Abstract
Globally, ∼1 in 15 men of reproductive age are infertile, yet the precise mechanisms underlying their gamete failure are unknown. Although a semen analysis is performed to determine fertilizing potential, the diagnostic suitability of this analysis has been questioned in several reports, as many men, classified as infertile according to their semen analysis, subsequently turn out to be fertile. Herein, we have used a quantitative (phospho)-proteomic analysis, using enrichment on titanium dioxide followed by ion-trap mass spectrometry (LC-MS/MS), to compare the semen of infertile versus fertile males. One protein, namely outer dense fiber 1 (ODF1), was dramatically reduced in infertile males. Using specific antibodies, we then screened the gametes of a cohort of suspected infertile men and demonstrated a reduction in the amount of ODF1 compared with fertile controls. Stress treatment of sperm deficient in ODF1 caused the head to decapitate, suggesting why these gametes fail to initiate fertilization. Interestingly, electron micrographs of ODF1-deficient spermatozoa revealed an abnormal connecting piece, indicating several developmental defects with both the implantation plate and the thin laminated fibers. In some cases, the implantation plate appeared to be reduced in size or was overburdened by granular material near the connecting piece. Hence, a strong reduction ODF1 is a marker of idiopathic male infertility and a potential driver of this condition.
Globally, in excess of 80 million people suffer from infertility (1), with ∼1 in every seven couples so affected (2). In at least half of these cases, a defect in one or more aspects of sperm function appears to be the cause (2). Understanding the contribution of each partner to a couple's infertility is critical in determining how this situation can be optimally addressed. In the case of the male, a semen analysis focusing on sperm motility, concentration, and morphology is often performed to determine the fertilizing potential. A set of guidelines for evaluating semen quality was originally published by the World Health Organization (WHO) in 1980 (3). However, so inadequate were these criteria in predicting infertility that revised values had to be released in 1987, 1992, 1999, and again in 2010 (4). Although a semen analysis is the best predictive test we have to date, it clearly falls short of a true diagnosis (5, 6). Indeed, several studies have shown that men with sperm numbers (7–9), morphology (8, 10), and motility (11–17) below the thresholds outlined by the WHO can be fertile. Furthermore, there are many instances of men with normal sperm parameters that are infertile (13, 18–20). Thus, these traditional diagnostic tests are limited in the information they generate and are poor predictors of male-factor infertility.
In order to define the cause of male infertility, attempts have been made to identify changes in the proteomic composition of normal and infertile spermatozoa (20–27). However, many of the proteins undergoing a quantitative change, for example, heat shock protein, are highly abundant in the sperm (21) and are likely to be a reflection of the condition, rather than the cause. Thus, the etiology of male infertility remains largely undefined.
It has been previously documented that 24% of men referred to assisted reproductive technology are classified as idiopathic (no obvious cause for infertility) (22). In order to understand the molecular basis of such, we have used a quantitative proteomic approach to compare the spermatozoa of normozoospermic (normal morphology of spermatozoa) idiopathic men to several known fertile donors. Herein, we show that the sperm-specific protein, outer dense fiber 1 (ODF1), was dramatically underexpressed within the infertile donors. As such, these sperm were prone to decapitation under freeze-thawing condition due to structural defects within their implantation plate and thin laminated fibers. Thus, the lack of ODF1 could explain why these men are infertile and is a biomarker that could be used for definitive diagnosis of male infertility.
EXPERIMENTAL PROCEDURES
Materials
All chemicals and antibodies were purchased from Sigma-Aldrich at the highest research grade, with the exception of albumin and ammonium persulphate (Research Organics, Cleveland, OH), Percoll (GE Healthcare, Castle Hill, Sydney, NSW Australia), HEPES (Gibco, Invitrogen Australia, Melbourne, VIC, Australia), and 10 × Ham's F-10 (MP Biomedical, Seven Hills, Sydney, NSW Australia). d-glucose, sodium hydrogen carbonate, sodium chloride, potassium chloride, calcium chloride, potassium orthophosphate, and magnesium sulfide were all AnalaR grade. Chloroform, methanol, and formaldehyde were purchased from Fronine (Riverstone, Sydney, NSW Australia) at the highest purity available. Ultrapure water was from Fluka (Castle Hill, Sydney, NSW Australia), Tris from ICN Biochemicals (Castle Hill, Sydney, NSW Australia), and the 4–20% precast-SDS gels were from NuSep, Ltd., Lane Cove, NSW, Australia. Anti-ODF1 and anti-CD45 were purchased from Abcam (Melbourne, Vic, Australia).
Preparation of Human Spermatozoa
Institutional and state government ethical approval were secured for the use of human semen samples in this research program. The study population was comprised of known fertile and suspected infertile (idiopathic) donors who were free of any detectable organic disease and were normozoospermic according to the conventional criteria of semen quality (4). A consent form was obtained from all donors. The semen samples were produced by masturbation and processed within 1 h of ejaculation using Percoll fractionation as described previously (23). The purified spermatozoa were obtained from the lower (80% Percoll) fraction. Sperm spermatozoa were washed three times in biggers-whitten-whittingham (BWW) medium and microscopically examined to ensure they were clear of any detectable round cells. The lack of leukocytes was ensured by probing with an anti-CD45 antibody in an immunoblot analysis.
Solubilization of Sperm Proteins and Trypsin Digestion
Approximately 400 μl of lysis buffer consisting of 4% (w/v) CHAPS, 7 m urea, 1/100 dilution of Halt™ phosphatase inhibitor (Pierce, Castle Hill, Sydney, NSW Australia) and 2 m thiourea were added to 1 × 107 sperm cells. The samples were lysed for 1 h at 4 °C with constant rotation then centrifuged (16,000 × g, 15 min, 4 °C) and the supernatant transferred to a new Eppendorf tube. An estimation of protein quantity was subsequently performed using a 2D quant kit (GE Healthcare). To 400 μg of protein, 10 mm DTT were added before incubation for 1 h at room temperature. After this, 50 mm idodoacetamide was added and allowed to incubate for 1 h in the dark (room temperature). These samples were then stored at −80 °C until needed. Before digestion, the sample was precipitated using methanol/chloroform as described elsewhere (24). Trypsin was then added in a ratio of 50:1 (protein:trypsin) in 25 mm ammonium bicarbonate containing 1 m urea, with constant shaking overnight at 37 °C.
Phosphopeptide Enrichment
Purification and enrichment of phosphopeptides from the tryptic digest (400 μg) was performed by a similar method to that previously described (25). Tryptic peptides were diluted fivefold in DHB1 buffer [350 mg/ml DHB, 80% (v/v) acetonitrile, 2% (v/v) trifluoroacetic acid (TFA)] and applied to dry TiO2 beads (200 μg). The sample was then washed 1 × in DHB buffer before being washed three times with wash buffer [80% acetonitrile (v/v), 2% TFA (v/v)] to remove the DHB. The sample was then directly eluted using 25 μl 2.5% ammonium hydroxide (pH ≥ 10.5) and immediately neutralized with 0.3 μl formic acid at a final concentration of 1.2%. All buffers used ultrapure water and were made fresh on the day of experimentation. The eluates, typically in 25 μl, were used immediately.
Ultra High Pressure Liquid Chromatography
For all experiments, an Ultimate3000 ultra high pressure liquid chromatography system (Dionex, Castle Hill, Sydney, Australia) was used, equipped with a ternary low pressure mixing gradient pump (LPG-3600), a membrane degasser unit (SRD-3600), a temperature-controlled pulled-loop autosampler (WPS-3000T), and a temperature controlled column oven with flow manager (FLM-3100). The LC experiments were performed using the preconcentration setup under the following conditions: Nanocolumn C18 PepMap100, 75 μm inner diameter × 150 mm, 3 μm, 100 Å; mobile phase A: 99.9% water + 0.1% formic acid (v/v); mobile phase B: 20/80 water/acetonitrile (v/v) + 0.08% formic acid; flow rate nanocolumn, 400 nl/min; gradient, 2–40% B over 45 min, 90% B for 5 min, 4% B for 30 min; loopsize: 5 μl; injection volume, 4 μl (FullLoop) by User Defined Program. The oven was set to 35 °C.
Mass Spectrometry
For the identification of phosphopeptides, separate collision induced dissociation (CID) and electron transfer dissociation (ETD) experiments were performed using an AmaZon ETD Ion Trap (Bruker Daltonik GmbH, Preston, Vic, Australia) equipped with an online-nanosprayer spraying from a 0.090 mm inner diameter and 0.02 mm inner diameter fused silica capillary to avoid any loss of phosphopeptides. Fine tuning was achieved using the smart parameter setting option (SPS) for 900 m/z, compound stability 60%, and trap drive level at 100% in normal mode resulted in the following mass spectrometric parameters: dry gas temperature, 180 °C; dry gas, 4.0 l min−1; nebulizer gas, 0.4 bar; electrospray voltage, 4500 V; high-voltage end-plate offset, −200 V; capillary exit, 140 V; trap drive, 57.4; funnel 1 in 100 V, out 35 V, and funnel 2 in 12 V, out 3.3 V; ion charge control (ICC) target, 500,000; maximum accumulation time, 50 ms. The sample was measured with the “Enhanced Scan Mode” at 8100 m/z per second (which allows monoisotopic resolution up to four charge stages), polarity positive, scan range from m/z 100–3000, five spectra averaged and rolling average of 2. The ETD reaction time was set to 100 ms using a reactant ICC of 500,000 allowing a maximum accumulation time for the reactant ion of 50 ms. The “Smart Decomposition” was set to “auto.” Acquired ETD/CID spectra were processed in DataAnalysis 4.0; deconvoluted spectra were further analyzed with BioTools 3.2 software and submitted to Mascot database search (Mascot 2.2.04, Swissprot database (Mascot 2.3.02, Swissprot database, 546, 000 sequences, 194,259,968 residues, release date January 10, 2014). The species subset was set at Homo sapiens parent peptide mass tolerance ± 0.8 Da, fragment mass tolerance ±0.8 Da; enzyme specificity trypsin with two missed cleavages considered. The following variable modifications have been used: fixed: carbamidomethylation (C); variable: deamidation (NQ), oxidation (M), phosphorylation (STY).
Bioinformatics
The derived mass spectrometry datasets on the three-dimensional-trap system were combined into protein compilations using the ProteinExtractor functionality of Proteinscape 2.1.0 573 (Bruker Daltonics, Bremen, Germany), which conserved the individual peptides and their scores, while combining them to identify proteins with much higher significance than achievable using individual searches. In order to exclude false positive identifications, peptides with Mascot scores below 35 (chosen on the basis of manual evaluation of the MS/MS data of peptides with scores below this number) were rejected. The only exception to this rule was the oxidized phosphopeptide KRSYKMNICK derived from ODF1, in which both the y-ion series and retention time could be compared with the nonoxidized form and determined to be correct, despite having a Mascot score of 25.5. The identified protein sequences were manually validated in BioTools (Bruker Daltonics, Bremen, Germany) on a residue-by-residue basis using the raw data to ensure accuracy. In brief, the ion series were inspected to ensure the peaks being selected were not simply baseline. The accuracy between the residues was less than 0.15 Da and that selected peaks had a signal above background. When run against a reversed database, and the spectra manually interpreted in a similar fashion, we produced less than 0.1% false discovery rates (26).
SDS-PAGE and Immunoblotting
SDS-PAGE was conducted on 1–10 μg solubilized sperm proteins using 4–20% precast polyacrylamide gels at 10 mA constant current per gel. The proteins were then transferred onto nitrocellulose hybond super-C membrane (Amersham Biosciences International, Sydney, Australia) at 350 mA constant current for 1 h. The membrane was blocked for 1 h at room temperature with Tris buffered saline (TBS;0.02 M Tris, pH 7.6, 0.15 m NaCl) containing 3% (w/v) BSA. The membrane was then incubated for 2 h at room temperature in a 1:1000 dilution of a monoclonal anti-ODF1, 1/1000 anti-CD45 or anti- β tubulin (Clone B-5–1-2) in TBS containing 1% (w/v) BSA and 0.1% (v/v) Tween. After incubation, the membrane was washed four times for 5 min with TBS containing 0.01% Tween-20 and then incubated for 1 h at room temperature with goat anti-mouse immunoglobulin G horseradish peroxidase (HRP) conjugate, at a concentration of 1:3000 in TBS containing 1% (w/v) BSA and 0.1% (v/v) Tween-20. The membrane was again washed as described above and then the detection was done using an enhanced chemiluminescence kit (Amersham Biosciences International, Sydney, Australia) according to the manufacturer's instructions.
In order to confirm equal loading of proteins, blots that had been probed were stripped and reprobed with an antibody against α-tubulin. For this procedure, ∼30 ml of stripping buffer, consisting of a 0.2 m NaOH solution was added to the membrane for 30 mins at room temperature. The membrane was then washed (3 × 10 min in TBS), blocked and probed with the primary antibody as described.
Experimental Design and Statistical Rationale
For the quantitative proteomics, we used five donations from fertile men. For the infertile sample, we used an infertile male who agreed to provide three separate donations for this study. Students t test were performed to identify phospho-peptide changes based on the integrated counts of the precursor peptide mass. As this work only reported one protein change, we could clearly validate this statistical model by performing immunoblotting against the original fertile and infertile sample. After this, we then screened 40 biobanked infertile samples for the presence of ODF1 using immunoblotting.
Immunocytochemistry
Slides were precoated with a 0.01% poly-l-lysine solution and then air-dried and washed in PBS. A 50 μl aliquot of spermatozoa was added to the coverslip for 10 min at room temperature. The cells were permeabilized using methanol for 10 min (4 °C) and excess solution was washed off with PBS. The cells were then blocked for 1 h using a 3% solution of BSA in PBS at 37 °C. To these cells, a 1:50 dilution of a monoclonal anti-ODF1 antibody in PBS containing 1% (w/v) BSA was added overnight at 4 °C. After incubation, cells were washed in PBS four times for 5 min and then incubated for 1 h at 37 °C with FITC-conjugated, goat anti-mouse immunoglobulin G at the concentration of 1:400 in PBS containing 1% (w/v) BSA. The cells were then washed as described above and mounted in the antifade medium, Mowiol. A fluorescence microscope (Axio Observer, Zeiss, Jena, Germany) was used to detect the FITC signal.
Electron Microscopy
Percoll purified sperm (∼ 1 × 106) were fixed in 50 μl of 2.5% glutaraldehyde in PBS 0.1 m (pH 7.4) for 2 h at 4 °C. The sample was then centrifuged at 3000 g for 15 min. The pellet was washed twice (10 min each). After each washing, sample was centrifuged at 3000 g for 1 min, supernatant discarded and pellet recovered in PBS 0.1 m (pH 7.4) mixing it by gentle agitation. Postfixation was performed using 1.33% osmium tetroxide in PBS 0.1 m (pH 7.4) for 1 h at 4 °C. The pellet was washed twice (10 min each) and the pellet resuspended in PBS 0.1 m (pH 7.4). In order to dehydrate the cells, the samples were resuspended following ascending ethanol solutions: 30% (10 min), 50% (twice for 10 min), 70% (twice for 10 min), 95% (twice for 10 min), and 100% (twice for 10 min). The dehydrated sample was centrifuged at 3000 g for 1 min to recover the pellet for the substitution procedure in 1.2 propylene oxide (twice for 20 min and two further centrifugations at 3000 g for 1 min). The pellet was the placed in a mixture of 50% 1.2 propylene oxide, 50% epoxy resin. Ultrathin sections were then taken on and used for transmission electron micrographs.
Freeze-Thaw Analysis
Spermatozoa from a fresh Percol-purified sample were taken and placed as a dry pellet within the −80 °C freezer and left for 1 h. After this, the cells were taken and medium BWW was added and the cells examined morphological for decapitation defects. More specifically, we looked for complete (normal) spermatozoa with both the head and the tail, compared with abnormal spermatozoa presented either with a head and no tail or a tail with no head.
RESULTS
Quantitative Phosphoproteomic Analysis of Fertile versus Infertile Samples
The semen analysis of the gametes of the initial male used in this study for the quantitative proteomic analysis is shown in Table I. Within his ejaculates, normal sperm cell numbers and motility were found; however, slightly low hyperactivation rates at 3.5 h were noted. In addition, the spermatozoa had acrosome defects (30% small, 16% absent) that were considered higher than normal. This patient was diagnosed as idiopathic (unknown cause of fertility failure). A pregnancy was achieved through conventional in vitro fertilization (IVF), suggesting that events leading up to sperm–egg interaction were likely to be the root cause behind the defect. As such, we immediately suspected that the process of capacitation was failing. Capacitation is the process whereby a freshly ejaculated spermatozoa undergoes protein modifications (in the female reproductive track) in order to fertilize. Capacitation is highly dependent on phosphorylation events, and therefore, we felt the best place to start was to look at phosphorylation patterns of the gametes of known fertile men, and compare them to the gametes of an idiopathic infertile male. We chose not to pool control samples since it was unclear if any change within one patient, would be reflected in other patient samples. Thus, phosphopeptides were enriched for from both fertile donors (n = 5 in total) and the infertile sample (three separate donations) using TiO2 and then run through liquid-chromatography coupled to ion-trap mass spectrometry (LC-MS/MS). Quantification of the samples demonstrated one major defect. In this case, three phosphopeptides from ODF1 were completely absent in the runs of the infertile samples, while present in all the samples derived from the fertile donors. Table II shows the individual peptides from ODF1. Column 1 shows the peptide sequence; note, the residues that are carbamidomethylated (C), phosphorylated (S), or oxidized (M) are underlined. Columns 2 and 3 show the measured and theoretical mass of the peptide, based on the charge state (z; column 5). The difference in mass between the measured and calculated is shown in parts per million (column 4). Using the ion-trap mass spectrometer, we could not detect a signal above backgroup in any of the infertile samples (column 6). Finally, the high and low values of the 95% confidence intervals, based on the integrated counts of the fertile biological replicates (n = 5) are given in columns 7 and 8, respectively. The last phosphopeptide derived from ODF1, shown in Table II (row 6), was not detected in the infertile male; however, we only detected the presence of this doubly phosphorylated peptide in two of the fertile controls, hence the confidence intervals were not determined. Supplemental Table 1 gives the tandem mass spectra for each peptide.
Table I. Semen analysis of the infertile male, compared to normal semen parameters.
| Semen Analysis | ||
|---|---|---|
| Sperm Parameters | Infertile Subject | Normal Reference Rangesa |
| Sperm concentration (×106/ml) | 227 | ≥15 |
| Total volume (ml) | 2.7 | ≥1.5 |
| % motile | 70 | ≥40 |
| % progressive | 36 | ≥32 |
| % Strict normal morphology | 19 | ≥4 |
| % small acrosome | 46 | <20% |
| % midpiece defect | 2 | <20% |
| % tail defect | 9 | <20% |
| Leukocytes (×106/ml) | 2.6 | ≤1 |
| Overnight survival % | 94 | ≥70 |
| % Hyperactivating 3 h | 6 | ≥10 |
| % Hyperactivating 6 h | 20 | ≥10 |
a Reference ranges adapted from WHO (2010) or validated by the Male Fertility Lab, University of Washington
Table II. Phosphopeptides from ODF1.
| Sequence | m/z meas. | Mr calc. | Δ m/z [ppm] | z | Infertile | Fertile: 95% Confidence Intervals |
|
|---|---|---|---|---|---|---|---|
| High | Low | ||||||
| DVTYSYGLGSCVK | 764.75 | 1527.63 | −91.66 | 2 | n/d | 371307891 | 156887226 |
| KYSYMNICK | 651.74 | 1301.51 | −36.13 | 2 | 1288386776 | 607661995 | |
| KYSYMNICK | 643.74 | 1285.52 | −37.42 | 2 | n/d | ||
| KYSYMNICK | 652.22 | 1302.50 | −57.58 | 2 | |||
| ILASSCCSSNILGSVNVCGFEPDQVK | 1461.33 | 2920.28 | 125.52 | 2 | n/d | 266457330 | 4109863 |
| ILAp(SSCCSSNILGS)VNVCGFEPDQVK | 1000.94 | 3000.24 | −143.44 | 3 | n/d | a | * |
n/d = not detected; meas. = measured; calc. = calculated.
a = only detected in two of the samples, therefore confidence intervals were not determined.
Extracted Ion Chromatogram of ODF1
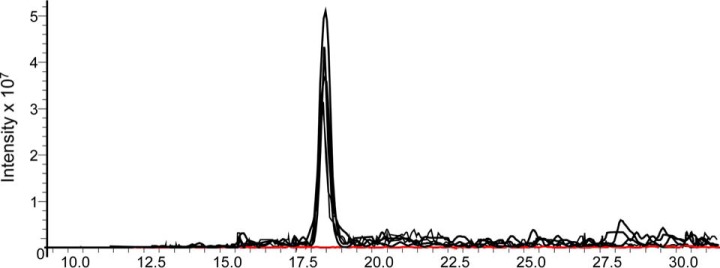
To visually describe the extent of the changes, an extracted ion chromatogram (EIC) for the peptide KYSYMNICK (residues modified underlined; measured m/z 651.74) is given in Fig. 1. The intensity of the peptide signals from five fertile donations are overlaid (black) As shown, just after 17.5 min, the peptide elutes in all five fertile samples (black); however, very little signal was detected in the gametes originating from the infertile male (EIC shown in red; n = 3). These data, in conjunction with Table II, strongly suggested that ODF1 phosphorylated peptides normally present within fertile samples, were missing from the spermatozoa of this infertile male.
Fig. 1.
Loss of phospho-ODF1 peptide from infertile male. Extracted ion chromatogram from the phosphopeptide KYSYMNICK (m/z 651.74) derived from ODF1. The signal intensity of the fertile (black; n = 5) and the infertile (red, n = 3) samples are shown.
Immunoblotting Shows Several Reduced Amounts of ODF1 Within Infertile Men
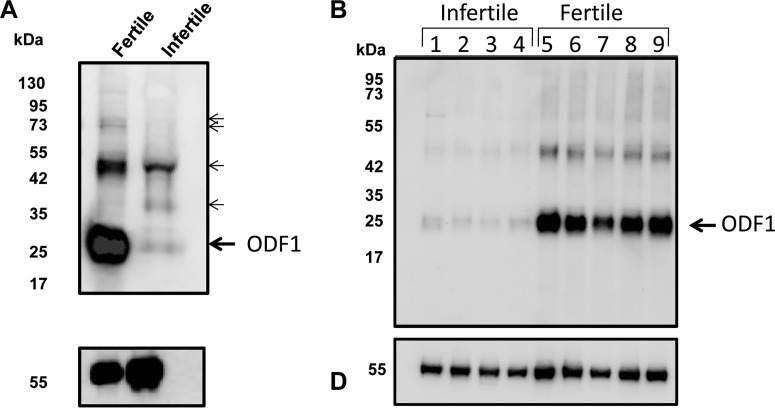
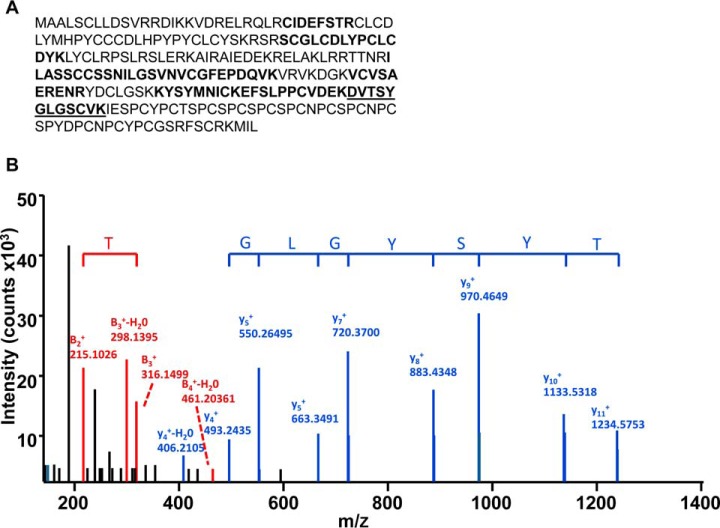
The loss of three phosphopeptides from one protein can be explained by at least one of two possibilities. First, kinase(s)/phosphatases that actively phosphorylate/dephosphorylate ODF1 may be occurring within the infertile gametes, which could explain the lack of phospho-peptides. Secondly, it could also be that the ODF1 protein itself may be absent from the infertile sample altogether, and as such, all three phospho-peptides are missing. In order to determine which is the case, we next performed immunoblotting using the ODF1 antibody on both the infertile and fertile samples. As shown in Fig. 2A, the antibody detected some cross-reacting bands across each sample; however, a strong signal at 27 kDa (the predicted size of ODF1;) can be seen (Fig. 2A, upper panel, arrow labeled ODF1). The presence of ODF1 in this band was confirmed by excising a corresponding gel plug from SDS-PAGE and running tryptic digest through LC-MS. In addition, several minor bands of higher mass were also detected at ∼35, 50 and 75–80 kDa (bands marked in Fig. 2A with a smaller arrow) within the immunoblots. As such, we also excised these regions from a corresponding gel and ran the tryptic digest through LC-MS. Importantly, LC-MS analysis of the 27 kDa band allowed the detection of seven unambiguous peptides corresponding to ODF1 (of note, 28 redundant peptides were found for ODF1). The nonredundant peptides within the ODF1 sequence is highlighted in Fig. 3A. In total, 36% sequence coverage was obtained for ODF1 within the 27 kDa band. A representative spectra of the peptide DVTSYGLGSCVK, which is derived from ODF1, together with the y and b ion series is shown in Fig. 3B. Despite finding 36% sequence coverage in the 27 kDa band, not a single peptide derived from ODF1 was found in the cross-reacting bands within the 35, 50 and 75–80 kDa region. Therefore, it is unclear why they reacted with ODF1 antibody.
Fig. 2.
Severe reduction in ODF1 from the gametes of the infertile male. (A) Western blotting analysis. Proteins were extracted from either a known fertile donor (lane 1) or the infertile male (lane 2). The proteins were run into SDS-PAGE, then transferred to nitrocellulose. The membrane was then probed for using anti-ODF1 antibody (top panel). After this, the membrane was stripped and reprobed using anti β-tubulin antibody (lower panel). The position of the molecular weight markers are shown on the left-hand side. The large arrow shows the expected position of ODF1. The smaller arrows higher molecular mass cross-reacting bands that were excised for nano-LC-MS/MS analysis. (B) Immunoblot using the anti-ODF1 against suspected idiopathic infertile men (lanes 1–4) or control fertile donors.
Fig. 3.
LC-MS/MS analysis. To verify the presence of ODF1, SDS-PAGE was run from fertile sperm lysates. Gel fragments corresponding to the arrows labeled in Fig. 2 were excised and trypsin digested before analysis in LC-MS/MS. (A) In the 27 kDa bands, we detected six unambiguous peptides corresponding to ODF1. This equated to 36% sequence coverage. The MS/MS spectra for peptide DVTSYGLGSCVK (boldfaced/underlined in A) is shown in (B).
Importantly, when we compared the fertile and infertile sample, markedly less cross-reactivity of ODF1 was detected in the 27 kDa region within the infertile sample (Fig. 2, lane 2). Control for protein equal loading was performed with anti β-tubulin antibody (Fig. 2, lower panel). These data clearly demonstrated that ODF1 protein was severely reduced in idiopathic infertile sperm when compared with fertile control.
Loss of ODF1 Is Common within Normozoospermic Idiopathic Samples
Given the data on the quantitative phosphoproteomics and immunoblotting approaches, we wanted to see how widespread the loss of ODF1 would be within the infertile male population. In order to achieve this, several sperm samples were taken from either known fertile donors (defined as having a baby within the last 2 years) or suspected male-factor infertile men who were chosen on the basis that all their semen characteristics were above the normal range for WHO criteria of motility, morphology, and sperm cell numbers; however, their female partner showed no obvious sign of infertility (female under 35 years of age, normal history and examination, normal diagnostic laparoscopy, and normal pattern of ovulation). To define the level of ODF1 within these samples, an anti-ODF1 immunoblot analysis was performed. Clearly, a signal arising from the fertile men was easily obtained (Fig. 3B, lanes 5–9). However, several infertile men were shown to have severally reduced amounts of the entire protein for ODF1.
Lack of ODF1 Leads to Decapitation when Sperm Are under Stress
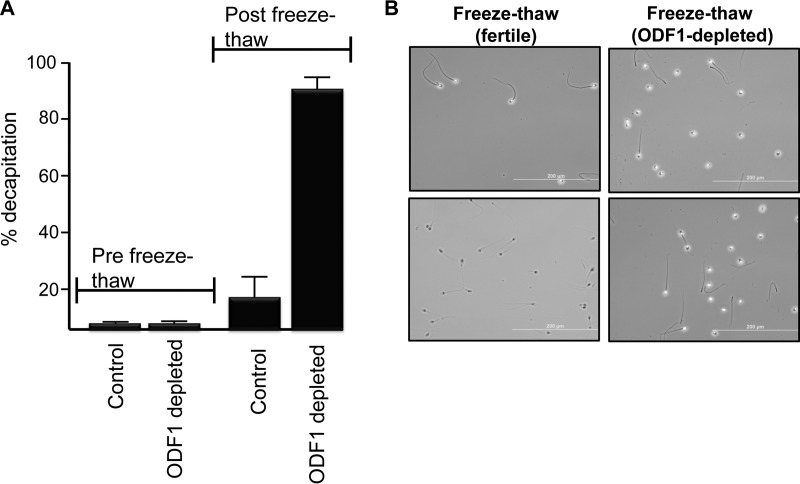
The role of ODFs in general and ODF1 specifically in spermatozoa is still unclear. The knockout male mice deficient in ODF1 have been shown to be completely infertile, as spermatozoa undergo decapitation when taken from the epididymis (27). Given that the samples we used were idiopathic (i.e. of unknown infertility origin), the same phenotype was not reciprocated within the human sperm samples that we characterized with severe reduction in the ODF1 protein. However, during a routine fixation procedure of sperm samples, we noted that many of the sperm from ODF1-deficient men, became decapitated. Hence, it appeared that ODF1 negative sperm may be weakened within the neck region. To test this hypothesis, spermatozoa from men known to lack ODF1 were subjected to one round of freeze-thawing (note: no glycerol was included is the freezing mixture and as such, all the sperm were immotile following thawing). As a control, spermatozoa from known, previously fertile men (pregnancy within the last 2 years) were also subjected to the same conditions. As shown (Fig. 4A), only a small percentage of sperm cells are decapitated before freezing in both controls in the ODF1-depleted samples. However, after one round of freeze-thaw, the ODF1 depleted sperm demonstrate a 90% decapitation rate, compared with ∼20% within the fertile controls. A phase contrast image of a population of sperm cells after the freeze-thawing process is shown (Fig. 4B). On the left-hand side are two images from control sperm after freeze-thawing. Clearly, the majority of cells remain intact. In contrast, on the right side are two images from ODF1-deficient samples after freeze-thawing show that a majority are decapitated. These data indicated that human spermatozoa lacking ODF1 were not decapitated upon ejaculation but required some form of stress, such as freeze-thawing, to reciprocate this condition.
Fig. 4.
Decapitation of sperm following freeze thawing in ODF1-deficient samples. Spermatozoa were placed in an Eppendorf tube and subjected to one round of freeze thawing cycle. After this, the cells were counted phase contrast microscopy. (A) The percentage of cells demonstrated decapitation before and after one round of freeze-thawing in both control and ODF1-depleted sperm. (B) Phase contrast image of spermatozoa from two fertile donors (left side) and ODF1-depleted samples (right side). The images show the status of the spermatozoa following one round of freeze-thawing. Scale bar represents 200 μm.
Electron Micrographs
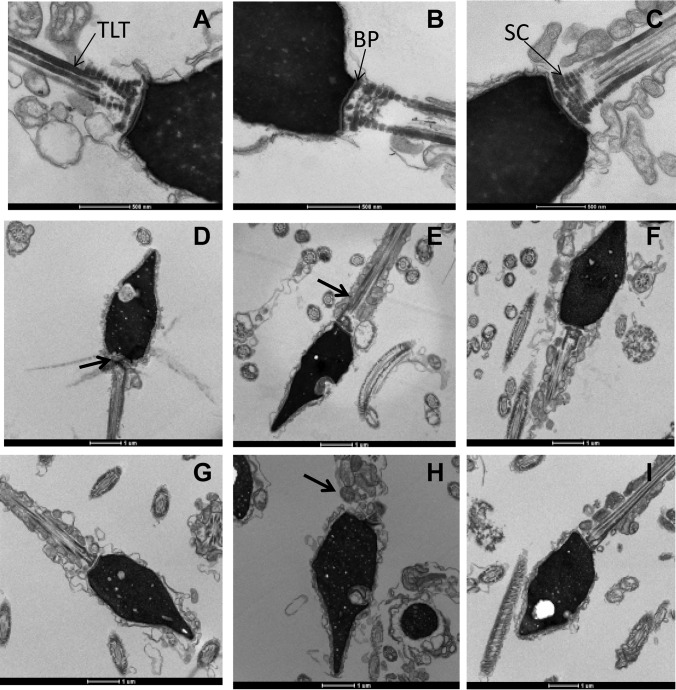
In order to investigate the neck region of ODF1-depleted human spermatozoa, we looked at electron micrographs of normal and ODF1-depleted cells. Electron pictographs taken of sperm cells from known fertile donors showed the characteristic implantation plate which consists of the thin filament fibers (Fig. 5A, TLT), basel plate (Fig. 5B, BP), and striated columns (Fig. 5C, SC). However, samples taken from patients lacking ODF1 showed several abnormalities. These included malformed or reduced size in the basal plate (Fig. 5D, arrow) and thin laminated fibers (Fig. 5E, arrow, D,H). In some cases, the implantation plate was overburdened by granular material near the connecting piece (Fig. 5H). Finally, we also observed a lack of, or poorly developed, striated columns in many of the spermatozoa (Figs. 5D–5I). The same observation has been made by Kumal et al., who showed within 16 male patients (all of which produced spermatozoa that easily decapitated) all had lower-density around the connecting piece (28). The micrographs show that a malformed connecting piece is the likely reason that spermatozoa lacking in ODF1 become decapitated under mild stressful conditions such as freeze-thawing.
Fig. 5.
Ultrastructure changes to the implantation plate of spermatozoa with reduced ODF1. The appearance of a normal implantation plate with fertile samples is shown (A–C). The thin laminated fiber (A;TLT), basel plate (B, BP), and striated columns (C, SC) are labeled. Within the several reduced ODF1 infertile sperm (D–I) a lack of the basel plate formation was observed (D, arrow), lack of or poorly developed striated columns (D–I), and absent or smaller than usual thin laminated fibers (D, E, H).
DISCUSSION
According to the World Health Organization, up to 15% of reproductive-aged couples are infertile, with 50% of these being male-factor driven (29). In some cases, a clear association with male infertility is established. For example, congenital abnormalities, antisperm antibodies, varicocele, glandular infections, and obstructive azoospermia are all known causes of male infertility. Nevertheless, these cases are relatively easy to diagnose, with either a lack of sperm in the ejaculate or poor sperm functionality, often presenting in a clinical environment. For the majority of couples, it is often difficult to diagnose male infertility due to a lack of biomarkers or clinical presentation. Thus, when a couple presents for assisted conception, understanding the contribution of each partner to a couple's infertility falls disproportionately on the female and often involves blood, hormone, frequency of ovulation, and invasive laparoscopies. This study has used a quantitative (phosho)-proteomic analysis to determine that ODF1 is dramatically reduced within the gametes of male infertile idiopathic patients, whose sperm parameters are considered normal according to the WHO criteria. The lack of ODF1 appeared to be associated with a failure in the development of the implantation plate and thin filament fibers, making the spermatozoa vulnerable to decapitation. Although the data are mostly correlative, the reduction in ODF1 may participate in the failure of these spermatozoa to fertilize in vivo.
A semen analysis of the initial infertile patient used in this study demonstrated that the sample had 46% small acrosomes, otherwise normal sperm cells numbers and motility were apparent. This same male achieved a successful outcome using conventional in vitro fertilization (IVF). As such, events preceding sperm–egg binding are a likely cause of fertilization failure. One of the most important of these events is capacitation. Capacitation is the term now used to describe the biochemical pathway sperm undergo inside the female reproductive tract. During capacitation, sperm alter their behavior of swimming and transit from a forward progressive to a hyperactivated state. After this, sperm also undergo the acrosome reaction—an exocytotic event whereby the outer portion of the sperm head releases proteases into the surrounding milieu and exposes key proteins for fertilization such as Izumo1 (30). As this entire process is underpinned by a series of phosphorylation events (31–34), we reasoned that a quantitative phosphoproteomic approach would reduce the dynamic range of proteins we were comparing and, thus, be a more sensitive manner in which to find changes within the infertile gametes. From this basis, we found a severe reduction in the protein ODF1.
The lack of ODF1 correlates to defects within the implantation plate and thin laminated fibers. Interestingly, mice lacking Odf1 produce normal numbers of spermatozoa with normal motility but are completely infertile (35). The gametes of these mice display fragile sperm connecting pieces and also a disorganized mitochondrial sheath (35). Furthermore, the spermatozoa of these mice are in a decapitated state. In our human samples, we initially observed that the sperm head and neck remained intact. However, after stress-induced freeze-thawing, the head became decapitated. One reason we may see a difference between the human and mouse is that, although the later are completely ODF1 deficient, in many of the human samples we tested, we could not detect a complete lack of the protein but rather a severe reduction in ODF1 as seen with the immunoblots (Figs. 2 and 3). Interestingly, electron micrographs showed that human ODF1-deficient sperm display a very similar phenotype to the mouse Odf1−/− mice. In our study, men lacking ODF1 show a weakness in the connecting piece (the same as ODF1 knockout mice), and their sperm are easily decapitated (same as ODF1 knockout mice). Therefore, our descriptive data in humans, added to the previously published data in mice, suggest a potential causative role for ODF1 deficiency in human infertility. Other reports exists on human sperm that present as decapitated or, in the words of the authors, have “loose tails and head” (36). In one such report, a 36-year-old infertile man with normal WHO semen parameters (35–46 × 106 sperm/ml, 60% motility), displayed decapitated heads, even though the tails were structurally normal (36). Interestingly, electron micrographs showed many of the sperm cells displayed an underdeveloped or rudimentary connecting piece (36) or a failure in the implantation fossa to differentiate, giving rise to sperm with loose heads. In two other case reports, men, both of whom were 33 years old, presented with normal sperm parameters, with the exception that 90% of the cells were decapitated (37). In both cases, the basal plate and subsequent implantation fossa were not formed (37, 38), suggesting a common mechanism in the etiology of this condition. Finally, Kamal et al., have reported several cases of infertile men in which they described the use of a micromanipulator to press the sperm gently against the bottom of the dish. Within 16 cases, they found that the head of the sperm was easily separated from the tail (28) compared with controls. Given the similarities of these data and our current findings, we suggest that a lack of ODF1 may be a driver underpinning male infertility in cases where the head and neck are loosely attached.
Based on the information gained from this report, semen testing in couples with a history of unexplained infertility could be extended to investigate lack of ODF1. The lack of suitable and definitive biomarkers for male infertility is one reason why, when a couple presents to an infertility clinic, diagnostic and therapeutic focus falls disproportionately on the female. In recognition of the limited ability of descriptive semen analyses to predict infertility, additional tests have been developed, which include DNA damage assessment (39), acrosome reaction tests (40), egg- and zona-penetration assays (41), antisperm antibody testing (42), aneuploidy screening (43), hyaluronic acid binding (44), and DNA methylation status (5). Although helpful, the predictive values of these tests are suboptimal (45), and the need for improved diagnostic tools to evaluate male infertility is widely accepted (46). We propose that assaying ODF1 in the semen and assessing one round of freeze-thawing cycle, could be used for testing the contribution of the male partner toward the couple's infertility.
Overall, this proteomic-based analysis identified the down-regulation of ODF1 in human semen as a marker and potential driver for male infertility. Future investigations are warranted to determine the clinical significance of these findings for the diagnosis of male infertility.
Supplementary Material
Footnotes
Author contributions: H.H., T.V., and M.A.B. designed the research; L.H., E.S., and T.V. performed research; C.H.M. contributed new reagents or analytic tools; C.S., D.D., H.H., and M.A.B. analyzed the data; and H.H. and M.A.B. wrote the paper.
* This work was supported by NHMRC Grants APP1101615 and APP1084750.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- DHB
- 2,3-dihydroxybenzoic acid
- TFA
- trifluoro-acetic acid
- TiO2
- titanium dioxide
- ODF1
- outer dense fiber 1
- PKA
- protein kinase A
- ETD
- electron transport dissociation
- CID
- collision induced dissociation
- ICC
- ion charge control
- IVF
- in vitro fertilization.
REFERENCES
- 1. Matzuk M. M., and Lamb D. J. (2008) The biology of infertility: Research advances and clinical challenges. Nat. Med. 14, 1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McLachlan R. I., and de Kretser D. M. (2001) Male infertility: The case for continued research. Med. J. Aust. 174, 116–117 [DOI] [PubMed] [Google Scholar]
- 3. Aitken R. J., Baker H. W. G., Barratt C., Beher H. M., Comhaire F., Cooper. T. G., De Jonge C., Eliasson R., Farley T. M., Griffen. P. D., Huhtaniemi I., Kruger T. F., Mbvizvo M. T., Overstreet. J. W., Sellaro L., Suomien. J., Waites G. M., and Wang C. C. (1980) Laboratory manual for the examination of human semen-cervical mucus interaction. Cambridge University Press, UK. [Google Scholar]
- 4. WHO. (2010) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interation, Cambridge University Press, UK. [Google Scholar]
- 5. Aston K. I., Uren P. J., Jenkins T. G., Horsager A., Cairns B. R., Smith A. D., and Carrell D. T. (2015) Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil. Steril. 104, 1388–1397 e1385 [DOI] [PubMed] [Google Scholar]
- 6. Esteves S. C., Zini A., Aziz N., Alvarez J. G., Sabanegh E. S. Jr., and Agarwal A. (2012) Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology 79, 16–22 [DOI] [PubMed] [Google Scholar]
- 7. David G., Jouannet P., Martin-Boyce A., Spira A., and Schwartz D. (1979) Sperm counts in fertile and infertile men. Fertil. Steril. 31, 453–455 [PubMed] [Google Scholar]
- 8. MacLeod J., and Wang Y. (1979) Male fertility potential in terms of semen quality: A review of the past, a study of the present. Fertil. Steril. 31, 103–116 [DOI] [PubMed] [Google Scholar]
- 9. van Zyl J. A., Menkveld R., van Kotze T. J., Retief A. E., and van Niekerk W. A. (1975) Oligozoospermia: A seven-year survey of the incidence, chromosomal aberrations, treatment and pregnancy rate. Int. J. Fertil. 20, 129–132 [PubMed] [Google Scholar]
- 10. Smith K. D., Rodriguez-Rigau L. J., and Steinberger E. (1977) Relation between indices of semen analysis and pregnancy rate in infertile couples. Fertil. Steril. 28, 1314–1319 [DOI] [PubMed] [Google Scholar]
- 11. Eliasson R. (2010) Semen analysis with regard to sperm number, sperm morphology and functional aspects. Asian J. Androl. 12, 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papillon-Smith J., Baker S. E., Agbo C., and Dahan M. H. (2015) Pregnancy rates with intrauterine insemination: comparing 1999 and 2010 World Health Organization semen analysis norms. Reprod. Biomed. Online 30, 392–400 [DOI] [PubMed] [Google Scholar]
- 13. Sánchez V., Wistuba J., and Mallidis C. (2013) Semen analysis: Update on clinical value, current needs and future perspectives. Reproduction 146, R249–R258 [DOI] [PubMed] [Google Scholar]
- 14. Wang C., and Swerdloff R. S. (2014) Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 102, 1502–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y., Yang J., Jia Y., Xiong C., Meng T., Guan H., Xia W., Ding M., and Yuchi M. (2014) Variability in the morphologic assessment of human sperm: Use of the strict criteria recommended by the World Health Organization in 2010. Fertil. Steril. 101, 945–949 [DOI] [PubMed] [Google Scholar]
- 16. Menkveld R., Wong W. Y., Lombard C. J., Wetzels A. M., Thomas C. M., Merkus H. M., and Steegers-Theunissen R. P. (2001) Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: An effort towards standardization of in-vivo thresholds. Hum. Reprod. 16, 1165–1171 [DOI] [PubMed] [Google Scholar]
- 17. Ombelet W., Wouters E., Boels L., Cox A., Janssen M., Spiessens C., Vereecken A., Bosmans E., and Steeno O. (1997) Sperm morphology assessment: Diagnostic potential and comparative analysis of strict or WHO criteria in a fertile and a subfertile population. Int. J. Androl. 20, 367–372 [DOI] [PubMed] [Google Scholar]
- 18. Ombelet W., Bosmans E., Janssen M., Cox A., Vlasselaer J., Gyselaers W., Vandeput H., Gielen J., Pollet H., Maes M., Steeno O., and Kruger T. (1997) Semen parameters in a fertile versus subfertile population: A need for change in the interpretation of semen testing. Hum. Reprod. 12, 987–993 [DOI] [PubMed] [Google Scholar]
- 19. Stern J. E., Luke B., Hornstein M. D., Cabral H., Gopal D., Diop H., and Kotelchuck M. (2014) The effect of father's age in fertile, subfertile, and assisted reproductive technology pregnancies: A population based cohort study. J. Assist. Reprod. Genet. 31, 1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zukerman Z., Rodriguez-Rigau L. J., Smith K. D., and Steinberger E. (1977) Frequency distribution of sperm counts in fertile and infertile males. Fertil. Steril. 28, 1310–1313 [DOI] [PubMed] [Google Scholar]
- 21. Baker M. A., Naumovski N., Hetherington L., Weinberg A., Velkov T., and Aitken R. J. (2013) Head and flagella sub-compartmental proteomic analysis of human spermatozoa. Proteomics 13, 61–74 [DOI] [PubMed] [Google Scholar]
- 22. Muller C. H. (1992) The andrology laboratory in an assisted reproductive technologies program. Quality assurance and laboratory methodology. J. Androl 13, 349–360 [PubMed] [Google Scholar]
- 23. Lessley B. A., and Garner D. L. (1983) Isolation of motile spermatozoa by density gradient centrifugation in Percoll. Gamete Res. 7, 49–61 [Google Scholar]
- 24. Baker M. A., Lane D. J., Ly J. D., De Pinto V., and Lawen A. (2004) VDAC1 is a transplasma membrane NADH:ferricyanide reductase. J. Biol. Chem. 279, 4811–4819 [DOI] [PubMed] [Google Scholar]
- 25. Larsen M. R., Thingholm T. E., Jensen O. N., Roepstorff P., and Jørgensen T. J. (2005) Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol. Cell. Proteomics 4, 873–886 [DOI] [PubMed] [Google Scholar]
- 26. Baker M. A., Naumovski N., Hetherington L., Weinberg A., Velkov T., and Aitken R. J. (2013) Head and flagella subcompartmental proteomic analysis of human spermatozoa. Proteomics 13, 61–74 [DOI] [PubMed] [Google Scholar]
- 27. Yang K., Meinhardt A., Zhang B., Grzmil P., Adham I. M., and Hoyer-Fender S. (2012) The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol. Cell. Biol. 32, 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamal A., Mansour R., Fahmy I., Serour G., Rhodes C., and Aboulghar M. (1999) Easily decapitated spermatozoa defect: A possible cause of unexplained infertility. Hum. Reprod 14, 2791–2795 [DOI] [PubMed] [Google Scholar]
- 29. Cui W. (2010) Mother or nothing: The agony of infertility. Bull. World Health Organ 88, 881–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inoue N., Ikawa M., Isotani A., and Okabe M. (2005) The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434, 234–238 [DOI] [PubMed] [Google Scholar]
- 31. Ficarro S., Chertihin O., Westbrook V. A., White F., Jayes F., Kalab P., Marto J. A., Shabanowitz J., Herr J. C., Hunt D. F., and Visconti P. E. (2003) Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J. Biol. Chem. 278, 11579–11589 [DOI] [PubMed] [Google Scholar]
- 32. Visconti P. E., Bailey J. L., Moore G. D., Pan D., Olds-Clarke P., and Kopf G. S. (1995) Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121, 1129–1137 [DOI] [PubMed] [Google Scholar]
- 33. Visconti P. E., Moore G. D., Bailey J. L., Leclerc P., Connors S. A., Pan D., Olds-Clarke P., and Kopf G. S. (1995) Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 121, 1139–1150 [DOI] [PubMed] [Google Scholar]
- 34. Visconti P. E., Muschietti J. P., Flawia M. M., and Tezon J. G. (1990) Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim. Biophys. Acta 1054, 231–236 [DOI] [PubMed] [Google Scholar]
- 35. Yang K., Meinhardt A., Zhang B., Grzmil P., Adham I. M., and Hoyer-Fender S. (2012) The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol. Cell. Biol. 32, 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perotti M. E., Giarola A., and Gioria M. (1981) Ultrastructural study of the decapitated sperm defect in an infertile man. J. Reprod. Fertil. 63, 543–549 [DOI] [PubMed] [Google Scholar]
- 37. Toyama Y., Kazama T., Fuse H., and Katayama T. (1995) A case of decapitated spermatozoa in an infertile man. Andrologia 27, 165–170 [DOI] [PubMed] [Google Scholar]
- 38. Saïas-Magnan J., Metzler-Guillemain C., Mercier G., Carles-Marcorelles F., Grillo J. M., and Guichaoua M. R. (1999) Failure of pregnancy after intracytoplasmic sperm injection with decapitated spermatozoa: Case report. Hum. Reprod. 14, 1989–1992 [DOI] [PubMed] [Google Scholar]
- 39. Zini A., Boman J. M., Belzile E., and Ciampi A. (2008) Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum. Reprod. 23, 2663–2668 [DOI] [PubMed] [Google Scholar]
- 40. Sanchez R., Toepfer-Petersen E., Aitken R. J., and Schill W. B. (1991) A new method for evaluation of the acrosome reaction in viable human spermatozoa. Andrologia 23, 197–203 [DOI] [PubMed] [Google Scholar]
- 41. Lee M. A., Trucco G., Bechtol K., Wummer N., Kopf G., Blasco L., and Storey B. (1987) Capacitation and acrosome reactions in human spermatozoa monitored by a chlortetracycline fluorescence assay. Fertil. Steril. 48, 649–658 [DOI] [PubMed] [Google Scholar]
- 42. Haas G. G. Jr., Cines D. B., and Schreiber A. D. (1980) Immunologic infertility: Identification of patients with antisperm antibody. N. Engl. J. Med. 303, 722–727 [DOI] [PubMed] [Google Scholar]
- 43. Burkman L. J., Coddington C. C., Franken D. R., Kruger T. F., Rosenwaks Z., and Hodgen G. D. (1988) The hemizona assay (HZA): development of a diagnostic test for the binding of human spermatozoa to the human hemizona pellucida to predict fertilization potential. Fertil. Steril. 49, 688–697 [PubMed] [Google Scholar]
- 44. Huszar G., Ozenci C. C., Cayli S., Zavaczki Z., Hansch E., and Vigue L. (2003) Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil. Steril. 79, 1616–1624 [DOI] [PubMed] [Google Scholar]
- 45. Sigman M., Baazeem A., and Zini A. (2009) Semen analysis and sperm function assays: what do they mean? Sem. Reprod. Med. pp. 115–123 [DOI] [PubMed] [Google Scholar]
- 46. Barratt C. L., Mansell S., Beaton C., Tardif S., and Oxenham S. K. (2011) Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J. Androl. 13, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.