Abstract
IMPORTANCE
Mechanical ventilation may be lifesaving, but in certain persons, such as those with advanced dementia, it may prolong patient suffering without a clear survival benefit.
OBJECTIVE
To describe the use and outcomes of mechanical ventilation and its association with the increasing numbers of intensive care unit (ICU) beds in the United States for patients with advanced dementia residing in a nursing home 120 days before that hospital admission.
DESIGN, SETTING, AND PARTICIPANTS
This retrospective cohort study evaluated Medicare beneficiaries with advanced dementia hospitalized from January 1, 2000, to December 31, 2013, using the Minimum Data Set assessments linked with Medicare part A claims. A hospital fixed-effect, multivariable logistic regression model examined the effect of changes in ICU beds within individual hospitals and the likelihood of receiving mechanical ventilation, controlling for patients’ demographic characteristics, function, and comorbidities.
MAIN OUTCOMES AND MEASURES
Mechanical ventilation.
RESULTS
From 2000 to 2013, a total of 635 008 hospitalizations of 380 060 eligible patients occurred (30.5% male and 69.5% female; mean [SD] age, 84.4 [7.4] years). Use of mechanical ventilation increased from 39 per 1000 hospitalizations in 2000 to 78 per 1000 hospitalizations in 2013 (P < .001, test of linear trend). As the number of ICU beds in a hospital increased over time, patients with advanced dementia were more likely to receive mechanical ventilation (ie, adjusted odds ratio per 10 ICU bed increase, 1.06; 95% CI, 1.05–1.07). In 2013, hospitals in the top decile in the number of ICU beds were reimbursed $9611.89 per hospitalization compared with $8050.24 per hospitalization in the lower decile (P < .001) without an improvement in 1-year mortality (65.2% vs 64.6%; P = 54).
CONCLUSIONS AND RELEVANCE
Among hospitalized nursing home residents with advanced dementia, we found an increase in the use of mechanical ventilation over time without substantial improvement in survival. This increase in the use of mechanical ventilation was associated with an increase in the number of ICU beds within a hospital.
At present, more than 5 million persons have dementia; by 2050, dementia will result in 1.6 million deaths.1 Intensive care units (ICUs) may be an appropriate location of care for some elderly patients, but the role of the ICU in the medical care of persons with advanced dementia raises concerns about whether this care is consistent with informed preferences, has the potential to improve the quality of life, and provides increased value from a societal perspective.2–4 The disease trajectory of advanced dementia is characterized by progressive cognitive and functional impairment, with 86% developing eating problems that often lead to malnutrition; recurrent infections are common and are often accompanied by burdensome patterns of hospitalizations before death.5
Striking variation in ICU use across hospitals persists even after adjustment for health status, expected mortality, race/ethnicity, and preferences of patients and their families.6,7 Despite the expense and potential burdens of ICU care, ICU admission remains one of the few Medicare sites of care without regulatory oversight.8 Although the number of hospital beds has decreased during the past decade, the number of ICU beds has grown, with few hospitals accounting for much of this growth.9 As noted by Gooch and Kahn,10 the increase in the number of ICU beds raises a concern that increased ICU bed capacity may result in the use of ICUs by patients unlikely to benefit, such as persons with advanced dementia.
Our goal is to characterize temporal trends in the use of mechanical ventilation, the survival of patients with advanced dementia, and the association of the increased use of mechanical ventilation in hospitals that increased their number of ICU beds. In addition, we describe the difference in the use of health care services and costs among these patients admitted to hospitals within the lowest and highest decile regarding the number of ICU beds in 2013.
Methods
We obtained our study sample from a national repository of the Minimum Data Set (MDS) assessments linked with Medicare part A claims from January 1, 2000, to December 31, 2013. The MDS is a federally mandated nursing assessment conducted on admission and at least quarterly that contains detailed demographic and clinical information on every resident living in all Medicare- or Medicaid-certified US nursing facilities. A retrospective cohort of hospitalized fee-for-service Medicare beneficiaries from 2000 to 2013 was examined to see whether they were in a nursing home during the 120 days immediately before that hospital admission. Based on that MDS assessment, we identified hospitalizations during which the nursing home resident had a preexisting diagnosis of dementia (the equivalent of an MDS Cognitive Performance Scale score of 5 or 6, indicating the most severe cognitive impairment),11 and documentation of impairments in 4 or more activities of daily living. This study was reviewed and approved by the institutional review board of Brown University, who waived the requirement for patient consent.
Definitions
Based on the procedure codes (96.70, 96.71, and 96.72 from the International Classification of Diseases, Ninth Revision, Clinical Modification) documented in the Medicare claims data, we identified hospitalizations during which a nursing home resident with advanced dementia received mechanical ventilation. A Danish registry found that coding for mechanical ventilation had a positive predictive value of 100%.12 The numbers of ICU and ICU step-down beds were based on the American Hospital Association (AHA) annual survey, a voluntary measure that most US hospitals complete. We used available surveys that were conducted in 2000, 2005, 2007, 2010, and 2013. Unlike using hospital cost reports to determine the number of ICU beds, only the AHA data are able to examine the use of ICU step-down beds.13
Individual Characteristics
Information on the sample’s sociodemographic characteristics was based on the Medicare Beneficiary Enrollment file that includes age, race/ethnicity, sex, and state of residence. Race/ethnicity is based on information collected by the Social Security Administration and was used in the analysis as a potential confounder. Medical diagnoses, function, cognitive status, and other patient characteristics were based on the assessment completed as part of the MDS within 120 days of that hospitalization. Because of a change in variables collected in the MDS, version 3.0, after 2010, information on do-not-resuscitate (DNR) orders and other restrictions on life-sustaining treatment were included only in sensitivity analyses using data from 2000 to 2010. A DNR order, as defined by the MDS, version 2.0, instruction manual,14 includes no intubation in the event of respiratory or circulatory failure.
Statistical Analysis
Descriptive statistics were used to characterize the changing use of mechanical ventilation from 2000 to 2001, 2004 to 2005, 2006 to 2007, 2009 to 2010, and 2012 to 2013 that corresponded to the years of available AHA annual survey data (2000, 2005, 2007, 2010, and 2013) on the number of ICU and ICU step-down beds in each hospital. Temporal trends were tested using a variance-weighted least squares model for bivariate associations and a multivariable model that adjusted for patients’ sociodemographic characteristics, cognitive status, and functional status with an indicator variable for year. To examine the association of change in the number of ICU and ICU step-down beds within each hospital, a multivariable hospital fixed-effect logistic model was used that adjusted for a similar set of patient-level covariates. The use of the fixed-effect model allows us to examine the change in ICU beds and ICU step-down beds within each hospital over time with the risk for persons with advanced dementia undergoing mechanical ventilation. The number of ICU step-down beds was entered into the model as a separate independent variable based on the hypothesis that improving staffing in an ICU step-down unit compared with a regular hospital floor may prevent the use of mechanical ventilation. We tested for a nonlinear association between ICU beds and the risk for mechanical ventilation with a cubic spline. Based on the MDS assessment completed 120 days before that hospitalization, we included in the model the following patient characteristics: (1) sociodemographic characteristics (age, sex, and race/ethnicity); (2) cognitive and functional status; (3) clinical diagnosis recorded on the MDS assessment; (4) whether the patient had a prior hospitalization in the preceding 120 days; and (5) the days from the MDS assessment to hospitalization.
In the first sensitivity analysis, we examined whether the association of ICU beds with the risk for mechanical ventilation held when adjusting for the presence of a DNR order, a variable that is available in the MDS from 2000 to 2010 but not in version 3.0 instituted in 2011. A second sensitivity test examined whether the association with growth in the number of ICU beds held when only admissions for pneumonia and/or septicemia were considered. These stratified analyses allowed us to exclude cases that were admitted for trauma or surgical conditions. A third sensitivity analysis examined whether the association held when we restricted the analyses to persons with the equivalent of an MDS Cognitive Performance Scale score of 6—the highest level of cognitive impairment—and persons impaired in all 7 activities of daily living using the data from 2000 to 2013. Finally, we completed 2 additional analyses to address concerns that, over time, hospitalizations of persons with advanced dementia have been reduced. We estimated the denominator of persons in a nursing home with advanced dementia who met our study criteria. Inverse probability weights were used to balance rates of risk factors across all years, ensuring that changes in outcomes over time are not owing to changes in patient risk profiles over time.
To better describe the clinical and financial implications of the variation in the number of ICU beds in 2013, we characterize the hospital length of stay and reimbursement for that hospitalization after adjustment for regional variation and graduate medical education payment. Statistical testing was 2-sided with a threshold of P < .05. All analyses were performed with STATA software, version 14 (StataCorp).
Results
A total of 635 008 hospitalizations of 380 060 patients (30.5% male and 69.5% female; mean [SD] age, 84.4 [7.4] years) occurred in the selected years of available AHA data. Table 1 reports the distribution of age, sex, race/ethnicity, and cognitive and functional status of these patients who were hospitalized within 120 days of that assessment. In 2013, 98.2% (95% CI, 98.1%–98.3%) of these patients with advanced dementia were bed bound, 23.2% (95% CI, 22.8%–23.6%) had a feeding tube, and 63.4% (95% CI, 62.9%–63.9%) of persons without a feeding tube were prescribed a mechanical soft diet. Over time, the proportion of patients who were black or Hispanic increased. The mean (SD) hospital length of stay declined from 7.1 days (7.0; interquartile range, 3–9 days) in 2000 to 6.3 days (6.0; interquartile range, 3–8 days) in 2013. Admissions to the ICU increased from 16.9% (95% CI, 16.7%–17.1%) in 2000 to 38.5% (95% CI, 38.1%–38.8%) in 2013, whereas the mean (SD) length of ICU stay increased from 5.0 days (6.2; interquartile range, 2–6 days) in 2000 to 5.4 days (5.7; interquartile range, 2–7 days) in 2013.
Table 1.
Characteristics of Hospitalized Nursing Home Residents With Advanced Dementia
| Characteristic | 2000–2001 | 2004–2005 | 2006–2007 | 2009–2010a | 2012–2013 |
|---|---|---|---|---|---|
| No. of hospitalizations | 169 071 | 147 339 | 136 096 | 87 730 | 94 772 |
| No. of hospitals | 3076 | 2882 | 2870 | 2441 | 2432 |
| Age, mean (SD) [IQR], y |
84.2 (7.3) [79–89] | 84.1 (7.3) [79–89] | 84.1 (7.3) [79–89] | 84.2 (7.4) [79–89] | 83.9 (7.6) [79–89] |
| Female, % (95% CI) | 70.5 (70.3–70.8) | 70.2 (70.0–70.4) | 69.1 (68.9–69.3) | 68.4 (68.2–68.8) | 65.5 (65.2–65.8) |
| Race/ethnicity, % (95% CI) |
|||||
| White | 76.4 (76.1–76.6) | 72.4 (72.1–72.6) | 71.9 (71.7–72.6) | 69.9 (69.6–70.2) | 67.6 (67.2–67.9) |
| Black | 20.0 (19.8–20.2) | 22.6 (22.4–22.8) | 22.3 (22.1–22.5) | 23.6 (23.3–23.9) | 24.2 (23.9–24.5) |
| Hispanic | 1.9 (1.8–2.0) | 2.6 (2.5–2.6) | 3.0 (2.9–3.1) | 3.5 (3.4–3.6) | 3.8 (3.7–4.0) |
| No. of ADL dependencies, mean (SD) [IQR] |
6.5 (0.8) [6–7] | 6.6 (0.8) [6–7] | 6.6 (0.8) [6–7] | 6.6 (0.7) [6–7] | 6.6 (0.7) [6–7] |
| 7 ADL dependencies present, % (95% CI) |
72.5 (72.3–72.7) | 74.3 (74.0–74.5) | 74.3 (74.1–74.5) | 74.0 (73.7–74.3) | 70.1 (69.8–70.4) |
| Bed bound, % (95% CI) | 94.8 (94.7–94.9) | 96.1 (96.0–96.2) | 96.7 (96.6–96.8) | 97.3 (97.2–97.4) | 98.2 (98.1–98.3) |
| CPS score of 6, % (95% CI)b | 69.1 (68.9–69.4) | 66.4 (62.1–66.6) | 62.5 (62.2–62.8) | 57.9 (57.6–58.2) | 46.8 (46.5–47.1) |
| Weight loss, % (95% CI) | 14.6 (14.4–14.8) | 13.1 (12.9–13.3) | 12.7 (12.5–12.9) | 11.4 (11.1–11.6) | 10.8 (10.6–11.0) |
| Feeding tube present, % (95% CI) |
35.2 (34.9–35.4) | 35.0 (34.7–35.3) | 33.4 (33.1–33.7) | 33.1 (32.8–33.4) | 23.2 (22.8–23.6) |
| Mechanical soft diet among those without feeding tube, % (95% CI) |
74.2 (73.9–74.5) | 69.4 (69.1–69.7) | 68.3 (68.0–68.6) | 66.5 (66.1–66.8) | 63.4 (62.9–63.9) |
| Hospital primary diagnosis, % (95% CI) |
|||||
| Pneumonia | 28.1 (28.0–28.4) | 24.4 (24.2–24.6) | 21.3 (21.2–21.6) | 18.4 (18.2–18.7) | 14.7 (14.4–14.9) |
| Dehydration | 5.8 (5.7–5.9) | 4.7 (4.6–4.8) | 4.1 (4.0–4.2) | 3.6 (3.5–3.8) | 3.2 (3.1–3.3) |
| Hip or limb fracture | 2.4 (2.3–2.5) | 2.2 (2.1–2.2) | 2.3 (2.2–24) | 2.3 (2.2–2.4) | 2.4 (2.3–2.5) |
| Renal failure | 1.3 (1.2–1.3) | 2.8 (2.7–2.9) | 3.7 (3.6–3.8) | 3.6 (3.5–3.7) | 4.6 (4.5–4.7) |
| Septicemia | 10.0 (9.9–10.2) | 14.8 (14.6–15.0) | 17.5 (17.3–17.7) | 21.1 (20.8–21.4) | 25.0 (24.7–25.2) |
| CHF | 2.6 (2.5–2.7) | 2.6 (2.5–2.7) | 2.6 (2.5–2.6) | 2.5 (2.4–2.6) | 2.1 (2.0–2.2) |
| Respiratory failure | 1.9 (1.8–2.0) | 2.9 (2.8–3.0) | 2.9 (2.8–3.0) | 2.1 (2.0–2.2) | 2.0 (1.9–2.1) |
| GI tract bleed | 2.4 (2.4–2.5) | 2.2 (2.3–2.2) | 2.3 (2.2–2.4) | 2.3 (2.2–2.4) | 2.4 (2.3–2.5) |
| Stroke | 2.5 (2.4–25) | 2.1 (2.1–2.2) | 2.2 (2.0–2.2) | 2.1 (2.0–2.2) | 2.1 (2.0–2.2) |
| CAD | 1.5 (1.4–1.5) | 1.5 (1.5–1.6) | 1.4 (1.3–1.4) | 1.2 (1.2–1.3) | 1.0 (1.0–1.1) |
| COPD | 1.3 (1.2–1.3) | 1.2 (1.2–1.3) | 1.2 (1.2–1.3) | 1.4 (1.4–1.5) | 1.1 (1.0–1.2) |
| Hospital length of stay, mean (SD) [IQR], d |
7.1 (7.0) [3–9] | 6.7 (6.7) [3–8] | 6.6 (6.3) [3–8] | 6.4 (6.6) [3–8] | 6.3 (6.0) [3–8] |
| ICU admission, % (95% CI) |
16.9 (16.7–17.1) | 22.3 (22.1–22.5) | 25.9 (25.6–26.1) | 31.1 (30.8–31.4) | 38.5 (38.1–38.8) |
| ICU length of stay, mean (SD) [IQR], d |
5.0 (6.2) [2–6] | 5.3 (6.1) [2–7] | 5.5 (6.2) [2–7] | 5.4 (5.7) [2–7] | 5.4 (5.7) [2–7] |
| DNR orders, % (95% CI)c | 49.5 (49.2–49.8) | 52.5 (52.3–52.8) | 51.9 (51.7–52.2) | 50.8 (50.5–51.1) | NA |
| No. of hospital beds, mean (SD) [IQR] |
151.6 (124.2) [65–200] |
164.8 (140.4) [73–228] |
173.3 (143.7) [73–228] |
191.5 (162.0) [82–244] |
204.7 (169.6) [90–266] |
| No. of ICU beds, mean (SD) [IQR] |
22.4 (23.3) [8–30] |
25.8 (24.8) [10–34] |
27.9 (28.3) [10–36] |
31.6 (32.2) [12–40] |
34.2 (35.6) [12–42] |
| No. of ICU step-down beds, mean (SD) [IQR] |
6.3 (21.7) [0-0] | 11.2 (43.9) [0-0] | 11.8 (51.6) [0-0] | 15.8 (67.1) [0-0] | 20.0 (84.4) [0–5] |
| Type of hospital ownership, % (95% CI) |
|||||
| Government | 14.6 (14.4–14.7) | 13.5 (13.3–13.7) | 14.0 (13.2–13.7) | 13.4 (13.2–13.6) | 11.6 (11.4–11.8) |
| Church | 14.3 (14.1–14.4) | 14.4 (14.3–14.7) | 13.6 (13.4–13.7) | 12.5 (12.3–12.7) | 12.2 (12.0–12.4) |
| Not-for-profit | 56.2 (56.0–56.5) | 57.7 (57.4–57.9) | 57.0 (56.7–57.2) | 59.1 (58.8–59.4) | 60.2 (60.0–60.6) |
| For-profit | 14.3 (14.1–14.4) | 14.4 (14.3–14.7) | 13.6 (13.4–13.7) | 12.5 (12.3–12.7) | 12.2 (12.0–12.3) |
Abbreviations: ADL, activities of daily living; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CPS, Cognitive Performance Scale; DNR, do not resuscitate; GI, gastrointestinal; ICU, intensive care unit; IQR, interquartile range; NA, not applicable.
Owing to the change from the Minimum Data Set (MDS) version 2.0 to version 3.0, 2010 was a partial year and numbers are smaller.
Scores range from 0 to 6, with higher scores indicating greater cognitive impairment.
Owing to a change in data collected for MDS assessment in 2011, information was not included for DNR orders.
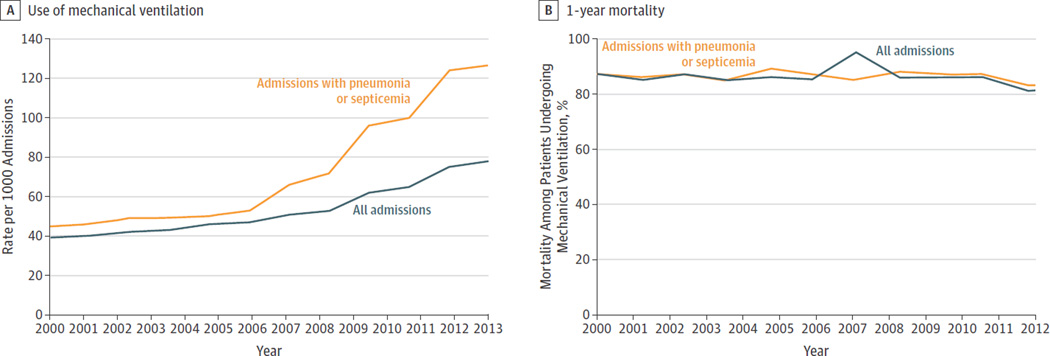
The Figure presents the change in the proportion of hospitalizations of study patients who underwent mechanical ventilation. In 2000, mechanical ventilation was used in 39 per 1000 hospital admissions with a steady increase to 78 per 1000 admissions in 2013 (P < .001, test of trend). Table 2 reports the mean monthly incidence rates of hospitalization and mechanical ventilation from 2000 to 2012 using the denominator of all nursing home residents with advanced dementia and 4 or more activities of daily living impairments. The incidence rate of mechanical ventilation increased from 0.19% in 2000 to 0.32% in 2012. When we restricted the sample to only those persons hospitalized with pneumonia or septicemia, the rate of increase in the use of mechanical ventilation grew from 44.8 per 1000 admissions in 2000 to 126.8 per 1000 admissions in 2013 (Figure) (P < .001, test of trend). The overall 1-year mortality for patients receiving mechanical ventilation remained consistently greater than 80% in all cohorts. In 2012, 51.1% of patients receiving mechanical ventilation died in the hospital or within 30 days of admission. In 2013, the cost of care for these patients was $95.3 million.
Figure. Mechanical Ventilation per 1000 Hospital Admissions of Patients and 1-Year Mortality of Patients With Advanced Dementia Receiving Mechanical Ventilation.
Mechanical ventilation and 1-year mortality are stratified by all admissions and those admissions with pneumonia or septicemia as the primary diagnosis.
Table 2.
Changes in the Rates of Hospitalizations and Mechanical Ventilation Among All Nursing Home Residents With Advanced Dementia
| Year | Mean Monthly Denominator, No. of Residents |
Mean Monthly Incidence Rate, % | |
|---|---|---|---|
| Hospitalizations | Mechanical Ventilation |
||
| 2000 | 210 279 | 5.05 | 0.19 |
| 2001 | 213 556 | 5.19 | 0.20 |
| 2002 | 210 941 | 5.24 | 0.21 |
| 2003 | 204 066 | 5.19 | 0.21 |
| 2004 | 195 005 | 5.06 | 0.22 |
| 2005 | 185 516 | 5.25 | 0.24 |
| 2006 | 177 173 | 5.07 | 0.25 |
| 2007 | 169 108 | 5.00 | 0.26 |
| 2008 | 160 854 | 5.05 | 0.27 |
| 2009 | 157 746 | 4.71 | 0.26 |
| 2010 | 157 452 | 4.76 | 0.28 |
| 2011 | 169 701 | 5.27 | 0.34 |
| 2012 | 164 858 | 4.76 | 0.32 |
Table 3 presents the multivariable hospital fixed-effect model. A fixed-effect model examines the association of changes in ICU beds with the risk for mechanical ventilation within each hospital over time. For each increase of 10 ICU beds per hospital, the adjusted odds ratio (AOR) is 1.06 (95% CI, 1.05–1.07), indicating a 6% increase in the receipt of mechanical ventilation among hospitalized nursing home residents with advanced dementia admitted to that hospital. An increase in ICU step-down beds did not have a protective effect, but mechanical ventilation had a slight increase (AOR, 1.01; 95% CI, 1.004–1.01). We tested for a nonlinear association of ICU bed growth with the risk for mechanical ventilation with cubic spline and found that P = .98, thus rejecting the hypothesis of nonlinearity.
Table 3.
Multivariable Analyses of the Association of Increase in ICU Beds With the Odds of Mechanical Ventilation in Hospitalized Nursing Home Residents With Advanced Dementiaa
| Analysis | Hospitalizations, OR (95% CI) | ||||
|---|---|---|---|---|---|
| All (n = 635 008) |
With Pneumonia or Septicemia Diagnosis Only (n = 238 074) |
With CPS Score of 6 and ADL Score of 7 Only (n = 367 740) |
2000–2010 Onlyb (n = 471 031) |
Use of Inverse Probability Weights (n = 635 008) |
|
| Increased odds of mechanical ventilation per 10 ICU bed increase (95% CI) |
1.06 (1.05–1.07) |
1.11 (1.09–1.13) |
1.06 (1.04–1.08) |
1.04 (1.02–1.05) |
1.06 (1.05–1.08) |
| Increased odds of mechanical ventilation per 10 ICU step-down bed increase (95% CI) |
1.01 (1.004–1.01) |
1.01 (1.01–1.02) |
1.01 (1.003–1.01) |
1.00 (0.98–1.01) |
1.01 (1.01–1.02) |
Abbreviations: ADL, activities of daily living; CPS, Cognitive Performance Scale; ICU, intensive care unit; OR, odds ratio.
The eMethods and eTables 1 through 5 in the Supplement provide the full model for the association of increase in the number of ICU beds with the risk for mechanical ventilation.
Indicates when information on do-not-resuscitate orders was collected.
We conducted 4 additional sensitivity analyses. When we restricted our analysis to hospitalizations for patients with a primary reason for admission of pneumonia or septicemia, we found the increase in ICU beds had an AOR of 1.11 (95% CI, 1.09–1.13). The second analysis restricting the sample to only those cases with MDS Cognitive Performance Scale scores of 6 and 7 activities of daily living impairments found an AOR of 1.06 (95% CI, 1.04–1.08). The third sensitivity analysis focused on 2000 to 2010 when the MDS assessment noted whether the patient had a DNR order. As reported in Table 3, the AOR was 1.04 (95% CI, 1.02–1.05) after adjusting for DNR status, suggesting the increase in the use of mechanical ventilation was not due to a difference in preferences for resuscitation. A final sensitivity analysis addressed the concern of changing characteristics of hospitalized patients (Table 3). Using inverse probability weights that balance risk factors across all years of the study, we found an AOR of 1.06 (95% CI, 1.05–1.08).
To illustrate differences in the care of persons with advanced dementia admitted to hospitals in the highest and lowest deciles of number of ICU beds, we examined 2013 data to document the care of 4171 persons admitted to hospitals in the highest decile of ICU beds compared with the 4333 persons admitted to hospitals in the lowest decile of ICU beds. Table 4 contrasts that proportion of persons receiving mechanical ventilation, hospital reimbursement, and length of stay. The health care reimbursement for admission to a hospital in the highest decile of ICU beds was $9611.89 per person compared with $8050.24 for persons hospitalized in the lowest decile without a change in 1-year mortality (65.2% vs 64.6%; P = .54).
Table 4.
Outcomes in Hospitals in the Lowest and Highest Decile of ICU Beds in 2013 Among Hospitalized Patients With Advanced Dementia and Severe Functional Impairmenta
| Outcome | Decile of No. of ICU Beds | P Value | |
|---|---|---|---|
| Lowest (n = 4333) |
Highest (n = 4171) |
||
| Use of mechanical ventilation, % | 4.5 | 10.6 | <.001 |
| Reimbursement per person, mean (SD), $ | |||
| All cases | 8050.24 (5639) | 9611.89 (8235) | <.001 |
| Cases with mechanical ventilation | 22 039.12 (15 463) | 23 413.46 (16 849) | .17 |
| Hospital length of stay, mean (SD), d | |||
| All cases | 5.4 (4.0) | 6.9 (6.6) | <.001 |
| Cases with mechanical ventilation | 9.6 (8.2) | 12.3 (12.3) | .005 |
| 1-y Mortality, %b | |||
| All cases | 65.2 | 64.6 | .54 |
| Cases with mechanical ventilation | 70.0 | 67.5 | .57 |
Abbreviation: ICU, intensive care unit.
Advanced dementia and severe functional impairment were based on (1) a diagnosis of dementia; (2) a Cognitive Performance Scale score of at least 5 (indicating cognitive impairment); and (3) impairment in at least 4 activities of daily living.
Based on analyses of cases in 2012 with complete survival data available.
Discussion
Mechanical ventilation may be lifesaving, but for some it merely prolongs their suffering without a clear benefit and with a substantial societal cost. Care in the ICU accounted for 13.2% of hospital costs and 4.1% of the National Health Expenditures in 2010.13 We document a 2-fold increase in the use of mechanical ventilation among nursing home residents with advance dementia admitted to the hospital without a substantial improvement in survival. In 2013, Medicare expended nearly $100 million in this high-intensity, low-value care. More important, the total number of hospital beds in the United States has decreased, whereas the number of ICU beds has increased. During times of a public health crisis, such as an earthquake or influenza epidemic, an excess capacity of ICU beds may be lifesaving, but multiple commentaries10,15,16 have expressed concerns that excess ICU bed capacity potentially creates a technological imperative to use those beds by persons who may not benefit from ICU care, such as those with advanced dementia. We found that persons with advanced dementia admitted to a hospital with an increase of 10 ICU beds during a 2-year period were 6% more likely to receive mechanical ventilation. Such care seems inconsistent with the findings by Mitchell and colleagues5 that 96% of health care proxies for persons with advanced dementia desire comfort to be the goal of care. These results raise important questions as to whether such care is consistent with informed preferences and about the value of this care from a societal perspective.
Although our analysis used a hospital fixed-effect multivariable model that compared changes in ICU beds and in mechanical ventilation within individual hospitals over time, we cannot conclude that simply reducing the number of ICU beds will reduce the use of mechanical ventilation for persons with advanced dementia. Rather, the increase of ICU beds may be a marker for norms that guide and determine decision making by clinicians, and perhaps patients and families, regarding the use of the ICU toward the end of life.6,17–19 For example, Barnato and colleagues17 conducted an ethnographic study comparing a hospital with high-intensity end-of-life care with a hospital with low-intensity end-of-life care: in the former, the cultural norm was to only discuss changing the goals of care late in the disease course, whereas in the hospital with low-intensity care, the cultural norm was to talk about the goals of care early in the hospital stay. Qualitative studies of hospitals in the United States and United Kingdom similarly found variability in communication about DNR orders, which highlight the importance of institutional norms.20 Therefore, multifaceted interventions probably will be needed to ensure that care is consistent with the informed preferences of the patient and his or her family.
Single-focused interventions will likely fail to address this complex social interaction.21 Multifaceted and sequential interventions are likely needed to address multiple targets, including enhancement of communication skills, provision of feedback to institutions and clinicians on their performance, and address of cultural norms.21 In addition, policy interventions seem likely to be important, including aligning financial incentives with quality as opposed to volume and developing a strategic plan to manage regional ICU bed capacity. At present, ICU beds are not regulated, resulting in differential regional growth. A national dialogue is needed to develop a regional strategy regarding ICU bed capacity to ensure appropriate access.
One of the advantages of the AHA data compared with hospital cost reports is that the AHA annual survey provides an estimate of ICU step-down beds. Although the number of ICU step-down beds has increased, research examining the effectiveness and value of step-down beds is limited. We hypothesized that having higher nurse staffing ratios than regular acute care beds but less ability to intervene technologically than an ICU might prevent mechanical ventilation for these patients. However, we found that an increase in the number of step-down beds did not result in a lower proportion of persons receiving mechanical ventilation. Further research is needed to understand the influence of the growing number of ICU step-down beds.
Several important limitations should be acknowledged in interpreting the findings of this study. First, our analyses are based on Medicare claims data and the MDS. Information on patient preferences is lacking with the exception of DNR orders from 2000 to 2010. Even after adjusting for the presence of DNR orders, the association of increasing ICU beds and use of mechanical ventilation persisted. Furthermore, prior research suggests that patient preferences do not explain this variability.22 Second, we relied on data from the AHA annual survey for information on the number of ICU beds and ICU step-down beds. Not all hospitals participate in the AHA annual survey. Hospitals excluded from this analysis were smaller, less likely to be affiliated with medical schools, and more likely to be critical access hospitals (eMethods and eTables 1 through 5 in the Supplement). Furthermore, hospitals completing the AHA annual survey may inaccurately report their number of ICU step-down beds. Third, the rate of hospital admissions for patients with advanced dementia decreased. This fact raises the question of whether sicker persons are being admitted to the hospital. Because of this concern, we conducted 3 sensitivity analyses. Each found the same association between the increase in the number of ICU beds and the use of mechanical ventilation for persons with advanced dementia. Finally, despite our sensitivity analyses examining preexisting DNR orders, patients with different preference patterns may have been admitted over time and not been captured by our data.
Conclusions
From 2000 to 2013, the use of mechanical ventilation for hospitalized persons with advanced dementia and severe functional impairment increased without substantial evidence of improved survival. These hospitalized patients were at higher risk for mechanical ventilation when they were admitted to hospitals that had increased their number of ICU beds. Our results call for reconsideration of the role that the excess supply of ICU beds23 plays in the ICU admission and subsequent mechanical ventilation of patients with advanced dementia. Furthermore, our results highlight the urgency of developing a multifaceted approach to address the increasing intensity of care for the growing population of patients with advanced dementia. Such an approach will need to align clinical care and financial incentives with patients’ informed preferences and provide the needed education and training for health care professionals24 to ensure that decisions to use mechanical ventilation in persons with advanced dementia are based on high-quality shared decision making that incorporates the goals of care of informed patients and their families.
Supplementary Material
Key Points.
Question
Among hospitalized patients with advanced dementia, how is the use of mechanical ventilation associated with the increasing number of intensive care unit (ICU) beds?
Findings
In this cohort study, mechanical ventilation increased 2-fold without improved survival. Nursing home residents admitted to a hospital that increased their ICU beds by 10 had an increased risk for mechanical ventilation.
Meaning
Increasing rates of mechanical ventilation raise the need for multifaceted interventions to improve advance care planning in nursing homes, decision making in the hospital, and a national strategic plan regarding regional ICU bed growth.
Acknowledgments
Funding/Support: This research was supported by program project grant P01 AG027296 from the National Institute on Aging (Dr Mor).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: Chris Bostrup-Jensen, BA, Brown University School of Public Health, provided database preparation as part of his job responsibility; he did not receive special compensation.
Footnotes
Author Contributions: Drs Teno and Gozalo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Study concept and design: Teno, Gozalo, Curtis, Meltzer, Mor.
Acquisition, analysis, or interpretation of data: Teno, Khandelwal, Curtis, Meltzer, Engelberg, Mor.
Drafting of the manuscript: Teno, Gozalo.
Critical revision of the manuscript for important intellectual content: Teno, Khandelwal, Curtis, Meltzer, Engelberg, Mor.
Statistical analysis: Teno, Gozalo, Mor.
Administrative, technical, or material support: Teno, Gozalo, Meltzer, Mor.
Study supervision: Teno, Gozalo.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the United States among persons with Alzheimer’s disease (2010–2050) Alzheimers Dement. 2014;10(2):e40–e46. doi: 10.1016/j.jalz.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Is care for the dying improving in the United States? J Palliat Med. 2015;18(8):662–666. doi: 10.1089/jpm.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright AA, Keating NL, Balboni TA, Matulonis UA, Block SD, Prigerson HG. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol. 2010;28(29):4457–4464. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med. 2015;43(5):1102–1111. doi: 10.1097/CCM.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146(3):573–582. doi: 10.1378/chest.13-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019–1026. doi: 10.1001/jamainternmed.2015.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenq G, Tinetti ME. Changes in end-of-life care over the past decade: more not better. JAMA. 2013;309(5):489–490. doi: 10.1001/jama.2013.73. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DJ, Angus DC, Seymour CW, Barnato AE, Kahn JM. Critical care bed growth in the United States: a comparison of regional and national trends. Am J Respir Crit Care Med. 2015;191(4):410–416. doi: 10.1164/rccm.201409-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooch RA, Kahn JM. ICU bed supply, utilization, and health care spending: an example of demand elasticity. JAMA. 2014;311(6):567–568. doi: 10.1001/jama.2013.283800. [DOI] [PubMed] [Google Scholar]
- 11.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 12.Blichert-Hansen L, Nielsson MS, Nielsen RB, Christiansen CF, Nørgaard M. Validity of the coding for intensive care admission, mechanical ventilation, and acute dialysis in the Danish National Patient Registry: a short report. Clin Epidemiol. 2013;5:9–12. doi: 10.2147/CLEP.S37763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpern NA, Pastores SM. Critical care medicine beds use, occupancy, and costs in the United States: a methodological review. Crit Care Med. 2015;43(11):2452–2459. doi: 10.1097/CCM.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services. Revised Long-Term Care Facility Resident Assessment Instrument User’s Manual, Version 2.0. Baltimore, MD: Centers for Medicare & Medicaid Services; 2009. p. 38. [Google Scholar]
- 15.Wunsch H. Is there a Starling curve for intensive care? Chest. 2012;141(6):1393–1399. doi: 10.1378/chest.11-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward NS, Chong DH. Critical care beds and resource utilization: current trends and controversies. Semin Respir Crit Care Med. 2015;36(6):914–920. doi: 10.1055/s-0035-1564876. [DOI] [PubMed] [Google Scholar]
- 17.Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886–1896. doi: 10.1007/s00134-012-2661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garland A, Connors AF. Physicians’ influence over decisions to forego life support. J Palliat Med. 2007;10(6):1298–1305. doi: 10.1089/jpm.2007.0061. [DOI] [PubMed] [Google Scholar]
- 19.Curtis JR, Barnato AE. Variability in decisions to limit life-sustaining treatments: is it all about the physician? Chest. 2014;146(3):532–534. doi: 10.1378/chest.14-0636. [DOI] [PubMed] [Google Scholar]
- 20.Dzeng E, Colaianni A, Roland M, et al. Influence of institutional culture and policies on do-not-resuscitate decision making at the end of life. JAMA Intern Med. 2015;175(5):812–819. doi: 10.1001/jamainternmed.2015.0295. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JR, Engelberg RA, Teno JM. Understanding variability of end-of-life care in the ICU for the elderly [published online March 31, 2016] Intensive Care Med. 2016 doi: 10.1007/s00134-016-4340-5. [DOI] [PubMed] [Google Scholar]
- 22.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences? a study of the US Medicare population. Med Care. 2007;45(5):386–393. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angus DC, Truog RD. Toward better ICU use at the end of life. JAMA. 2016;315(3):255–256. doi: 10.1001/jama.2015.18681. [DOI] [PubMed] [Google Scholar]
- 24.Arnold RM, Back AL, Barnato AE, et al. The Critical Care Communication project: improving fellows’ communication skills. J Crit Care. 2015;30(2):250–254. doi: 10.1016/j.jcrc.2014.11.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.