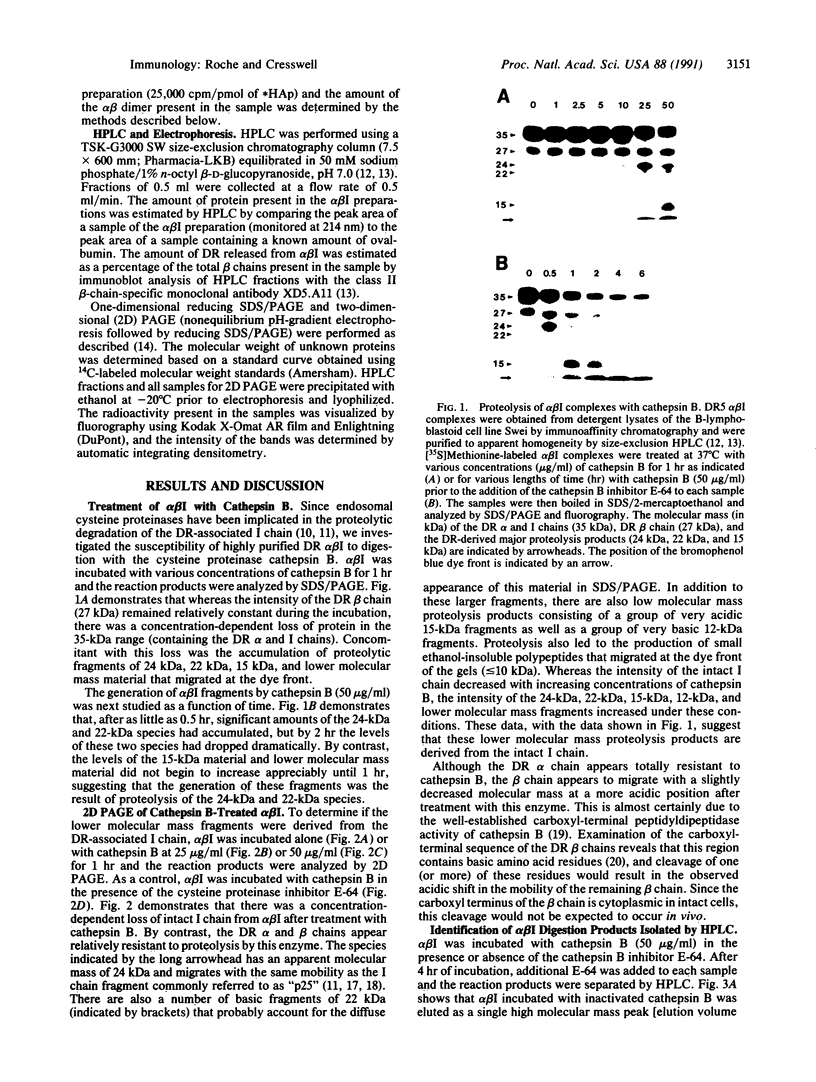
Abstract
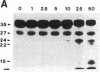
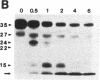
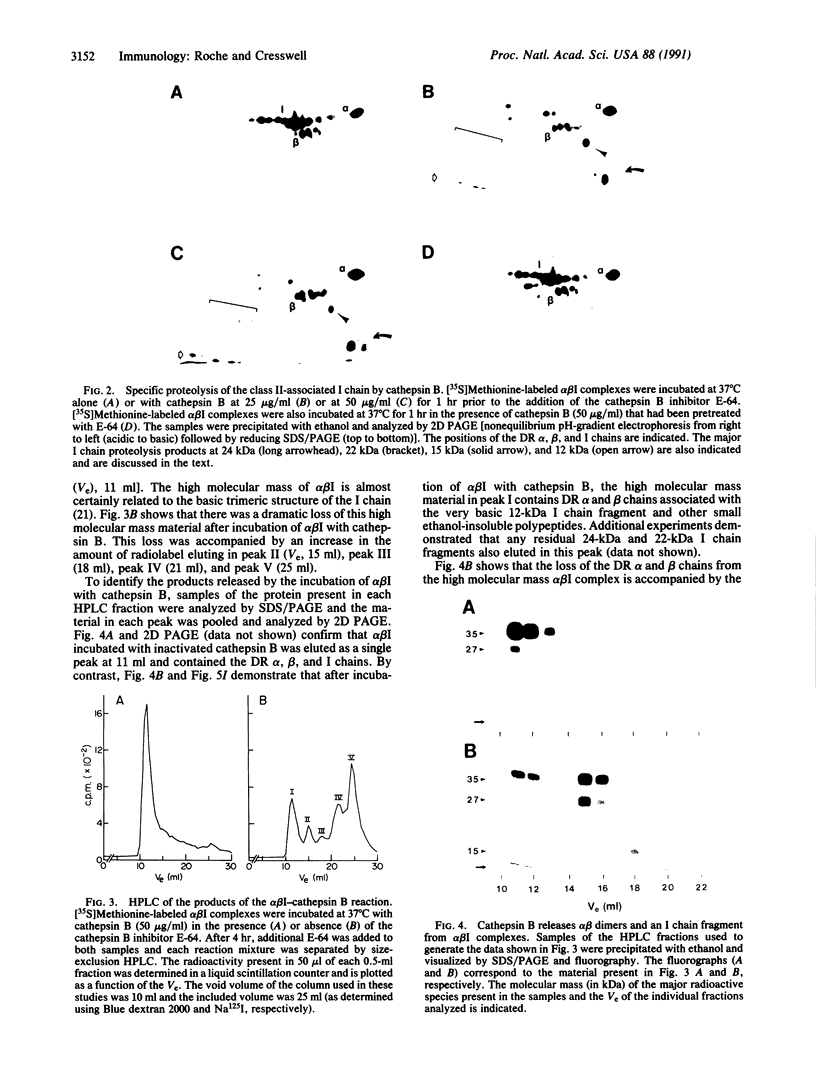
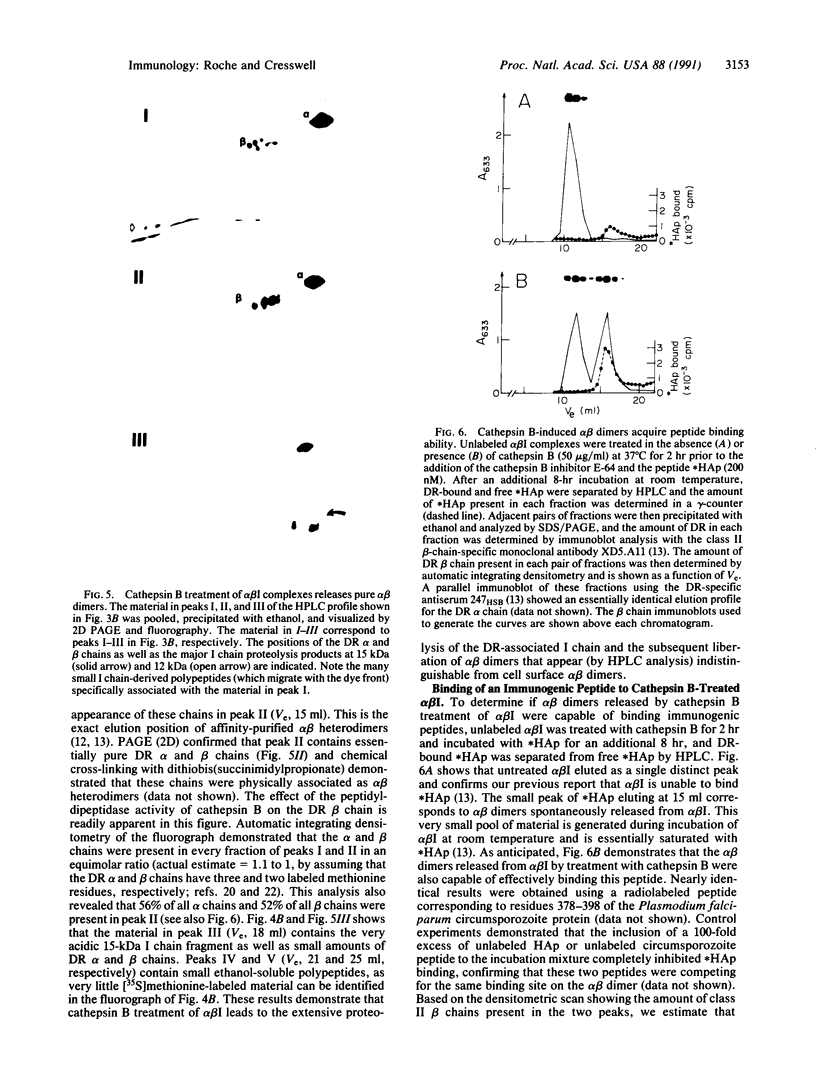
HLA-DR molecules are heterodimeric transmembrane glycoproteins that associate intracellularly with a polypeptide known as the invariant (I) chain. Shortly before expression of the HLA-DR alpha beta dimer on the cell surface, however, the I chain is removed from the intracellular alpha beta I complex by a mechanism thought to involve proteolysis. In this report, we show that treatment of purified alpha beta I with the cysteine proteinase cathepsin B results in the specific proteolysis of the HLA-DR-associated I chain in vitro. As a consequence of this, the I chain is removed and free alpha beta dimers are released from alpha beta I. Although alpha beta I fails to bind an immunogenic peptide, the released alpha beta dimers acquire the ability to bind the peptide after proteolysis of the I chain. These results suggest that the I chain inhibits immunogenic peptide binding to alpha beta I early during intracellular transport and demonstrate that proteolysis is likely to be the in vivo mechanism of I chain removal.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Brett S. J., Streicher H. Z., Takahashi H. Antigen processing for presentation to T lymphocytes: function, mechanisms, and implications for the T-cell repertoire. Immunol Rev. 1988 Dec;106:5–31. doi: 10.1111/j.1600-065x.1988.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Blum J. S., Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. P., Madrigal A., Parham P. Cytotoxic T cell recognition of an endogenous class I HLA peptide presented by a class II HLA molecule. J Exp Med. 1990 Sep 1;172(3):779–788. doi: 10.1084/jem.172.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P. Intracellular class II HLA antigens are accessible to transferrin-neuraminidase conjugates internalized by receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8188–8192. doi: 10.1073/pnas.82.23.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Shuman S., Abruzzini L. F., Gorski J., Watling D. L., Tieber V. L., Schwartz B. D. Characterization of two distinct DR beta chain alleles at the beta III locus of the DR5 haplotype: beta III alleles are highly conserved. J Immunol. 1986 Oct 15;137(8):2627–2631. [PubMed] [Google Scholar]
- Guagliardi L. E., Koppelman B., Blum J. S., Marks M. S., Cresswell P., Brodsky F. M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990 Jan 11;343(6254):133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D., Marti M., Long E. O. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990 Sep 1;172(3):947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner D. N., Cresswell P. Biosynthetic intermediates of HLA class II antigens from B lymphoblastoid cell lines. J Immunol. 1986 Oct 15;137(8):2632–2639. [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Travers P. J., Carey J., Grosveld F., Jenkins J., Bodmer W. F. Sequence of an HLA-DR alpha-chain cDNA clone and intron-exon organization of the corresponding gene. Nature. 1982 Oct 21;299(5885):750–752. doi: 10.1038/299750a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- Marks M. S., Blum J. S., Cresswell P. Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens. J Cell Biol. 1990 Sep;111(3):839–855. doi: 10.1083/jcb.111.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Cresswell P. Invariant chain associates with HLA class II antigens via its extracytoplasmic region. J Immunol. 1986 Apr 1;136(7):2519–2525. [PubMed] [Google Scholar]
- Neefjes J. J., Stollorz V., Peters P. J., Geuze H. J., Ploegh H. L. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990 Apr 6;61(1):171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Nguyen Q. V., Knapp W., Humphreys R. E. Inhibition by leupeptin and antipain of the intracellular proteolysis of Ii. Hum Immunol. 1989 Mar;24(3):153–163. doi: 10.1016/0198-8859(89)90056-6. [DOI] [PubMed] [Google Scholar]
- Nowell J., Quaranta V. Chloroquine affects biosynthesis of Ia molecules by inhibiting dissociation of invariant (gamma) chains from alpha-beta dimers in B cells. J Exp Med. 1985 Oct 1;162(4):1371–1376. doi: 10.1084/jem.162.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchtern J. G., Biddison W. E., Klausner R. D. Class II MHC molecules can use the endogenous pathway of antigen presentation. Nature. 1990 Jan 4;343(6253):74–76. doi: 10.1038/343074a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990 Jun 14;345(6276):615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Sweetser M. T., Morrison L. A., Braciale V. L., Braciale T. J. Recognition of pre-processed endogenous antigen by class I but not class II MHC-restricted T cells. Nature. 1989 Nov 9;342(6246):180–182. doi: 10.1038/342180a0. [DOI] [PubMed] [Google Scholar]
- Teyton L., O'Sullivan D., Dickson P. W., Lotteau V., Sette A., Fink P., Peterson P. A. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990 Nov 1;348(6296):39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- Thomas L. J., Nguyen Q. V., Elliott W. L., Humphreys R. E. Proteolytic cleavage of Ii to p25. J Immunol. 1988 Apr 15;140(8):2670–2674. [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Viguier M., Dornmair K., Clark B. R., McConnell H. M. The invariant chain forms complexes with class II major histocompatibility complex molecules and antigenic peptides "in vivo". Proc Natl Acad Sci U S A. 1990 Sep;87(18):7170–7174. doi: 10.1073/pnas.87.18.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]