Abstract
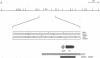
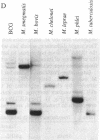
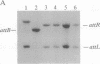
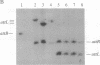
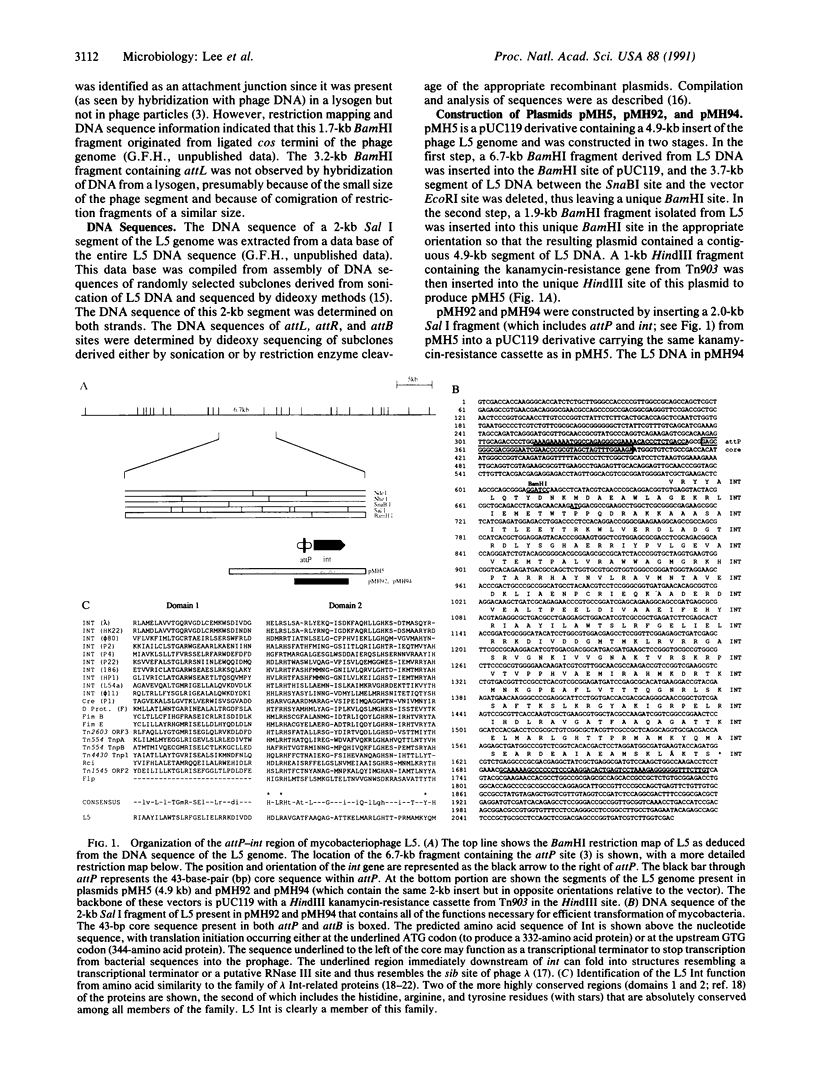
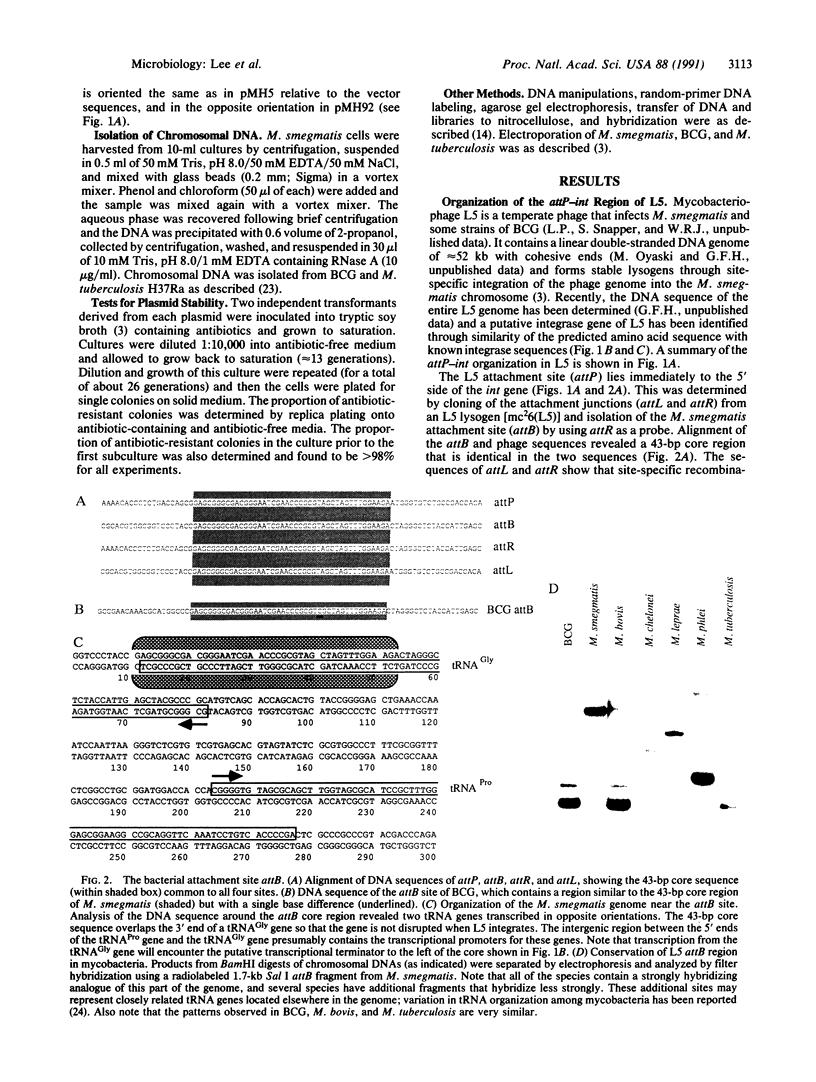
Mycobacteriophage L5, a temperate phage of mycobacteria, integrates site-specifically into the Mycobacterium smegmatis chromosome. We have identified the int gene and attP site of L5, characterized the chromosomal attachment site (attB), and constructed plasmid vectors that efficiently transform M. smegmatis through stable site-specific integration of the plasmid into the bacterial genome. These integration-proficient plasmids also efficiently transform slow-growing mycobacteria such as the pathogen Mycobacterium tuberculosis and the vaccine strain bacille Calmette-Guérin (BCG). The ability to easily generate stable recombinants in these slow-growing mycobacteria without the requirement for continual selection is of particular importance for the construction of recombinant BCG vaccines and for the isolation and characterization of mycobacterial pathogenic determinants in animal model systems. Integration vectors of this type should be of general use in a number of additional bacterial systems where temperate phages have been identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhargava S., Tyagi A. K., Tyagi J. S. tRNA genes in mycobacteria: organization and molecular cloning. J Bacteriol. 1990 Jun;172(6):2930–2934. doi: 10.1128/jb.172.6.2930-2934.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Goldschmidt R. M., Fletchall N. B., Kelly S. M. Avirulent Salmonella typhimurium delta cya delta crp oral vaccine strains expressing a streptococcal colonization and virulence antigen. Vaccine. 1988 Apr;6(2):155–160. doi: 10.1016/s0264-410x(88)80020-3. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M., Gulig P. A., Nakayama K. Stable recombinant avirulent Salmonella vaccine strains. Adv Exp Med Biol. 1989;251:33–47. doi: 10.1007/978-1-4757-2046-4_4. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Weiss W. R., Norris K. A., Seifert H. S., Kumar S., So M. Generation of a cytotoxic T-lymphocyte response using a Salmonella antigen-delivery system. Mol Microbiol. 1990 Dec;4(12):2111–2118. doi: 10.1111/j.1365-2958.1990.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Goodman S. D., Scocca J. J. Nucleotide sequence and expression of the gene for the site-specific integration protein from bacteriophage HP1 of Haemophilus influenzae. J Bacteriol. 1989 Aug;171(8):4232–4240. doi: 10.1128/jb.171.8.4232-4240.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskinsky C. M., Jacobs W. R., Jr, Clark-Curtiss J. E., Bloom B. R. Genetic relationships among Mycobacterium leprae, Mycobacterium tuberculosis, and candidate leprosy vaccine strains determined by DNA hybridization: identification of an M. leprae-specific repetitive sequence. Infect Immun. 1989 May;57(5):1535–1541. doi: 10.1128/iai.57.5.1535-1541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D., Attridge S., van den Bosch L., Hackett J. A chromosomal integration system for stabilization of heterologous genes in Salmonella based vaccine strains. Microb Pathog. 1988 Dec;5(6):407–418. doi: 10.1016/0882-4010(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Tuckman M., Bloom B. R. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature. 1987 Jun 11;327(6122):532–535. doi: 10.1038/327532a0. [DOI] [PubMed] [Google Scholar]
- Landy A. Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Annu Rev Biochem. 1989;58:913–949. doi: 10.1146/annurev.bi.58.070189.004405. [DOI] [PubMed] [Google Scholar]
- Martin C., Timm J., Rauzier J., Gomez-Lus R., Davies J., Gicquel B. Transposition of an antibiotic resistance element in mycobacteria. Nature. 1990 Jun 21;345(6277):739–743. doi: 10.1038/345739a0. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Stein D., Cohen S. N. Site-specific insertion of biologically functional adventitious genes into the Streptomyces lividans chromosome. J Bacteriol. 1988 May;170(5):2174–2184. doi: 10.1128/jb.170.5.2174-2184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. Molecular characterization of two proteins involved in the excision of the conjugative transposon Tn1545: homologies with other site-specific recombinases. EMBO J. 1989 Aug;8(8):2425–2433. doi: 10.1002/j.1460-2075.1989.tb08373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranes M. G., Rauzier J., Lagranderie M., Gheorghiu M., Gicquel B. Functional analysis of pAL5000, a plasmid from Mycobacterium fortuitum: construction of a "mini" mycobacterium-Escherichia coli shuttle vector. J Bacteriol. 1990 May;172(5):2793–2797. doi: 10.1128/jb.172.5.2793-2797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzier J., Moniz-Pereira J., Gicquel-Sanzey B. Complete nucleotide sequence of pAL5000, a plasmid from Mycobacterium fortuitum. Gene. 1988 Nov 30;71(2):315–321. doi: 10.1016/0378-1119(88)90048-0. [DOI] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Yeats S. Transfer RNA genes frequently serve as integration sites for prokaryotic genetic elements. Nucleic Acids Res. 1989 Mar 11;17(5):1907–1914. doi: 10.1093/nar/17.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Lugosi L., Jekkel A., Melton R. E., Kieser T., Bloom B. R., Jacobs W. R., Jr Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6987–6991. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990 Nov;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugnell R. A., Maskell D., Fairweather N., Pickard D., Cockayne A., Penn C., Dougan G. Stable expression of foreign antigens from the chromosome of Salmonella typhimurium vaccine strains. Gene. 1990 Mar 30;88(1):57–63. doi: 10.1016/0378-1119(90)90059-z. [DOI] [PubMed] [Google Scholar]
- Yagil E., Dolev S., Oberto J., Kislev N., Ramaiah N., Weisberg R. A. Determinants of site-specific recombination in the lambdoid coliphage HK022. An evolutionary change in specificity. J Mol Biol. 1989 Jun 20;207(4):695–717. doi: 10.1016/0022-2836(89)90238-6. [DOI] [PubMed] [Google Scholar]
- Ye Z. H., Buranen S. L., Lee C. Y. Sequence analysis and comparison of int and xis genes from staphylococcal bacteriophages L54a and phi 11. J Bacteriol. 1990 May;172(5):2568–2575. doi: 10.1128/jb.172.5.2568-2575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. H., Lee C. Y. Nucleotide sequence and genetic characterization of staphylococcal bacteriophage L54a int and xis genes. J Bacteriol. 1989 Aug;171(8):4146–4153. doi: 10.1128/jb.171.8.4146-4153.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]