Abstract
In this review, a brief account of the historical perspective of the discovery of the first cellular receptor and co-receptor of the prototype adeno-associated virus serotype 2 (AAV2) will be presented. The Subsequent discovery of a number of AAV serotypes, and attempts to identify the cellular receptors and co-receptors for these serotype vectors has had significant implications in their use in human gene therapy. As additional AAV serotypes are discovered and isolated, a detailed understanding of their tropism is certainly likely to play a key role in all future studies, both basic science as well as clinical.
Introduction
Adeno-associated virus (AAV) is small, naked icosahedral virus, which was first discovered in 1965 [1]. In addition to being a single-stranded DNA containing virus, AAV remains the only virus that has not been conclusively proven to be the etiologic agent of any human disease to date. On the contrary, recombinant AAV vectors have been used in a number of Phase I/II clinical trials, and in some cases, have shown clinical efficacy in the potential gene therapy of several human diseases [2–10]. Although many of the steps in the life cycle of AAV have been studied extensively, details at the molecular level continue to emerge. In addition, in recent years, a number additional AAV serotypes have been isolated, and their use as vectors is likely to further greatly expand the landscape for their optimal use for therapeutic purposes. In spite of these exciting developments, the molecular bases of the varied tissue-tropisms of the AAV serotype vectors have not been fully delineated. In this chapter, we will attempt to shed light on this aspect of AAV vector biology.
Main text
Discovery of the cellular receptor for AAV2
As stated above, AAV2 was discovered in 1965 [1]. However, because AAV2 tropism transcended the species barrier, the conventional wisdom for nearly three decade was that AAV2 infection was non-specific. In 1996, Ponnazhagan et al [11] identified the first human cell line that could not be infected by the wild-type AAV2, or transduced by recombinant AAV2 vectors, and suggested that AAV2 infection of human cells was receptor-mediated.
The search for the putative cellular receptor intensified. In 1996, Mizukami et al [12] reported that a 150-kDa protein present in membranes could bind to AAV2, and suggested that it might be the cellular receptor for AAV2, but provided no corroborating evidence. In 1998, Summerford and Samulski [13] identified heparan sulfate proteoglycan (HSPG) as the cellular receptor for AAV2. This provided an explanation for the wide tropism of AAV2 since all cells across the species barrier express HSPG, except for the first human cell type identified by Ponnazhagan et al [11]. The discovery of the cellular receptor for AAV2 also provided the explanation why the very first Phase I clinical trial performed by Flotte and colleagues [14] for the potential gene therapy of cystic fibrosis, although established the safety of recombinant AAV2 vectors in humans, did not achieve clinical efficacy, even though that was not the primary objective. The elegant studies by Duan et al [15] documented that HSPG is expressed predominantly on the baso-lateral surface, rather than the apical surface, of primary human airway epithelial cells, and thus AAV2 vectors failed to efficiently transduce these cells.
Discovery of the cellular co-receptors for AAV2
Soon after the discovery of the first cellular receptor for AAV2, it also became apparent that HSPG, which is required for binding of AAV2 to the cellular membrane, is not sufficient for the viral entry into cells. In 1999, Qing et al [16] identified the human fibroblast growth factor receptor 1 (FGFR1) as the first cellular co-receptor for AAV2. Simultaneously, Summerford et al [17] also identified αVβ5 as yet another co-receptor for AAV2.
Based on these studies, a clearer pictured emerged of the underlying mechanism of AAV2 binding and entry into target cells. However, Chen et al [18] reported the isolation of AAV sequences from various tissues, predominantly tonsils, from children, and showed that 7% of these “AAV2-like” sequences shared ~98% identity with the wild-type AAV2. Interestingly, these AAV2-like viruses lacked the HSPG-binding site, and failed to bind to the cellular receptor. These studies suggested that either the use of HSPG as a receptor by AAV2 was a consequence of in vitro propagation of AAV2 in culture, or alternatively, AAV2 utilizes multiple putative cellular receptors. Indeed, recombinant AAV2 vectors lacking the HSPG-binding site have been shown to exhibit efficient and widespread transduction in murine brain and retinal tissues [19,20]. Similarly, in addition to FGFR1 and αVβ5, at least four additional cellular co-receptors, hepatocyte growth factor receptor (HGFR) [21], α5β1 integrin [22]; laminin receptor (LamR) [23]; and CD9 [24] have been shown to be utilized by AAV2 by as cellular co-receptors to date.
Discovery of additional AAV serotypes
Multiple AAV serotypes have been isolated from tissue culture stocks, humans, as well as non-human primates [25–33]. Following their development as recombinant vectors, their efficacy has been evaluated in various tissue culture cell lines. To date, 13 distinct AAV serotype vectors (AAV1 – AAV13) have been described, but this number is certainly likely to grow. In general, whereas AAV1 – AAV6 serotype vectors transduce tissue culture cells to various degrees of efficiency, for the most part, AAV7 – AAV13 serotype vectors transduce tissue culture cells poorly in vitro, but these serotype vectors efficiently transduce various tissues and organs in various animal models in vivo.
Although the precise mechanism of tissue-tropism of other AAV serotype vectors in vivo remains unknown, it has become increasingly clear that attachment to putative cell surface receptors is the initial step for successful transduction. It has also become clear that the attachment of most of the AAV serotype vectors is first mediated by binding to various cell surface glycans, which serve as primary receptors. To date, 23 different glycan receptors for AAV serotype vectors have been identified, such as: α2-3 and α2–6 N-linked sialic acid (SIA) for AAV1 [34,35]; HSPG for AAV2, AAV3, and AAV13 [13,33,36]; α2-3 O-linked and α2-3 N-linked SIAs for AAV4 and AAV5, respectively [37–39]; HSPG and α2-3 and α2-6 N-linked SIA for AAV6 [35,40,41]; and termimal N-linked galactose (GAL) of SIA for AAV9 [42,43]. The primary cellular receptors for AAV7, AAV8, AAV9, AAVrh10, AAV11, AAV12, and AAV13 remain unknown. In general, AAV serotype vectors can be grouped into 3 categories with respect to their glycan receptor usage: HSPG for AAV2, AAV3, AAV6, and AAV13; SIA for AAV1, AAV4, AAV5, and AAV6; GAL for AAV9.
As with AAV2, binding to the primary cellular receptors is most likely not sufficient for AAV serotype vectors to gain entry into cells, and additional cell surface as co-receptors are required. The following co-cellular receptors identified thus far include: FGFR1 [16], αVβ5 [17] and α5β1 [22] integrins for AAV2; a putative integrin for AAV9 [44]; FGFR1 for AAV2 [16] and AAV3 [45]; hepatocyte growth factor receptor (HGFR) for AAV2 [21] and AAV3 [46]; platelet-derived growth factor receptor (PDGFR) for AAV5 [47]; epidermal growth factor receptor (EGFR) for AAV6 [48]; and laminin receptor (LamR) for AAV2, AAV3, AAV8, and AAV9 [23].
Following binding to the primary receptors, and interactions with the secondary co-receptors, AAV serotype vectors are internalized through endosomal pathways including clathrin-coated vesicles and/or clathrin-independent carriers/GPI-anchored-protein-enriched endosomal compartments (CLIC/GEEC) [49].
Discovery of AAVR
In 2016, using a genome-wide screen, Pillay et al [50] reported the identification of a trans-membrane protein, which was designated as an essential receptor for AAV2 infection (AAVR). AAVR was shown to bind directly to AAV2, and was capable of endocytosis of AAV from plasma membrane and trafficking to the trans-Golgi network. Deletion of AAVR rendered various mammalian cell types resistant to infection by AAV2. More interestingly, AAVR was found to be a critical factor for infection by several AAV serotypes, and AAVR-knockout mice were resistant to AAV infection. Based on these data, it was claimed that AAVR is a universal receptor for AAV infection, but it remains to be seen what role, if any, AAVR plays in large animal models, and especially in humans.
Animal models for AAV vector transduction
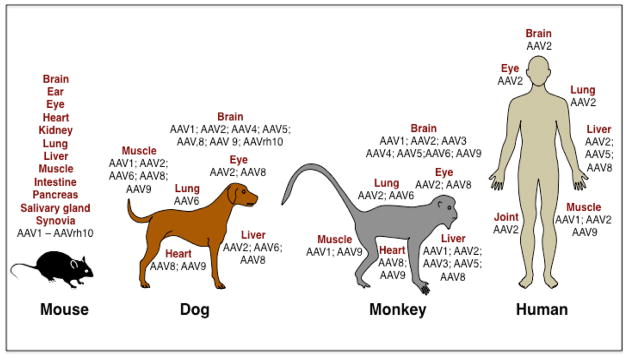
A large body of information has been gleaned from studies in mice, where different AAV serotype vectors have been shown to exhibit distinct tropism for various tissues and organs [51]. The efficacy of some of the AAV serotype vectors has also been evaluated in other animals, small and large, such as rats, gerbils, hamsters, rabbits, cats, dogs, horses, and non-human primates. For example, the first evidence of transduction by AAV2 vectors and long-term gene expression in the murine brain was reported by Kaplitt et al in 1994 [52]. In 1996, first successful transduction of the mouse retina [53] and muscle [54] was reported. AAV2 vector-mediated gene transfer to the guinea pig cochlea [55] and to the primate lung [56] was also reported in 1996. In 1997, several groups first reported the transduction of the mouse liver [57–59], and hematopoietic stem cells [60]. Successful transduction of the rabbit lung [61,62], and the rat carotid arteries [63] by AAV2 vectors was also reported in 1997. Subsequently, various AAV serotype vector-mediated gene transfer in various cell cells and tissues, such as intestinal epithelial cells [64], pancreatic beta cells [65], salivary glands [66], maxillary sinus [67] and temporomandibular joints [68], and in various animal models, such as hamster [69], Japanese quail [70], gerbil [71], cat [72], dog [73], and cynomolgus monkeys [74] and were also reported. A representative example of some of these studies with the various AAV serotype vectors in the most commonly used animal models is depicted in Figure 1.
Figure 1. Schematic representation of the use of recombinant AAV serotype vectors in various animal models and in humans.
Schematic representation of the most commonly used animal models for evaluating the efficacy and safety of recombinant AAV serotype vectors. Various routes of administration of AAV vectors to target various tissues and organs have been utilized. In several Phase I/II, and one Phase III, clinical trials in humans with various AAV serotype vectors to target the indicated organs are also depicted.
Human clinical trials with AAV vectors
As stated above, Flotte and colleagues were the first to perform a Phase I/II clinical trial with AAV2 vectors for the potential gene therapy of cystic fibrosis in 1996 [14]. The next two Phase I trials for the potential gene therapy of hemophilia B with AAV2 vectors were also performed, one muscle-directed [75], and one liver-directed [76]. The first trial did not lead to therapeutic levels of Factor IX, and the second trial was complicated by the host immune response. In 2007, a Phase I trial for the potential gene therapy of arthritis was also attempted, but was halted due to death of a patient, which was unrelated to the AAV2 vector used [77]. The first successful Phase I trials were performed by three independent groups with AAV2 vectors in which clinical efficacy was achieved in patients with Leber’s congenital amaurosis (LCA) [2–5]. Four additional successful Phase I/II clinical trials have since been reported for as diverse diseases as lipoprotein lipase deficiency with AAV1 vectors [8], hemophilia B with AAV8 vectors [6,78], aromatic amino acid decarboxylase deficiency with AAV2 vectors [7], and choroideremia AAV2 vectors [9]. A number of additional Phase I/II clinical trials are currently being pursued with AAV1 vectors for the potential gene therapy of α1 anti-trypsin deficiency [79] and Pompe disease [80]. AAV9 vectors are also being used for the potential gene therapy of Pompe disease [81]. AAV8 and AAV5 vectors also currently being used for the potential gene therapy of hemophilia B and hemophilia A, respectively. Although some efficacy has been achieved, several pre-clinical studies suggest that AAV3 serotype vectors may prove to be significantly more efficient in targeting human liver diseases [82–84] since AAV3 utilizes human HGFR as a cellular co-receptor to specifically target primary human hepatocytes [46]. Thus, although AAV1, AAV2, AAV5, AAV8, and AAV9 serotype vectors have also been, or are currently being used, in 162 Phase I/II clinical trials in humans to date [85] (http://www.wiley.com/legacy/wileychi/genmed/clinical/), further studies are warranted to gain a better understanding of the in vivo tissue-tropism of AAV serotype vectors.
Conclusions
Despite little interest for nearly four decades by the scientific community at large, the sustained efforts of a handful of investigators, focused on the basic molecular biology of AAV, led to the development of recombinant AAV vectors. In the past decade, AAV vectors have taken center stage as an ever-increasing number of human diseases have been targeted by academia as well as industry, both small biotechnology companies and big pharma. The well-established safety of AAV vectors in 162 Phase I/II clinical trials in humans to date, and clinical efficacy in at least 6 human diseases, essentially ensures that with the availability of a vast repertoire of AAV serotype vectors, which is certainly likely to expand, the coming decades will witness their successful use in curing a wide variety of human diseases, both genetic and acquired. Furthermore, the future outlook appears even more optimistic, given the currently ongoing efforts to design and optimize novel AAV serotype vectors capable of targeting specific tissues and organs [86]. However, it is important to emphasize that efforts to pursue the basic molecular biology of AAV in general, and AAV vectors in particular, must also continue, which will, most assuredly, continue to pay rich dividends.
Highlights.
AAV is a non-pathogenic virus, and recombinant AAV vectors have proven to be highly efficient for gene delivery to a wide variety of cell types, tissue, and organs in small and large animal models.
A number of AAV serotype vectors have now become available, with distinct tissue-tropism, and long-term transgene expression, and this repertoire is likely to expand.
An ever increasing number of rationally designed and optimized novel AAV serotype vectors capable of targeting specific tissues and organs, is likely to further expand their therapeutic landscape.
The safety of recombinant AAV vectors has been established in 162 Phase I/II/III clinical trials to date, and clinical efficacy has also been achieved in at least 6 human diseases.
Acknowledgments
This work was supported in part by Public Health Service grants R01 HL-097088, and R21 EB-015684 from the National Institutes of Health; a grant from the Children’s Miracle Network; and support from the Kitzman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149(3685):754–756. doi: 10.1126/science.149.3685.754. This paper, and the next three papers, describe the first succesful use of recombinant AAV2 vectors to achieve clinical efficacy in Phase I trials in patients with Leber’s congenital amaurosis. [DOI] [PubMed] [Google Scholar]
- 2.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 3.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase i trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–2365. doi: 10.1056/NEJMoa1108046. This paper describes the first succesful use of recombinant AAV8 vectors to achieve clinical efficacy in a Phase I trials in patients with hemophilia B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwu WL, Muramatsu S, Tseng SH, Tzen KY, Lee NC, Chien YH, Snyder RO, Byrne BJ, Tai CH, Wu RM. Gene therapy for aromatic l-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4(134):134ra161. doi: 10.1126/scitranslmed.3003640. [DOI] [PubMed] [Google Scholar]
- 8.Gaudet D, Methot J, Dery S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447x) gene therapy for lipoprotein lipase deficiency: An open-label trial. Gene Ther. 2013;20(4):361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuer WJ, Schiffman JC, Davis JL, Porciatti V, Gonzalez P, Koilkonda RD, Yuan H, Lalwani A, Lam BL, Guy J. Gene therapy for leber hereditary optic neuropathy: Initial results. Ophthalmology. 2016;123(3):558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Ponnazhagan S, Wang XS, Woody MJ, Luo F, Kang LY, Nallari ML, Munshi NC, Zhou SZ, Srivastava A. Differential expression in human cells from the p6 promoter of human parvovirus B19 following plasmid transfection and recombinant adeno-associated virus 2 (AAV) infection: Human megakaryocytic leukaemia cells are non-permissive for AAV infection. J Gen Virol. 1996;77(Pt 6):1111–1122. doi: 10.1099/0022-1317-77-6-1111. This paper describes the first human cell line that could not be infected by AAV2, or transduced by recombinant AAV2 vectors, suggesting for the first time that AAV2 infection of human cells was receptor-mediated. [DOI] [PubMed] [Google Scholar]
- 12.Mizukami H, Young NS, Brown KE. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217(1):124–130. doi: 10.1006/viro.1996.0099. [DOI] [PubMed] [Google Scholar]
- 13*.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72(2):1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. This paper describes the fidentification of the first cellular receptor for AAV2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7(9):1145–1159. doi: 10.1089/hum.1996.7.9-1145. This paper describes the first use of recombinant AAV2 vectors in a Phase I clinical trial in patients with cystic fibrosis. This trial established the safety of recombinant AAV2 vectors in humans. [DOI] [PubMed] [Google Scholar]
- 15.Duan D, Yue Y, Yan Z, McCray PB, Jr, Engelhardt JF. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9(18):2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- 16*.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5(1):71–77. doi: 10.1038/4758. This paper describes the identification of the first cellular co-receptor for AAV2. [DOI] [PubMed] [Google Scholar]
- 17.Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: A co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5(1):78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 18.Chen CL, Jensen RL, Schnepp BC, Connell MJ, Shell R, Sferra TJ, Bartlett JS, Clark KR, Johnson PR. Molecular characterization of adeno-associated viruses infecting children. J Virol. 2005;79(23):14781–14792. doi: 10.1128/JVI.79.23.14781-14792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzyczka N, Warrington KH., Jr Custom adeno-associated virus capsids: The next generation of recombinant vectors with novel tropism. Hum Gene Ther. 2005;16(4):408–416. doi: 10.1089/hum.2005.16.408. [DOI] [PubMed] [Google Scholar]
- 20.Boye SL, Bennett A, Scalabrino ML, McCullough KT, Van Vliet K, Choudhury S, Ruan Q, Peterson J, Agbandje-McKenna M, Boye SE. The impact of heparan sulfate binding on transduction of retina by rAAV vectors. J Virol. 2016 doi: 10.1128/JVI.00200-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, Nakamura T, Watanabe M, Oshimi K, Daida H. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79(1):609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J Virol. 2006;80(18):8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80(19):9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurzeder C, Koppold B, Sauer G, Pabst S, Kreienberg R, Deissler H. CD9 promotes adeno-associated virus type 2 infection of mammary carcinoma cells with low cell surface expression of heparan sulphate proteoglycans. Int J Mol Med. 2007;19(2):325–333. [PubMed] [Google Scholar]
- 25.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73(5):3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muramatsu S, Mizukami H, Young NS, Brown KE. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221(1):208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 27.Chiorini JA, Yang L, Liu Y, Safer B, Kotin RM. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant aav4 particles. J Virol. 1997;71(9):6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bantel-Schaal U, Delius H, Schmidt R, zur Hausen H. Human adeno-associated virus type 5 is only distantly related to other known primate helper-dependent parvoviruses. J Virol. 1999;73(2):939–947. doi: 10.1128/jvi.73.2.939-947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than aav type 2. J Virol. 1998;72(1):309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–11859. doi: 10.1073/pnas.182412299. This paper describes the discovery of novel AAV serotype vectors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori S, Wang L, Takeuchi T, Kanda T. Two novel adeno-associated viruses from cynomolgus monkey: Pseudotyping characterization of capsid protein. Virology. 2004;330(2):375–383. doi: 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): A novel aav serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82(3):1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt M, Govindasamy L, Afione S, Kaludov N, Agbandje-McKenna M, Chiorini JA. Molecular characterization of the heparin-dependent transduction domain on the capsid of a novel adeno-associated virus isolate, AAV(VR-942) J Virol. 2008;82(17):8911–8916. doi: 10.1128/JVI.00672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S, Kapturczak M, Loiler SA, Zolotukhin S, Glushakova OY, Madsen KM, Samulski RJ, Hauswirth WW, Campbell-Thompson M, Berns KI, Flotte TR, et al. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum Gene Ther. 2005;16(2):235–247. doi: 10.1089/hum.2005.16.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 n-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80(18):9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handa A, Muramatsu S, Qiu J, Mizukami H, Brown KE. Adeno-associated virus (AAV)-3-based vectors transduce haematopoietic cells not susceptible to transduction with aav-2-based vectors. J Gen Virol. 2000;81(Pt 8):2077–2084. doi: 10.1099/0022-1317-81-8-2077. [DOI] [PubMed] [Google Scholar]
- 37.Kaludov N, Handelman B, Chiorini JA. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Hum Gene Ther. 2002;13(10):1235–1243. doi: 10.1089/104303402320139014. [DOI] [PubMed] [Google Scholar]
- 38.Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, Zabner J. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276(23):20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- 39.Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with aav4 more efficiently than AAV5. J Biol Chem. 2002;277(26):23709–23713. doi: 10.1074/jbc.M200292200. [DOI] [PubMed] [Google Scholar]
- 40.Seiler MP, Miller AD, Zabner J, Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17(1):10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- 41.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol Ther. 2006;14(3):316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011;286(15):13532–13540. doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell CL, Gurda BL, Van Vliet K, Agbandje-McKenna M, Wilson JM. Identification of the galactose binding domain of the adeno-associated virus serotype 9 capsid. J Virol. 2012;86(13):7326–7333. doi: 10.1128/JVI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen S, Berry GE, Castellanos Rivera RM, Cheung RY, Troupes AN, Brown SM, Kafri T, Asokan A. Functional analysis of the putative integrin recognition motif on adeno-associated virus 9. J Biol Chem. 2015;290(3):1496–1504. doi: 10.1074/jbc.M114.608281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackburn SD, Steadman RA, Johnson FB. Attachment of adeno-associated virus type 3B to fibroblast growth factor receptor 1. Arch Virol. 2006;151(3):617–623. doi: 10.1007/s00705-005-0650-6. [DOI] [PubMed] [Google Scholar]
- 46.Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, Cheng B, Gee SW, McGoogan KE, Govindasamy L, Zhong L, et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther. 2010;21(12):1741–1747. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, Chiorini JA. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9(10):1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- 48.Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med. 2010;16(6):662–664. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nonnenmacher M, Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10(6):563–576. doi: 10.1016/j.chom.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS, Carette JE. An essential receptor for adeno-associated virus infection. Nature. 2016;530(7588):108–112. doi: 10.1038/nature16465. This paper describes the identification of a cell membrane receptor invoved in the entry of multiple AAV serotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of aav serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 52.Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8(2):148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 53.Ali RR, Reichel MB, Thrasher AJ, Levinsky RJ, Kinnon C, Kanuga N, Hunt DM, Bhattacharya SS. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum Mol Genet. 1996;5(5):591–594. doi: 10.1093/hmg/5.5.591. [DOI] [PubMed] [Google Scholar]
- 54.Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, Kurtzman GJ, Byrne BJ. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci U S A. 1996;93(24):14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lalwani AK, Walsh BJ, Reilly PG, Muzyczka N, Mhatre AN. Development of in vivo gene therapy for hearing disorders: Introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3(7):588–592. [PubMed] [Google Scholar]
- 56.Conrad CK, Allen SS, Afione SA, Reynolds TC, Beck SE, Fee-Maki M, Barrazza-Ortiz X, Adams R, Askin FB, Carter BJ, Guggino WB, et al. Safety of single-dose administration of an adeno-associated virus (aav)-cftr vector in the primate lung. Gene Ther. 1996;3(8):658–668. [PubMed] [Google Scholar]
- 57.Koeberl DD, Alexander IE, Halbert CL, Russell DW, Miller AD. Persistent expression of human clotting factor ix from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1997;94(4):1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Ponnazhagan S, Mukherjee P, Yoder MC, Wang XS, Zhou SZ, Kaplan J, Wadsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: Organ-tropism and expression of transduced sequences in mice. Gene. 1997;190(1):203–210. doi: 10.1016/s0378-1119(96)00576-8. This paper describes the hepatotropic nature of recombinant AAV2 vectors. [DOI] [PubMed] [Google Scholar]
- 59.Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, Gown AM, Winther B, Meuse L, Cohen LK, Thompson AR, et al. Persistent and therapeutic concentrations of human factor ix in mice after hepatic gene transfer of recombinant aav vectors. Nat Genet. 1997;16(3):270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 60.Ponnazhagan S, Yoder MC, Srivastava A. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J Virol. 1997;71(4):3098–3104. doi: 10.1128/jvi.71.4.3098-3104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubenstein RC, McVeigh U, Flotte TR, Guggino WB, Zeitlin PL. Cftr gene transduction in neonatal rabbits using an adeno-associated virus (aav) vector. Gene Ther. 1997;4(5):384–392. doi: 10.1038/sj.gt.3300417. [DOI] [PubMed] [Google Scholar]
- 62.Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW, Miller AD. Transduction by adeno-associated virus vectors in the rabbit airway: Efficiency, persistence, and readministration. J Virol. 1997;71(8):5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rolling F, Nong Z, Pisvin S, Collen D. Adeno-associated virus-mediated gene transfer into rat carotid arteries. Gene Ther. 1997;4(8):757–761. doi: 10.1038/sj.gt.3300465. [DOI] [PubMed] [Google Scholar]
- 64.Polyak S, Mah C, Porvasnik S, Herlihy JD, Campbell-Thompson M, Byrne BJ, Valentine JF. Gene delivery to intestinal epithelial cells in vitro and in vivo with recombinant adeno-associated virus types 1, 2 and 5. Dig Dis Sci. 2008;53(5):1261–1270. doi: 10.1007/s10620-007-9991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prasad KM, Yang Z, Bleich D, Nadler JL. Adeno-associated virus vector mediated gene transfer to pancreatic beta cells. Gene Ther. 2000;7(18):1553–1561. doi: 10.1038/sj.gt.3301279. [DOI] [PubMed] [Google Scholar]
- 66.Voutetakis A, Kok MR, Zheng C, Bossis I, Wang J, Cotrim AP, Marracino N, Goldsmith CM, Chiorini JA, Loh YP, Nieman LK, et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci U S A. 2004;101(9):3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner JA, Messner AH, Moran ML, Daifuku R, Kouyama K, Desch JK, Manley S, Norbash AM, Conrad CK, Friborg S, Reynolds T, et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope. 1999;109(2 Pt 1):266–274. doi: 10.1097/00005537-199902000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Dai J, Rabie AB. Direct AAV-mediated gene delivery to the temporomandibular joint. Front Biosci. 2007;12:2212–2220. doi: 10.2741/2224. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Dressman D, Tsao YP, Sakamoto A, Hoffman EP, Xiao X. rAAV vector-mediated sarcogylcan gene transfer in a hamster model for limb girdle muscular dystrophy. Gene Ther. 1999;6(1):74–82. doi: 10.1038/sj.gt.3300830. [DOI] [PubMed] [Google Scholar]
- 70.Lin CY, Ho CH, Hsieh YH, Kikuchi T. Adeno-associated virus-mediated transfer of human acid maltase gene results in a transient reduction of glycogen accumulation in muscle of japanese quail with acid maltase deficiency. Gene Ther. 2002;9(9):554–563. doi: 10.1038/sj.gt.3301672. [DOI] [PubMed] [Google Scholar]
- 71.Shimazaki K, Urabe M, Monahan J, Ozawa K, Kawai N. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: Prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 2000;7(14):1244–1249. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- 72.Vite CH, Passini MA, Haskins ME, Wolfe JH. Adeno-associated virus vector-mediated transduction in the cat brain. Gene Ther. 2003;10(22):1874–1881. doi: 10.1038/sj.gt.3302087. [DOI] [PubMed] [Google Scholar]
- 73.Monahan PE, Samulski RJ, Tazelaar J, Xiao X, Nichols TC, Bellinger DA, Read MS, Walsh CE. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5(1):40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- 74.Mori S, Takeuchi T, Enomoto Y, Kondo K, Sato K, Ono F, Iwata N, Sata T, Kanda T. Biodistribution of a low dose of intravenously administered AAV-2, 10, and 11 vectors to cynomolgus monkeys. Jpn J Infect Dis. 2006;59(5):285–293. [PubMed] [Google Scholar]
- 75*.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, et al. AAV-mediated factor ix gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. This paper describes the first use of recombinant AAV2 vectors in a Phase I trial for musle-directed gene delivery. [DOI] [PubMed] [Google Scholar]
- 76*.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. This paper describes the first use of recombinant AAV2 vectors to in a Phase I trial for liver-directed gene delivery. [DOI] [PubMed] [Google Scholar]
- 77.Kaiser J. Clinical trials. Gene transfer an unlikely contributor to patient’s death. Science. 2007;318(5856):1535. doi: 10.1126/science.318.5856.1535. [DOI] [PubMed] [Google Scholar]
- 78**.Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, Riddell A, et al. Long-term safety and efficacy of factor ix gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994–2004. doi: 10.1056/NEJMoa1407309. This paper describes the long-term safety and efficacy of recombinant AAV8 vectors in patients with hemophilia B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, Campbell-Thompson M, Yachnis AT, Sandhaus RA, McElvaney NG, Mueller C, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: Interim results. Hum Gene Ther. 2011;22(10):1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Byrne PI, Collins S, Mah CC, Smith B, Conlon T, Martin SD, Corti M, Cleaver B, Islam S, Lawson LA. Phase I/II trial of diaphragm delivery of recombinant adeno-associated virus acid alpha-glucosidase (rAAV1-CMV-GAA) gene vector in patients with pompe disease. Hum Gene Ther Clin Dev. 2014;25(3):134–163. doi: 10.1089/humc.2014.2514. [DOI] [PubMed] [Google Scholar]
- 81.Corti M, Cleaver B, Clement N, Conlon TJ, Faris KJ, Wang G, Benson J, Tarantal AF, Fuller D, Herzog RW, Byrne BJ. Evaluation of readministration of a recombinant adeno-associated virus vector expressing acid alpha-glucosidase in pompe disease: Preclinical to clinical planning. Hum Gene Ther Clin Dev. 2015;26(3):185–193. doi: 10.1089/humc.2015.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Li S, Ling C, Zhong L, Li M, Su Q, He R, Tang Q, Greiner DL, Shultz LD, Brehm MA, Flotte TR, et al. Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3B vectors. Mol Ther. 2015;23(12):1867–1876. doi: 10.1038/mt.2015.174. This paper describes that recombinant AAV3 vectors selectively and efficiently transduce the liver in non-human primates following intravenous delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Bell P, Somanathan S, Wang Q, He Z, Yu H, McMenamin D, Goode T, Calcedo R, Wilson JM. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol Ther. 2015;23(12):1877–1887. doi: 10.1038/mt.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84**.Vercauteren K, Hoffman BE, Zolotukhin I, Keeler GD, Xiao JW, Basner-Tschakarjan E, High KA, Ertl HC, Rice CM, Srivastava A, de Jong YP, et al. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther. 2016;24(6):1042–1049. doi: 10.1038/mt.2016.61. This paper describes the first direct comaprison of the efficiencies of recombinant AAV3, AAV5, AAV8, and AAV9 vectors in primary human hepatocytes in a humanized mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. J Gene Med. 2013;15(2):65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 86.Srivastava A. Adeno-associated virus: The naturally occurring virus versus the recombinant vector. Hum Gene Ther. 2016;27(1):1–6. doi: 10.1089/hum.2015.29017.asr. [DOI] [PMC free article] [PubMed] [Google Scholar]