Significance
We describe an approach based on cytokine therapeutics to enhance the persistence and effectiveness of T-cell–based immunotherapies using chimeric antigen receptors (CARs). This strategy is effective without the use of high-dose exogenous cytokines that are typically associated with toxicities. Moreover, we report that the persistence of the least differentiated memory T cell, the T-memory stem cell, was promoted by signaling induced by a membrane-bound chimeric IL-15 cytokine-fusion molecule. These findings may contribute to improving the safety and therapeutic efficacy of CAR-based immunotherapies of patients with advanced cancer.
Keywords: adoptive immunotherapy, CAR, IL-15, T-memory stem cell, T-cell persistence
Abstract
Adoptive immunotherapy retargeting T cells to CD19 via a chimeric antigen receptor (CAR) is an investigational treatment capable of inducing complete tumor regression of B-cell malignancies when there is sustained survival of infused cells. T-memory stem cells (TSCM) retain superior potential for long-lived persistence, but challenges exist in manufacturing this T-cell subset because they are rare among circulating lymphocytes. We report a clinically relevant approach to generating CAR+ T cells with preserved TSCM potential using the Sleeping Beauty platform. Because IL-15 is fundamental to T-cell memory, we incorporated its costimulatory properties by coexpressing CAR with a membrane-bound chimeric IL-15 (mbIL15). The mbIL15-CAR T cells signaled through signal transducer and activator of transcription 5 to yield improved T-cell persistence independent of CAR signaling, without apparent autonomous growth or transformation, and achieved potent rejection of CD19+ leukemia. Long-lived T cells were CD45ROnegCCR7+CD95+, phenotypically most similar to TSCM, and possessed a memory-like transcriptional profile. Overall, these results demonstrate that CAR+ T cells can develop long-term persistence with a memory stem-cell phenotype sustained by signaling through mbIL15. This observation warrants evaluation in clinical trials.
Chimeric antigen receptors (CARs) that redirect T-cell specificity to desired tumor-associated antigens (TAAs) (1) are engineered to activate T cells for survival, serial killing, and cytokine production only upon contacting TAA (2). Adoptive transfer of CAR T cells can achieve durable complete responses in some patients; successful outcomes are associated with engraftment and long-term persistence of CAR T cells (3). Long-term immunosurveillance by persisting CAR T cells is likely key to achieving durable responses in adoptive cell therapy (ACT). Memory T-cell subsets appear to exist along a gradient of differentiation characterized by reciprocal potentials for longevity and effector function (4). Indeed, adoptively transferred effector CD8+ T cells derived from central memory (TCM) or naive (TN) T-cell subsets in murine and nonhuman primate models demonstrated increased therapeutic potential. Thus, T-cell subsets corresponding to an immature state of differentiation are appealing for their potential to provide superior clinical utility (5, 6).
T-memory stem cells (TSCM), so far the least differentiated memory T-cell subset identified, can be generated under specific ex vivo culture conditions (e.g., IL-7, IL-15, or small molecules targeting metabolic or developmental pathways) (7–9). This memory subset possesses the highest self-renewal capacity and therapeutic potential. Due to superior persistence in the absence of antigen-driven stimulation, TSCM are suggested to be the primary precursors of T-cell memory once antigen is cleared in an immune response (10). Furthermore, only the frequency of CD8+CD45RA+CCR7+ TSCM-like cells in the infusion product correlated with expansion of CD19-specific CAR T cells (11). Because TSCM represent only a small percentage (2–3%) of peripheral blood mononuclear cells (PBMCs) (8), strategies to manufacture TSCM suitable for human applications are essential and under development (9).
Endogenous and administered T cells receive prosurvival signals through the common cytokine receptor γ-chain, such as those signals mediated by IL-2 and IL-15, independent of native or introduced immunoreceptors. ACT trials have provided exogenous IL-2, which causes dose-limiting toxicities at high doses (12), whereas low doses may favor peripheral tolerance and regulatory T-cell production (13). Moreover, IL-2 signaling drives effector T-cell proliferation, promoting terminal differentiation and senescence (14). Conversely, IL-15 is a prosurvival cytokine that is required for homeostatic maintenance of long-lived CD8+ memory T cells (15), inhibits activation-induced cell death (AICD) (16), enhances in vivo antitumor activity (17), and reverses T-cell anergy (18). High IL15 expression in the tumor microenvironment correlates with elevated infiltration of CD3+ T cells, correlating with improved survival of patients with colorectal cancer (19). Moreover, IL-15 is required for the generation of innate-like T cells that participate in immunosurveillance and impede tumor growth (20).
IL-15 is recognized as an immunotherapeutic agent for cancer treatment (21) and is being coinfused with natural killer (NK) or T cells. Monomeric IL-15 is a small unstable protein with a short serum t1/2, necessitating supraphysiological dosing to attain in vivo responses (22). However, physiological transpresentation of IL-15 uses the IL-15 receptor alpha chain (IL-15Rα) (23, 24). Recombinant soluble IL-15 bound to a recombinant soluble IL-15Rα (referred to as IL-15 complex) augments T-cell antitumor activity (22), likely due to the qualitatively different signaling achieved upon mimicking transpresentation (25). Thus, IL-15 complex and ALT-803 (a fusion protein of IL-15 superagonist and IL-15Rα sushi domain) have enhanced the antitumor effect of responding T cells (26, 27), leading to testing in clinical trials.
To improve the persistence and potential for memory of infused T cells, we sought to harness IL-15 transpresentation by engineering a recombinant tethered variant of IL-15 coexpressed with a second-generation CAR using the Sleeping Beauty (SB) system (28). The SB system is the most clinically advanced nonviral approach to gene therapy, with recent trials demonstrating promising data related to survival of infused CD19-specific CAR T cells and recipients with B-cell malignancies (29). Signaling from membrane-bound IL-15 (mbIL15) generated CD19-specific CAR T cells with long-term persistence and superior in vivo antitumor activity. The mbIL15-CAR T cells, despite being recursively cocultured with γ-irradiated activating and propagating cells (AaPCs), exhibited an immature state of differentiation upon antigen removal, leading to sustained in vitro and in vivo persistence without aberrant T-cell proliferation. Indeed, combining expression of mbIL15 with CAR generated T cells that retained memory potential with a CD45ROnegCCR7+CD95+ TSCM-like phenotype. Because mbIL15-CAR T-cell survival appears independent of antigen, this immunotherapy may be of use not only for treating and preventing relapse when antigen burden is high but also for treating malignant diseases with low or sequestered tumor burdens.
Results
AaPC-Based Platform Generates Numerous T Cells Stably Coexpressing CAR and MbIL15 with Enhanced Signaling via Phosphorylated Signal Transducer and Activator of Transcription 5.
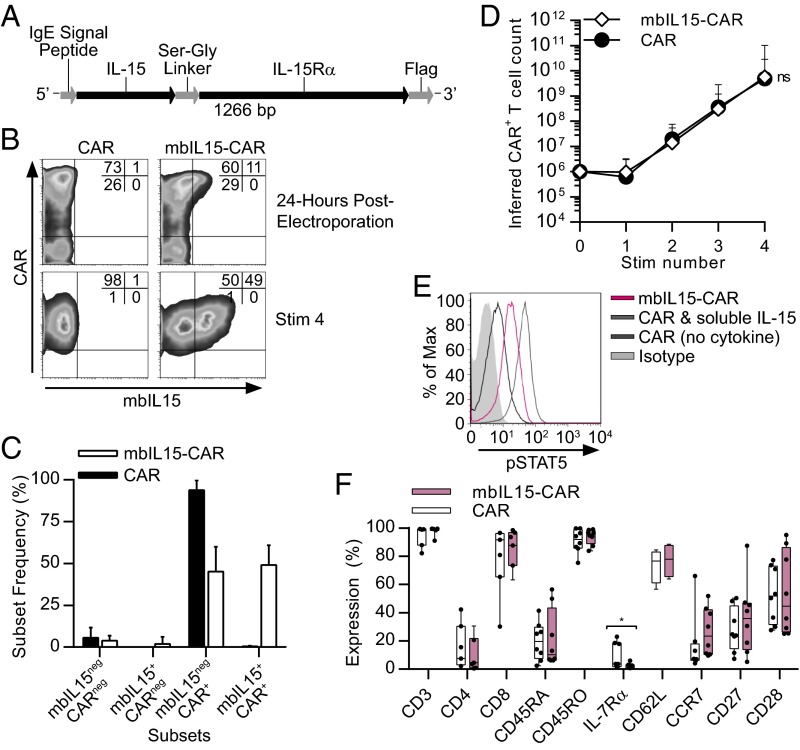
Second-generation CARs mediate signal 1 (via CD3-ζ) and signal 2 (e.g., via CD28) for T-cell activation. We designed mbIL15 for cell-surface expression to provide signal 3 (cytokine stimulation), with and without CAR-mediated signaling. To generate mbIL15, the full-length native IL-15 peptide was fused to the full-length IL-15Rα sequence via a flexible linker (Fig. 1A), mimicking presentation of IL-15 in context with IL-15Rα (30, 31). T cells from PBMCs were coelectroporated with DNA plasmids coding a CD19-specific CAR transposon (designated CD19RCD28), with or without the mbIL15 transposon and with the SB-11 transposase. These cells were propagated with small modifications from previously described methods adapted for human application (28) (SI Appendix, Fig. S1). CAR T cells generated with the SB platform were recursively stimulated using K562-derived AaPCs ex vivo to propagate genetically modified T cells in an antigen-dependent fashion to yield a high frequency of cells expressing CAR in the final product. To expand out T cells selectively coexpressing both mbIL15 and CAR after electroporation (Fig. 1B), we added recombinant soluble IL-21 along with AaPCs during ex vivo coculture to capitalize on the synergistic effect of combining IL-21 with IL-15 upon T-cell proliferation (32). After four 8- to 10-d cycles of γ-irradiated K562-derived AaPCs (clone 9), CAR expression on the CAR and mbIL15-CAR T cells was not significantly different [94% ± 6% (mean ± SD) and 94% ± 7%, respectively; P = 0.9748, n = 10; Fig. 1B). Electrotransfer of mbIL15 and CAR from two separate plasmids resulted in coexpression of mbIL15 and CAR at 51% ± 14% (mean ± SD; n = 10) on the antigen-stimulated cells, with minimal expression of mbIL15 alone (Fig. 1 B and C). Ex vivo expansion of mbIL15-CAR T cells yielded comparable inferred cell numbers to control CAR T cells (Fig. 1D). Western blotting confirmed mbIL15 protein expression with the anticipated molecular weight as a fusion protein (SI Appendix, Fig. S2A). Validation of mbIL15 function assessed phosphorylation levels of signal transducer and activator of transcription 5 (STAT5). The mbIL15-CAR T cells cultured without exogenous cytokines and CAR T cells exposed to exogenous IL-15 exhibited elevated pSTAT5 expression, relative to CAR T cells without cytokine (Fig. 1E). Other than low expression of IL-7Rα, mbIL15 expression did not alter the phenotype of ex vivo expanded CAR T cells (Fig. 1F). The mbIL15-CAR product produced by coculture with AaPCs and IL-21 was predominantly CD8+CD45RO+ T cells expressing moderate levels of CD62L and CD28 (Fig. 1F). Antigen-specific cytolysis and IFN-γ production remained intact and comparable between CAR and mbIL15-CAR T cells (SI Appendix, Fig. S2 D and E). Thus, clinically large numbers of functional mbIL15-CAR T cells possessing elevated pSTAT5 can be generated.
Fig. 1.
T cells stably coexpressing mbIL15 and CAR display enhanced costimulation via pSTAT5. (A) Diagram of the mbIL15 coding insert used within the SB DNA expression vector [IL15-IL15Ra-Flag(CoOP)/pSBSO]. (B) Transient (24-h postelectroporation) and stable [stimulation 4 (Stim 4)]) expression of mbIL15 and CAR on SB-modified T cells. A representative flow plot from one of 10 normal donors is shown. Quadrant statistics display the percentage of gated cells. (C) Frequencies of mbIL15+ and/or CAR+ subsets of CAR and mbIL15-CAR T cells. Data are mean ± SD (n = 10). (D) Inferred numeric expansion of CAR and mbIL15-CAR T cells upon serial coculture with γ-irradiated AaPC (“Stim number”) and cytokines. Data are expressed as mean ± SD (n = 6), two-way repeated measures ANOVA (Bonferroni posttest). (E) Functionality of mbIL15 was validated using phosflow of pSTAT5 expression in CAR and mbIL15-CAR T cells under serum- and cytokine-starved conditions. Positive control CAR T cells were treated with 100 ng/mL IL-15 for 1 h before fixation. A representative experiment is shown for one of six donors. Max, maximum. (F) Phenotype of CAR and mbIL15-CAR T cells was assessed by flow cytometry for T-cell markers and surface markers associated with memory and differentiation state after Stim 4 expansion. Horizontal lines indicate mean values, and dots represent individual donors. *P = 0.047 (n > 4), paired t test.
MbIL15-CAR T Cells Sustain Functional IL-15 Signaling and Antigen-Specific Response in Vitro Even in the Long-Term Absence of CAR-Mediated Activation.
Activation via IL-15 should sustain T-cell persistence, which was initially assessed in vitro by withdrawing CD19 antigen and exogenous cytokine. Propagated mbIL15-CAR T cells underwent prolonged culture without restimulation or exogenous cytokines, whereas control CAR T cells received either IL-15 complex or no exogenous cytokine (SI Appendix, Fig. S3A). As anticipated, CAR T cells without cytokine supplementation did not persist (SI Appendix, Fig. S3B). CAR T cells receiving IL-15 complex initially expanded, and then maintained cell numbers. In contrast, mbIL15-CAR T cells did not expand, but persisted without unrestricted growth, and cell viability of the groups was similar at day 65 (P > 0.05, Student’s t test; SI Appendix, Fig. S3 B and C). The mbIL15-CAR T cells maintained a constant cell number and did not grow in an unrestricted or autonomous manner. T cells persisting at and beyond 65 d under antigen-withdrawal (AWD) conditions maintained CAR expression (SI Appendix, Fig. S3 D and E). Although low surface expression of mbIL15 was observed on AWD-mbIL15-CAR T cells compared with day 0, intracellular levels were detected (72% ± 23%) (SI Appendix, Fig. S3 D and E). Moreover, we observed sustained expression of pSTAT5 and Bcl-2, a prosurvival molecule downstream of STAT5, confirming continued signaling pathway activation (SI Appendix, Fig. S3F). Both AWD-CAR (cytokine supplemented) and AWD-mbIL15-CAR T cells remained antigen-responsive, producing comparable IFN-γ levels upon coculture with CD19+ targets (P > 0.05; SI Appendix, Fig. S3G). Thus, long-term persisting mbIL15-CAR T cells retain functional mbIL15 and effector responses in the absence of CD19 TAA.
Long-Term in Vitro Antigen-Independent Persistence of MbIL15-CAR T Cells Exhibits a Molecular Profile and Phenotype Associated with TSCM.
Because IL-15 plays a crucial role in CD8+ memory T-cell homeostasis (15), we postulated that mbIL15 could support long-lived TSCM in vitro in the absence of immunoreceptor signaling. To assess whether mbIL15 conferred a survival advantage via molecular programming, we compared the mRNA levels of 479 lymphocyte-specific genes between cultured CAR and mbIL15-CAR T cells, both products of four AaPC stimulations, using a digital gene expression analysis with a lymphocyte-specific probe set (SI Appendix, Table S1). Surprisingly, we found only four differentially expressed genes (SI Appendix, Table S2), with the difference in IL7RA expression corroborated by cell-surface phenotyping (SI Appendix, Fig. S2C), indicating no initial overt molecular survival advantage for mbIL15-CAR T cells.
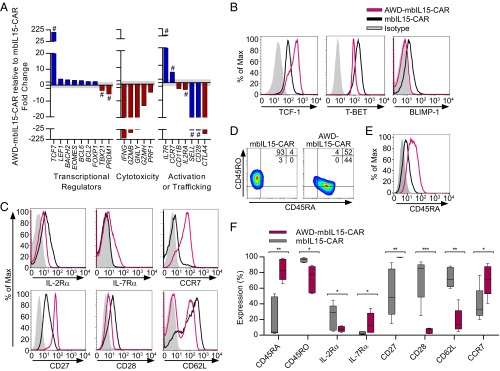
Differences emerged when comparing persisting AWD-mbIL15-CAR cells with mbIL15-CAR T cells, where 98 genes were significantly differentially expressed (SI Appendix, Fig. S4). Transcripts encoding key regulators of effector differentiation and related cytotoxic molecules showed reduced expression in AWD-mbIL15-CAR T cells: TBX21 (encoding T-BET), PRDM1 (encoding BLIMP-1), IL2RA, IFNG, PRF1, GNLY, GZMH, GZMB, CD11B, and CTLA4. Inversely, a promemory transcriptional profile indicating an immature state of differentiation was present in AWD-mbIL15-CAR T cells: TCF7 (encoding TCF-1), EOMES, BCL2, BACH2, LEF1, BCL6, FOXP1, CCR7, and IL7R (8, 33) (Fig. 2A and SI Appendix, Fig. S4). Some of these transcription factors function in pairs, with graded reciprocal activity controlling effector and memory T-cell differentiation. The ratio of TBET and EOMES is one such pairing, with the ratio lowest in memory T-cell stages (33). We observed that mbIL15-CAR and AWD-mbIL15-CAR T cells had altered mRNA ratios of TBET and EOMES (20:1–94:1 and 2:1–9:1 TBET/EOMES, respectively), with AWD-mbIL15-CAR T cells having the low ratio associated with memory phenotypes.
Fig. 2.
Prolonged in vitro antigen-independent persistence of mbIL15-CAR T cells creates a molecular profile and phenotype associated with an immature differentiation state. After four stimulation cycles with γ-irradiated AaPCs (35 total days of culture), mbIL15-CAR T cells were placed under antigen and exogenous cytokine withdrawal conditions for 65 d (100 total days of culture after electroporation). T cells persisting in culture for >65 d are referred to as “AWD-T cells.” The CD4/CD8 ratio of starting cell populations was 1:39.9 ± 58.6 (mean ± SD). (A) Comparison of the top significantly differentially expressed genes (twofold cutoff, P < 0.01, false discovery rate q value <0.05). Classified by function, these genes are associated with immature differentiation (blue bars) or more differentiated (red bars) effector T-cell characteristics. Bars represent mean fold change of AWD-mbIL15-CAR T cells relative to mbIL15-CAR T cells (n = 3). The shaded gray area denotes less than twofold change. #, verification of protein expression by flow cytometry. (B) Representative histograms of transcription factor expression associated with differentiation status. (C) Representative histograms of surface-expressed molecules associated with differentiation status. (D) Representative flow plots showing coexpression of CD45RA and CD45RO. Quadrant frequencies are displayed in the top right corner of the plots. (E) Representative histogram of CD45RA expression on CCR7+ gated cells. (F) Frequencies of respective markers from C and D. Data are plotted as box and whiskers, with the horizontal bar denoting the median. Data in B–F are representative of greater than five donors. *P < 0.05, **P < 0.01, ***P < 0.001; paired t test.
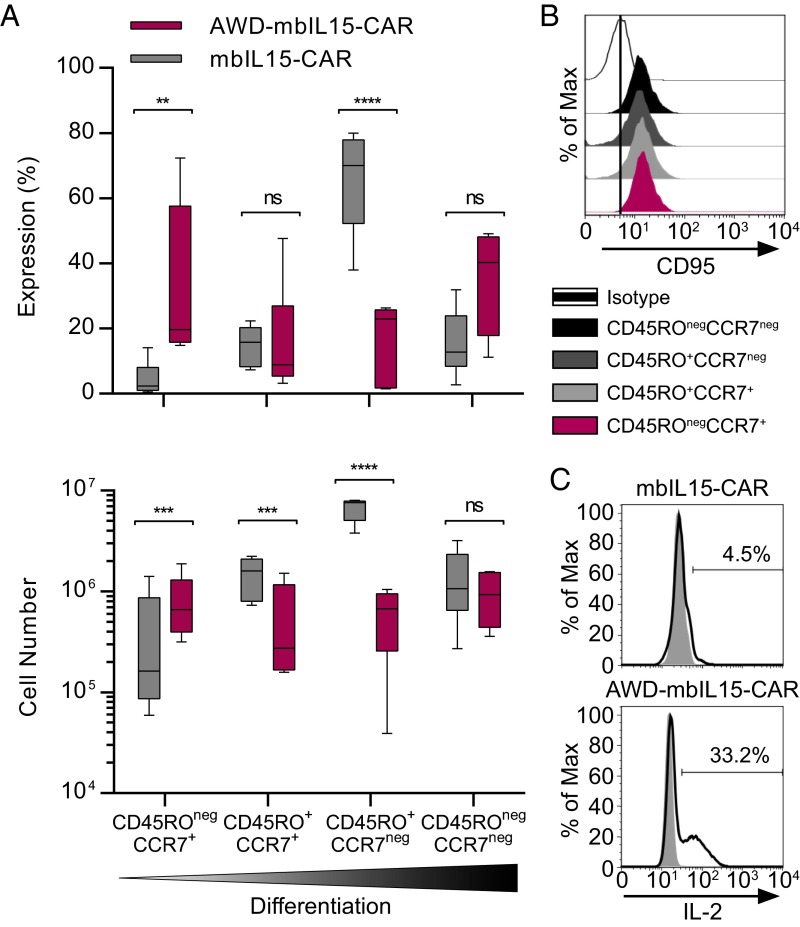
The protein expression of specific transcription factors and surface markers associated with the status of T-cell differentiation corroborated the molecular profile of a less differentiated state for AWD-mbIL15-CAR T cells, with increased expression of cell-surface markers associated with memory (IL-7Rα, CD27, and CCR7; Fig. 2 B, C, and F). CD62L and CD28 expression on AWD-mbIL15-CAR T cells was diminished compared with mbIL15-CAR T cells (Fig. 2 C and F). AWD-mbIL15-CAR T cells highly expressed CD45RA, but the majority of cells were CD45RA+CD45RO+ (Fig. 2 D and F). However, compared with mbIL15-CAR T cells, AWD-mbIL15-CAR T cells had a significant population of CD45RA+CD45ROneg cells [31% ± 17%, (mean ± SD); P = 0.0233, Student’s t test; Fig. 2D] with nearly all CCR7+ T cells coexpressing CD45RA [89% ± 10% (mean ± SD); Fig. 2E]. With an observed high coexpression of CD45RA and CD45RO, we defined T-cell memory subsets by CD45RO and CCR7 expression (34, 35). Thus, it appears that ex vivo numerical expansion (with AaPCs and IL-21) generates predominantly differentiated effector memory mbIL15-CAR T cells with a CD45RO+CCR7neg phenotype (Fig. 3A). Upon the withdrawal of antigen and exogenous cytokine, mbIL15 supported two T-cell memory subsets. Differentiated CD45ROnegCCR7neg AWD-mbIL15-CAR T-cell numbers were maintained, whereas the least differentiated CD45ROnegCCR7+ AWD-mbIL15-CAR T cells increased as much as eightfold, and the remaining two memory subsets significantly declined (Fig. 3A). All four memory subsets observed in AWD-mbIL15-CAR T cells coexpressed CD95 (Fig. 3B), thus delineating the CD45ROnegCCR7+ population as a TSCM subset. The gain of this immature T-cell subset corresponded with improved capacity for IL-2 production (Fig. 3C), because 2–8% of mbIL15-CAR and 25–46% of AWD-mbIL15-CAR T cells produced IL-2 in response to stimulation, a cardinal feature of memory cells (35). These data support our contention that mbIL15-CAR T cells contain and/or produce TSCM.
Fig. 3.
Emergence of a TSCM-like subset after prolonged in vitro antigen-independent persistence of mbIL15-CAR T cells. After four stimulation cycles with γ-irradiated AaPCs (35 total days of culture), mbIL15-CAR T cells were placed under antigen and exogenous cytokine withdrawal conditions for 65 d (100 total days of culture after electroporation). T cells persisting in culture for >65 d are referred to as “AWD-T cells.” The CD4/CD8 ratio of starting cell populations was 1:9.6 ± 10.0 (mean ± SD). (A) Memory subset (based on CD45RO and CCR7) composition (Top) and cell counts (Bottom). Data are plotted as box and whiskers, with the horizontal bar denoting the median, and are representative of greater than five donors. **P < 0.01, ***P < 0.001, ****P < 0.0001, two-way ANOVA (Bonferroni’s posttest). ns, not significant. (B) At the end time point, AWD-mbIL15-CAR T cells were assessed for CD95 expression to dissect the CD45ROnegCCR7+ population further and delineate TSCM from TN subsets. Representative histogram of one of four donors showing CD95 expression on cells gated on memory subsets from A. The vertical bar in the plot denotes a reference line based on isotype staining. (C) Representative histograms of IL-2 intracellular staining after stimulation with lymphocyte activation cocktail (black line) or no stimulation (gray) (n = 6).
To determine whether the promotion of TSCM was due to mbIL15, we conducted a modification of the withdrawal assay comparing AWD-mbIL15-CAR T cells with AWD-CAR T cells that had IL-2 or IL-15 complex support during antigen withdrawal (SI Appendix, Fig. S5A). CD45RA expression was uniformly high among all treatment groups, whereas CCR7 expression was only high on AWD-mbIL15-CAR T cells (69% ± 23%), which also had significantly reduced Annexin V levels compared with control CCR7+ AWD-CAR T cells cultured with soluble IL-2 or IL-15 complex (P < 0.05; SI Appendix, Fig. S5 B–E). Thus, mbIL15 supports the survival of CD45ROnegCCR7+ T cells, thereby supporting the persistence and/or emergence of TSCM in our system.
MbIL15-CAR T Cells Persisting in Vivo in the Absence of Antigen Demonstrate the Emergence of a CD45ROnegCCR7+ TSCM Subset.
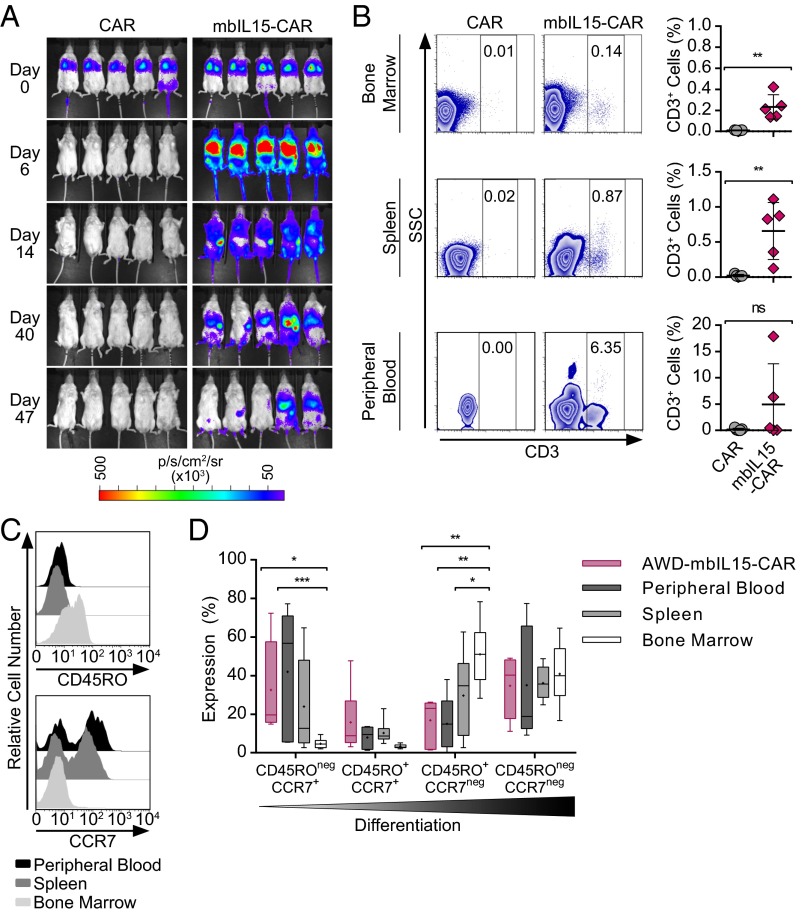
To assess if mbIL15-CAR T cells maintain prolonged persistence in vivo without CAR-mediated signaling, genetically modified T cells [mbIL15±-CAR-firefly luciferase (ffLuc)] were administered to mice without tumor or exogenous cytokine support and monitored for 48–50 d. Control CAR T cells did not persist, whereas mbIL15-CAR T cells persisted without apparent autonomous growth and were detected primarily in the bone marrow (BM) and spleen (Fig. 4 A and B and SI Appendix, Fig. S6A). Thus, it appears that mbIL15 provides sufficient support to sustain T-cell persistence in a CAR-independent manner.
Fig. 4.
Long-term in vivo persistence of mbIL15-CAR T cells facilitates the emergence of a CD45ROnegCCR7+ subset. NSG mice were injected i.v. with 2 × 107 ffLuc+CAR T cells with or without coexpression of mbIL15. The infused T-cell number was based on CAR T cells. The CD4/CD8 ratio of injected cells was CAR = 1:12 and mbIL15-CAR = 1:161. sr, steradian. (A) Longitudinal bioluminescent imaging (BLI) was performed to monitor T-cell persistence from days 0–47. Images represent photon flux from T-cell–derived ffLuc activity. (B, Left) Representative flow plots show frequencies of CD3 in the BM, spleen, and peripheral blood from tissues and blood harvested on day 48. (B, Right) Plotted frequencies of CD3+ cells in tissues and blood. Data are represented as mean ± SD (n = 5) and are representative of two experiments. *P < 0.05, **P < 0.01; unpaired t test. (C) Peripheral blood, spleen, and BM of mice were analyzed after 48 d to assess the differentiation of adoptively transferred T cells persisting long term in the absence of CAR activation. Representative histograms of CD45RO (Upper) and CCR7 (Lower) expression. (D) Memory subset composition of the in vivo T cells in reference to in vitro AWD-mbIL15-CAR T cells. Data are plotted as box and whiskers showing the median (horizontal bar) and mean (cross). *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA (Tukey’s posttest).
The phenotype and ability of mbIL15-CAR T cells to redirect specificity to CD19 were evaluated 48 d after adoptive transfer into nonobese diabetic/severe combined immunodeficiency/gc-/- (NSG) mice without tumor or exogenous cytokine support. Recovered T cells exhibited tissue-specific distribution with reciprocal expression of CD45RO and CCR7, with the highest levels observed in BM and peripheral blood, respectively (Fig. 4C). T-cell differentiation in the peripheral blood and spleen recapitulated our in vitro observations of the preferential persistence of CD45ROnegCCR7+ T cells, whereas the BM contained the highest proportion of more differentiated CD45RO+CCR7neg T cells (Fig. 4D). Similar to in vitro observations, T cells in the blood expressed moderate levels of CAR and reduced levels of cell-surface mbIL15 (SI Appendix, Fig. S6 B and C). After ex vivo numeric expansion, T cells from these tissues retained their antigen-specific function and produced IFN-γ in response to CD19+ targets (SI Appendix, Fig. S6D). Together, these data suggest that mbIL15-CAR T cells persist long term in vivo, with a substantial subset exhibiting a clinically desirable TSCM phenotype and continuing to demonstrate specificity for CD19 TAA.
We also evaluated the safety of long-term mbIL15-CAR T-cell persistence, focusing on potential for transformation or autonomous proliferation. The mbIL15-CAR T cells were maintained in continuous in vitro culture for up to 2 y in the absence of antigen restimulation and exogenous cytokine. These T cells did not demonstrate aberrant unrestricted proliferation, and all samples tested (n = 4) maintained a normal karyotype as well as polyclonal Vα/Vβ repertoires (SI Appendix, Fig. S7). In vivo studies of mice with long-term (125 d after T-cell infusion) engraftment of mbIL15-CAR T cells in the absence of tumor demonstrated no host toxicity and no evidence of aberrant proliferation of infused genetically modified T cells. Indeed, splenomegaly was not observed, and <1% of human CD3+ cells were observed at day 125 (SI Appendix, Fig. S8).
MbIL15-CAR T Cells Prevent Leukemia Engraftment and Sustain Persistence After Clearance of Tumor.
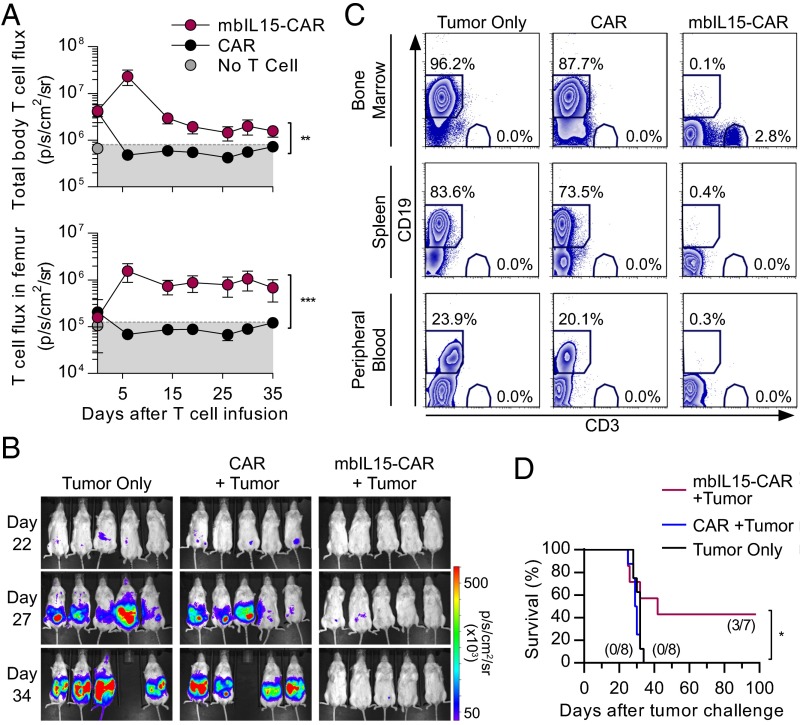
We assessed survival of mbIL15-CAR T cells in a model of minimal residual disease (MRD or low TAA burden), where adoptively transferred T cells may not immediately encounter and be stimulated by TAA, such as CD19. This assessment was achieved using a model to mimic MRD whereby CAR T cells expressing ffLuc and mbIL15-CAR-ffLuc T cells were engrafted in NSG mice, followed 6 d later by i.v. injection of CD19+NALM-6 cells. Only mbIL15-CAR T cells persisted throughout the experiment. In vivo persistence of mbIL15-CAR T cells leveled off 19 d after administration, with stable BM engraftment (Fig. 5 A and C). The mbIL15-CAR T cells displayed potent antitumor activity (Fig. 5B), with no tumor (<0.5% of CD19+ cells) detected by flow cytometry in the BM, spleen, or peripheral blood (Fig. 5C). In another experiment, mice receiving mbIL15-CAR T cells had improved survival: 43% at 98 d compared with 100% mortality in all other treatments by day 34 (Fig. 5D). These data indicate that mbIL15-CAR T cells were capable of engrafting in the absence of CD19 antigen or exogenous cytokine, mounting an antitumor response in a low TAA environment and persisting after tumor clearance, thereby making these cells more therapeutically effective in mice than conventional CAR T cells.
Fig. 5.
MbIL15-CAR T cells prevent leukemia engraftment and persist after tumor clearance. NSG mice were infused i.v. with 2 × 107 ffluc+CAR T cells with or without coexpression of mbIL15. Infused T-cell number was based on CAR+ T cells. Six days later, 1 × 105 rLuc+NALM-6 was i.v. injected The CD4/CD8 ratio of injected cells was CAR = 1:12 and mbIL15-CAR = 1:161. (A) Longitudinal plotting of T-cell flux derived from regions of interest encompassing the body (Upper) and the femurs (Lower). Background BLI (gray area) was derived by imaging mice without ffLuc+ T cells that were treated with d-luciferin. Data represent mean ± SD (n = 4–5). **P < 0.01, ***P < 0.001; unpaired t test. (B) Longitudinal BLI was performed to monitor NALM-6 tumor cell persistence. Images represent photon flux from NALM-6 cell–derived rLuc activity. (C) At day 36, tissues and blood were analyzed by flow cytometry for the presence of CD3+ T cells and CD19+ tumor cells in the three treatment cohorts. Representative flow plots show frequencies of CD3 and CD19 in the peripheral blood (n = 3–5), spleen, and BM (n = 4–5). (D) Similar experiment was continued to day 98 to assess survival (n = 7–8 per group). CD4/CD8 ratio of injected cells was CAR = 1:31 and mbIL15-CAR = 1:3. Values in parentheses represent the fraction of mice surviving to day 98. *P < 0.05 for mbIL15-CAR versus CAR T-cell treatment, log-rank (Mantel–Cox).
MbIL15-CAR T Cells Reject Established Leukemia and Sustain Persistence After Clearance of Tumor.
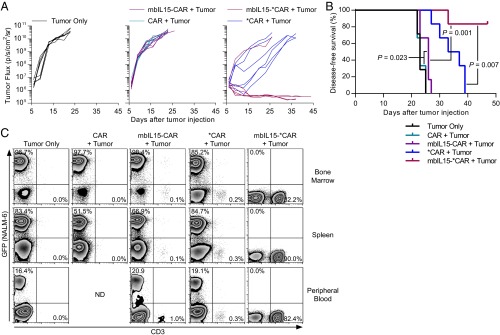
Recently, it was reported that the IgG4-Fc extracellular domain within CAR structures, such as CD19RCD28 in our initial clinical trials, may bind to innate Fcγ receptors (FcγRs), leading to deleterious clearance and reduced efficacy of infused CAR T cells (36). We hypothesized that mbIL15 can continue to support CAR T-cell persistence and act additively with an improved CAR design to augment antitumor efficacy further. Another CAR was engineered (referred as *CAR) with a mutation to eliminate FcγR binding. We reassessed mbIL15±-CAR and mbIL15±-*CAR T-cell persistence and antitumor efficacy using a model of established leukemia. NSG mice were injected with ffLuc+NALM-6, followed 6 d later by genetically modified T cells (not modified with ffLuc). The *CAR T cells demonstrated greater antitumor activity than did CAR T cells, significantly increasing survival (P = 0.0007; Fig. 6 A and B). The *CAR T-cell persistence at the experimental end point was low (Fig. 6C). Conversely, the mbIL15-*CAR T cells demonstrated superior antitumor activity, reducing tumor burden to background levels, with tumor flux significantly lower than in mice treated with *CAR T cells (P = 0.006, two-tailed Student’s t test, day 26 after T-cell administration). The *CAR T cells induced only temporary tumor regression, followed by aggressive relapse (Fig. 6A). Notably, 83% of mice treated with mbIL15-*CAR T cells exhibited tumor elimination, because no responders relapsed during the experiment and flow cytometry corroborated that these mice were tumor-free in the BM, spleen, and peripheral blood (Fig. 6 A and C). Some mice in this group began dying 28 d after T-cell injection with no tumor measured by bioluminescence and flow cytometry. Likely, these deaths were from xenograft-versus-host-disease, as noted by others using cytokine-producing CAR T cells (37). To compensate, we report disease-free survival, rather than overall survival, which was significantly greater for mice treated with mbIL15-*CAR T cells compared with *CAR T cells (P = 0.007; Fig. 6B). In vivo persistence of genetically modified T cells was only slightly enhanced by the substitution of CAR with *CAR. Substantial improvement was made when mbIL15 was coexpressed with *CAR, whereby these mice did not bear tumor at the time of sampling (Fig. 6C). These data indicate that mbIL15-*CAR T cells were capable of engrafting, mounting an antitumor response in an environment with abundant TAA, and persisting after tumor clearance with no evidence of relapse, thereby making these cells more therapeutically effective in mice irrespective of the potency of the CAR.
Fig. 6.
MbIL15-CAR T cells potently reject established systemically disseminated leukemia and persist beyond tumor clearance. NSG mice (n = 7, tumor only group; n = 6, all other T-cell treatments) were injected i.v. with 1.5 × 104 NALM-6 coexpressing GFP and ffLuc. After 6 d of engraftment, 1 × 107 genetically modified T cells were given via intracardiac injection. Infused cells were CD19-specific CAR T cells (expressing CAR or *CAR) with or without coexpression of mbIL15, and infused cell number was based on CAR+ T cells. The CD4/CD8 ratio of injected cells was CAR = 1:3, mbIL15-CAR = 1:65, *CAR = 1:25, and mbIL15-*CAR = 1:48. (A) Quantified tumor burden (ffLuc activity) was measured by BLI. Each line represents an individual animal. (B) Kaplan–Meier survival curves show tumor-free survival whereby mice were considered tumor-free if tumor flux was below background. Significance was determined by log rank (Mantel–Cox). (C) Representative flow plots of blood and tissue samples showing frequency of GFP+ (tumor) and human CD3+ (genetically modified T cells) cells harvested from mice (left to right: n = 3, n = 1, n = 2, n = 2, and n = 4).
Discussion
Adoptive transfer of CD19-specific CAR T cells has demonstrated significant antitumor effects as an investigational treatment for leukemias and lymphomas, in which therapeutic potential appears to correlate with sustained in vivo persistence (11, 38–41). Moreover, elevated IL15 expression in the tumor microenvironment correlates with improved patient survival (19). We demonstrated that, in contrast to T cells solely expressing a second-generation CD19-specific CAR, coexpression of CAR with a tethered variant of IL-15 augmented T-cell survival in vitro and in vivo that resulted in superior antitumor effects and was associated with the outgrowth of TSCM. Thus, it appears that mbIL15 mimics the transpresentation of endogenous IL-15, signaling via STAT5, without leading to aberrant proliferation.
Second-generation CARs are designed with modular costimulatory endodomains to coordinate two signals to mediate CAR-dependent T-cell activation (42), but this design may not be sufficient in all cases. When TAA is abundant, as when treating relapsed B-cell malignancies, the CAR design may be sufficient to mitigate malignant disease. Striking results have been observed in several second-generation CAR T-cell clinical trials that treated acute lymphoblastic leukemia, where complete responses were observed in 70–90% of patients (43–45). However, long-term results were not as durable, because the overall survival was reported to be 65–78% at 6–12 mo (43–45). In parallel, our mice with established leukemia treated with *CAR T cells showed 50% initial remission but 100% eventually relapsed. This is in contrast to no mice that relapsed following infusion of mbIL15-*CAR T cells (Fig. 6). This difference suggests that an mbIL15-mediated signal 3 can bolster the effects of the more potent CAR designs, and may augment the T-cell response by inhibiting AICD (16). Alternatively, mbIL15 may favor the persistence of T cells with a less differentiated state throughout the multiple activation events of an antitumor response. Conversely, when TAA is limited, such as with MRD, then activation solely through the CAR may be insufficient to maintain persistence (41). Indeed, in our model with a low leukemia burden, the survival of infused T cells and mice depended on coexpression of mbIL15 with CAR. The mbIL15-CAR T cells persisted in the absence of CD19 TAA, presumably due to sustained STAT5 phosphorylation. These observations agree with studies where engraftment was enhanced by T cells engineered to (i) express constitutively active STAT5 (46); (ii) express a chimeric cytokine receptor mediating constitutive signaling through CD122 (47); (iii) secrete monomeric recombinant IL-15 (37, 48); and (iv) express miR155, which increases AKT and STAT5 signaling (49). Thus, it appears that coordinated signaling with mbIL15 and CAR provides a pathway to improving antitumor effects across a spectrum of tumor burden. The approach of combining signals 1 and 2 via CAR with signal 3 from mbIL15 may be preferred to coinfusing CAR T cells with soluble recombinant IL-15 due to toxicity (50).
Differentiation status is a predictor of T-cell persistence with therapeutic preference given to a biological product that has not entered into terminal differentiation or replicative senescence (8). It is not clear whether adoptively transferred CAR T cells modified to receive additional costimulation (e.g., via constitutively active pSTAT5 or secreted cytokines) can modulate their activation to form or maintain memory, or if they remain perpetually supraphysiologically activated, which may raise concerns for long-term safety. We observed that mbIL15-CAR T cells harvested from TAA-free mice after 50 d persisted in a spectrum of differentiation states, including a substantial population exhibiting immature differentiation, delineated as CD45ROnegCCR7+ and consistent with the TSCM phenotype (Fig. 4). The in vivo observations recapitulated the in vitro data from long-term cultured AWD mbIL15-CAR T cells (Fig. 3) that were CD45RA+ and coexpressed CD27 and IL-7Rα, had elevated TCF-1, and were capable of IL-2 production (Fig. 2). Some transcription factors function in pairs, with graded reciprocal activity controlling effector and memory T-cell differentiation. The ratio of TBET and EOMES is one such pairing, with the ratio lowest in early memory T-cell differentiation states (33, 51), a pattern observed in mbIL15-CAR and AWD-mbIL15-CAR T cells. Long-lived CAR T cells genetically modified to secrete monomeric IL-15 had increased expression of CD45RA, and a small subset of CD45RA+CCR7+ cells (∼6%) persisted in the spleen after an antitumor response (37). We observed that ∼25% of engrafted splenic T cells were CD45ROnegCCR7+ (Fig. 4). In contrast to our results, antigen-specific T cells modified to express constitutively active STAT5 demonstrated an effector phenotype 20 d after infusion characterized by elevated T-BET levels, effector molecule expression, and diminished IL-2 production (52). However, another study that used transgenic (Tg) mice expressing a constitutively activated form of STAT5 in the T-lymphocyte compartment showed an increased number of CD8+ T cells phenotypically similar to memory-like or homeostatically proliferating T cells (53). This finding suggests that mbIL15-mediated activation of the STAT5 pathway enables the emergence of the early memory phenotype we observed. Thus, it appears that mbIL15 provides signaling to CAR T cells that delivers an advantage over other approaches to STAT5-mediated activation, possibly due to its ability to generate TSCM.
One concern for extensively ex vivo-propagated T cells is the potential for generating an exhausted product with limited antitumor efficacy. Unlike the protective effects of chimeric 4-1BB costimulation, chimeric CD28 apparently exacerbates exhaustion induced by chronic CAR signaling, whether by high tumor loads, ex vivo numeric expansion, or antigen-independent mechanisms (54). Exhausted CAR T cells express higher levels of inhibitory receptors (e.g., PD-1, TIM-3, LAG-3) and increased exhaustion-associated transcription factors (e.g., T-bet, Blimp-1). Such cells exhibit poor production of IL-2 and IFN-γ, increased expression of CD25, and decreased levels of CD27 and CD127 (54). The AWD-mbIL15-CAR T cells possessed reciprocal features: reduced LAG3 and CTLA4 transcripts (SI Appendix, Fig. S4); low T-BET, BLIMP-1, and IL2RA expression; and increased CD27 and CD127 expression (Fig. 2). The AWD-mbIL15-CAR T cells also possessed antigen-dependent IFN-γ production (SI Appendix, Fig. S3), enhanced IL-2 production (Fig. 3), and antigen-independent persistence (SI Appendix, Fig. S3). Our data indicate that mbIL15 maintains a memory subset of CAR T cells consistent with TSCM that also preserve their CAR-mediated function. Thus, although chimeric CD28 costimulation participates in T-cell activation, differentiation, and exhaustion, it appears that IL-15 signaling may block these downstream effects. Additional experiments will be needed to understand this interaction and the possible effect of mbIL15 on activating CAR T cells via chimeric CD137.
The cytokines IL-7 and IL-15 appear integral to generating CD45RA+CCR7+ early memory populations (7). We demonstrated that mbIL15, and not IL-2 or soluble IL-15 complex, helps preserve the CCR7+ subset (SI Appendix, Fig. S5). Different signaling patterns are mediated between soluble and transpresented IL-15 (25); thus, the surface expression level and specific signaling induced by the tethered mbIL15 may provide necessary and sufficient signaling to maintain the CCR7+ T-cell subset. This finding contrasts with a report that both soluble and membrane-bound IL-15Ra complexes with IL-15 (but not soluble monomeric IL-15) supported expansion of CD62L+ and CCR7+ central memory antigen-specific T cells using an in vitro cell-based cytokine production system (55). Soluble cytokines present at supraphysiological levels may induce T-cell differentiation (56), which may have been a factor contributing to the observations within our in vitro system. Nonetheless, although mechanisms for generating less differentiated T cells remain to be elucidated fully, we show that these subsets can arise in our system from signal 3 transmitted by mbIL15. This preserved persistence potential appears analogous to early memory, and, as demonstrated by others, the least differentiated subsets of adoptively transferred T cells exhibit the greatest therapeutic potential (7, 8).
Bona fide TSCM (CD45RA+CD45ROnegCD62L+CCR7+IL-7Rα+CD122+CD95+) have been generated in vitro from TN cells using a glycogen synthase kinase 3β-inhibitor to activate the WNT pathway, combined with IL-2 (8) or IL-7 and IL-21 (9) cytokine supplementation. TSCM, albeit CD45RO+, have also been produced using IL-7 and IL-15 ex vivo (7). Our TSCM population of mbIL15-CAR T cells (CD45RA+CD45ROnegCCR7+CD122+CD95+) had exposure to IL-21– and IL-15–mediated signaling during ex vivo numeric expansion, and then only to IL-15 (in the form of mbIL15) signaling, during the 65-d antigen and cytokine withdrawal assay to generate AWD-mbIL15-CAR T cells. Furthermore, TSCM recovered from mice apparently only had access to mbIL15. Although it cannot be ruled out that IL-21 could have a programming effect on the cells, it was primarily upon removal of IL-21 and antigen (presented by AaPCs) that we observed the promotion and outgrowth of the mbIL15-CAR TSCM subset. Previously, it has been shown that IL-15 alone could produce a small population of TSCM, but combination with IL-7 improved these numbers (7, 57). Thus, we contend that the desired TSCM subset can arise solely upon IL-15–mediated signaling as induced by mbIL15.
In contrast to observations of IL-7– and IL-15–generated TSCM with no apparent up-regulation of the WNT pathway (7), we noted increased expression of genes in this pathway (TCF7, LEF1, and AXIN2; SI Appendix, Fig. S4), which are regulated by Akt (51, 58). Moreover, because AXIN2 is a direct target of the WNT pathway through TCF-1 and LEF-1 activity, its up-regulation suggests that WNT signaling is active in AWD-mbIL15-CAR T cells. Furthermore, Akt signal strength regulates the WNT pathway, where weaker signaling sustains a memory phenotype (51), suggesting that molecular pharmacology may be beneficial when using exogenous cytokines or priming conditions that can strongly activate Akt (8, 9, 59). Moreover, our data demonstrated the promotion of an mbIL15-CAR TSCM-like subset, suggesting that mbIL15 only weakly activates Akt.
Our study has benefited from an approach to genetically modifying a population of T cells, which has appeal for the manufacture of a clinical grade mbIL15-CAR product. In our antigen and cytokine withdrawal assay, we observed a decline in CD45RO+CCR7+ T-cell numbers with a concomitant increase of the CD45ROnegCCR7+ TSCM pool. This finding may be due to cell death in the CD45RO+CCR7+ subpopulation with proliferation in the CD45ROnegCCR7+ subset. However, this process appears unlikely, considering reports that IL-15 is critical to supporting TCM cells (60). Alternatively, it has been shown that a proportion of sorted TCM cells dedifferentiated to a CD45ROnegCCR7+ phenotype in the presence of IL-7 and IL-15 and in the absence of antigen (57). At this time, we find dedifferentiation to be a plausible and likely predominant mechanism generating the mbIL15-CAR TSCM-like cells rather than preferential propagation of small numbers of TSCM in the genetically modified founder population.
Antigen-experienced T cells can drive precocious differentiation of TN cells within mixed populations that impairs antitumor immunity (61). In our system, we modify, ex vivo expand, and infuse cell products containing a mixed grouping of memory T-cell subsets. The heterogeneous mbIL15-CAR T-cell cultures show the emergence of TSCM both in vitro and in vivo when removed from antigen. This preserved potential may have clinical appeal, because the least differentiated T-cell subset mediated the greatest therapeutic potential (7, 8). It is currently not feasible to treat patients with naturally occurring TSCM due to their low circulating frequency; thus, identification of strategies to generate, or preserve the potential of, clinically relevant numbers of genetically modified TSCM would likely contribute to the advancement of CAR T-cell–based therapies. The results presented herein show that, in our system, mbIL15 promoted a TSCM-like population of CAR-modified T cells, and thus bolster the rationale for using mbIL15 in further developing clinically compliant strategies to augment this desirable T-cell subset in immunotherapy infusion products.
Supporting T-cell longevity via IL-15 may have risk, because chronic high exposure to IL-15 could cause aberrant T-cell proliferation or toxicities. In humans, dysregulated IL-15 production, elevated serum levels, or abnormal IL-15 signaling has been associated with autoimmune disease and may be involved in the pathogenesis of large granular lymphocytic leukemia (62) and cutaneous T-cell lymphoma (63). This concern is heightened, given that Tg mice engineered to overexpress IL-15 developed leukemia (64, 65); another form of mbIL15 led to autonomous growth of NK cells (66); a T-cell clone modified to secrete IL-15 led to aberrant autonomous T-cell growth (67); and in IL-15Rα-IL-15 double-Tg mice, cis IL-15Rα expression on leukemic CD8 T cells was required for the development of uncontrolled lymphoproliferation (65). Additionally, it has been reported that excessive IL-15 can cause malignant transformation of CD4 T cells via inhibition of a negative autoregulatory loop (63). The common thread among these studies is T-cell supraphysiological activation from elevated levels of IL15-Rα and/or soluble IL-15. However, we provide an alternative to IL-15 hyperstimulation. We demonstrated that mbIL15-CAR T cells had submaximal pSTAT5 levels relative to control CAR T cells stimulated with exogenous IL-15 and that these cells attained a desired less differentiated phenotype. In other studies, T cells constitutively expressing activated STAT5 demonstrated enhanced persistence but not transformation (46), despite an activated effector phenotype (46, 52). In summary, contrasting the previous studies (62, 63, 65, 67), our assessments of AWD-mbIL15-CAR T cells did not demonstrate (i) log growth in vitro, (ii) uncontrolled expansion in vivo, (iii) aneuploidy, (iv) a homogeneous differentiated or activated population, or (v) clonal outgrowth, all of which predict favorable human application of mbIL15-CAR T cells. It is significant that our study demonstrated long-term engraftment of T cells without dysregulated proliferation or activation and no phenotypic, functional, or chromosomal anomalies.
In summary, we demonstrate that CAR T cells can be engineered to coexpress a tethered form of IL-15 that supports in vivo persistence and maintenance of an immature state of differentiation through in vitro culture and in vivo engraftment. The signal 3 provided by mbIL15 alone gave rise to CD45ROnegCCR7+ TSCM despite multiple rounds of ex vivo T-cell activation. Our observations suggest that mbIL15 may provide a desired signaling enabling infused T cells to prolong persistence, thereby providing durable immune surveillance and therapeutic potential.
Methods
Animal studies were performed in accordance with guidelines set forth by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee (A3343-01, protocol 03-06-04333). Human mononuclear cell buffy coats from anonymized healthy donors were obtained from a US Food and Drug Administration-approved vendor or blood bank (Key Biologics or the Gulf Coast Regional Blood Center). Cell lines and their propagation, genetic modification of T cells using SB, immunophenotyping, chromium release assay, and BLI were previously described (28, 68–71). Detailed information about experimental procedures, antibodies, mice, and statistical analyses can be found in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Carl June (University of Pennsylvania) for helping to generate the K562-derived AaPC clone 9 cell line and Dr. Perry Hackett (University of Minnesota) for his help with the SB system. Our appreciation goes to Dr. George McNamara (M. D. Anderson Cancer Center) and Dr. Judy Moyes (M. D. Anderson Cancer Center) for editing. Based on this work, L.V.H. was a recipient of the Andrew Sowell-Wade Huggins Scholarship in Cancer Research (Cancer Answers Foundation) and Center for Clinical and Translational Sciences T32 Grant Trainee support (NIH). This work was supported by funding from the Center for Clinical and Translational Sciences, which is funded by the National Center for Advancing Translational Sciences of the NIH under Award TL1TR000369; Cancer Center Core Grant (CA16672); Research Project Grants R01 (CA124782, CA120956, and CA141303); Research Program Project P01 (CA148600); Specialized Programs of Research Excellence Grants (CA100632, CA136411, and CA00632); Albert J. Ward Foundation; Alex’s Lemonade Stand Foundation; Burroughs Wellcome Fund; Cancer Prevention and Research Institute of Texas; Charles B. Goddard Foundation of Texas; CLL Global Research Foundation; Energy Transfer Partners; Estate of Noelan L. Bibler; Gillson Longenbaugh Foundation; Harry T. Mangurian, Jr., Fund for Leukemia Immunotherapy; Khalifa Bin Zayed Al Nahyan Foundation; Kleberg Foundation; Leukemia and Lymphoma Society; Lymphoma Research Foundation; Miller Foundation; Mr. Herb Simons; Mr. and Mrs. Joe H. Scales; Mr. Thomas Scott; National Foundation for Cancer Research; Pediatric Cancer Research Foundation; Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy; University of Texas M. D. Anderson Cancer Center Sister Institution Network Fund and Moon Shot Fund; and William Lawrence and Blanche Hughes Children’s Foundation. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement: Some of the technology described was advanced through research conducted at the M. D. Anderson Cancer Center by L.J.N.C. The M. D. Anderson Cancer Center, L.J.N.C., L.V.H., K.C.S., H.S., T.M., S.M., S.O., B.R., M.-A.F., H.H., A.M.N., R.E.C., and D.A.L. all have or had a financial interest in ZIOPHARM Oncology, Inc., and Intrexon Corporation. On May 7, 2015, L.J.N.C. was appointed as the Chief Executive Officer at ZIOPHARM. L.J.N.C. is now a Visiting Scientist at the M. D. Anderson Cancer Center. A patent application based on this manuscript has been filed.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610544113/-/DCSupplemental.
References
- 1.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savoldo B, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: Building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12(10):671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger C, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinrichs CS, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117(3):808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cieri N, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 8.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatino M, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. 2016;128(4):519–528. doi: 10.1182/blood-2015-11-683847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugli E, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123(2):594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123(24):3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alwan LM, et al. Comparison of acute toxicity and mortality after two different dosing regimens of high-dose interleukin-2 for patients with metastatic melanoma. Target Oncol. 2014;9(1):63–71. doi: 10.1007/s11523-013-0276-7. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka K, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra43. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pipkin ME, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 16.Marks-Konczalik J, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97(21):11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebanoff CA, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101(7):1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teague RM, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12(3):335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 19.Mlecnik B, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med. 2014;6(228):228ra37. doi: 10.1126/scitranslmed.3007240. [DOI] [PubMed] [Google Scholar]
- 20.Dadi S, et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell. 2016;164(3):365–377. doi: 10.1016/j.cell.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 22.Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177(9):6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, et al. IL-15 superagonist-mediated immunotoxicity: Role of NK cells and IFN-γ. J Immunol. 2015;195(5):2353–2364. doi: 10.4049/jimmunol.1500300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112(12):4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci USA. 2007;104(2):588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kermer V, Baum V, Hornig N, Kontermann RE, Müller D. An antibody fusion protein for cancer immunotherapy mimicking IL-15 trans-presentation at the tumor site. Mol Cancer Ther. 2012;11(6):1279–1288. doi: 10.1158/1535-7163.MCT-12-0019. [DOI] [PubMed] [Google Scholar]
- 27.Rhode PR, et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res. 2016;4(1):49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh H, et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS One. 2013;8(5):e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kebriaei P, et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest. 2016;126(9):3363–3376. doi: 10.1172/JCI86721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortier E, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem. 2006;281(3):1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 31.Rowley J, Monie A, Hung CF, Wu TC. Inhibition of tumor growth by NK1.1+ cells and CD8+ T cells activated by IL-15 through receptor beta/common gamma signaling in trans. J Immunol. 2008;181(12):8237–8247. doi: 10.4049/jimmunol.181.12.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360(6401):264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 36.Jonnalagadda M, et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther. 2015;23(4):757–768. doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115(17):3508–3519. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins PF, et al. Cutting edge: Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173(12):7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowolik CM, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 43.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JH, et al. Implications of minimal residual disease negative complete remission (MRD-CR) and allogeneic stem cell transplant on safety and clinical outcome of CD19 targeted 19-28z CAR modified T cells in adult patients with relapsed, refractory B-cell ALL. Blood. 2015;126(23):682. [Google Scholar]
- 46.Grange M, et al. Activated STAT5 promotes long-lived cytotoxic CD8+ T cells that induce regression of autochthonous melanoma. Cancer Res. 2012;72(1):76–87. doi: 10.1158/0008-5472.CAN-11-2187. [DOI] [PubMed] [Google Scholar]
- 47.Hunter MR, et al. Chimeric γc cytokine receptors confer cytokine independent engraftment of human T lymphocytes. Mol Immunol. 2013;56(1-2):1–11. doi: 10.1016/j.molimm.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Hoyos V, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji Y, et al. miR-155 augments CD8+ T-cell antitumor activity in lymphoreplete hosts by enhancing responsiveness to homeostatic γc cytokines. Proc Natl Acad Sci USA. 2015;112(2):476–481. doi: 10.1073/pnas.1422916112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conlon KC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim EH, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188(9):4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grange M, et al. Active STAT5 regulates T-bet and eomesodermin expression in CD8 T cells and imprints a T-bet-dependent Tc1 program with repressed IL-6/TGF-β1 signaling. J Immunol. 2013;191(7):3712–3724. doi: 10.4049/jimmunol.1300319. [DOI] [PubMed] [Google Scholar]
- 53.Burchill MA, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: Development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171(11):5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 54.Long AH, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan AN, et al. Soluble and membrane-bound interleukin (IL)-15 Rα/IL-15 complexes mediate proliferation of high-avidity central memory CD8(+) T cells for adoptive immunotherapy of cancer and infections. Clin Exp Immunol. 2016;186(2):249–265. doi: 10.1111/cei.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castro I, Yu A, Dee MJ, Malek TR. The basis of distinctive IL-2- and IL-15-dependent signaling: Weak CD122-dependent signaling favors CD8+ T central-memory cell survival but not T effector-memory cell development. J Immunol. 2011;187(10):5170–5182. doi: 10.4049/jimmunol.1003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101(11):4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 58.Wu D, Pan W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Waart AB, et al. Inhibition of Akt signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood. 2014;124(23):3490–3500. doi: 10.1182/blood-2014-05-578583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyman O, Krieg C, Homann D, Sprent J. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci. 2012;69(10):1597–1608. doi: 10.1007/s00018-012-0968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klebanoff CA, et al. (2015) Klebanoff CA, et al. (2016) Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest 126(1):318–334. [DOI] [PMC free article] [PubMed]
- 62.Mishra A, et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell. 2012;22(5):645–655. doi: 10.1016/j.ccr.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mishra A, et al. Mechanism, consequences, and therapeutic targeting of abnormal IL15 signaling in cutaneous T-cell lymphoma. Cancer Discov. 2016;6(9):986–1005. doi: 10.1158/2159-8290.CD-15-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fehniger TA, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193(2):219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato N, et al. Development of an IL-15-autocrine CD8 T-cell leukemia in IL-15-transgenic mice requires the cis expression of IL-15Rα. Blood. 2011;117(15):4032–4040. doi: 10.1182/blood-2010-09-307504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imamura M, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124(7):1081–1088. doi: 10.1182/blood-2014-02-556837. [DOI] [PubMed] [Google Scholar]
- 67.Hsu C, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109(12):5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denman CJ, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1):e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh H, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71(10):3516–3527. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper LJ, et al. T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 71.Singh H, et al. Combining adoptive cellular and immunocytokine therapies to improve treatment of B-lineage malignancy. Cancer Res. 2007;67(6):2872–2880. doi: 10.1158/0008-5472.CAN-06-2283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.