Abstract
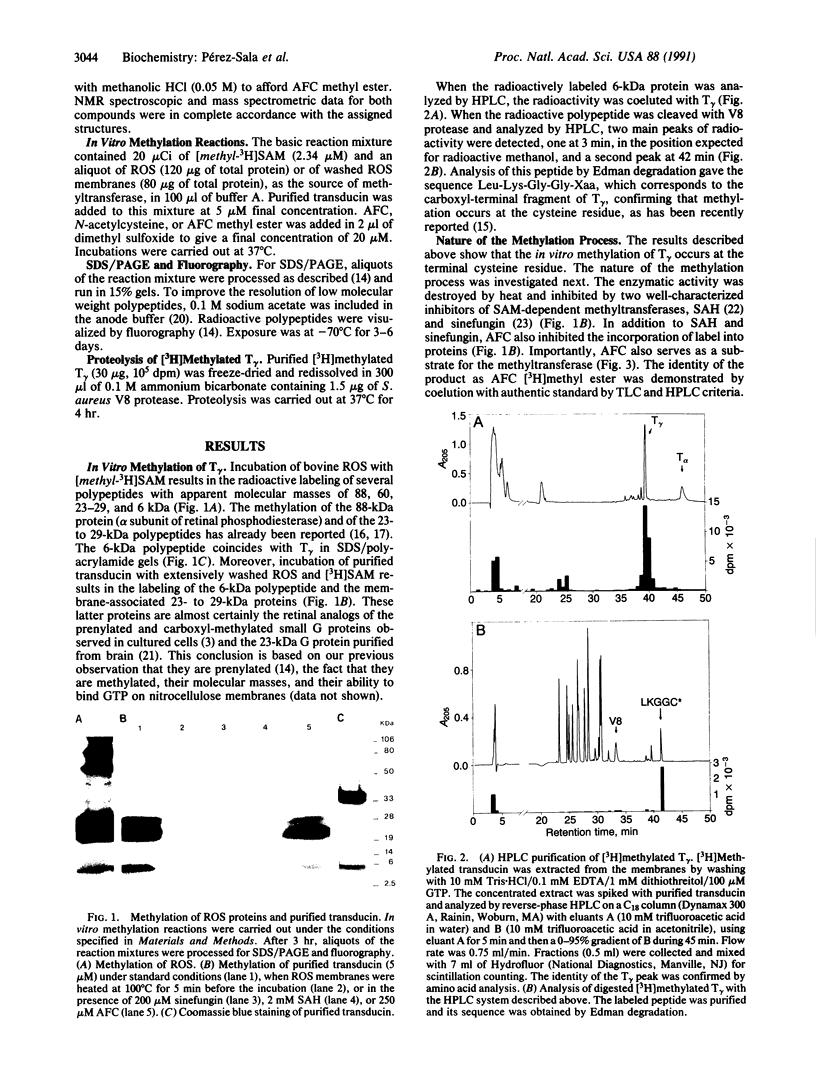
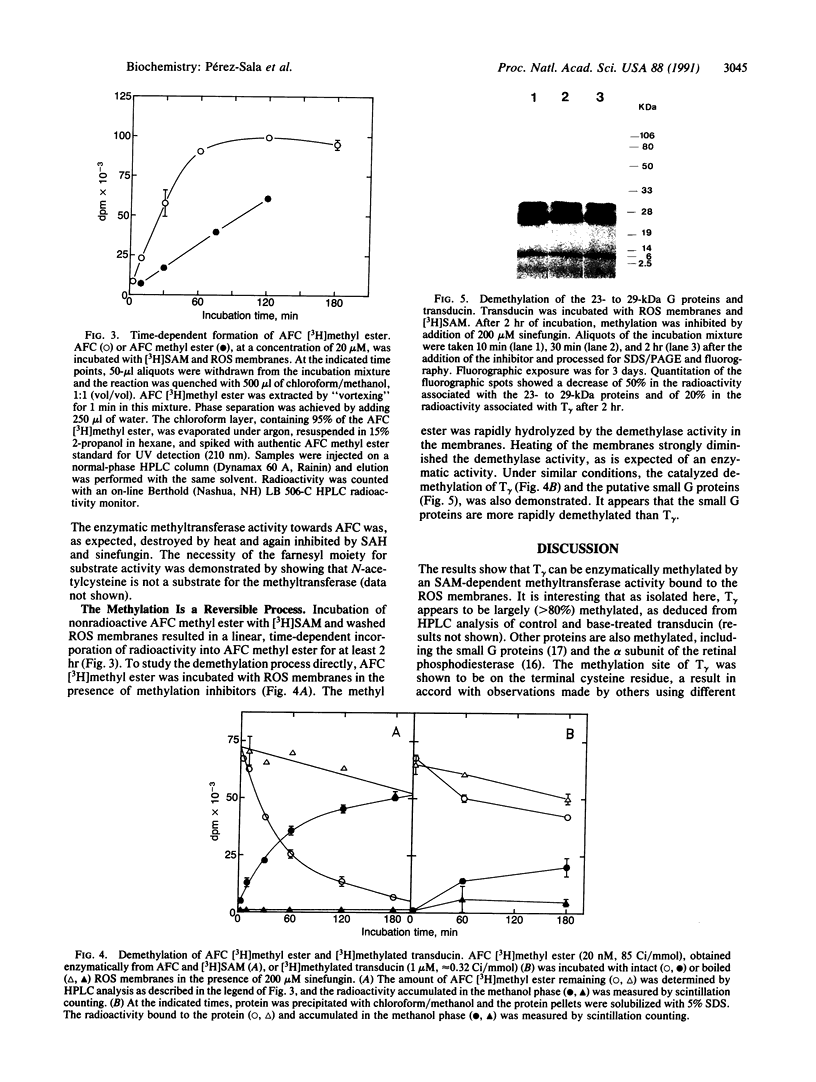
Retinal transducin was previously shown to be farnesylated on its gamma subunit. This farnesylation reaction on a cysteine residue near the carboxyl terminus is followed by peptidase cleavage at the cysteine. Thus the modified cysteine becomes the carboxyl terminus. It is shown here that the free carboxyl group can be methylated by an S-adenosyl-L-methionine-dependent methyltransferase associated with the rod outer segment membranes. This process can be inhibited by S-adenosyl-L-homocysteine and sinefungin. Moreover, synthetic N-acetyl-S-farnesyl-L-cysteine, but not N-acetyl-L-cysteine, is a substrate for the enzyme. Rapid demethylation of N-acetyl-S-farnesyl-L-cysteine methyl ester can be observed in the membranes. Transducin is also enzymatically demethylated by the rod outer segment membranes. Moreover, the 23- to 29-kDa small G proteins are methylated and demethylated in this system. These data suggest that methylation/demethylation may play a regulatory role in visual signal transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Barber J. R., Clarke S. Inhibition of protein carboxyl methylation by S-adenosyl-L-homocysteine in intact erythrocytes. Physiological consequences. J Biol Chem. 1984 Jun 10;259(11):7115–7122. [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy K. G., Jr, LaTart D. B., Osterhoudt H. W. Modifications for SDS-PAGE of proteins. Biotechniques. 1989 Jul-Aug;7(7):692–693. [PubMed] [Google Scholar]
- Clarke S. Protein carboxyl methyltransferases: two distinct classes of enzymes. Annu Rev Biochem. 1985;54:479–506. doi: 10.1146/annurev.bi.54.070185.002403. [DOI] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Gelb M. H., Glomset J. A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science. 1990 Jan 19;247(4940):320–322. doi: 10.1126/science.2296721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Lai R. K., Perez-Sala D., Cañada F. J., Rando R. R. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M. Isoprenylated proteins in cultured cells: subcellular distribution and changes related to altered morphology and growth arrest induced by mevalonate deprivation. J Cell Physiol. 1987 Dec;133(3):471–481. doi: 10.1002/jcp.1041330307. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M., Repko E. M., Erdman R. A. Post-translational modification of low molecular mass GTP-binding proteins by isoprenoid. J Biol Chem. 1990 Feb 5;265(4):2148–2155. [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota I. M., Clarke S. Enzymatic methylation of 23-29-kDa bovine retinal rod outer segment membrane proteins. Evidence for methyl ester formation at carboxyl-terminal cysteinyl residues. J Biol Chem. 1989 Aug 5;264(22):12879–12884. [PubMed] [Google Scholar]
- Pugh C. S., Borchardt R. T., Stone H. O. Sinefungin, a potent inhibitor of virion mRNA(guanine-7-)-methyltransferase, mRNA(nucleoside-2'-)-methyltransferase, and viral multiplication. J Biol Chem. 1978 Jun 25;253(12):4075–4077. [PubMed] [Google Scholar]
- Reiss Y., Goldstein J. L., Seabra M. C., Casey P. J., Brown M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990 Jul 13;62(1):81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Swanson R. J., Applebury M. L. Methylation of proteins in photoreceptor rod outer segments. J Biol Chem. 1983 Sep 10;258(17):10599–10605. [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Allosteric behavior in transducin activation mediated by rhodopsin. Initial rate analysis of guanine nucleotide exchange. J Biol Chem. 1987 Mar 15;262(8):3697–3705. [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. K., Fung B. K. The membrane-binding domain of a 23-kDa G-protein is carboxyl methylated. J Biol Chem. 1989 Nov 25;264(33):20100–20105. [PubMed] [Google Scholar]




