Abstract
Mycobacterium tuberculosis (M.tb) imposes a large global health burden as the airborne agent of tuberculosis. M.tb has been flourishing in human populations for millennia and is therefore highly adapted to the lung environment. Alveolar macrophages (AMs), a major host cell niche for M.tb, not only phagocytose inhaled microbes and particulate matter but are also crucial in catabolizing lung surfactant, a lipid-protein complex that lines the alveolar spaces. Since macrophage host defense properties can be regulated by surfactant and M.tb can use host lipids as a carbon source during infection, we sought to determine the receptor(s) involved in surfactant lipid uptake by human macrophages and whether the presence of those lipids within macrophages prior to infection with M.tb enhances bacterial growth. We show that preformed scavenger receptor CD36 is redistributed to the cell membrane following exposure to surfactant lipids and surfactant protein A (SP-A). Subsequently, surfactant lipids and/or SP-A enhance CD36 transcript and protein levels. We show that CD36 participates in surfactant lipid uptake by human macrophages, as CD36 knockdown reduces uptake of dipalmitoylphosphatidylcholine (DPPC), the most prevalent surfactant lipid species. Finally, exposing human macrophages to surfactant lipids prior to infection augments M.tb growth in a CD36-dependent manner. Thus, we provide evidence that CD36 mediates surfactant lipid uptake by human macrophages and that M.tb exploits this function for growth.
Introduction
Mycobacterium tuberculosis (M.tb), the bacterium responsible for tuberculosis (TB), is a prominent global health threat: TB causes a human death every twenty seconds (1). In conjunction with this alarming death rate, M.tb’s escalating ability to resist antibiotic treatment necessitates increasing our understanding of the host response during TB. M.tb resides latently within a third of the world’s population and circulates among humans by airborne transmission. Thus, characterizing endogenous lung processes which are encountered by M.tb upon inhalation into the alveolar spaces is crucial, because homeostatic mechanisms of the healthy lung are likely exploited by M.tb to enhance its success early during infection.
Alveolar macrophages (AMs), the initial host cell niche for M.tb (2), exhibit a tissue-specific immunoregulatory phenotype (3–5) which is shaped in part by their exposure to lung surfactant, a lipid-protein complex which is necessary for normal pulmonary function (6–21). Surfactant lines the alveolar spaces and lowers surface tension across the tissue, thereby reducing the energy required for inhalation and preventing alveolar collapse during expiration (22). Surfactant is a phospholipid monolayer predominantly composed of phosphocholine-based lipids, primarily dipalmitoylphosphatidylcholine (DPPC), with smaller amounts of other phospholipids and cholesterol. The four surfactant proteins (SP-A – D) comprise roughly ten percent of the total composition of surfactant. SP-B and SP-C are small hydrophobic proteins and contribute to proper spreading of the phospholipid monolayer (23). SP-A and SP-D are larger hydrophilic proteins with important functions in pulmonary innate immune responses (22).
AM phenotype and behavior are influenced by surfactant exposure, which has major implications for AM-mediated immune responses in pulmonary tissue. For example, SP-A inhibits signaling through toll-like receptor 2 (TLR2), a pattern recognition receptor which is important in the host response to M.tb infection (11). Surfactant lipids inhibit NF-κβ activation and increase macrophage production of the anti-inflammatory cytokine IL-10 following LPS exposure (10). In addition to AM immunoregulation by surfactant components, AMs are active in surfactant lipid catabolism (24, 25) and are indispensable for surfactant homeostasis (26, 27). Due to the prominent role of surfactant in shaping the AM immune response repertoire and the contributions of AMs to pulmonary homeostasis, it is crucial to incorporate aspects of the lung environment, such as surfactant, into experiments addressing macrophage responses to M.tb, a predominantly pulmonary pathogen. Furthermore, characterizing AM-surfactant interactions in the absence of infection will enable us to understand the environment into which M.tb is inhaled and successfully establishes a pulmonary infection. An important gap in our knowledge regarding the endogenous lung environment is the mechanism(s) of surfactant lipid uptake by AMs. Although surfactant lipid uptake by AMs is receptor-mediated (28), the receptor(s) involved have yet to be identified. We therefore sought to identify which receptor(s) may contribute to surfactant lipid uptake by human macrophages.
Scavenger receptor CD36 mediates uptake of palmitate by type II pneumocytes (29), the cells which synthesize surfactant. CD36 imports fatty acids into a variety of tissues, such as cardiac and skeletal muscle (30), and is the major receptor for the uptake of oxidized low density lipoprotein (oxLDL), thereby contributing to the development of foamy macrophages (FMs) during atherosclerosis (31). CD36 also interacts with various TLRs to mediate their location and cellular signaling responses during both infection and sterile inflammation (32–35).
CD36 is expressed on AMs (36–38) and has been implicated in the establishment and progression of TB in several animal models (39, 40), although data indicating that CD36 is dispensable for long-term control of TB in the murine model have been reported as well (41). In contrast to potentially enabling pulmonary colonization by M.tb, CD36 seems to provide a host-protective function during infections with other pathogens (42–45). This discrepancy between the roles of CD36 during TB relative to other infectious diseases led us to hypothesize that CD36 performs a homeostatic function in the lung, such as constitutive surfactant lipid uptake by macrophages, which M.tb capitalizes on early during airborne infection.
M.tb uses host lipids as a carbon source (46–48), which may contribute to the bacterium’s successful persister strategy (49, 50). TB results in disrupted host lipid metabolism during later stages of disease (51) and post-primary reactivation TB has historically been described as lipid pneumonia (52). During in vitro infection, M.tb induces macrophages to acquire a lipid-laden “foamy” phenotype (48). However, we speculate that the surfactant lipid-rich lung environment constitutes an endogenously favorable setting for M.tb, thereby necessitating less manipulation of the host by the bacteria during early infection. Due to their role in surfactant catabolism, AMs are an endogenously lipid-filled cell, a tissue-specific phenotype which was described decades ago (53, 54). We therefore hypothesized that CD36 functions as an uptake receptor for surfactant lipids and that constitutively present surfactant lipids and metabolites enable enhanced M.tb growth in macrophages during early infection.
Herein we show that surfactant lipids and SP-A increase CD36 surface expression by inducing translocation of preformed CD36 to the cell membrane. Subsequently, these surfactant components increase CD36 transcript and protein levels over days in culture. We further show that CD36 knockdown macrophages have a significantly diminished ability to acquire DPPC. Finally, we show that exposure of macrophages to surfactant lipids prior to M.tb infection enhances bacterial growth, but only if CD36 is present. Thus, CD36 location and expression are regulated by surfactant and the presence of CD36 and surfactant lipids enhances M.tb growth in human macrophages.
Materials and Methods
Reagents and antibodies
RPMI 1640 +L-Glutamine was purchased (Life Technologies, Carlsbad, CA, USA) and supplemented with donor autologous serum for cell culture or with 20mM HEPES buffer (Sigma Chemical Co, St. Louis, MO, USA), pH 7.2 and 1 mg/mL human serum albumin (HSA) (Calbiochem Corp., La Jolla, CA, USA) during M.tb infection. For confocal microscopy, anti-CD36 (sc-7309, Santa Cruz) and anti-SR-A (AB5486, EMD Millipore) Abs were used, followed by AlexaFluor (AF) 488- or 647-conjugated goat-anti-mouse IgM or AF568-conjugated goat-anti-rabbit IgG (Invitrogen, Waltham, MA, USA). Isotype controls were mouse IgM and rabbit IgG (Ancell Corporation, Stillwater, MN, USA). Survanta (bovine-derived surfactant lipids lacking SP-A and SP-D) was from Abbott pharmaceuticals (Abbott Park, IL, USA). SP-A was purified from the bronchoalveolar lavage of alveolar proteinoisis patients as described (55). DiI oxLDL was from Alta Aesar (Haverhill, MA, USA), DiI acLDL from ThermoFisher Scientific (Waltham, MA, USA) and 1-palmitoyl-2-{12-[(7-nitro-2-1,3-benzoxadiazole-4-yl)amino] dodecanoyl}-sn-glycero-3-phosphocholine (hereafter referred to as NBD-DPPC) and 1-palmitoyl-2-{12-[(7-nitro-2-1,3-benzoxadiazole-4-yl)amino] dodecanoyl}-sn-glycero-3-[phospho-rac-(1-glycerol)] (hereafter referred to as NBD-PG) from Avanti Polar Lipids (Alabaster, AL, USA). Pam3Cys was purchased from Calbiochem (EMD Biosciences, La Jolla, CA, USA). Human anti-TNFα and anti-IL-6 DuoSet ELISA development kits were purchased from R&D Systems (Minneapolis, MN, USA).
Human monocyte-derived macrophage and alveolar macrophage isolation and cultivation
Human peripheral blood mononuclear cells (PBMCs) were isolated from the heparinized blood of healthy donors on a Ficoll-Hypaque (Amersham, Pittsburgh, PA, USA) cushion as described (56). PBMCs were then cultured in Teflon wells in RPMI 1640 + 20% donor autologous serum for 5 days at 37°C, 5% CO2 (56). During this time, monocytes differentiate into monocyte-derived-macrophages (MDMs). Experiments were conducted in duplicate or triplicate wells using MDM monolayers in tissue culture plates. Human alveolar macrophages (HAMs) were isolated from the BAL of healthy human donors (8). PBMC and HAM protocols were approved by The Ohio State University (OSU) IRB.
Confocal Microscopy
MDMs (1.5 x 105) adhered to glass coverslips in 24-well tissue culture plates were exposed to SP-A (10 μg/mL), Survanta (100 μg/mL) or both for various times. Monolayers were fixed with 4% PFA for 10 min in the dark at room temperature, washed and left intact or permeabilized by a one minute methanol exposure (6). Coverslips were blocked overnight at 4° (PBS + 5% BSA + 10% FBS) and labeled with anti-CD36 (1:200 for 1h at room temperature) or SR-A (1:50 for 1h at room temperature) primary Abs and AF secondary Abs (1:500 for 1h at room temperature). HAMs (1 x 105) were adhered to coverslips for 2h, washed, fixed with 4% PFA, blocked overnight at 4° and immunolabeled for CD36. Coverslips were mounted on glass slides using ProLong Gold AntiFade Mounting media plus DAPI (Invitrogen Life Technologies) and viewed using a FluoView 1000 Laser Scanning Confocal microscope (Olympus). For MDM experiments, the mean fluorescence intensity (MFI) of random confocal images was quantified using pixel intensity measurement (NIH Image J program). The MFI was calculated for approximately 150 MDMs per coverslip, from duplicate slides for each experiment. To evaluate intracellular lipid content, HAMs and MDMs on coverslips were labeled with 2.5 μg/mL Bodipy (Life Technologies) for 30 min in the dark at room temperature and imaged by confocal microscopy.
CD36 knockdown in human macrophages and exposure to surfactant lipids
Day 6 MDMs were incubated with 25 nM of CD36, SR-A or scramble control siRNA (SMARTPool, Dharmacon, Lafayette, LA, USA) in Mirus TransitX2 transfection solution (Mirus Bio LLC, Madison, WI, USA) per the manufacturer’s protocol for 72h. Knockdown efficacy was determined by confocal microscopy and Western blotting (WB). MDMs were washed once with warm RPMI and exposed to 10 μg/mL DiI oxLDL or DiI acLDL, or 20 μg/mL NDB-DPPC or NBD-PG in RPMI + 2% donor autologous serum for 30 min, followed by fixation and labeling for confocal microscopy.
Quantitative real time PCR
Macrophage RNA was isolated using TRIzol reagent (Invitrogen Life Technologies) (57). RNA purity and quality were determined using a NanoDrop 1000 spectrophotometer (ThermoFisher Scientific). Total RNA was reverse transcribed to cDNA using SuperScriptIII reverse transcriptase (Invitrogen Life Technologies) (57). Quantitative real-time PCR (qRT-PCR) was conducted using human CD36 TaqMan gene expression systems (Applied Biosystems). Negative controls consisted of no-reverse transcriptase and no-template reactions. All samples were run in triplicate using a cfx96 real-time system (Bio-Rad) and analyzed using the threshold cycle (2−ΔΔct) method (58). Gene expression was normalized against β–actin.
M.tb strains and macrophage infection
M.tb H37Rv (ATCC #25618) and a luciferase expressing reporter strain (M.tb-Lux) containing the plasmid pMV306hsp+Lux (59) were used (56). Day 6 MDMs (4 x 105/ml) were seeded in a 24 well tissue culture plate and either exposed to Survanta (100 μg/ml) for 48h prior to infection or transfected with scramble or CD36 siRNA for 48h and then exposed to Survanta for 24h. MDMs were infected with M.tb H37Rv or M.tb-Lux (MOI 1:1) at 37°C with 5% CO2 on a platform shaker for 30 min, followed by 90 min incubation without shaking. Monolayers were washed, repleted with RPMI containing 2% autologous serum and incubated up to 72h. Bacterial growth was measured by luciferase activity (59) or supernatants were collected for ELISAs.
ELISAs
Day 6 MDMs were exposed to Survanta (100 μg/mL) or left resting for 48h prior to infection with M.tb H37Rv at an MOI of 1:1 as described above. Cell free supernatants were collected 24, 48 and 72h after infection. Alternatively, day 6 MDMs were transfected with SC or anti-CD36 siRNA via the MirusX2 transfection system for 72h. Monolayers were washed and exposed to Pam3Cys (100 ng/mL) and cell free supernatants were collected after 1 or 24h. ELISAs were conducted to evaluate TNFα and IL-6 release per the manufacturer’s protocol (R&D Systems) using triplicate supernatants from a minimum of three independent experiments.
Statistical Analysis
Experiments were conducted in duplicate (confocal, infections) or triplicate (gene expression, ELISAs) using MDMs from a minimum of three different donors. Results were converted to fold or percent change relative to internal controls for each experiment, due to the variability in absolute levels among donors. Prism-5 software (Version 5.04; GraphPad) was used to determine the statistical significance of differences in the means of experimental groups using an unpaired, one-tailed Student t-test. p values < 0.05 were considered significant.
Results
Surfactant exposure induces translocation of preformed CD36 from an intracellular pool to the cell membrane of human macrophages
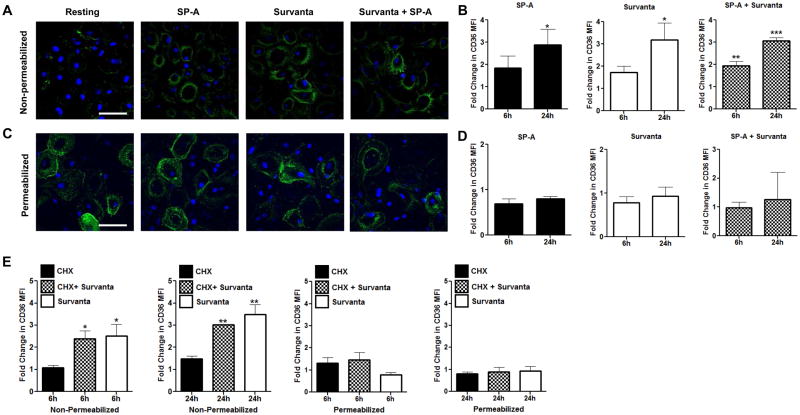
Previous publications using rat skeletal muscle and cardiac myocytes demonstrated that preformed CD36 is redistributed to the cell surface from an intracellular pool following exposure to various stimuli (60, 61) and MDMs have been shown to contain preformed CD36 (36). We therefore initially investigated whether surfactant alters the cellular distribution of preformed CD36 in MDMs by evaluating CD36 levels and location in permeabilized and non-permeabilized macrophages which had been exposed to surfactant components. MDMs were cultured in SP-A (10 μg/mL), Survanta (100 μg/mL), both or left untreated as a control for 6 or 24h and surface localization of CD36 in non-permeabilized MDMs was determined by confocal microscopy. Although untreated MDMs demonstrate low level CD36 surface expression, surface localization is increased by 3–4 fold 24h after surfactant exposure (figure 1A, B). Regardless of exposure to surfactant, MDMs in tissue culture for 24h contain preformed intracellular CD36 as determined by quantifying CD36 abundance in permeabilized MDMs (figure 1C, D). Cyclohexamide treatment (9) does not impair redistribution of CD36 to the cell surface after Survanta exposure (figure 1E) indicating that protein synthesis is not needed prior to surfactant-induced redistribution. Therefore, we conclude that surfactant induces trafficking of preformed CD36 to the plasma membrane of human macrophages.
FIGURE 1. Surfactant induces redistribution of preformed CD36 to the cell surface.
MDMs on coverslips were exposed to SP-A (10 μg/mL), Survanta (100 μg/mL), both or left untreated for 6 or 24h. Monolayers were fixed with PFA, labeled with anti-CD36 primary Ab followed by AlexaFluor488 secondary Ab and imaged using confocal microscopy. (A) Representative image for the 24h time point shows increased CD36 (green) surface localization relative to untreated control cells. Scale bar, 50 μm. (B) Surface exposed CD36 graphed as fold change in mean fluorescence intensity (MFI) relative to untreated cells. (C) Representative image of total CD36, determined by permeabilizing monolayers prior to labeling with anti-CD36 Ab. Scale bar, 50 μm. (D) Results of permeabilization experiments are graphed as fold change in CD36 MFI relative to untreated cells. (E) MDMs were exposed to CHX for 60 min prior to Survanta. CD36 surface expression on MDMs is graphed as fold change relative to DMSO control. All graphs show cumulative results of 3 (C–E) or 4 (A, B) independent experiments conducted in duplicate (mean ± SEM).
Surfactant exposure increases CD36 transcript and protein levels over days in culture
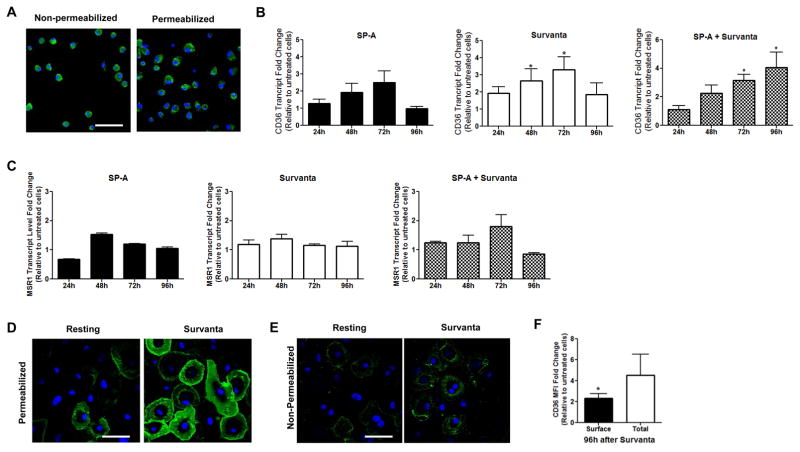
The phenotype of AMs is influenced by surfactant components (9, 11) and HAMs express CD36 protein (36, 37; figure 2A). Due to the cost and accessibility of HAMs and the ephemeral nature of their phenotype following isolation from the lungs, we used surfactant-cultured MDMs for the majority of the experiments in the present work. Experimental evidence indicates that surfactant-treated MDMs are a tractable model for studying AM phenotype and functions (7–11, 14, 16). Additionally, as opposed to HAMs which are chronically exposed to total surfactant, the use of MDMs enabled us to investigate distinctions between the effects of surfactant proteins versus lipids, as well as the effects of initial exposure to individual surfactant lipids. In order to determine whether CD36 expression could be an aspect of the AM phenotype which is regulated by surfactant, we cultured MDMs in surfactant (Survanta and/or SP-A) over several days. CD36 transcript levels increase up to four-fold relative to untreated controls at 72h (figure 2B). This increase is specific to CD36; other scavenger receptors (MARCO, SR-B1 and SR-A) did not change over this time period (data not shown and figure 2C). Consistent with the increase in CD36 transcript levels, MDMs cultured in surfactant for 96h contain five times more CD36 protein than untreated controls (figure 2D, F) and express twice as much CD36 at their cell membranes (figure 2E, F). No increase in CD36 protein levels was observed prior to 72h in culture with surfactant (data not shown). Since CD36 expression is regulated by surfactant components, we speculated that CD36 could be involved in surfactant lipid uptake by macrophages.
FIGURE 2. Freshly isolated HAMs contain preformed CD36 and exposure of MDMs to surfactant increases CD36 expression over days in culture.
(A) Freshly isolated HAMs were adhered to coverslips, fixed with PFA, left intact or permeabilized with methanol and labeled with anti-CD36 Ab followed by AlexaFluor488. Scale bar, 50 μm. (B, C) MDMs were left untreated or were exposed to SP-A (10 μg/mL), Survanta (100 μg/mL) or both up to 96h. Monolayers were lysed with TriZOL. CD36 transcript levels (B) or SR-A transcript levels (C) were determined via qRT-PCR. (D, E) MDMs on coverslips were left untreated or exposed to Survanta (100 μg/mL) for 96h. Monolayers were fixed with PFA and were (D) permeabilized by methanol or (E) left intact and labeled with anti-CD36 Ab. Scale bars, 50 μm. (F) Fold change in CD36 MFI in Survanta-exposed MDMs relative to untreated cells 96h after Survanta exposure. Experiments were conducted in duplicate from 3 (A, C–F) independent donors or in triplicate from 4 (B) independent donors (mean ± SEM).
CD36 knockdown impairs human macrophage acquisition of surfactant lipid DPPC
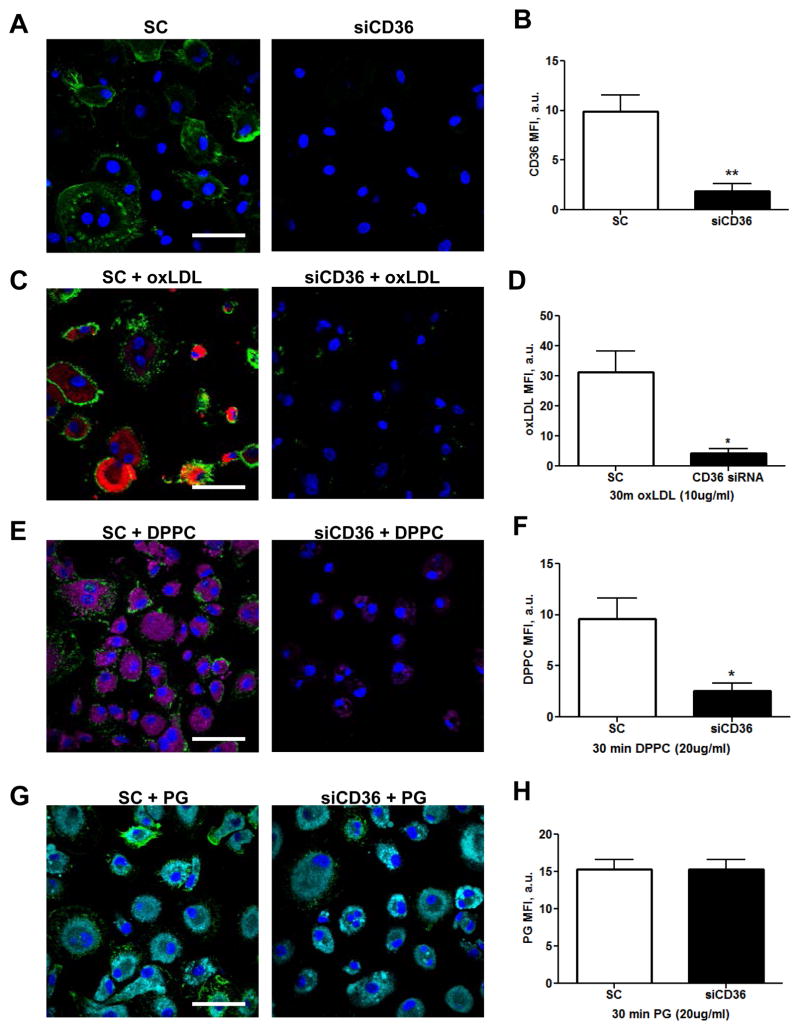
Although surfactant uptake by AMs is receptor-mediated (28), no receptor(s) have been identified to date. CD36 transports palmitate into type II alveolar epithelial cells (29) and has a well characterized role in the uptake of fatty acids by a variety of tissues (30, 31). We therefore investigated whether CD36 contributes to surfactant lipid uptake by human macrophages using CD36 knockdown, which is 80% effective relative to scramble control (SC) treated MDMs (figure 3A, B) and significantly inhibits DiI-oxLDL uptake (figure 3C, D). We investigated the acquisition of DPPC, which comprises ~40% of the total surfactant lipids (22). siCD36 or SC MDMs were exposed to NBD-DPPC for 30 min and uptake was determined by confocal microscopy. As shown in figure 3E and F, CD36 knockdown MDMs acquire five-fold less NBD-DPPC relative to scramble control exposed cells. To determine whether this phenomenon is specific to DPPC uptake, we evaluated the uptake of NBD-PG, the second most abundant lipid in surfactant (22). CD36 knockdown and scramble control MDMs acquire comparable levels of NBD-PG (figure 3G, H), indicating that CD36 specifically imports DPPC into macrophages.
FIGURE 3. CD36 knockdown inhibits uptake of DPPC.
(A) MDMs on coverslips were treated with SC or siCD36 siRNA for 72h using the MirusX2 transfection system, fixed with 4% PFA and permeabilized with methanol prior to labeling with anti-CD36 Abs. (B) CD36 knockdown is 80% effective. (C) SC or siCD36 MDMs were exposed to 10 μg/mL oxLDL (red) for 30 min and association was analyzed by confocal microscopy. (D) oxLDL MFI in SC or siCD36 exposed MDMs. (E) MDMs were treated with SC or CD36 siRNA prior to exposure to 20 μg/mL NBD-DPPC (purple) for 30 min. (F) DPPC MFI in SC or siCD36 exposed MDMs. (G) SC or siCD36 MDMs were exposed to 20 μg/mL NBD-PG (cyan) for 30 min. (H) PG MFI in SC or siCD36 exposed MDMs. Representative images and cumulative results from 7 (A, B), 4 (C, D, E, F) or 3 (G, H) different experiments conducted in duplicate (mean ± SEM). Scale bars, 50 μm.
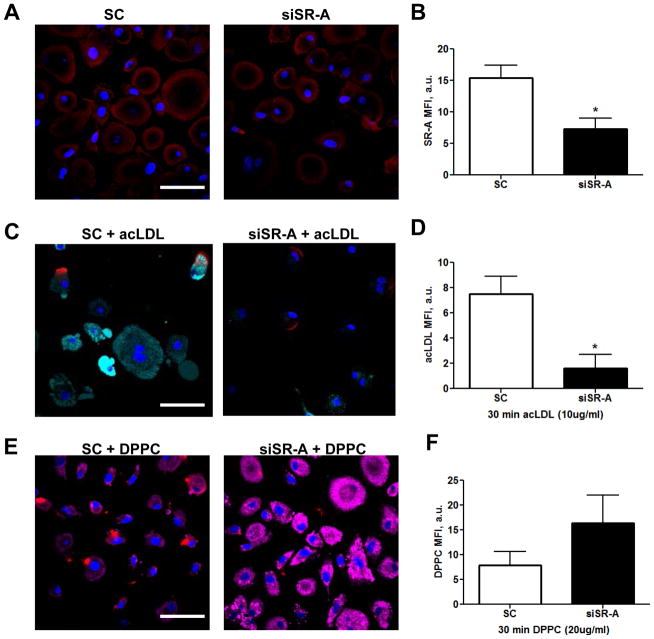
To confirm that DPPC uptake by human macrophages is mediated by CD36 and not scavenger receptors in general we next knocked down scavenger receptor A (SR-A) (figure 4A, B), which is also expressed by AMs (44) and mediates uptake of acetylated (ac)-LDL (62, 63). As a positive control, we verified that SR-A knockdown significantly reduces macrophage acquisition of acLDL (figure 4C, D). In contrast to the results following CD36 knockdown, DPPC acquisition is increased following SR-A knockdown (figure 4E, F). CD36 and SR-A are known to have compensatory expression profiles (64) and we observed a 40% increase in CD36 expression following SR-A knockdown (supplemental figure 1A–C) which may account for the observed increase in DPPC acquisition.
FIGURE 4. SR-A knockdown increases uptake of DPPC.
(A) MDMs were exposed to SC or anti-SR-A siRNA via Mirus X2 for 72h. (B) SR-A knockdown is 60% effective. (C) SC or siSR-A MDMs were exposed to 10 μg/mL acLDL (cyan) for 30 min and association was analyzed by confocal microscopy. (D) acLDL MFI in SC and siSR-A MDMs. (E) SC or siSR-A (red) treated MDMs were exposed to 20 μg/mL NBD-DPPC for 30 min. (F) NBD-DPPC MFI in SC or siSR-A exposed MDMs. Representative images and cumulative graphs from 3 independent experiments conducted in duplicate (mean ± SEM). Scale bars, 50 μm.
Exposure to surfactant lipids prior to M.tb infection enhances bacterial growth in a CD36- dependent manner
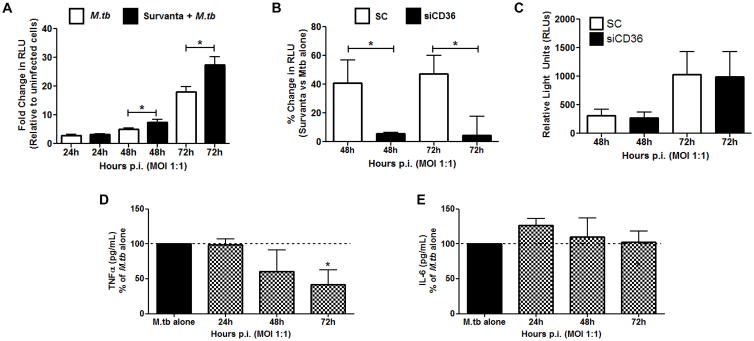
AMs constitutively consume surfactant lipids (53, 54) and display tissue-specific lipid bodies which are absent from resting MDMs (supplemental figure 1D, E). M.tb thrives inside of AMs (2) and has been shown to use host lipids as a carbon source in other contexts (46–48). We therefore hypothesized that the presence of surfactant lipids within MDMs prior to infection with M.tb would enhance bacterial growth. In fact, M.tb growth is increased significantly when MDMs are cultured in surfactant lipids for 48h prior to infection (figure 5A). As measured by relative light units (RLUs), this growth advantage equates to 60% more bacteria in surfactant-cultured macrophages 48 and 72h after infection. In the absence of CD36 (by knockdown) this growth advantage is abolished (figure 5B), although M.tb uptake is not affected (data not shown). The difference in growth is predicated on the presence of surfactant lipids, as SC and siCD36 MDMs contain comparable bacterial burdens in the absence of Survanta (figure 5C). Thus, CD36-mediated uptake of surfactant lipids is beneficial for the bacteria during the early stages of human macrophage infection by M.tb.
FIGURE 5. Culturing MDMs in surfactant lipids enhances M.tb growth in a CD36-dependent manner.
(A) MDMs were left untreated or were exposed to Survanta (100 μg/mL) for 48h prior to infection with M.tb-lux bacteria (MOI 1:1). Infection proceeded for up to 72h and M.tb growth was measured by luminometry (relative light units, RLUs). Graph shows fold change in RLUs relative to uninfected MDMs. (B) MDMs were treated with scramble control or CD36 siRNA for 48h. Monolayers were then exposed to Survanta (100 μg/mL) for 24h prior to infection with M.tb-lux (MOI 1:1). Infection proceeded for up to 72h and M.tb growth was measured using luminometry. Graph shows percent increase in RLUs when MDMs were exposed to Survanta prior to infection, relative to infection alone. (C) CD36 knockdown alone does not affect M.tb growth in human macrophages as determined by luminometry. (D, E) Survanta inhibits M.tb-induced TNFα but not IL-6. Supernatants from resting or Survanta-cultured MDMs were collected 24–72h post-infection (p.i.) and TNFα (D) or IL-6 (E) release was determined by ELISA. Experiments conducted in duplicate from 3 (A) or 4 (B, C) different donors or conducted in triplicate (D, E) from 3 different donors (mean ± SEM).
TNFα release is inhibited by surfactant lipid exposure but is not affected by CD36 knockdown
We hypothesize that surfactant lipids not only provide a carbon source for M.tb but also inhibit macrophage microbicidal responses. In order to investigate the possibility that the observed surfactant-mediated growth advantage is in part due to immunosuppression, we evaluated the release of the pro-inflammatory cytokines TNFα and IL-6 which are both induced by M.tb infection (65, 66). Based on previous publications (67–69), we hypothesized that Survanta would inhibit the release of both cytokines. As shown in figure 5D and E, culturing MDMs in Survanta prior to infection significantly inhibits release of M.tb-induced TNFα, but not IL-6. TNFα blocking therapeutics can lead to both TB reactivation and increased susceptibility to mycobacterial infection (70). In contrast, M.tb-induced IL-6 has been shown to be beneficial for the bacteria (66). Therefore, surfactant-mediated inhibition of TNFα potentially contributes to the enhanced bacterial growth that we observed.
TNFα can be induced through TLR2 (32, 34, 35), M.tb has TLR2 ligands (71) and CD36 has been shown to contribute to host responses in conjunction with TLR2 (32, 34, 35). We therefore investigated whether the absence of CD36 affects the TLR2-dependent release of TNFα. MDMs were transfected with SC or anti-CD36 siRNA as described above and were then exposed to the TLR2 ligand Pam3Cys (100 ng/mL). SC and siCD36 MDMs release comparable levels of TNFα following Pam3Cys exposure (supplemental figure 2A and B), suggesting that the absence of CD36 does not affect TLR2-dependent cytokine production in M.tb-infected MDMs. Therefore, although surfactant lipids appear to specifically inhibit TNFα production in response to M.tb infection, CD36 does not appear to contribute to this phenomenon.
Discussion
Understanding aspects of host cell physiology which render AMs endogenously susceptible to infection by M.tb will amplify our ability to develop novel therapeutics effective in the pulmonary environment. In the absence of infection, AMs indispensably contribute to surfactant catabolism to maintain lung homeostasis. We contend that this function of AMs creates a host cell which is naturally susceptible to M.tb infection due to both the immunosuppressive properties of surfactant and the presence of surfactant lipid metabolites as a readily available carbon source for this host-adapted pathogen. Therefore, minimal manipulation of host cell metabolism may be required for the bacteria to thrive in the endogenously lipid-rich environment of the AM.
As mentioned above, the increased bacterial growth that we observed may be attributable to the immunosuppressive properties of surfactant. Exposure to surfactant proteins and lipids prior to LPS increases expression of negative regulators of inflammation, while decreasing LPS-mediated release of TNFα and IL-6 (10). SP-A decreases pro-inflammatory TLR activity (11) and hampers microbicidal functions by inhibiting NADPH oxidase assembly (7). In addition, surfactant proteins A and D are both necessary for maintaining lung homeostasis and proper immune function in this tissue (12, 13). The role of tissue factors which are unique to the lung environment (e.g., surfactant components) is an aspect of M.tb infection which is often overlooked in experimental studies, yet most likely plays a defining role in TB pathogenesis.
We report suppression of M.tb-induced TNFα by surfactant lipids, a host response which may augment bacterial growth. This possibility is in keeping with the well-known effects of TNFα inhibitors on TB reactivation (70). However, because CD36 knockdown did not alter TLR2-dependent TNFα release, CD36 does not appear to contribute to this phenomenon, a finding which is supported by previous publications indicating that CD36 is dispensable for Pam3Cys-induced TLR2-dependent TNFα production (32, 34, 35). However, because TLR2 recognizes an array of established ligands and CD36 has also been reported to sense diacylglycerides in conjunction with TLR2 (32), we cannot rule out the possibility that CD36 is involved in the TLR2 response to one or more M.tb ligands; pursuing this possibility is beyond the scope of the current manuscript.
The exact role(s) of CD36 during TB pathogenesis remain somewhat obscure, as conflicting data regarding the importance of CD36 during mycobacterial infection in the mouse model have been reported (40, 41). However, temporal discrepancies in the experiments may partially explain this dissonance. Court et al found that a double knockout of SR-A and CD36 did not impact the control of chronic M.tb infection, while Hawkes et al demonstrated that CD36 knockout mice are less susceptible to mycobacterial colonization and distal organ dissemination early during infection. The exact role of CD36 during infection remains to be revealed, since ex vivo experiments using macrophages from CD36 knockout mice did not exhibit differences in phagocytosis or reactive oxygen species production. Furthermore, the authors did not attribute the differential growth to the cytokine response during infection (40). Although CD36 may not be regulating cytokine production during mycobacterial infection, the present study provides evidence for the role of CD36 in the uptake of surfactant lipids by human macrophages. We posit that the presence of surfactant lipids dampens protective cytokine production and may also provide a ready carbon source for M.tb upon entry into an AM. The latter possibility is the focus of ongoing experiments.
During in vitro infection, M.tb up-regulates genes involved in lipid metabolism (6, 49) and utilizes host lipids as a carbon source (46–48, 50). However, the source of the host lipids used by the bacteria remains less clear. There is evidence that the ability to metabolize cholesterol contributes to intracellular growth (47, 72, 73), although this appears to be more crucial during persistent stages of infection (74, 75). Cholesterol metabolism by M.tb is not required to establish infection in the lung (75) and there is some evidence that cholesterol is not a necessary carbon source for survival within a host (76). Therefore, identifying essential nutrient sources for the bacteria during early infection remains of great importance. Our ongoing experiments are investigating whether M.tb can acquire and utilize surfactant lipid metabolites from macrophages.
Herein we show that the location and expression of scavenger receptor CD36 is regulated by surfactant lipids and proteins, and that CD36 contributes to surfactant lipid uptake by human macrophages, conferring a bacterial growth advantage during the early stages of M.tb infection at least in part by surfactant lipid-mediated reduction in the host protective cytokine TNFα. These findings enhance our knowledge regarding the contributions of the lung environment during the establishment of M.tb infection and ongoing experiments will determine whether this bacterial growth advantage is also due to the presence of surfactant lipid-based carbon sources.
Supplementary Material
Acknowledgments
We thank the Ohio State University Biological Safety Level 3 staff and the Campus Microscopy and Imaging Facility (Columbus, OH) for their support in mycobacterial and confocal microscopy experiments.
Grant support: This work was supported by NIH AI059639 (LSS) and the HHMI MED into GRAD fellowship program (CED).
Abbreviations used
- acLDL
acetylated low-density lipoprotein
- AM
alveolar macrophage
- DPPC
dipalmitoylphosphatidylcholine
- HAMs
human alveolar macrophages
- MDM
monocyte-derived macrophages
- MFI
mean fluorescence intensity
- M.tb
Mycobacterium tuberculosis
- NBD
nitrobenzoxadiazole
- oxLDL
oxidized low-density lipoprotein
- PFA
paraformaldehyde
- PG
phosphatidylglycerol
- SC
scramble control
- SP-A
surfactant protein A
- SR-A
scavenger receptor A
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.WHO. Global Tuberculosis Report 2014. 2014. [Google Scholar]
- 2.Suter E. The multiplication of tubercle bacilli within normal phagocytes in tissue culture. J Exp Med. 1952;96(2):137–150. doi: 10.1084/jem.96.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajaram MVS, Ni B, Dodd CE, Schlesinger LS. Macrophage immunoregulatory pathways in tuberculosis. Sem Immunol. 2014;26:471–485. doi: 10.1016/j.smim.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussell T, Bell T. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14(2):81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson JS, Schlesinger LS. Pulmonary surfactant in innate immunity and the pathogenesis of tuberculosis. Tubercle and Lung Disease. 2000;80:173–184. doi: 10.1054/tuld.2000.0242. [DOI] [PubMed] [Google Scholar]
- 6.Guirado E, Mbawuike U, Keiser TL, Arcos J, Azad AK, Wang SH, Schlesinger LS. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. mBio. 2014;6(1):e02537–14. doi: 10.1128/mBio.02537-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowther JE, Kutala EK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;171:6866–6874. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor C, McCormack F, Voelker D, McGowan S, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155(11):5343–5351. [PubMed] [Google Scholar]
- 9.Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169(7):3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen HA, Rajaram MVS, Meyer DA, Schlesinger LS. Pulmonary surfactant protein A and surfactant lipids regulate IRAK-M, a negative regulator of TLR-mediated inflammation in human macrophages. Am J Lung Physiol. 2012;303(7):608–16. doi: 10.1152/ajplung.00067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180(12):7847–7858. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrod KS, Trapnell BC, Otake K, Korfhagen TR, Whitsett JA. SP-A enhances viral clearance and inhibits inflammation after pulmonary adenoviral infection. Am J Physiol. 1999;277(3):580–588. doi: 10.1152/ajplung.1999.277.3.L580. [DOI] [PubMed] [Google Scholar]
- 13.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KBM. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol. 2002;169:2892–2899. doi: 10.4049/jimmunol.169.6.2892. [DOI] [PubMed] [Google Scholar]
- 14.Henning LN, Azad AK, Parsa KVL, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A: A key regulator of Toll-Like Receptor expression and activity in human macrophages. J Immunol. 2008;180:7847–7858. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis–macrophage interactions and the capacity to control infection. J Immunol. 2011;187:372–381. doi: 10.4049/jimmunol.1100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisomes-proliferator activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185(2):929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilsher ML, Parker DJ, Haslam PL. Immunosuppression by pulmonary surfactant: mechanisms of action. Thorax. 1990;45(1):3–8. doi: 10.1136/thx.45.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol, Lung, Cell and Mol Biol. 2009;296(6):936–946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita CM, Veldhuizen RAW, Gill SE. Alveolar macrophages and pulmonary surfactant: More than just friendly neighbors. OA Biol. 2013;1(1):6. [Google Scholar]
- 20.Schlesinger LS. Mononuclear phagocytes in tuberculosis. Lung Macrophages and Dendritic Cells in Health and Disease for the series Lung Biology in Health and Disease. 1997;102:437–480. [Google Scholar]
- 21.Carlson TK, Brooks MN, Rajaram MVS, Henning LN, Meyer DA, Schlesinger LS. Pulmonary innate immunity: soluble and cellular host defenses of the lung. In: Marsh, Hunter, Tridandapani, editors. Regulation of innate immune function. Chapter 7. Transworld Research Signpost, STM Books; 2010. pp. 167–211. [Google Scholar]
- 22.Goerke J. Pulmonary surfactant: Functions and molecular composition. Mol Bas Dis. 1998;2(3):79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Ann Rev Physiol. 2001;63:555–578. doi: 10.1146/annurev.physiol.63.1.555. [DOI] [PubMed] [Google Scholar]
- 24.Wright JR, Youmans DC. Degradation of surfactant lipids and surfactant protein A by alveolar macrophages in vitro. Am J Physiol. 1995;268(5):772–780. doi: 10.1152/ajplung.1995.268.5.L772. [DOI] [PubMed] [Google Scholar]
- 25.Eckert H, Lux M, Lachmann B. The role of alveolar macrophages in surfactant turnover. Lung. 1983:213–218. doi: 10.1007/BF02713866. [DOI] [PubMed] [Google Scholar]
- 26.Baker AD, Malur A, Barna BP, Ghosh S, Kavuru MS, Malur AG, Thomassen MJ. Targeted ppar-gamma deficiency in alveolar macrophages disrupts surfactant catabolism. J Lip Res. 2010;51(6):1325–1331. doi: 10.1194/jlr.M001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forbes, Pickell AM, Foroughian M, Yao L-J, Lewis J, Veldhuizen R. Alveolar macrophage depletion is associated with increased surfactant pool sizes in rats. J App Physiol. 2007;103(3):637–645. doi: 10.1152/japplphysiol.00995.2006. [DOI] [PubMed] [Google Scholar]
- 28.Poelma DL, Ju MR, Bakker SC, Zimmerman LJ, Lachmann BF, Iwaardan JFV. A common pathway for the uptake of surfactant lipids by alveolar cells. Am J Respir Cell and Mol Biol. 2004;30:751–758. doi: 10.1165/rcmb.2003-0127OC. [DOI] [PubMed] [Google Scholar]
- 29.Guthmann F, Haupt R, Looman AC, Spener F, Rustow B. Fatty acid translocase/CD36 mediates the uptake of palmitate by type II pnuemocytes. Am J Physiol. 1999;277:191–196. doi: 10.1152/ajplung.1999.277.1.L191. [DOI] [PubMed] [Google Scholar]
- 30.Koonen DPY, Glatz JFC, Bonen A, Luiken JJFP. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta. 2005;1736:163–180. doi: 10.1016/j.bbalip.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Glatz J, Luiken J, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol Rev. 2010;90(1):367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 32.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zähringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433(7025):523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 33.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdman LK, Cosio G, Helmers AJ, Gowda DC, Grinstein S, Cain KC. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. J Immunol. 2009;183(10):6452–6459. doi: 10.4049/jimmunol.0901374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Won WJ, Bachman MF, Kearney JF. CD36 is differentially expressed on B cell subsets and in response to antigen. J Immunol. 2008;180:230–237. doi: 10.4049/jimmunol.180.1.230. [DOI] [PubMed] [Google Scholar]
- 36.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: Potential role of CD36 in foam cell formation. Blood. 1996;87(5):2020–2028. [PubMed] [Google Scholar]
- 37.Asada K. Antiinflammatory roles of peroxisome proliferator-activated receptor in human alveolar macrophages. Am J Respir Crit Care Med. 2003;169(2):195–200. doi: 10.1164/rccm.200207-740OC. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: Implications for understanding lung disease in humans. J Immunol. 2014;192(6):2821–2829. doi: 10.4049/jimmunol.1302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palanisamy G, Kirk NM, Ackart DF, Obregon-Henau A, Shenley CA, Orme IM, Basaraba RJ. Uptake and Accumulation of Oxidized Low-Density Lipoprotein during Mycobacterium tuberculosis Infection in Guinea Pigs. PLoS ONE. 2012:E34148–E34148. doi: 10.1371/journal.pone.0034148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawkes M, Li X, Crockett M, Diasitti A, Finney C, Min-Oo G, Lilies C, Liu J, Cain KC. CD36 deficiency attenuates experimental mycobacterial infection. BMC Infect Dis. 2010;10:299. doi: 10.1186/1471-2334-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Court N, Vasseur V, Vacher R, Fremond C, Shebzukhov Y, Yeremeev W, Maillet I, Nodosposov SA, Gordon S, Fallon PG, Suzuki H, Ryffel B, Quiesniaux VF. Partial redundancy of the pattern recognition receptors, scavenger receptors, and C-type lectins for the long-term control of Mycobacterium tuberculosis infection. J Immunol. 2010;184(12):7057–7070. doi: 10.4049/jimmunol.1000164. [DOI] [PubMed] [Google Scholar]
- 42.Sharif O, Matt U, Saluzo S, Lakovitz K, Haslinger I, Furtner T, Doninger B, Knapp S. The scavenger receptor CD36 downmodulates the early inflammatory response while enhancing bacterial phagocytosis during pneumococcal pneumonia. J Immunol. 2013;190(11):5640–5648. doi: 10.4049/jimmunol.1202270. [DOI] [PubMed] [Google Scholar]
- 43.Stuart LM, Deng J, Silver JM, Moore KJ, Tseng AA, Hennessey EJ, Ezekowitz RAB. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170(3):477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanchet C, Jouvion G, Fitting C, Cavaillon J, Adib-Conquy M. Protective or deleterious role of scavenger receptors SR-A and CD36 on host resistance to Staphylococcus aureus depends on site of infection. PLoS One. 2014 doi: 10.1371/journal.pone.0087927. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 46.Lee W, VanderVen BC, Fahey RJ, Russell DG. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem. 2013;8(288):6788–6800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandey AK, Sassetti C. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci. 2008;105(11):4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniel J, Maamar H, Deb C, Sirakova T, Kolattukudy P. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Path. 2011:E1002093–E1002093. doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez JG, Hernandez AC, Helgeura-Repetto C, Ayala DA, Guadarrama-Medina R, Anzola JM, Bustos JR, Zambrano MM, Gonzales-y-Merchand J, Garcia MJ, Del Portillo P. Global adaptation to a lipid environment triggers the dormancy-related phenotype of Mycobacterium tuberculosis. Am Soc Microbiol. 2014;5(3):e01125–14. doi: 10.1128/mBio.01125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peyron, Vabourgiex PJ, Poquet Y, Lavillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona P-J, de Chastellier C, Altare F. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathogens. 2008;4(11):e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim M, Wainright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Weihart U, Tsenova L, Kaplan G, Russell DG. Caseation of human tuberculosis granuloma correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2(7):258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter RL, Jaqannath C, Actor JK. Pathology of post-primary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis. 2007;87(4):267–278. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Nichols B. Normal rabbit alveolar macrophages, I. The phagocytosis of tubular myelin. J Exp Med. 1976;144(4):906–919. doi: 10.1084/jem.144.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichols B. Normal rabbit alveolar macrophages, II. Their primary and secondary lysosomes as revealed by electron microscopy and cytochemistry. J Exp Med. 1976;144(4):920–932. doi: 10.1084/jem.144.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuroki Y, Mason RI, Voelker DR. Pulmonary surfactant apoprotein A structure and modulation of surfactant secretion by rat alveolar type II cells. J Biol Chem. 1988;263:3388. [PubMed] [Google Scholar]
- 56.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 57.Rajaram MVS, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, Schoenberg DR, Torrelles JB, Schlesinger LS. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci U S A. 2011;108:17408–17413. doi: 10.1073/pnas.1112660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 59.Salunke SB, Azad AK, Kapuriya NP, Balada-Llasat JM, Pancholi P, Schlesinger LS, Chen CS. Design and analysis of novel anti-tuberculosis agents from the celecoxib pharmacore. Bioorg & Med Chem. 2015;23(9):1935–1943. doi: 10.1016/j.bmc.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 60.Luiken JJ, Dyck DJ, Han XX, Tandon NN, Arumugen Y, Glatz JF, Bonen A. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am J Physiol Endocrinol & Metabol. 2002;282(2):491–495. doi: 10.1152/ajpendo.00419.2001. [DOI] [PubMed] [Google Scholar]
- 61.Chabowski A, Gorski J, Calles-Escandon J, Tandon NN, Bonen A. Hypoxia-induced fatty acid transporter translocation increases fatty acid transport and contributes to lipid accumulation in the heart. FEBS Letters. 2006;580:3617–3623. doi: 10.1016/j.febslet.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 62.Gough PJ, Greaves DR, Suzuki H, Hakkinen T, Haltunen MO, Talrunen MP, Herttuala SY, Kodama T, Gordon S. Analysis of scavenger receptor a (SR-A) expression in human aortic atherosclerotic lesions. Arterioscl, Throm and Vasc Biol. 19:461–471. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- 63.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proceedings of the National Academy of Sciences U S A. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, Millstone DS, Mortensen RM, Spiegelman BM, Freeman MW. The role of PPAR-y in macrophage differentiation and cholesterol uptake. Nat Med. 2001;7:41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 65.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J Immunol. 1990;145:149–154. [PubMed] [Google Scholar]
- 66.Nagabhushanam V, Solachi A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-γ. J Immunol. 2003;171:4750–4757. doi: 10.4049/jimmunol.171.9.4750. [DOI] [PubMed] [Google Scholar]
- 67.Allen JN, Moore SA, Pope-Harman AL, Marsh CB, Wewers MD. Immunosuppressive properties of surfactant and plasma on alveolar macrophages. J Lab Clin Med. 1995;125:356–369. [PubMed] [Google Scholar]
- 68.Antal JM, Divis LT, Erzurum SC, Wiedemann HP, Thomassen MJ. Surfactant suppresses NF-kappa B activation in human monocytic cells. Am J Respir Cell Mol Biol. 1996;14:374–379. doi: 10.1165/ajrcmb.14.4.8600942. [DOI] [PubMed] [Google Scholar]
- 69.Thomassen MJ, Antal JM, Barna BP, Divis LT, Meeker DP, Wiedemann HP. Surfactant downregulates synthesis of DNA and inflammatory mediators in normal human lung fibroblasts. Am J Physiol. 1996;270:159–L163. doi: 10.1152/ajplung.1996.270.1.L159. [DOI] [PubMed] [Google Scholar]
- 70.Harris J, Hope JC, Keane J. Tumor necrosis factor blockers influence macrophage responses to Mycobacterium tuberculosis. J Infect Dis. 2008;198(12):1842–1850. doi: 10.1086/593174. [DOI] [PubMed] [Google Scholar]
- 71.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced pro-inflammatory signaling in macrophages. Proc Natl Acad Sci. 1999;96(25):14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nesbitt NM, Yang X, Fontan P, Kolesnikova I, Smith I, Sampson NS, Dubnau E. A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Infect Immun. 2010;78(1):275–282. doi: 10.1128/IAI.00893-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.VanderVen BC, Fahey RJ, Lee W, Liu Y, Abramovich RB, Memmott C, Crowe AM, Eltis LD, Perola E, Deininger DD, Wang T, Locher CP, Russell DG. Novel inhibiters of cholesterol metabolism in Mycobacterium tuberculosis reveal how the bacterium’s metabolism is constrained by the intracellular environment. PLoS Path. 2015 doi: 10.1371/journal.ppat.1004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffin JE, Pandey AK, Gilmore SA, Sasetti CM, McKinney JD, Bertozzi CR. Cholesterol Catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem & Biol. 2012;19:218–227. doi: 10.1016/j.chembiol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pandey AK, Sassetti C. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci. 2008;105(11):4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang X, Gao J, Smith I, Dubnau E, Sampson N. Cholesterol is not an essential source of nutrition for Mycobacterium tuberculosis during infection. J Bacteriol. 2011;193(6):1473–1476. doi: 10.1128/JB.01210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.