Abstract
Salt stress inhibits plant growth and development. We investigated the importance of cell cycle regulation in mediating the primary root growth response of Arabidopsis to salt stress. When seedlings were transferred to media with increasing concentrations of NaCl, root growth rate was progressively reduced. At day 3 after transfer of seedlings to growth medium containing 0.5% NaCl the primary roots grew at a constant rate well below that prior to the transfer, whereas those transferred to control medium kept accelerating. Kinematic analysis revealed that the growth reduction of the stressed roots was due to a decrease in cell production and a smaller mature cell length. Surprisingly, average cell cycle duration was not affected. Hence, the reduced cell production was due to a smaller number of dividing cells, i.e. a meristem size reduction. To analyze the mechanism of meristem size adaptation prior to day 3, we investigated the short-term cell cycle events following transfer to saline medium. Directly after transfer cyclin-dependent kinase (CDK) activity and CYCB1;2 promoter activity were transiently reduced. Because protein levels of both CDKA;1 and CDKB1;1 were not affected, the temporary inhibition of mitotic activity that allows adaptation to the stress condition is most likely mediated by posttranslational control of CDK activity. Thus, the adaptation to salt stress involves two phases: first, a rapid transient inhibition of the cell cycle that results in fewer cells remaining in the meristem. When the meristem reaches the appropriate size for the given conditions, cell cycle duration returns to its default.
Due to their sessile lifestyle, plants have to be extremely adept to adjust their growth to environmental conditions. Consequently, growth regulation is a central theme in plant research. Surprisingly, there is still no consensus on the role of elemental growth processes, cell division and expansion, in the regulation of whole organ and plant level responses. Considering the organ as a whole, the rate at which an organ grows is determined by the size of the region where expansion occurs and by the specific expansion rates in this region. Conceivably, both of these parameters are specified by positional controls acting on the expansion process (Cooke and Lu, 1992; Kaplan, 1992). Alternatively, we can view the organ as a large population of cells and the growth of the organ as the result of the production of new cells by cell division and their subsequent expansion (Bradford and Trewavas, 1994). Both perspectives are not incompatible and presumably interact (Beemster et al., 2003).
As cells are first produced prior to their expansion, it is plausible that the rate of cell production is a determining factor for the rate at which an organ grows. Evidence from transgenic plants overproducing cell cycle genes supports this contention (Doerner et al., 1996; Cockcroft et al., 2000; De Veylder et al., 2001b, 2002). The rate of cell production in a meristem is determined both by the number of dividing cells and the average time it takes to progress through the cell cycle.
Cell cycle regulators presumably control both cell cycle duration and the number of dividing cells. Experimental evidence suggests that the control of these parameters is largely independent. Ontogenetic acceleration of in vitro grown Arabidopsis roots was accompanied by an increasing number of cells in the meristem while average cell cycle duration remained constant (Beemster and Baskin, 1998). In contrast, the inhibitory effect of the synthetic auxin homolog 2,4-D was mediated by an inhibition of division rate, whereas the number of dividing cells remained approximately constant (Beemster and Baskin, 2000). Cell cycle progression is controlled by checkpoints that mediate the entry into S-phase and mitosis, respectively (for review, see De Veylder et al., 2003). The progression through these checkpoints is catalyzed by the cyclin-dependent kinases (CDKs), whose activity depends on the association with cyclins (for review, see Dewitte and Murray, 2003). These regulatory proteins determine substrate specificity of the CDK complexes both in a temporal and a spatial manner. Multiple cyclins have been identified in Arabidopsis and they are classified into A-, B-, D-, and H-type cyclins, based mainly on sequence similarity (Vandepoele et al., 2002). Furthermore, the activity of the CDK/cyclin complexes is regulated by the binding of CKS (De Veylder et al., 2001a), a putative docking factor, and of Kip-related proteins (KRPs), which are inhibitors of CDK activity (reviewed by Dewitte and Murray, 2003). Superimposed on this, the phosphorylation state of various conserved residues of the CDK protein itself also determines its activity (Russo et al., 1996; De Veylder et al., 2003).
In Arabidopsis, several classes of CDKs have been identified, of which the A-type and B-types have been best characterized (Vandepoele et al., 2002). The A-type CDK CDKA;1 is constitutively expressed during the cell cycle. The activity of its protein product is maximal at the G1-to-S and G2-to-M transitions, implying an involvement in the transition through both checkpoints. The plant-specific B-type CDKB1;1 is transcribed in the S and G2 phases and the activity of the corresponding protein peaks only at G2-to-M transition (Porceddu et al., 2001; Sorrell et al., 2001). The expression of both genes is linked to dividing cells, whereas CDKA;1 is also found in cells that do not divide but are competent to do so (for review, see Mironov et al., 1999). Very limited data are available to date about the involvement of components of the cell cycle machinery specifically in the regulation of the number of dividing cells or the average division rate. In roots it was shown that overexpression of CKS1At increased cell cycle duration and at the same time reduced the size of the meristem (De Veylder et al., 2001a). In contrast, overexpression of CYCD2;1At reduced cell cycle duration and an increased population of dividing cells (Cockcroft et al., 2000). In Arabidopsis leaves, overexpression of the CDK inhibitor KRP2 led to a pronounced inhibition of the rate at which cells divide. The timing of transition from division to expansion and subsequently to mature phase was remarkably conserved, suggesting that this gene may be specific for the regulation of cell cycle duration and not cell cycle exit (Wang et al., 2000; De Veylder et al., 2001b).
To assess the importance of cell cycle regulation in the adaptation to environmental conditions, we investigated the involvement of the cell cycle regulatory system in root growth response to adverse conditions, specifically salt stress. Saline soil is an important agronomic problem, particularly on irrigated fields in arid climates. It has two major physiological effects on plants: (1) it decreases the water potential, thus hindering soil water uptake, and (2) in the plant it disturbs cellular ion homeostasis resulting in an inhibition of several metabolic processes (for review, see Hasegawa et al., 2000). As a consequence, plant growth is reduced, which leads to decreased crop yield.
It has previously been shown that impaired root growth caused by osmotic stress conditions was associated with a reduced cell division activity (Sacks et al., 1997; Samarajeewa et al., 1999). This implies an important role of the meristematic activity in the response of root growth to these stress conditions. Nevertheless, little is known about the mechanisms by which abiotic stress conditions affect the cell cycle regulation. In wheat (Triticum aestivum) leaves water stress caused a shortening of the meristem size and prolonged cell cycle duration as a result of reduced CDK-kinase activity due to an inhibitory phosphorylation (Schuppler et al., 1998). In maize (Zea mays) leaves grown under mild water deficit conditions a reduction in cell division rate was observed. Also p34cdc2a kinase activity was reduced by approximately 45% in all leaf zones, although the amount of p34cdc2a was not affected (Granier et al., 2000). In Arabidopsis severe salt stress conditions transiently reduced the CYCA2;1 and CYCB1;1 expression (Burssens et al., 2000).
Here we show that response to salt stress involves inhibition of cell production by inhibition of cell cycle progression and reduction of meristem size. The primary and transient response involves a rapid decline of cell cycle activity throughout the meristem, which in turn reduces the meristem size. After 3 d on 0.5% NaCl local cell cycle activities return to control values, but a reduced size of the meristem now leads to fewer cells being produced.
RESULTS
Root Elongation
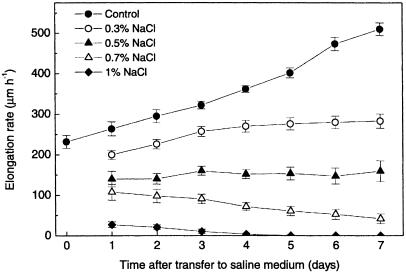
We determined the growth rate at the primary root tip of Arabidopsis thaliana (Col-0) seedlings growing on agar solidified medium by marking daily the position of the root tip on the plates. By measuring the distances between these marks, we calculated average daily growth rates. Transferring roots by itself transiently affected growth rates for less than 1 h (data not shown); thereafter root growth accelerated over time (Fig. 1) as described earlier (Beemster and Baskin, 1998). Increasing concentrations of NaCl reduced the growth rate immediately after transfer and subsequent growth acceleration (slope of the curve in Fig. 1). Root growth on 0.3% NaCl accelerated over time, but to a lesser extent than the control roots. In presence of 0.5% NaCl, root growth remained approximately steady-state over time, while with 0.7% NaCl it decreased. One percent NaCl fully inhibited development within 4 d after transfer.
Figure 1.
Elongation response of the primary root of wild-type (Col-0) plants to different NaCl concentrations. Symbols are means ± se of 24 seedlings.
Local Cell Elongation
To investigate the effect of salt stress on cell production and expansion, we performed a kinematic analysis. This analysis is one-dimensional, essentially simplifying the root to a single representative file of a particular cell type, in this case the cortical cell file. The use of this one-dimensional approach was justified as we did not detect the morphological and structural changes in the root tip, such as swelling, observed by Burssens et al. (2000). This is probably due to two reasons: (1) our analysis was performed on plants grown in plates placed vertically at an inclination of ±85°, allowing the roots to slide over the agar-solidified medium surface, and (2) the use of a lower concentration of NaCl.
The kinematic analysis was performed at day 3 after transfer to a NaCl concentration of 0 or 0.5% (w/w), respectively. Without salt, conditions the growth of primary roots accelerates with time after germination. This is the result of an increasing number of dividing cells, leading to an increase of the number of cells that flow in the elongation zone per unit of time and hence an accelerating root elongation rate. Therefore, cell length profiles at additional timepoints have to be determined for the control roots. In the presence of 0.5% NaCl, root growth is approximately steady-state, which simplified the analysis. As there is no increase in number of dividing cells, no local rate of change of cell density needs to be determined (Beemster and Baskin, 1998).
Although cell division is presumably involved in growth regulation, this is only achieved by cell expansion as only enlargement of cellular volume rather than its subdivision constitutes growth. Thus, one can consider the growth of the organ as a whole in a spatial manner without considering its constituent cells as such. This is the Eulerian or spatial view in opposition to the Lagrangian or cellular perspective (Kaplan, 1992). To gain insight in cell expansion, we used time-lapse analysis of roots to determine the spatial velocity profile throughout the growth zone relative to the quiescent center (QC), from which the cell files originate (Dolan et al., 1993).
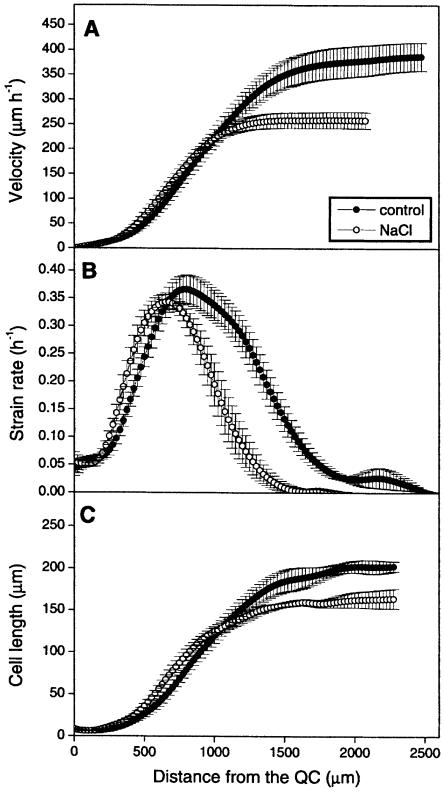
This profile has a typical shape in which three zones can be distinguished (Fig. 2A): most apically a zone of slow acceleration, followed by a region of fast acceleration, and finally a zone where the velocity has reached a constant value, which equals root elongation rate. At the end of the growth zone the velocity in the control roots and the roots growing on 0.5% NaCl reached a velocity of 388 ± 31 μm h−1 and of 263 ± 17 μm h−1, respectively (Table I). These values were not significantly different from the elongation rate of undisturbed roots in the same experiment, showing absence of an effect of experimental handling for microscopic observations.
Figure 2.
Kinematic analysis of the primary root growth of wild-type (Col-0) plants grown in the presence of 0.5% NaCl. Symbols are means ± se (n = 5). A, Velocity profile; B, strain rate; C, cell length.
Table I.
The effect of salt stress on kinematic parameters
| CONTROL
|
0.5% NaCl
|
|||
|---|---|---|---|---|
| Parameter | Mean ± SE | Mean ± SE | Difference % | P-Values |
| Total root growth rate E (μm h−1) | 388 ± 31 | 263 ± 17 | −32 | 0.008 |
| Mature cell length lmat (μm) | 205 ± 9 | 156 ± 7 | −24 | 0.002 |
| Cell length at meristem boundary ldiv(μm) | 45 ± 11 | 26 ± 8 | −43 | NS |
| Maximum strain rate R (h−1) | 0.370 ± 0.025 | 0.351 ± 0.014 | −5 | NS |
| Total cell production P (cells h−1μm−1) | 2.06 ± 0.15 | 1.69 ± 0.10 | −18 | 0.074 |
| Average cell cycle duration Tc (h) | 18.8 ± 2.5 | 18 ± 2 | −4 | NS |
| Average residence time in the meristem Tdiv (h) | 111 ± 15 | 99 ± 12 | −11 | NS |
| Average duration of post-meristematic elongation Tel(h) | 8 ± 2 | 10 ± 2 | −23 | NS |
| Number of dividing cells (Ndiv) | 60 ± 2 | 43 ± 3 | −28 | 0.002 |
| Length of meristematic zone Ldiv(μm) | 625 ± 52 | 405 ± 54 | −35 | 0.019 |
| Length of growth zone Lgz (μm) | 2,730 ± 254 | 1,655 ± 143 | −39 | 0.006 |
Data represent averages ± se for cortical cells (n = 5). P-values were determined by ANOVA analysis of variance using Microsoft EXCEL.
Strain Rate Profile
The increase of the local velocity in function of the position on the root axis is the result of cell expansion. Inversely, the local rate of cell expansion can be determined as the local derivative of the velocity function. Its profile is typically bell-shaped with a maximum near the center of the curve (Fig. 2B). The curve resulting from growth on 0.5% NaCl extended over a shorter distance from the QC compared to the control roots, but the maximal strain rate was not significantly affected.
The size of the growth zone, i.e. the distance between the QC and the position on the root axis where the strain rate becomes zero, was 2,730 ± 254 μm for the control roots and 1,655 ± 143 μm for the roots on saline medium, a reduction of approximately 40% (Table I). Thus, from a spatial perspective the reduced growth rate of the roots under salt stress is associated with a shortening of the growth zone.
Cell Division Parameters
To gain insight into the cellular perspective, we measured cortical cell length in function of position along the root axis in the apical region on the same roots directly following the time-lapse analysis. Typically, cell length is roughly constant in the most apical region of the growth zone (i.e. 0–200 μm from the QC), increases rapidly in the expansion zone, and is constant in the mature part of the root (Fig. 2C). In the apical region, we observed no significant difference in cell length between salt stressed and control roots, implying that the size at which cells divide is unaffected by the treatment. However, in correspondence to the expansion rates, cell length started to increase closer to the tip of the salt-grown roots, suggesting a decrease in the size of the meristem. Consequently, cells were significantly larger between approximately 200 μm and approximately 900 μm from the QC in the salt stressed roots than in the control roots. In salt stressed roots mature cell length is reduced (156 ± 7 μm) compared to the control roots (205 ± 9 μm; Table I) and consistent with the shortening of the growth zone, this reduced cell length is reached closer to the root tip.
The size of the meristem was determined as the distance between the QC and the position where local cell flux, calculated as the ratio between velocity and cell length, becomes constant (Beemster and Baskin, 1998). We found that the meristem covers almost one-quarter of the entire growth zone and that the presence of 0.5% NaCl reduced the size of the meristem proportionally to the reduction of the size of the growth zone (Table I). Under steady-state conditions total cell production per cell file was calculated as the ratio of root elongation rate and mature cell length. When growth is not steady, as in the control roots, the change in cell density in the meristem, determined from additional measurements of cell length distribution on day 1 and 5, was added to this ratio to calculate the appropriate cell production rate (Beemster and Baskin, 1998). Thus we found that total cell production in the salt stressed roots was reduced by nearly 20% compared to the control roots (Table I). Cell production depends on the number of dividing cells (Ndiv) and their average cell cycle duration (Tc). The number of cells in the meristem can be assessed from meristem size and cell length distribution and was 28% lower in salt stressed than in control roots (Table I). Surprisingly, cell cycle duration was unaffected by the saline conditions (Table I).
Mature cell length depends on the length of cells leaving the meristem (ldiv), residence time of the cell in the elongation zone (Tel) and the rate of expansion while they are in the elongation zone. Maximal strain rate (Rmax) and Tel were not affected by the presence of NaCl (Table I), suggesting that the dynamics of cell expansion when cells have left the meristem were essentially unaffected. Instead, the length of cells leaving the meristem was approximately 40% shorter in salt stressed roots (Table I). Therefore it appears that NaCl induces cells to stop dividing at a smaller size than they normally do, and that this initial reduction in size is maintained as cells undergo a very similar expansion program in the elongation zone.
Thus, at day 3 after transfer reduced root growth was linked to fewer and smaller cells being produced in the meristem. The lower cell production was exclusively due to a reduced number of dividing cells in the meristem and the smaller size of mature cells to smaller cells leaving the meristem. This focused our attention on the changes occurring in the meristem.
We reasoned that the reduced meristem size might have been caused by a temporary inhibition of cell cycle duration in the days prior to our analysis. To investigate this possibility, we analyzed in more detail the effect of transition at 0, 2, 6, 12, 24, and 72 h after transfer to the salt-containing medium. As our kinematic approach does not have a high degree of temporal resolution, we resorted to alternative parameters of cell cycle behavior.
p10CKSat-Bound Kinase Activity and Western Analysis of CDK Protein Level
Cell cycle progression is regulated to a great extent by the activity of CDK complexes (Mironov et al., 1999). To investigate the effect of saline medium on the activity of the CDK/cyclin complexes, we determined the kinase activity of CDKs by measuring the rate of histone H1 phosphorylation. For this, total protein extracts were isolated from the apical 5-mm tip of approximately 250 primary roots for each treatment at various times after transfer. CDK complexes were isolated from the total protein extract by means of affinity purification using p10CKSat, which has a high affinity for both A-type and B-type CDKs. The apical 5-mm region of the root includes root cap, meristem, elongation zone, and mature cells. Because mature cells have large vacuoles, proteins from mature tissue presumably constitute only a minor fraction of the samples. More importantly, we have assured there is no interference with other meristematically active tissues such as the lateral root meristems, which only appear at further distance from the tip.
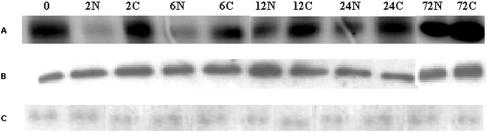
The histone H1 phosphorylation rate of control roots was roughly constant throughout the experiment (Fig. 3A). In contrast, the CDK activity in the salt stressed roots showed a strong reduction after 2 h, followed by a gradual recovery to near control levels at 72 h. Because all samples obtained from the apical 5 mm of the root contain similar amounts of total protein, the samples of salt stressed roots presumably contain a higher fraction of protein from mature tissue as a consequence of the smaller meristem size. The kinase assay is therefore an integrated measure of total cell production rather than division rates per se. This presumably explains why the kinase activity remained slightly lower in the salt stressed roots at 72 h after transfer, when cell cycle duration is no longer affected.
Figure 3.
p10CKSat-bound kinase activity (A), protein levels of CDKA;1 (B), and CDKB1;1 (C) in the primary root tip of Arabidopsis after transfer to media containing 0 or 0.5% NaCl. 0, 0 h; 2 n, 2 h on 0.5% NaCl; 2C, 2 h control; 6 n, 6 h on 0.5% NaCl; 6C, 6 h control; 12 n, 12 h on 0.5% NaCl; 12C, 12 h control; 24 n, 24 h on 0.5% NaCl; 24C, 24 h control; 72 n, 72 h on 0.5% NaCl; 72C, 72 h control.
Western analysis of the same samples, using anti-CDKA;1 and anti-CDKB1;1 specific antibodies, showed that the protein level of both CDKs was not significantly affected by the transfer to the saline medium (Fig. 3, B and C). Thus, the reduction in CDK activity in the stressed roots is not the result of a lower abundance of CDKs but due to posttranslational control.
Expression Analysis of pCYCB1;2::GUS
To investigate one aspect of CDK activation, we also analyzed the promoter activity of the mitotic cyclin CYCB1;2. Therefore, we used a transgenic line carrying a construct with GUS fused to the CYCB1;2 promoter and the region that includes the cyclin destruction box of CYCB1;2 (Donnelly et al., 1999). The destruction box is required for the degradation of the cyclin by the ubiquitin-dependent pathway, thus allowing the exit from mitosis (Donnelly et al., 1999). Due to the presence of this destruction box the dynamics of the β-glucuronidase (GUS) protein degradation is presumably similar to that of the cyclin itself. This construct can effectively be used to monitor cell cycle activity at high spatial and temporal resolution.
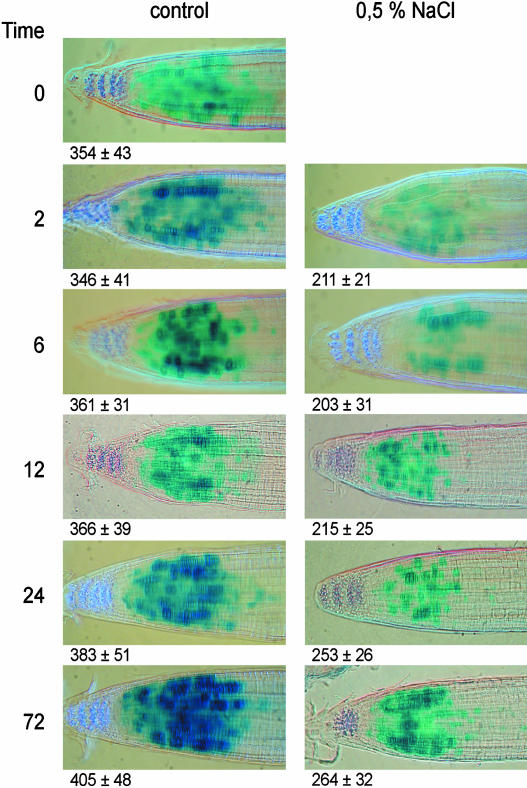
The histochemical detection of the GUS protein shows a patchy pattern, which is the result of cell cycle phases being more or less random in cells and the construct being transcribed during only a small part of the cell cycle and then rapidly degraded (Fig. 4).
Figure 4.
CYCB1;2 promoter activity in the primary root tip of Arabidopsis as determined by histochemical GUS-staining at different times after transfer to media containing 0 or 0.5% NaCl. The numbers indicate the average distance (n = 10 ± se) between the QC and the basal region of the meristem measured by image analysis.
Although some root-to-root variation occurs, the GUS expression pattern remained essentially unaltered over time under control conditions. In contrast, 2 h after transfer to the saline medium, the size of the zone of cells expressing the GUS gene is reduced by about 50%, whereas in the remaining zone a smaller fraction of cells were stained. This suggests a severely decreased mitotic activity in the meristem, particularly in the basal half, where it is completely abolished (Fig. 4). In the salt stressed roots, the density of cells expressing the reporter gene gradually increases (Fig. 4) and is roughly similar to that of the control roots after 72 h. However, at the same time the size of the region of stained cells does not recover, explaining the reduction of the meristem size measured on day 3 by the kinematic analysis (compare Figs. 2 and 4).
DISCUSSION
Role of Elongation Versus Cell Division Activity in Response to Salt Stress
Plant growth responses to environmental conditions have always intrigued physiologists. It is well known that adverse conditions inhibit root growth and that cell division and cell cycle regulation are involved in this response (Kurth et al., 1986; Sacks et al., 1997; Samarajeewa et al., 1999; Burssens et al., 2000). Also, particularly under higher concentrations of salt, organs are often observed to swell (Burssens et al., 2000), which we didn't observe under these relatively mild stress conditions. Our data showed that 0.5% NaCl reduced the root growth rate by 32%. There are two alternative views on how root growth rate is determined (Silk and Erickson, 1979; Silk, 1984). The spatial (Eulerian) view regards organ growth rate as being determined by the integration of relative expansion rate (strain rate, r[x]) over the full length of the growth zone. In this view cell division merely accompanies elongation by partitioning the organismal space created by expansion activity. When our data are interpreted according to this view, the reduced root growth is due to a shortening of the growth zone. How the sizes of the meristem and elongation zone are specified spatially is not clear, but some unknown gradient of positional information might determine them (Barlow, 1976). Shrinkage of the growth zone is also observed in Arabidopsis roots treated with 2,4-D, zeatin, as well as in the stp1 mutant (Beemster and Baskin, 2000). However, it is noteworthy that the maximal strain rate was not significantly changed by the presence of salt, which implies that the capacity of cellular expansion is not affected. A similar effect is observed in roots treated with 2,4-D (Beemster and Baskin, 2000). These findings suggest that hormones may be the positional signal or are at least a part of it.
The cellular (Lagrangian) view considers individual cells as the regulatory units that determine the behavior of the organ as a whole. In this view growth is the result of the number of cells produced in the meristem per unit of time and the final length they reach at the end of the growth zone (Ivanov and Dubrovsky, 1997). According to this model (and in contrast to the spatial view), cell division partially determines organ elongation rate through control of the number of dividing cells and the average cell cycle duration. From this viewpoint salt stress decreases the rate of root elongation by reducing mature cell size and lowering the number of dividing cells, thus shortening meristem size.
As the cells in the salt stressed roots start to undergo rapid elongation closer to the root tip, salt stress appears to stimulate the transition from cell division to elongation, resulting in a shortened meristem and cells stopping division at a smaller size. A similar effect was seen in the cortical root cells of rice under salt stress (Samarajeewa et al., 1999). Early expansion of cells could be an adaptive response to NaCl as it involves vacuolation. Vacuolar compartmentalization of sodium and chloride ions is known as one of the main adaptive strategies used by plants to reduce their toxic effect on vital physiological processes in the cytoplasm. In excised pea roots in culture, differentiation of vacuolated cells is noted much closer to the root apex under salt stress (Solomon et al., 1986). Also in barley (Hordeum vulgare) roots, vacuolation is advanced by the presence of salt (Huang and Van Steveninck, 1990).
Besides a spatial aspect, the root axis also constitutes a time gradient representing the relative age of cells, as they are moving away from the root tip. Hence, the time to cross each region of the root represents the duration of the corresponding developmental stage for the constituent cells. The residence times for each cell in the meristem and the elongation zone do not differ significantly between salt stressed and control roots. This would support the hypothesis that a cell has a programmed duration both for division and subsequent elongation. A constant timing of elongation, but not division, was also observed in the primary root of Arabidopsis under different treatments (Beemster and Baskin, 1998, 2000). As salt stress decreased cell production in the meristem, fewer cells leave the meristem per unit of time. Because the time they are present in the elongation zone is not affected by the treatment, there will be fewer cells in the elongation zone due to the effects exerted primarily on the meristem. Moreover, the smaller size of cells entering the elongation zone is not compensated (similar expansion rates) and thus average cells in the expansion zone of salt stressed roots will be smaller. Taken together, our observations are most easily interpreted with the cellular model where fewer and smaller cells are produced, both caused by effects on cell division in the meristem, resulting in a shorter elongation zone and also a shortening of the growth zone as a whole.
Short-Term Versus Long-Term Responses
The kinematic analysis did not reveal an effect on cell cycle duration at day 3 after transfer to saline medium, indicating that at that time cell cycle progression is not inhibited. However, given the shortening of the meristem it is likely that cell cycle progression is most probably affected at earlier time points. It is shown that oxidative stress exerts its effect on the cell cycle shortly after the stress was imposed (Reichheld et al., 1999). Therefore, we studied changes in mitotic activity in function of time after transfer to the saline medium.
B-type cyclins are good markers for cell proliferation as their expression patterns are specific to the G2/M phase of the cell cycle (Hemerly et al., 1992; Ferreira et al., 1994a; Donnelly et al., 1999). We analyzed a transgenic line containing a CYCB1;2::GUS construct as a reporter line for mitotic activity. In situ hybridization experiments with Arabidopsis tissue and analysis of GUS reporter expression in synchronous tobacco BY-2 cell cultures show that the CYCB1;2 transcripts are most abundant during G2 and M phases of the cell cycle (Hemerly et al., 1992; Shaul et al., 1996). During plant development CYCB1;2 expression is associated with cell division as it is found in meristematic regions or in tissues in which active cell division occurs (Ferreira et al., 1994b). Already 2 h after the application of the stress, a decrease in the meristem size and in the fraction of cells showing CYCB1;2 promoter activity was observed, indicating a severe disruption of mitotic activity. This is consistent with a model in which the cells become blocked at the G2/M transition. Additionally, the relatively low expression levels of the GUS-positive cells at 2 h in NaCl may also indicate that the expression level of the cyclin itself is affected, which might possibly prevent or delay entry into mitosis. The density of the staining and the fraction of stained cells gradually recovered to control levels. However, even after 72 h the size of the zone in which the reporter gene is expressed remains reduced, indicating a shortening in meristem size consistent with the kinematic data. This transient effect of cell cycle gene expression is also observed in the shoot apex and the root tip by Burssens et al. (2000) and is put here in the context of the adaptation of the growth of the organ as a whole. Due to the presence of the destruction box in the used construct, we observed an overall staining pattern, which is more patchy than the more smeared pattern observed by Burssens et al. (2000). Overall, the zone of staining was smaller than the meristem size as calculated in the kinematic analysis. This can be contributed to the fact that the overall root growth rate of the reporter line is lower (approximately 45% of the Col-0 root growth rate, but otherwise responding similarly; data not shown). Moreover, only cells that are at the entry of mitosis are histochemically stained, while the kinematic analysis is based on cells that possess a fully developed (microscopically visual) cell wall, thus cells which have already left the M-phase. During the time between these two events the cells will move a considerable distance given that the velocity at the end of the meristem is around 108 μm/h for control roots and 86 μm/h for salt stressed roots (Fig. 2).
The kinetics of the CDK activity mirrored CYCB1;2 promoter activity. A strong correlation between kinase activity and cell division activity in response to different environmental conditions was also observed in maize leaves (Granier et al., 2000), in wheat leaves under water stress (Schuppler et al., 1998), and in a comparative study of root growth of different Arabidopsis ecotypes (Beemster et al., 2002). If we consider the kinematic data, the recovery of CDK activity is only partial because of a reduced number of dividing cells and most probably does not reflect a delayed cell cycle progression.
The relative amount of CDKA and CDKB proteins was not affected by the stress, indicating that the protein level of CDKs is not limiting their activity. This is not surprising as in tobacco (Nicotiana tabacum) BY-2 cell suspension cultures it was shown that most of the CDKA protein is present in its inactive monomeric form (Porceddu et al., 2001). As the expression of cyclins could be a limiting factor for the mitotic activity of the CDK kinase (Ferreira et al., 1994b), this would suggest a pivotal role of CYCB1;2 in the activation of CDKs under salt stress conditions. Support for the fact that CDK activity is mainly regulated on a posttranslational level comes from transgenic lines overexpressing different cell cycle genes. It has indeed been shown that the overexpression of positive regulators such as B-type cyclins and D-type cyclins, can increase plant growth rates (Doerner et al., 1996; Cockcroft et al., 2000). However, other CDK interacting molecules such as KRPs could also play a role as was demonstrated by the overexpression of the CDK inhibitor KRP2, which reduced both kinase activity and cell division rate in Arabidopsis leaves (De Veylder et al., 2001b). Thus, a more comprehensive analysis of all positive and negative regulators will be required to gain a conclusive answer.
Analogous to the results presented here, Beemster et al. (1996) found that in wheat the first leaves of plants that were already present in the embryo when grown in soils with high mechanical resistance to penetration had lengthened cell cycle duration. However, in the later leaves grown on plants that were exposed longer the number of dividing cells was reduced and cell cycle duration was even somewhat shorter than that of control plants. A transient blocking of cell cycle progression with a concomitant activation of defense genes was also observed in tobacco BY-2 cell suspension cultures submitted to oxidative stress (Reichheld et al., 1999) and in UV-irradiated as well as fungal elicitor-treated parsley cell suspension cultures (Logemann et al., 1995). Put together, these results suggest the involvement of a general mechanism, wherein stress first rapidly blocks cell cycle progression, presumably to prevent entry into stages where the cell is vulnerable to damage (e.g. M-phase) and to allow the cellular defense system to be activated. Once adapted to the stress, which in the case of organs involves adaptation of meristem size, cell cycle can progress at default rates. Thus, a model is suggested wherein both regulation of cell cycle duration is primarily involved in rapid responses to possible transient changes in the environment, whereas in the longer term meristem size mediates responses to persisting conditions. One could envisage this system to have evolved so that plants are able to respond to emergencies and at the same time to maintain adequate growth irrespective of transient changes, such as for example diurnal fluctuations in the environment.
Moreover, our results emphasize the importance of taking into consideration the time of exposure to any treatment under investigation and distinguishing between the contribution of cell cycle timing and that of meristem size when analyzing the influence of environmental conditions upon cell division activity from an organ growth perspective.
MATERIALS AND METHODS
Root Growth Conditions
Seeds of Arabidopsis thaliana L. Heynh. ecotype Columbia (Col-0) were surface sterilized with 15% sodium hypochloride for 10 min, washed with sterile water and plated on 12 × 12-cm2 petri dishes containing 50 mL of agar-solidified culture medium. This medium contains 1× Murashige and Skoog micro- and macro-nutrient solution (Duchefa, Haarlem, The Netherlands) supplemented with 0.1% Suc and 0.8% plant tissue culture agar (Lab M, Bury, UK). The plates were sealed with Urgopore tape (Laboratoire Urgo, Chenoves, France) and kept at 4°C for 1 d and then (= day 1) placed at an inclination of ±85° in a growth chamber under constant conditions (16-h/8-h photoperiod at 22°C and 80 μmol m−1 s−1 of light). On day 10 the seedlings were transferred to the same culture medium containing either 0.5% or no NaCl (Merck Eurolab, Leuven, Belgium), and immediately placed back in the same growth chamber (= day 0 after transfer).
Kinematic Analysis of Cell Division and Cell Elongation in Primary Roots
Root elongation rates were determined by marking the bottom of the plate with a razor blade at the position of the root tip once a day at known times. Seven days after transfer to the NaCl-containing medium, the plates were scanned for imaging (Scanjet 4C/T; Hewlett-Packard, Palo Alto, CA). From the digitized images the increase in the daily length was determined by measuring the distance between the successive marks along the root axis by using the scionimage image analysis program (WinNT version beta3b; Scion, Frederick, MD). Average growth rate was calculated by dividing daily growth by the time interval between the corresponding marks.
Kinematic analysis of cell division and elongation was performed on day 3 after transfer using the methods described earlier (Beemster and Baskin, 2000). For measurements of velocity, selected roots on several plates were marked with toner particles, and a series of overlapping images of the root surface was captured hourly through a horizontally mounted Axiolab microscope (Zeiss, Jena, Germany) by a CCD camera connected to a computer fitted with a frame grabber board. Subsequently, a composite image was created for each observation to analyze the displacement velocity [v(x)] of individual particles for each observation interval. These data were adjusted for the distance between the tip of the root and the base of the quiescent center (see below). The subsequent data were smoothed and interpolated into 25-μm spaced data points using a kernel-smoothing routine described earlier (Beemster and Baskin, 1998) implemented as a macro in Microsoft Excel (version 97). This routine also calculated the derivative of the fitted function [δv/δx] that equals the local relative rate of cell expansion [r(x)].
Directly after the time-lapse observation, the roots were mounted under a microscope (Axioscop, Zeiss) fitted with differential interference contrast optics. A series of overlapping images of all cortical cell files spanning the most apical 4 mm of the root was obtained. From these images, the distance from the base of the quiescent center to the tip of the root was determined and the length of all cortical cells was measured as a function of their position relative to the quiescent center [l(x)]. The combined data of all files were then also smoothed and interpolated into 25-μm spaced data points and subsequently converted into density (the inverse of the cell length). For nonsteady-state growth, additional measurements of cell length were made at day 1 and 5. The continuity equation (Silk and Erickson, 1979; Gandar, 1980) was then used to calculate cell production rate as a function of position:
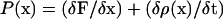 |
in which the local cell flux F(x) is the rate at which cells pass at a given position in each file [F(x) = v(x)/l(x)]. The second factor, the local change in cell density over time, equals zero at steady-state growth as occurred in the roots under salt stress. Analogous to earlier analyses (Beemster and Baskin, 1998) the roots on control medium accelerated over time, where the observations of cell density profiles 2 d prior to and after the observation of the velocity data were used. The function of P(x) of individual roots was used to determine the size of the meristem (Xdiv = distance between the quiescent center and the position where P goes to zero). The number of cells in the meristem was estimated as the cumulative density at Xdiv. The average cell production rate over the whole of the meristem (Pav) was calculated as follows (Beemster and Baskin, 2000):
 |
Average cell division rate over the whole of the meristem (Dav) was then calculated by dividing Pav by the number of meristematic cells (cumulative number of cells at Xdiv). Finally the average cell cycle duration was determined as Tc = ln(2)/Dav.
GUS Expression Analysis
Whole-mount GUS staining was performed on Arabidopsis transgenic plants, carrying the reporter construct pCYCB1;2::GUS (Donnelly et al., 1999) with a destruction box, that were grown as described above. Plants of each line were harvested at 0, 2, 6, 12, 24, and 72 h after transfer to control or 0.5% NaCl-containing medium and fixed for 30 min in 90% acetone at 4°C. Subsequently, they were rinsed three times in Tris buffer (100 mm Tris [USB, Cleveland] with 50 mm NaCl [Merck Eurolab, Leuven, Belgium], pH 7.4), incubated for 30 min at 37°C in ferricyanate buffer (Tris buffer containing 0.5 mm ferricyanide [Sigma, St. Louis]) and placed in staining buffer (Tris buffer, containing 0.5 mm ferricyanide, 1 mg/mL 5-bromo-4-chloro-3-indolyly-β-d-GlcUA [UCB, Leuven, Belgium]) until blue staining was visible. Afterward they were rinsed three times in Tris buffer, cleared in lactic acid, and mounted on microscopy slides. Images were obtained using a microscope (Leica DMLB, Wetzlar, Germany) fitted with a digital camera (Olympus Camedea C3040 Zoom, Hamburg, Germany) and enhanced using Photoshop software (version 5.0; Adobe, San Jose, CA).
p10CKSat-Bound Kinase Activity Assay
At 0, 2, 6, 12, 24, and 72 h after transfer the most apical 5-mm region of 200 to 250 root tips/treatment were harvested and immediately frozen in Eppendorf tubes cooled with liquid nitrogen. Thirty micrograms of total protein was isolated and analyzed according to the procedure described by De Veylder et al. (1997).
Western-Blot Analysis
From the same samples as used for the kinase assay, 30 μg of total protein was suspended in 10 μL of SDS loading buffer, boiled, and subsequently separated on a 12.5% SDS-polyacrylamide gel and electroblotted onto nitrocellulose membranes (Hybond C+; Amersham Pharmacia Biotech, Uppsala). Filters were blocked overnight with 3% skimmed milk in phosphate-buffered saline (PBS), washed three times for 15 min with PBS containing 0.5% Tween 20 (PBST). Subsequently, they were probed for 2 h with specific antibodies for CDKA;1 (1:5000 dilution) or CDKB1;1 (1:2500 dilution) in PBST and 3% skimmed milk. Then the filters were washed three times for 15 min with PBST and incubated for 2 h with peroxidase-conjugated secondary antibody (1:10000 dilution; Amersham Pharmacia Biotech) in PBST and 3% skimmed milk. The filters were washed three times for 15 min with PBST. Proteins were detected by chemiluminescence procedure (Pierce Chemical, Rockford, IL).
Acknowledgments
The authors thank Karel Spruyt for help with the images and the colleagues of the Plant Systems Biology department for insightful comments.
This work was supported by the Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen (predoctoral fellowship to G.W.), by grants from the Interuniversity Poles of Attraction Programme (Belgian State, Prime Minister's Office-Federal Office for Scientific, Technical and Cultural Affairs; P5/13), and by the European Commission Quality of Life and Management of Living Resources program (QLK5–CT–2001–01871).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040022.
References
- Barlow PW (1976) Towards an understanding of the behavior of root meristems. J Theor Biol 57: 433–451 [DOI] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI (1998) Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116: 515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI (2000) STUNTED PLANT 1 mediates effects of cytokinin, but not of auxin, on cell division and expansion in the root of Arabidopsis. Plant Physiol 124: 1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, De Vusser K, De Tavernier E, De Bock K, Inze D (2002) Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol 129: 854–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Fiorani F, Inzé D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8: 154–158 [DOI] [PubMed] [Google Scholar]
- Beemster GTS, Masle J, Williamson RE, Farquhar GD (1996) Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L.): kinematic analysis of leaf elongation. J Exp Bot 47: 1663–1678 [Google Scholar]
- Bradford KJ, Trewavas AJ (1994) Sensitivity thresholds and variable time scales in plant hormone action. Plant Physiol 105: 1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burssens S, Himanen K, van de Cotte B, Beeckman T, Van Montagu M, Inzé D, Verbruggen N (2000) Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta 211: 632–640 [DOI] [PubMed] [Google Scholar]
- Cockcroft CE, den Boer BG, Healy JM, Murray JA (2000) Cyclin D control of growth rate in plants. Nature 405: 575–579 [DOI] [PubMed] [Google Scholar]
- Cooke TJ, Lu B (1992) The independence of cell shape and overall form in multicellular algae and land plants: cells do not act as building blocks for constructing plant organs. Int J Plant Sci 153: S7–S27 [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, Krols L, Terras F, Landrieu I, Van Der Schueren E, Maes S, Naudts M, Inzé D (2001. b) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, de Almeida Engler J, Ormenese S, Maes S, Naudts M, Van Der Schueren E, Jacqmard A, Engler G, et al (2002) Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-Dpa transcription factor. EMBO J 21: 1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beemster GTS, Beeckman T, Inzé D (2001. a) CKS1At overexpression in Arabidopsis thaliana inhibits growth by reducing meristem size and inhibiting cell-cycle progression. Plant J 25: 617–626 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Joubès J, Inzé D (2003) Plant cell cycle transitions. Curr Opin Plant Biol 6: 536–543 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Segers G, Glab N, Casteels P, Van Montagu M, Inzé D (1997) The Arabidopsis Cks1At protein binds to the cyclin-dependent kinases Cdc2aAt and Cdc2bAt. FEBS Lett 412: 446–452 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JA (2003) The plant cell cycle. Annu Rev Plant Biol 54: 235–264 [DOI] [PubMed] [Google Scholar]
- Doerner P, Jorgensen J-E, You R, Steppuhn J, Lamb CJ (1996) Cyclin expression limits root growth and development. Nature 380: 520–523 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organization of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215: 407–419 [DOI] [PubMed] [Google Scholar]
- Ferreira P, Hemerly A, de Almeida Engler J, Bergounioux C, Burssens S, Van Montagu M, Engler G, Inzé D (1994. a) Three discrete classes of Arabidopsis cyclins are expressed during different intervals of the cell cycle. Proc Natl Acad Sci USA 91: 11313–11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira PCG, Hemerly AS, de Almeida Engler J, Van Montagu M, Engler G, Inzé D (1994. b) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6: 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandar PW (1980) The analysis of growth and cell production in root apices. Bot Gaz 141: 131–138 [Google Scholar]
- Granier C, Inzé D, Tardieu F (2000) Spatial distribution of cell division rate can be deduced from that of p34cdc2 kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol 124: 1393–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51: 463–499 [DOI] [PubMed] [Google Scholar]
- Hemerly A, Bergounioux C, Van Montagu M, Inze D, Ferreira P (1992) Genes regulating the plant cell cycle: isolation of a mitotic-like cyclin from Arabidopsis thaliana. Proc Natl Acad Sci USA 89: 3295–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CX, Van Steveninck RFM (1990) Salinity induced structural changes in meristematic cells of barley roots. New Phytol 115: 17–22 [Google Scholar]
- Ivanov VB, Dubrovsky JG (1997) Estimation of the cell cycle duration in the root apical meristem: a model of linkage between cell cycle duration, rate of cell production and rate of root growth. Int J Plant Sci 158: 757–763 [Google Scholar]
- Kaplan DR (1992) The relationship of cells to organisms in intact plants: problem and implications of an organismal perspective. Int J Plant Sci 153: S28–S37 [Google Scholar]
- Kurth E, Cramer GR, Lauchi A, Epstein E (1986) Effects of NaCl and CaCl2 on cell enlargement and cell production in cotton roots. Plant Physiol 82: 1102–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann E, Wu S-C, Schröder J, Schmelzer E, Somssich IE, Hahlbrock K (1995) Gene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-related genes. Plant J 8: 865–876 [DOI] [PubMed] [Google Scholar]
- Mironov V, De Veylder L, Van Montagu M, Inzé D (1999) Cyclin-dependent kinases and cell division in plants-The nexus. Plant Cell 11: 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porceddu A, Stals H, Reichheld J-P, Segers G, De Veylder L, de Pinho Barrocco R, Casteels P, Van Montagu M, Inzé D, Mironov V (2001) A plant-specific cyclin dependent kinase is involved in the control of G2/M progression in plants. J Biol Chem 276: 36354–36360 [DOI] [PubMed] [Google Scholar]
- Reichheld J-P, Vernoux T, Lardon F, Van Montagu M, Inzé D (1999) Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J 17: 647–656 [Google Scholar]
- Russo AA, Jeffrey PD, Pavletich NP (1996) Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol 3: 696–700 [DOI] [PubMed] [Google Scholar]
- Sacks MM, Silk WK, Burman P (1997) Effect of water stress on cortical cell division rates within the apical meristem of primary roots of maize. Plant Physiol 114: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarajeewa PK, Barrero RA, Umeda-Hara C, Kawai M, Uchimiya H (1999) Cortical cell death, cell proliferation, macromolecular movements and rTip1 expression patterns in roots of rice (Oryza sativa L.) under NaCl stress. Planta 207: 354–361 [Google Scholar]
- Schuppler U, He P-H, John PCL, Munns R (1998) Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves. Plant Physiol 117: 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O, Mironov V, Burssens S, Van Montagu M, Inzé D (1996) Two Arabidopsis cyclin promoters mediate distinctive transcriptional oscillation in synchronized tobacco BY-2 cells. Proc Natl Acad Sci USA 93: 4868–4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk WK (1984) Quantitative descriptions of development. Annu Rev Plant Physiol 35: 479–518 [Google Scholar]
- Silk WK, Erickson RO (1979) Kinematics of plant growth. J Theor Biol 76: 481–501 [DOI] [PubMed] [Google Scholar]
- Solomon M, Gedalovich E, Mayer AM, PolJakoff-Mayber A (1986) Changes induced by salinity to the anatomy and morphology of excised pea roots in culture. Ann Bot 68: 47–53 [Google Scholar]
- Sorrell DA, Menges M, Healy JMS, Deveaux Y, Amano C, Su Y, Nakagami H, Shinmyo A, Doonan JH, Sekine M, et al (2001) Cell cycle regulation of cyclin-dependent kinases in tobacco cultivar Bright Yellow-2 cells. Plant Physiol 126: 1214–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D (2002) Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14: 903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Gilmer S, Whitwill S, Fowke LC (2000) Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J 24: 613–623 [DOI] [PubMed] [Google Scholar]