salience, which can sharply increase upon detection of a cue (20), differs from the long-established slow accumulation of sodium appetite mediated by angiotensin II and circumventricular organs, including the lamina terminalis (1, 2). Several putative mechanisms through which the CeA might increase incentive salience have been suggested, involving direct and indirect modulation of mesocorticolimbic (33, 34) and brainstem circuits (25, 35). Interestingly, in addition to driving sodium and sucrose wanting, the CeA also plays a role in inhibiting feeding in response to satiety cues, conditioned taste aversion, and other anorexigenic signals (36); however, whether these roles involve inhibiting MOR signaling or downstream effects in this region remains to be determined. Intrinsic enkephalin neurons are the major source of endogenous ligand for MORs within the CeA (37, 38). The present findings suggest that these neurons receive information pertaining to sodium depletion, which may be derived indirectly via the circumventricular organs responding to circulating angiotensin II (39) and/or HSD2 neurons (which express the 11-β-hydroxysteroid dehydrogenase type 2 enzyme) within the NTS that detect plasma aldosterone, project to the CeA, are activated during sodium depletion, and promote sodium appetite (40).
Interestingly, the present studies revealed that within the CeA, the medial portion is preferentially activated in response to sodium gratification. This finding fits with studies that demonstrate the mCeA is a major output region for this structure and is particularly important for selecting and initiating different behavioral strategies in response to cues (41). This ability of the CeA to direct and refine instinctive behaviors in response to learnt cues and experience has obvious evolutionary advantage. In the context of sodium appetite, although it is important that a gradual decline in homeostatic sodium levels is detected by the brain via the circumventricular organs (1, 2, 39), an ability to sharply elevate sodium wanting upon detection of a sodium source represents a desirable survival strategy. From a technical perspective, although our local infusion studies targeted the mCeA, it is likely that the entire CeA was within the boundaries of drug/vehicle injections. Finally, we report here that the rNTS and LPB were also activated in response to sodium gratification; however, such a finding was expected given the known role of these structures in receiving and processing sodium taste information (42, 43).
In conclusion, our studies have revealed that endogenous MOR signaling within the CeA increases sodium appetite in mice. This finding builds upon the established role of the CeA in contributing to the control of sodium appetite and in influencing food consumption more broadly. Like other instinctive behaviors that once performed adaptive roles but are now often ill-suited to modern society, overconsumption of sodium can contribute to hypertension and cardiac dysfunction (21). The present study makes a significant contribution to furthering our understanding of the circuitry that underlies sodium consumption and has implications for both basic science and human health.
Materials and Methods
Animals.
All experimental procedures were approved by The Florey Institute of Neuroscience and Mental Health Animal Ethics Committee. Adult (>8 wk of age) male C57BL/6J mice were obtained either from a breeding colony at the Florey Institute of Neuroscience and Mental Health or from the Australian Research Centre. Mice were single-housed in a room maintained at ∼21 °C on a 12 h light:dark cycle (lights on at 0700–1900), with ad libitum access to water and standard laboratory chow unless otherwise stated. Four individual cohorts of mice (each n = 5–8) were used for i.p. studies, and two additional cohorts were used for Fos (n = 18) and intra-CeA studies (n = 20).
Morphine and Opioid Receptor Antagonists.
Naltrexone hydrochloride (cat. no. 0677, Tocris Bioscience; i.p. 1 mg/kg), naloxonazine dihydrochloride (cat. no. 0591, Tocris Bioscience; i.p. 5 mg/kg, intra-CeA 250 ng/nl), nor-BNI dihydrochloride (cat. no. 0347, Tocris Bioscience; i.p. 10 mg/kg), naltrindole hydrochloride (cat. no. 0740; i.p. 5 mg/kg), and morphine hydrochloride (Glaxo Australia Pty Ltd.; i.p. 1.5 mg/kg) were dissolved in vehicle consisting of either 0.9% NaCl (i.p. studies) or artificial cerebrospinal fluid (147 mM NaCl, 4 mM KCl, 0.85 mM MgCl2, and 2.3 mM CaCl2, for intra-CeA studies). Mice were allowed at least 7 d of recovery between infusions. For i.p. studies, 0.1 mL/10 g injection volumes were used.
Intra-CeA Infusions.
Stereotaxic implantation of indwelling intra-CeA guide cannula.
Mice were anesthetized via isoflurane inhalation [Delvet; 4% (vol/vol) at 0.2 L/min for induction and thereafter maintained at 2% (vol/vol)] and the head secured in a small animal stereotaxic frame (Kopf). Analgesic was provided (Meloxicam, Troy Laboratories, 20 mg/kg i.p.), and an incision was made to expose the skull, which was cleaned and dried with 6% (vol/vol) hydrogen peroxide. Three small screws (No. 087800, McCann Optical Parts) were secured into pits drilled into the skull surrounding the location of the cannula pedestal to anchor it in place, and small holes were drilled into the skull dorsal to the mCeA (relative to bregma, anterior–posterior, –1.4 mm; medial–lateral, ±2.5 mm). The bilateral guide cannula and pedestal (C235G/5.0/SPC, cut to 5.0 mm length, PlasticsOne) was lowered into place (dorsal–ventral, –3.5 mm) such that the ventral ends of the guide cannulae were 1.25 mm dorsal to the center of the mCeA. The cannula and screws were fixed in place with self-curing acrylic dental cement (Vertex-Dental), and a dummy cannula (C235DC/5.0/SPC, cut to protrude guide cannula by 1.25 mm, PlasticsOne) was inserted and secured with a cap (363DC, PlasticsOne). Mice were allowed to recover for 1 wk before testing, and dummy cannulae were loosened every 2–3 d to prevent blockage.
Intra-CeA infusions.
Mice were carefully restrained, the dummy cannula removed, and an injector (bilateral infusion internal cannula, C235I/5.0/SPC, cut to protrude guide cannula by 1.25 mm, PlasticsOne) inserted that was connected to a double polyethylene tubing connector assembly (C230C/SPC, PlasticsOne) and two 1 µL microsyringes (Hamilton Instruments) mounted on an infusion pump (11-Plus, Harvard). Simultaneous bilateral infusions of 250 nL were delivered over 1 min and the injector left in place for a further 20 s before being removed and replaced with the dummy cannula.
Histological target validation.
At the conclusion of the studies, mice were culled via i.p. infusion of pentobarbital (Nembutal, 100 mg/kg) and decapitated. Brains were removed from the skull and fixed in 4% (wt/vol) paraformaldehyde for 24 h, before being cryoprotected in 20% (wt/vol) sucrose for 24 h, frozen over dry ice, and stored at –80 °C. Sections 40 µm thick were cut using a cryostat, mounted onto glass microscope slides, and counterstained with eosin (1%, Amber Scientific), which was washed off with phosphate buffer (PB; 2.7 mM KCl, 11.2 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) after 20 s. Slides were dried for 20 min, mounting media (Dako) added, and coverslipped. Bright field micrographs were acquired, and the location of infusions determined by an observer blinded to identity.
Sodium Depletion and Repletion.
As mice display consistent sodium gratification behavior across multiple rounds of sodium depletion/repletion (18), cross-over experimental design was used such that vehicle (control) and drug responses were assessed within the same individual animals. For each round, mice were maintained on low-sodium food [0.02% (wt/wt) sodium, SF02-020, Specialty Feeds Pty Ltd.] for 7 d before experimentation and provided with a second water sipper containing 0.3 M NaCl that was removed 48 h before experimentation. Additionally, mice were i.p. injected with the diuretic furosemide (Lasix, Sanofi-Aventis; 1.2 mg in 0.12 mL) at 48 h and 24 h before experimentation. On the experimental day, mice were infused with either opioid receptor antagonist or morphine or vehicle (between 1000 and 1100). Thirty minutes later, the 0.3 M NaCl sipper was reintroduced, and the volume consumed was recorded every 15 min for 2 h.
Consummatory and Locomotor Activity Controls.
To assess the effects of naltrexone on other consummatory behaviors, mice (sodium replete; maintained on standard chow without a 0.3 M NaCl sipper) were deprived of either water or food for 24 h. Thirty minutes after i.p. naltrexone infusion, water or food was then reintroduced and the amount drunk/consumed was recorded every 15 min for 2 h. To determine whether intra-CeA naloxonazine infusion alters locomotor activity, 30 min after infusions mice (sodium replete) were placed in automated locomotor cells (Internal Length × Width, 27 × 27 cm; Tru Scan Photobeam Arena, Coulbourn Instruments) for 1 h, and the floor plane distance traveled was acquired in 5-min time bins using Tru Scan 2.0 software.
Fos Studies.
Tissue collection.
Three groups of mice (each n = 6) were culled either sodium-replete, following sodium depletion, or 90 min after sodium-depleted mice were allowed 10 min of 0.3 M NaCl access (sodium gratification) to allow for optimal Fos protein accumulation. To recapitulate the treatment schedule of the previous i.p. studies, mice in this latter group were i.p. infused with vehicle 30 min before 0.3 M NaCl was reintroduced, and to maintain consistency, sodium-replete and sodium-depleted groups were also i.p. injected with vehicle 130 min before being culled. Mice were culled by i.p. pentobarbital (100 mg/kg) and transcardially perfused with 12 mL PB followed by 60 mL paraformaldehyde [4% (wt/vol), dissolved in PB] at a rate of 8 mL/min. Brains were then removed, postfixed in ice-cold 4% (wt/vol) PFA for 1 h, cryoprotected in 20% (wt/vol) sucrose at 4 °C overnight, dried, frozen over dry ice, and stored at –80 °C. We cut 40-µm sections on a cryostat and stored them free-floating in cryoprotectant [30% (vol/vol) glycerol, 30% (vol/vol) ethylene glycol, 40% (vol/vol) PB] at –20 °C.
Fos immunohistochemistry.
Cryoprotectant was washed off and sections permeabilized via 4 × 10 min washes in PB containing 0.3% Triton X-100 and a further 4 × 10 min in PB. Sections were blocked for 1 h in PB with 10% (vol/vol) normal horse serum (NHS) before being transferred to PB containing 2% (vol/vol) NHS and rabbit anti–c-Fos primary antiserum (cat. no. sc-52, Santa Cruz, 1:4,000 dilution) overnight. The next morning, sections were washed 3 × 10 min in PB and transferred to PB containing 2% (vol/vol) NHS and biotinylated goat anti-rabbit secondary antiserum (Vectastain Elite, Vector Laboratories; 1:200 dilution) for 1 h. Sections were again washed 3 × 10 min in PB and transferred to PB containing avidin/biotin ABC peroxidase complex (Vectastain, 1:100 dilution) for 1 h. Following 3 × 10-min washes in PB, sections were incubated in PB containing 0.01% H2O2 and 0.0625 mg/mL 3,3′-diaminobenzidine (Sigma-Aldrich) for 30 min, before 3 × 10-min washes in PB. Sections were then transferred to dH2O with 1.8 g/L gelatin, mounted onto glass microscope slides and dried overnight, before being dehydrated and cleared in ethanol and xylene and cover-slipped with safety mount mounting media (cat. no. 11 067, Trajan).
Imaging and counting.
Brightfield digital images were captured using a Nikon Microphot SA microscope, and Fos-positive cells were automatically counted by a blinded observer using ImageJ software (1.47v) and the ITCN plugin using threshold settings that provided counts that matched manual scoring in initial validation studies. One or two 40-µm thick representative coronal sections (location-matched across all subjects; n = 4–6 mice per group) were assessed for each midline and bilateral brain region.
Statistical Analysis.
Prism V6.01 software (GraphPad) was used to generate all graphs and determine statistical significance (alpha P < 0.05), and all data are expressed as mean ± SEM. Time course data were analyzed using two-way repeated-measures ANOVA, and within time point between treatment comparisons were made using Sidak’s post hoc tests. Fos data were analyzed via one-way ANOVAs and Tukey post hoc tests for each brain region.
Acknowledgments
This research was supported by National Health and Medical Research Council of Australia Project Grant 1079891 (to A.J.L.) and Research Fellowship 1020737 (to A.J.L.), plus grants from the Pratt and Besen Family Foundations (to A.J.L.) and the Victorian Government Operational Infrastructure Support Programme. This research was also supported by grants from The Mathers Charitable Foundation of New York, The Search Foundation, Ms. Diana Gibson, Mr. S. Baillieu Myer, Dr. Mark Nelson, Mr. Robert Albert, Mrs. Jeanne Pratt, and Mr. Tim Jones.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616664113/-/DCSupplemental.
References
- 1.Denton DA. The Hunger for Salt. An Anthropological, Physiological and Medical Analysis. Springer; Berlin: 1983. [Google Scholar]
- 2.Hurley SW, Johnson AK. The biopsychology of salt hunger and sodium deficiency. Pflugers Arch. 2015;467(3):445–456. doi: 10.1007/s00424-014-1676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper H, et al. Liquorice and soy sauce, a life-saving concoction in a patient with Addison’s disease. Ann Clin Biochem. 2007;44(Pt 4):397–399. doi: 10.1258/000456307780945624. [DOI] [PubMed] [Google Scholar]
- 4.Denton DA, Sabine JR. The selective appetite for Na ions shown by Na ion-deficient sheep. J Physiol. 1961;157:97–116. doi: 10.1113/jphysiol.1961.sp006708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf G. Refined salt appetite methodology for rats demonstrated by assessing sex differences. J Comp Physiol Psychol. 1982;96(6):1016–1021. [PubMed] [Google Scholar]
- 6.Denton D, McBurnie M, Ong F, Osborne P, Tarjan E. Na deficiency and other physiological influences on voluntary Na intake of BALB/c mice. Am J Physiol. 1988;255(6 Pt 2):R1025–R1034. doi: 10.1152/ajpregu.1988.255.6.R1025. [DOI] [PubMed] [Google Scholar]
- 7.Kelley AE, et al. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 8.Hubbell CL, McCutcheon NB. Opioidergic manipulations affect intake of 3% NaCl in sodium-deficient rats. Pharmacol Biochem Behav. 1993;46(2):473–476. doi: 10.1016/0091-3057(93)90382-4. [DOI] [PubMed] [Google Scholar]
- 9.Dhawan BN, et al. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48(4):567–592. [PubMed] [Google Scholar]
- 10.Bodnar RJ, Glass MJ, Koch JE. Analysis of central opioid receptor subtype antagonism of hypotonic and hypertonic saline intake in water-deprived rats. Brain Res Bull. 1995;36(3):293–300. doi: 10.1016/0361-9230(94)00205-f. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento AI, Ferreira HS, Cerqueira DR, Fregoneze JB. Blockade of central delta-opioid receptors inhibits salt appetite in sodium-depleted rats. Peptides. 2014;55:110–119. doi: 10.1016/j.peptides.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento AI, Ferreira HS, Saraiva RM, Almeida TS, Fregoneze JB. Central kappa opioid receptors modulate salt appetite in rats. Physiol Behav. 2012;106(4):506–514. doi: 10.1016/j.physbeh.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Martin TJ, et al. Regional differences in mu and kappa opioid receptor G-protein activation in brain in male and female prairie voles. Neuroscience. 2015;311:422–429. doi: 10.1016/j.neuroscience.2015.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erbs E, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2015;220(2):677–702. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 2009;161(3):718–733. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavan CG, et al. Activation of μ opioid receptors in the LPBN facilitates sodium intake in rats. Behav Brain Res. 2015;288:20–25. doi: 10.1016/j.bbr.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 17.Hurley SW, Zhang Z, Beltz TG, Xue B, Johnson AK. Sensitization of sodium appetite: Evidence for sustained molecular changes in the lamina terminalis. Am J Physiol Regul Integr Comp Physiol. 2014;307(12):R1405–R1412. doi: 10.1152/ajpregu.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liedtke WB, et al. Relation of addiction genes to hypothalamic gene changes subserving genesis and gratification of a classic instinct, sodium appetite. Proc Natl Acad Sci USA. 2011;108(30):12509–12514. doi: 10.1073/pnas.1109199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AK, de Olmos J, Pastuskovas CV, Zardetto-Smith AM, Vivas L. The extended amygdala and salt appetite. Ann N Y Acad Sci. 1999;877:258–280. doi: 10.1111/j.1749-6632.1999.tb09272.x. [DOI] [PubMed] [Google Scholar]
- 20.Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221(3):407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris MJ, Na ES, Johnson AK. Salt craving: The psychobiology of pathogenic sodium intake. Physiol Behav. 2008;94(5):709–721. doi: 10.1016/j.physbeh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galaverna O, De Luca LA, Jr, Schulkin J, Yao SZ, Epstein AN. Deficits in NaCl ingestion after damage to the central nucleus of the amygdala in the rat. Brain Res Bull. 1992;28(1):89–98. doi: 10.1016/0361-9230(92)90234-o. [DOI] [PubMed] [Google Scholar]
- 23.Franchini LF, Rubinstein M, Vivas L. Reduced sodium appetite and increased oxytocin gene expression in mutant mice lacking β-endorphin. Neuroscience. 2003;121(4):875–881. doi: 10.1016/s0306-4522(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, et al. Activation of μ-opioid receptors in the central nucleus of the amygdala induces hypertonic sodium intake. Neuroscience. 2013;233:28–43. doi: 10.1016/j.neuroscience.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Yan JB, et al. Natriorexigenic effect of DAMGO is decreased by blocking AT1 receptors in the central nucleus of the amygdala. Neuroscience. 2014;262:9–20. doi: 10.1016/j.neuroscience.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Na ES, Morris MJ, Johnson AK. Opioid mechanisms that mediate the palatability of and appetite for salt in sodium replete and deficient states. Physiol Behav. 2012;106(2):164–170. doi: 10.1016/j.physbeh.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MJ, Warlow SM, Berridge KC. Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J Neurosci. 2014;34(50):16567–16580. doi: 10.1523/JNEUROSCI.2013-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiFeliceantonio AG, Berridge KC. Which cue to ‘want’? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res. 2012;230(2):399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29(20):6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menani JV, De Luca LA, Jr, Johnson AK. Role of the lateral parabrachial nucleus in the control of sodium appetite. Am J Physiol Regul Integr Comp Physiol. 2014;306(4):R201–R210. doi: 10.1152/ajpregu.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grondin ME, Gobeil-Simard A, Drolet G, Mouginot D. Na+ appetite induced by depleting extracellular fluid volume activates the enkephalin/mu-opioid receptor system in the rat forebrain. Neuroscience. 2011;192:398–412. doi: 10.1016/j.neuroscience.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Dadam FM, et al. Effect of sex chromosome complement on sodium appetite and Fos-immunoreactivity induced by sodium depletion. Am J Physiol Regul Integr Comp Physiol. 2014;306(3):R175–R184. doi: 10.1152/ajpregu.00447.2013. [DOI] [PubMed] [Google Scholar]
- 33.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 34.Ahn S, Phillips AG. Independent modulation of basal and feeding-evoked dopamine efflux in the nucleus accumbens and medial prefrontal cortex by the central and basolateral amygdalar nuclei in the rat. Neuroscience. 2003;116(1):295–305. doi: 10.1016/s0306-4522(02)00551-1. [DOI] [PubMed] [Google Scholar]
- 35.Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: Bidirectional connections with the central nucleus of the amygdala. J Comp Neurol. 2006;497(4):646–657. doi: 10.1002/cne.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17(9):1240–1248. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496(6):859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- 38.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18(3):292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 40.Koneru B, Bathina CS, Cherry BH, Mifflin SW. Mineralocorticoid receptor in the NTS stimulates saline intake during fourth ventricular infusions of aldosterone. Am J Physiol Regul Integr Comp Physiol. 2014;306(1):R61–R66. doi: 10.1152/ajpregu.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badrinarayan A, Prater KE, Orsini CA. The role of the central amygdala in selecting circuits and responses. J Neurosci. 2012;32(25):8431–8433. doi: 10.1523/JNEUROSCI.1820-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boughter JD, Jr, Gilbertson TA. From channels to behavior: An integrative model of NaCl taste. Neuron. 1999;22(2):213–215. doi: 10.1016/s0896-6273(00)81082-x. [DOI] [PubMed] [Google Scholar]
- 43.Cho YK, Li CS, Smith DV. Gustatory projections from the nucleus of the solitary tract to the parabrachial nuclei in the hamster. Chem Senses. 2002;27(1):81–90. doi: 10.1093/chemse/27.1.81. [DOI] [PubMed] [Google Scholar]