Abstract
Wood is an important raw material and environmentally cost-effective renewable source of energy. However, the molecular biology of wood formation (i.e. secondary growth) is surprisingly understudied. A novel experimental system was employed to study the molecular regulation of secondary xylem formation in Arabidopsis. First, we demonstrate that the weight carried by the stem is a primary signal for the induction of cambium differentiation and the plant hormone, auxin, is a downstream carrier of the signal for this process. We used Arabidopsis whole-transcriptome (23 K) GeneChip analysis to examine gene expression profile changes in the inflorescent stems treated for wood formation by cultural manipulation or artificial weight application. Many of the genes up-regulated in wood-forming stems had auxin responsive cis-acting elements in their promoter region, indicating auxin-mediated regulation of secondary growth. We identified 700 genes that were differentially expressed during the transition from primary growth to secondary growth. More than 40% of the genes that were up-regulated (>5×) were associated with signal transduction and transcriptional regulation. Biological significance of these regulatory genes is discussed in light of the induction and development of secondary xylem.
Growth and differentiation of apical meristems lead to the development of sets of primary tissues such as epidermis, vascular bundles, pith, and leaves. In addition to the primary tissues, tree species produce a secondary tissue (i.e. wood) from the vascular cambium in a process called secondary growth (Mauseth, 1998). Secondary growth is an important biological process. Its product is of primary importance to humans as timber for construction and pulp for paper manufacturing. It is also the most environmentally cost-effective renewable source of energy.
Wood is formed by the successive addition of secondary xylem, which differentiates from the vascular cambium. The vascular cambium originates from the procambium, which is derived from the apical meristem. The transition from procambium to cambium is currently not well understood. Phloem and xylem differentiate radially on each side of the vascular cambium. Periclinal and anticlinal divisions of the vascular cambium increase the diameter and circumference of an axis, respectively. The cells on the xylem side of the cambium go through four distinct cellular processes—cell division, expansion, maturation, and programmed cell death (Chaffey, 1999; Plomion et al., 2001). The resulting mature secondary xylem includes xylem parenchyma, fibers, vessels, and tracheary elements. Positional information appears to coordinate the growth and differentiation of cambium by defining the width of the cambial zone and regulating the radial number of dividing cells (Uggla et al., 1996, 1998; Zhong and Ye, 2001).
While several plant hormones have been implicated in the regulation of vascular tissue formation, considerable evidence indicates that auxin is the major signal involved in several aspects of the ontogeny of the vascular system (for review, see Aloni, 1987; Sachs, 2000). When supported by cytokinins, indole-3-acetic acid (IAA) can induce xylem tracheary element differentiation in suspension culture cells of suitable species (Fukuda, 1997a). Auxin-overproducing transgenic plants have increased amounts of vascular tissues (Klee et al., 1987), IAA application can replace leaf primordia in inducing vascular connections in stems (Jacobs, 1952), and local auxin sources can induce the formation of new vascular strands from parenchymatic cells (Sachs, 1981). However, vascular differentiation in response to IAA application does not occur readily in all genotypes, suggesting that further factors are often required. These factors probably include other plant hormones. A vascular-differentiation-promoting influence of cytokinins, ethylene, GAs, and brassinosteroids has indeed been reported (Aloni, 1987, 1995; Fukuda, 1997b; Sachs, 2000). In Arabidopsis, several mutants that interfere with various aspects of vascular development have been isolated (Dengler and Kang, 2001). Some of these mutants have been described with auxin transport or auxin signaling defects, and loss of tissue continuity within the vascular system (Hardtke and Berleth, 1998; for review, see Berleth and Sachs, 2001).
Several research groups have successfully analyzed a large number of expressed sequence tags from the wood-forming tissues of poplar (Populus; Sterky et al., 1998; Hertzberg et al., 2001), black locust (Robinia; Yang et al., 2003), and pine (Pinus; Allona et al., 1998; Kirst et al., 2003). However, study of the molecular biology of secondary growth is hampered by the inherent problems of tree species such as long generation time, large size, and lack of genetically pure lines. In recent years, Arabidopsis, the most well-studied herbaceous model plant species, has been shown to undergo secondary growth when it is kept from flowering by repeated removal of inflorescences (i.e. decapitation; Lev-Yadun, 1994; Zhao et al., 2000; Little et al., 2002; Oh et al., 2003). However, the decapitation treatment introduces wounding effects that complicate the data analyses and interpretation. We developed an experimental system for induction of secondary growth in Arabidopsis, which does not involve decapitation treatment. The analysis of whole-transcriptome in wood forming stems provided novel global gene expression profiles that define the metabolic and physiological processes characteristic of wood formation in Arabidopsis. In addition, the genes discovered from this Arabidopsis system are serving as a springboard from which we are able to further identify candidate genes involved in secondary growth.
RESULTS
Development of Secondary Xylem Was Correlated with the Height of the Plant
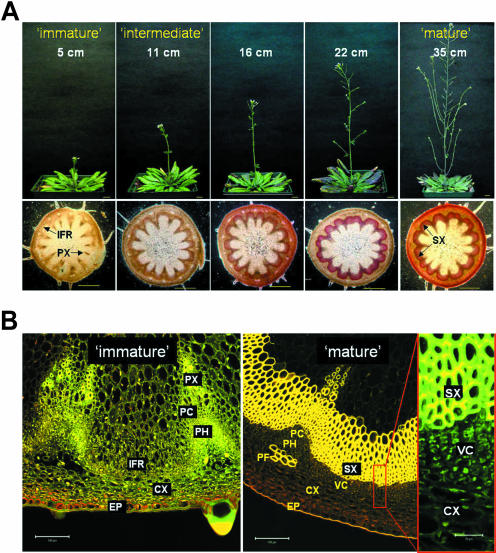
When this facultative long-day plant is grown for 8 weeks under the short-day (8 h light/16 h dark) condition, the Arabidopsis plant sustains vegetative growth and grows very large compared to the long-day (16 h light/8 h dark) grown plants (data not shown). In order to produce large quantities of secondary xylem tissues, we induced thick inflorescence stems by subjecting short-day grown plants to a brief long-day treatment. It is possible to obtain same-age plants with various stem heights by adjusting the long-day treatment period (5 to 10 d; Fig. 1A). The stem area located immediately above the rosette (basal level) was cross-sectioned by hand and stained with 2% phloroglucinol-HCl, which selectively reacts with cinnamaldehyde in the lignified secondary xylem cells. The red color staining in the interfascicular region was used as our primary estimation of the secondary xylem tissue. Since the parenchyma cells in the interfascicular region undergo liginification and form primary fibers that can be stained with the chemical (Turner and Somerville, 1997; Lev-Yadun and Flaishman, 2001), we confirmed the wood formation in the stems by using confocal laser microscopy (Fig. 1B). From the synchronized plants, we learned that the development of secondary xylem was correlated with the height of the plant (Fig. 1A). Secondary xylem development did not occur in stems shorter than 10 cm, regardless of stem thickness.
Figure 1.
Secondary xylem tissue development of Arabidopsis stem. A, Secondary xylem development is related to the plant stem height. All plants are the same age (8 weeks old) and have similar stem thickness, but differ in the height growth of inflorescence stems. The heights of the stems are indicated in upper panels. Basal level of the stem was cross-sectioned and stained with 2% phloroglucinol-HCl to visualize secondary xylem as red color in the lower section. Scale bars represent 1 cm in upper sections and 0.5 cm in lower sections. B, Secondary xylem developed from vascular cambium of the mature Arabidopsis stem. Detailed structure of immature and mature stem cross-section was obtained from confocal laser microscopy (see “Materials and Methods”). EP, epidermis; IFR, interfascicular region; PC, procambium; PH, phloem; PX, primary xylem; VC, vascular cambium; PF, phloem fiber; SX, secondary xylem. Bar indicates 0.2 mm of length.
Plant Body Weight Triggers the Induction of Cambium Differentiation in Vivo
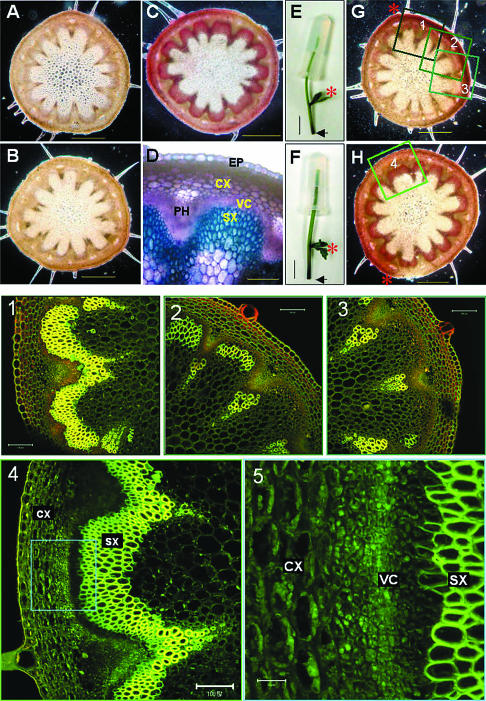
From these observations we hypothesized that the initiation of cambium differentiation (i.e. transition from primary growth to secondary growth) in Arabidopsis is triggered by the weight carried by the stem. To test this hypothesis, we applied artificial weights to the immature stems that had no obvious secondary xylem formation (Fig. 1B). After 3 d of the weight application under short-day condition, the stems that received artificial weight treatment (2.5 g, which is about the weight of a 25-cm tall stem) had produced significant amounts of secondary xylem tissues while the untreated control plants produced no observable secondary tissue (Fig. 2, A, C, and D).
Figure 2.
Artificially-applied weight promotes secondary xylem development. All of the plants used were approximately 5 cm tall (immature) and treated for 3 d. A, Basal level cross-section of intact plant (11 cm tall). B, Basal level cross-section of decapitated immature plants. C, Basal level cross-section of the stem that received 2.5 g-weight treatment and (D) its magnified view. E and F, Weights (2.5 g) were applied to the top part of the decapitated immature stem without (E) or with (F) 5 μm NAA. Plants cut at the basal level are shown and the arrows indicate the basal level. G and H, Basal level cross-sections of E and F, respectively. Red asterisks indicate the auxiliary branch developed during the treatment. All staining was performed using 2% phloroglucinol-HCl except (D), which was stained with 0.05% toluidine blue O. 1, 2, 3, and 4, Detailed confocal laser microscopy images of the insets in G and H. 5, Magnified view of inset in 4 showing the vascular cambium cells. Scale bars represent 0.5 mm (A, B, C, G, and H); 100 μm (D, 1, 2, 3, and 4); 1 cm (E and F); and 20 μm (5).
Auxin Is Required for the Weight-Triggered Secondary Growth
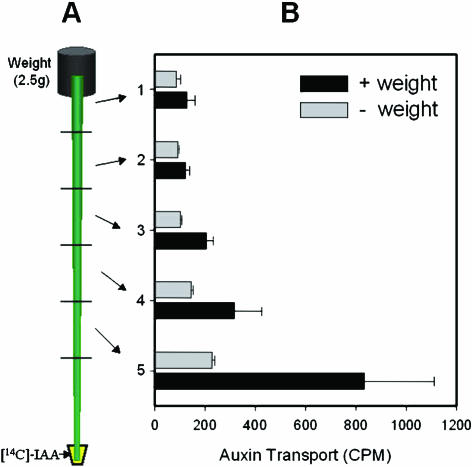
Our next question was what mediates this weight signaling in xylogenesis. Numerous studies have shown that auxin, either endogenous or exogenously supplied, stimulates cambial cell growth. Is auxin involved in the weight signal transduction pathway for induction of secondary growth? To answer this question, we carried out agar block (2.5 g with or without 5 μm naphthylacetic acid [NAA], a synthetic auxin) tests with decapitated plants (Fig. 2, E–H). Decapitated control plants with no agar block did not produce secondary xylem (Fig. 2B). Likewise, the plants that received only agar block treatments developed no visible secondary xylem (Fig. 2G). The discrepancy between this finding and the weight-triggered secondary growth described above may be due to the lack of shoot apical meristem (i.e. no endogenous auxin source) in the decapitated stem. In fact, this explanation is supported by the observation that the lower parts of the stem in Figure 2G produced a small amount of secondary tissues asymmetrically. Secondary xylem formation was observed exclusively on the side of axillary branch. Confocal image analysis clearly shows the gradient of wood formation in this stem (Fig. 2, 1–3). This is likely due to the effect of endogenous auxin, which is produced in the newly emerging axillary shoot apex and transported basipetally. In contrast, the plants that received the auxin-containing agar block treatment developed large quantities of secondary xylem throughout their stems (Fig. 2, H, 4, and 5). Also, the auxin-containing agar block treatment made the stem thicker and more rigid when compared to the agar block alone. These results show that auxin is required for the weight-triggered secondary growth and may serve as a down stream signal transducer. In fact, weight application treatment indeed facilitates auxin transport in the stem (Fig. 3). Thus, we suggest that the weight stimulus may facilitate auxin transport and subsequently promote the development of secondary xylem. Previously, Edwards and Gray (1977) reported that hypergravity (2–25 g) created by centrifugation increased auxin transport and subsequently stimulated stem growth. On the other hand, microgravity generated by horizontal clinostat decreased polar auxin transport about 60% compared to 1 g-force control (Miyamoto et al., 1999).
Figure 3.
Artificially-applied weight increases basipetal auxin transport. A, Diagram of the experimental design. Weight (2.5 g) was applied on top of the stem placed upside down in the 15-mL conical tube containing [14C]IAA (see “Materials and Methods”). B, Basipetal [14C]IAA movement was facilitated by weight treatment. Error bar means se (n = 3).
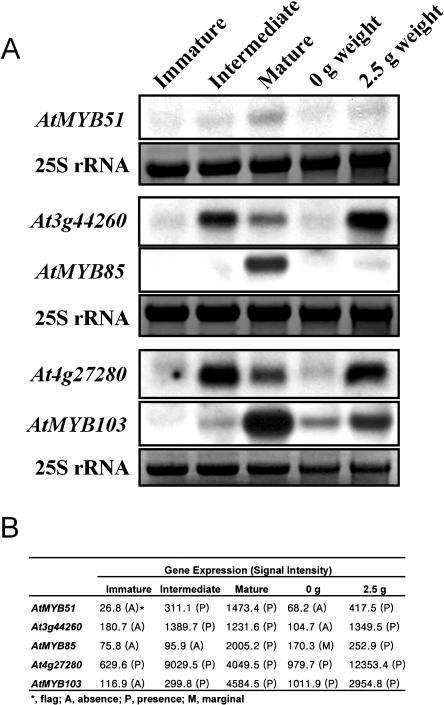
Figure 4.
Confirmation of signal intensity of GeneChip data with conventional northern-blot analysis. A, Northern-blot analysis. RNA gel-blot analysis was performed with 15 μg of total RNA extracted from each sample indicated above and hybridized with the randomly labeled cDNA probes indicated on the left side. All of the RNA samples are duplicates of the RNAs used in the GeneChip analysis. Exposed to Kodak x-ray film for 1 d (Rochester, NY). 25S rRNA serves as a RNA loading control. B, Signal intensity of the GeneChip data.
Figure 5.
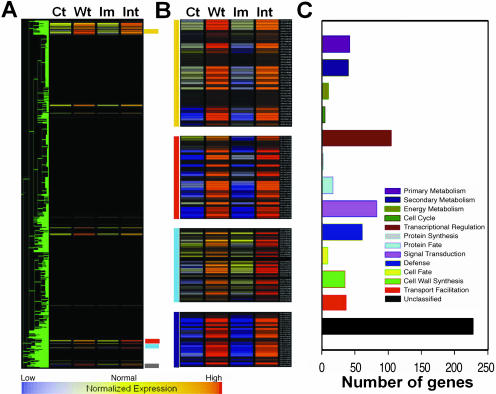
Comparison of gene expression patterns with weight treatment and intermediate stem. A, Gene finding using hierarchical clustering; 700 genes have similar expression pattern (with minimum correlation of 0.95). Normalization and K-Mean clustering was done by GeneSpring 4.2.1 software (Silicon Genetics, Redwood City, CA). The expression patterns of the selected 700 genes are selectively shown. B, Magnifying view of each cluster in (A). Supplemental Table provides the list of genes. Ct, control; Wt, 2.5-g weight treatment; Im, immature; Int, intermediate stem. C, Functional classifications. Classification was based on the Munich Information Center for Protein Sequences (http://mips.gsf.de/proj/thal/db/tables/tables_func_frame.html).
Whole-Transcriptome GeneChip Analysis
To investigate the global steady-state mRNA levels of Arabidopsis plants at three different stages of stem development (immature, intermediate, and mature), we used the GeneChip Arabidopsis ATH1 Genome Array (Affymetrix, Santa Clara, CA) that contains 22,620 genes. Plants were divided into those developmental groups based on the height of the plant and the extent of secondary xylem development. Plants in the immature stage (about 5 cm tall) had no visible secondary xylem and lignification in the interfascicular region. The intermediate stage (10–15 cm) plants showed the beginning of lignification in the interfascicular region. Plants in the mature stage (>25 cm) clearly showed well-developed secondary xylem in the interfascicular region.
The individual gene expression value was obtained as signal intensity and ranged from approximately 40 to 90,000 (with the average value of 2,015). The determination of the difference between the duplicate experiments (D for decrease, MD for marginal decrease, I for increase, MI for marginal increase, and NC for no change) was made by using Affymetrix GeneChip Analysis Suite version 5.0 with default parameters. Only data with NC (no significant changes between the duplicated samples) calling were selected for further analysis. Over 97% of the genes on the chip had NC calling between the duplicate experiments from all of the samples used. Although the GeneChip technology is well known for its reliability and fidelity (Harmer et al., 2000; Lockhart and Winzeler, 2000; Tepperman et al., 2001; Che et al., 2002), we first confirmed the GeneChip analysis results by comparing the signal intensity from the analysis with the signals obtained using the conventional northern hybridization. The results showed that the signal intensity highly corresponded to the northern signal (Fig. 4, A and B).
Identification of the Genes Involved in the Transition from Primary to Secondary Growth
In an attempt to identify the genes involved in the transition from primary growth to secondary growth, we identified the early responsive (i.e. up-regulated) genes to wood formation treatment by analyzing and comparing the transcription profile obtained from the immature stems (undecapitated as in Fig. 2A) that had received 2.5-g weight treatment for 24 h to that of intermediate stage stems. The intermediate stems are thought to be at the development stage where the transition from primary growth to secondary growth occurs. A total of 16,538 genes were expressed in the stem tissues. Eighty-two percent (13,707) of them showed no significant changes in their expression among no weight control (same as immature stem), weight-treated, immature, and intermediate stage stems. Very interestingly, more than 4.2% (700) of the 16,663 genes had a similar expression pattern (correlation coefficient >0.96) between the weight-treated and intermediate stems (Fig. 5, A and B; Supplemental Table, which can be viewed at www.plantphysiol.org, provides the list of genes). This suggests that weight treatment can induce gene expression changes comparable to those of stem developmental shift from immature to intermediate stage. Of the 700 genes, 228 are currently of unknown function and the genes involved in the transcriptional regulation and signal transduction make up a high proportion (15% and 12%, respectively; Fig. 5C).
Of the initial 700 genes having similar expression patterns, 79 genes were up-regulated 3-fold or higher and 56 of them were annotated to known functions (Table I). It is notable that more than one-half of the 56 genes encode proteins involved in transcriptional regulation (32%) and signal transduction (21%). Three calcium-binding proteins, including TCH2, and eight protein kinases (two of them are calcium-dependent protein kinases) are also included in the list. Expression of the TCH2 (At5g37770) gene of Arabidopsis is strongly induced by mechanical stimuli such as touch and wind (Braam, 1992). The expression of this gene is also induced in the intermediate stem that received no external mechanical stimuli, suggesting that the growing plant body itself makes stimuli internally and that these calcium-binding proteins could transduce this signal. Therefore, it is possible that these genes may play an important role in the signal transduction and the commitment step in secondary xylem formation. In addition, TCH4 (xyloglucan endotransglycosylase; At5g57560), expansin-like protein (At-EXPL3), and putative pectinesterase, which are known to be involved in cell expansion, were up-regulated in both weight treatment and intermediate stage stems. Furthermore, the list of candidate genes includes secondary metabolism related genes such as cinnamoyl coenzyme A (CoA) reductase and cytochrome P450 and a putative cell division-associated gene (centrin, At4g27280). Dihydrofolate reductase, a small enzyme that plays a supporting but essential role in the building of DNA, and two putative auxin induced genes (At3g09870 and At4g02380) were expressed more than 5-fold in both of the stems.
Table I.
Selected genes from the initial 700 genes that have similar expression patterns in both of weight-treated and intermediate stems
| AGIa | Gene Description | CCb | Wt/Ctc | Int/Immd | FnCe |
|---|---|---|---|---|---|
| At5g57560 | Xyloglucan endotransglycosylase (TCH4) protein | 0.953 | 34.6 | 15.9 | CW |
| At3g55980 | Putative protein zinc finger transcription factor (PEI1) | 0.976 | 26.1 | 14.2 | RT |
| At5g58120 | Disease resistance protein RPP1-WsA | 0.959 | 21.4 | 13.7 | DF |
| At2g32140 | Putative disease resistance protein | 0.981 | 20.3 | 7.0 | DF |
| At3g50930 | BCS1 protein-like protein | 0.970 | 18.7 | 10.1 | EN |
| At1g09950 | Similar to Nicotiana tumor-related protein | 0.973 | 16.0 | 26.1 | RT |
| At3g09870 | Similar to auxin-induced proteins | 0.976 | 14.9 | 10.3 | RT |
| At2g41100 | Calmodulin-like protein | 0.982 | 13.8 | 7.0 | ST |
| At1g63030 | Transcription factor DREB1A | 0.990 | 13.7 | 4.3 | RT |
| At3g44260 | CCR4-associated factor 1-like protein | 0.988 | 12.9 | 7.7 | RT |
| At4g27280 | Putative protein centrin | 0.971 | 12.6 | 14.3 | CC |
| At5g51190 | Similarity to ethylene responsive element binding factor | 0.962 | 10.7 | 6.5 | RT |
| At1g80820 | Cinnamoyl CoA reductase | 0.974 | 10.3 | 3.7 | SM |
| At1g72520 | Putative lipoxygenase | 0.977 | 9.8 | 30.0 | PM |
| At4g23810 | AtWRKY53, WRKY-type DNA binding protein | 0.954 | 9.8 | 4.0 | RT |
| At2g38470 | AtWRKY33, WRKY-type DNA binding protein | 0.988 | 9.4 | 7.9 | RT |
| At2g17040 | NAM (no apical meristem)-like protein | 0.973 | 9.2 | 6.1 | RT |
| At5g61600 | DNA binding protein-like, EREBP-4 | 0.979 | 8.5 | 5.7 | RT |
| At3g26210 | Putative cytochrome P450 | 0.990 | 8.2 | 4.8 | SM |
| At3g45960 | Putative protein cim1 induced allergen (At-EXPL3) | 0.968 | 8.0 | 7.1 | CW |
| At2g34930 | Putative disease resistance protein | 0.990 | 7.6 | 3.1 | DF |
| At1g72920 | Similar to virus resistance protein | 0.990 | 7.2 | 3.4 | DF |
| At3g50060 | AtMYB77, R2R3-MYB transcription factor | 0.994 | 7.1 | 3.9 | RT |
| At5g37770 | CALMODULIN-RELATED PROTEIN 2 (TCH2) | 0.969 | 6.5 | 4.8 | ST |
| At3g57530 | Calcium-dependent protein kinase | 0.994 | 6.2 | 5.1 | ST |
| At1g18570 | AtMYB51, R2R3-MYB transcription factor | 0.979 | 6.1 | 11.6 | RT |
| At5g37110 | Putative helicase PIF1 protein | 0.984 | 6.1 | 7.2 | TN |
| At4g24380 | Putative dihydrofolate reductase | 0.987 | 5.9 | 5.6 | SM |
| At4g02380 | Similar to IAA induced protein ARG | 0.996 | 5.7 | 5.4 | RT |
| At2g40140 | Putative CCCH-type zinc finger protein | 0.986 | 5.7 | 5.5 | RT |
| At3g52400 | Syntaxin-like protein | 0.983 | 5.3 | 5.3 | CO |
| At4g17500 | Ethylene responsive element binding factor 1 | 0.997 | 5.3 | 5.5 | RT |
| At2g31880 | Putative receptor-like protein kinase | 0.975 | 5.1 | 4.3 | ST |
| At5g66210 | Calcium-dependent protein kinase | 0.996 | 4.8 | 4.1 | ST |
| At4g02410 | Putative protein kinase | 0.996 | 4.8 | 6.4 | ST |
| At4g08950 | Putative phi-1-like phosphate-induced protein | 0.962 | 4.7 | 9.1 | UN |
| At1g66090 | Similar to disease resistance protein RPP1-WsA | 0.983 | 4.6 | 3.3 | DF |
| At1g63750 | Similar to disease resistance protein (RPP1-WsC) | 0.974 | 4.5 | 4.7 | DF |
| At5g06320 | Harpin-induced protein-like | 0.990 | 4.3 | 4.2 | DF |
| At5g39670 | Calcium-binding protein-like cbp1 | 0.988 | 4.3 | 3.8 | ST |
| At3g49530 | NAC2-like protein | 0.999 | 4.1 | 3.7 | RT |
| At4g23180 | Ser/Thr kinase-like protein | 0.998 | 4.0 | 5.4 | ST |
| At2g01180 | Putative phosphatidic acid phosphatase | 0.988 | 3.9 | 3.2 | PM |
| At5g41740 | Disease resistance protein-like | 0.992 | 3.8 | 6.7 | DF |
| At3g08860 | Putative beta-Ala-pyruvate aminotransferase | 0.997 | 3.7 | 3.1 | PM |
| At5g47960 | RAS superfamily GTP-binding protein-like | 0.988 | 3.5 | 4.1 | ST |
| At1g52560 | Chloroplast-localized small heat shock protein | 0.983 | 3.3 | 7.8 | DF |
| At4g02200 | Similar to drought-induced-19 | 0.999 | 3.3 | 4.0 | DF |
| At3g08870 | Putative Ser/Thr protein kinase | 0.998 | 3.3 | 3.2 | ST |
| At3g45640 | Mitogen-activated protein kinase 3 | 0.997 | 3.3 | 6.4 | ST |
| At5g11650 | Lysophospholipase homolog LPL1 | 0.984 | 3.2 | 3.1 | PM |
| At4g04540 | Putative receptor-like protein kinase | 0.996 | 3.2 | 4.7 | ST |
| At2g32150 | Putative hydrolase | 0.998 | 3.1 | 3.0 | PM |
| At5g37540 | Putative protein nucleoid DNA-binding protein cnd41 | 0.974 | 3.1 | 3.3 | RT |
| At2g45220 | Putative pectinesterase | 0.959 | 3.1 | 7.1 | CW |
| At3g59080 | Putative protein CND41 | 0.996 | 3.0 | 5.3 | RT |
Arabidopsis Gene Index (AGI) number.
Correlation coefficient calculated by GeneSpring software.
Fold change: signal intensity of 2.5-g weight treated stem over control (immature) stem.
Fold change: signal intensity of intermediate stem over immature stem.
Functional classification using MIPS (http://mips.gsf.de/proj/thal/db/tables/tables_func_frame.html); RT, regulation of transcription; ST, signal transduction; CW, cell wall; DF, defense; CC, cell cycle; PM, primary metabolism; SM, secondary metabolism; EN, energy metabolism; TN; transcription; UN, unclassified.
Transcriptional Regulators Involved in the Initiation of Secondary Growth
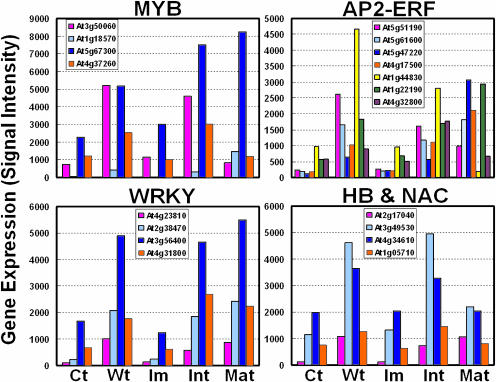
As a first step toward identifying the crucial transcription factors responsible for the initiation of wood formation, we examined the expression profiles of all transcription factors from the initial 700 genes. Many genes belonging to several transcription factor families were highly up-regulated in both weight-treated and intermediate stage stems. R2R3-type MYB transcription factors control many aspects of plant secondary metabolism, as well as the identity and fate of plant cells (Stracke et al., 2001). Four MYB genes up-regulated in both weight-treated and intermediate stage stems are AtMYB77 (At3g50060), AtMYB73 (At4g37260), AtMYB44 (At5g67300), and AtMYB51 (At1g18570; Fig. 6). Two MYB genes (AtMYB77, At3g50060 and AtMYB44, At5g67300) were reported to be up-regulated in the late stages of embryogenesis (Kirik et al., 1998). However, these two had differential expression patterns in mature stems (Fig. 6). While AtMYB44 showed the highest expression at the mature stage, AtMYB77 was down-regulated. This suggests that the two may play different regulatory roles. AP2 (APETALA2) and ethylene-response factors (ERFs) are transcription factors unique to plants and form a large multigene family. They play a variety of roles throughout the plant life cycle from being key regulators of several developmental processes, like floral organ identity determination or control of leaf epidermal cell identity, to forming part of defense mechanisms against various types of biotic and environmental stresses (Riechmann and Meyerowitz, 1998). Seven AP2/ERF genes had differential expression in wood-forming stems. Four (AtERF1, At4g17500; AtERF2, At5g47220; RAP2, At1g22190; At5g61600) of them were expressed at a higher level in mature stem than in intermediate stem. The expressions of the other three genes (At5g51190, At1g44830, and At4g32800) were peaked in intermediate stem and increased by the weight treatment, suggesting these three AP2/ERF genes may have different roles than the others such as AtERF1, AtERF2, and RAP2. However, the functions of the proteins are currently not characterized. The WRKY superfamily of transcription factors comprise up to 100 genes in Arabidopsis. Many WRKYs are reported to be involved in the regulation of various physiological programs that are unique to plants, including pathogen defense, senescence, and trichome development (Eulgem et al., 2000). We found four WRKY factors were up-regulated in wood-forming stems (Fig. 6). Three of them (WRKY33, At2g38470; WRKY18, At4g31800; and WRKY53, At4g23810) were reported to be highly responsive to the pathogen and salicylic acid treatment (Dong et al., 2003). However, WRKY70 (At3g56400), showing the highest up-regulation in both weight-treated and intermediate stage stems, did not respond to pathogen and salicylic acid treatments (Dong et al., 2003). This suggests that WRKY70 might have a specific role other than defense signaling in stem development. NACs (NAM, ATAF, and CUC family) are plant specific gene families and are found to play a role in a diverse set of developmental processes. Two NAC proteins (At2g17040 and At3g49530) showed similar expression patterns between the weight-treated and intermediate stems. However, their functions are still unknown. Two unknown Homeodomain-Leu zipper homeobox gene (HB) proteins showed high expression in both the weight treatment and intermediate stage stems (Fig. 6). In addition, 10 AtHB genes were expressed in Arabidopsis stems. Among them, AtHB-1 (At3g01470), AtHB-6 (At2g22430), AtHB-7 (At2g46680), AtHB-8 (At4g32880), and AtHB-12 (At3g61890) genes were expressed highly at mature stage (Fig. 7).
Figure 6.
Expression profiles of transcription factor candidates might be involved in the transition from primary to secondary growth. Gene expression (y axis) designates the mean value of signal intensity of each gene. Note the different scope. Ct, control; Wt, 2.5-g weight treatment; Im, immature; Int, intermediate stem; Mat, mature stem.
Figure 7.
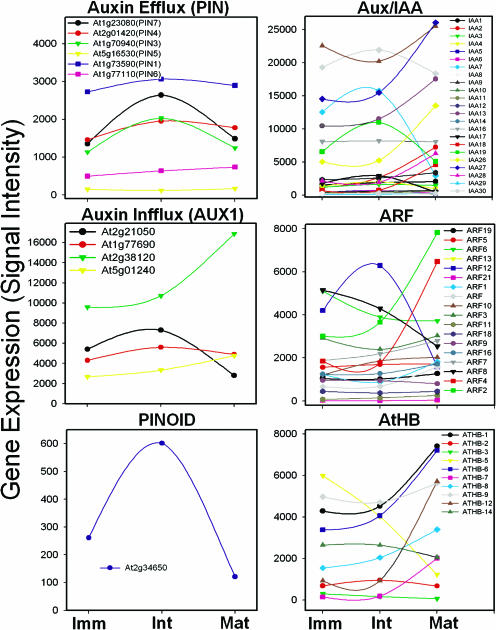
Expression profiles of auxin-modulating genes during the development of wood-forming stems. Gene expression (y axis) designates the mean value of signal intensity of each gene. Note the different scope. Imm, immature; Int, intermediate; Mat, mature stem.
Auxin-Mediated Regulation of Gene Expression during Secondary Growth
We examined the expression patterns of the genes that are possibly regulated by auxin. The expression of auxin efflux carrier genes (AtPIN3, AtPIN4, and AtPIN7) was found to peak at the intermediate stem (Fig. 7). Four AUX1 and AUX1-like genes, known as auxin influx carriers, were expressed in the stem tissues. The expression of one gene (At2g21050) was highest in intermediate stem, while the other (At2g38120) peaked in mature stem (Fig. 7). Two related protein families, Aux/IAA proteins and auxin response factors (ARFs), are key regulators of auxin-modulated gene expression (Guilfoyle et al., 1998a, 1998b; Walker and Estelle, 1998). The Arabidopsis genome may encode as many as 29 Aux/IAAs (Liscum and Reed, 2002) and 23 ARFs (Hagen and Guilfoyle, 2002). The expression of 4 IAAs (IAA8, IAA7, IAA17, and IAA19) peaked in the intermediate stem, while 6 IAAs (IAA2, IAA13, IAA18, IAA26, IAA27, and IAA28) were up-regulated in the mature stem (Fig. 7). Several ARF genes were differentially regulated during xylogenesis. Three ARF genes (ARF2, ARF4, and ARF12) had the most dramatic expression changes, indicating their putative roles in xylogenesis (Fig. 7).
Auxin responsive cis-acting elements (AuxREs) have been identified from the promoter regions of the genes that are rapidly and specifically activated by auxin (Guilfoyle et al., 1998a). We surveyed the promoter regions of the genes that are highly up-regulated in wood-forming stems for the presence of such AuxREs (Table II). Four genes related to cell wall biosynthesis, 8 genes regulating gene expression, 6 genes related to secondary metabolism, and 4 genes involved in signal transduction have biologically active AuxREs (Table II). In fact, examination of the existing cDNA microarray data compiled at the Stanford Microarray Database (http://genome-www5.stanford.edu/MicroArray/SMD/) revealed that 21 genes out of 24 genes for which microarray data were available are highly responsive to auxin. These findings suggest that many of the genes up-regulated in wood-forming stems might be regulated by auxin.
Table II.
The mature stem up-regulated genes having AuxREs in their promoter region
| AGIa | Gene Description | FnCb | Foldc | Auxin Responsive Cis-Elementsd | Auxin Responsee |
|---|---|---|---|---|---|
| At4g26220 | Caffeoyl-CoA O-methyltransferase | SM | 95.3 | ARFAT, 2 CATATGGMSAUR | Y (26716, 26717) |
| At1g32770 | Similar to OsNAC7 | RT | 82.0 | AUXRETGA1GMGH3 | ND |
| At4g02280 | Putative Suc synthetase | CW | 43.6 | CACGCAATGMGH3, CATATGGMSAUR | ND |
| At4g22680 | AtMYB85, myb transcription factor | RT | 26.5 | CACGCAATGMGH3 | Y (26715, 19775) |
| At4g12480 | pEARLI 1 | CW | 24.4 | AUXRETGA1GMGH3, CATATGGMSAUR | ND |
| At2g43060 | Similar to transcription factor | RT | 24.1 | ARFAT, DR5GMGH3 AUXRETGA2GMGH3 | Y (3749, 6338, 7229) |
| At1g09970 | Similar to receptor-like protein kinase | ST | 19.4 | ARFAT, AUXRETGA1GMGH3 | N |
| At4g08300 | Nodulin-like protein | UN | 18.6 | ARFAT, AUXRETGA1GMGH3 | Y (26716, 3743, 7204, 26715, 19775) |
| At3g05200 | Putative RING-H2 zinc finger protein | RT | 18.4 | ARFAT, AUXRETGA1GMGH3, CATATGGMSAUR | Y (26715) |
| At5g17420 | Cellulose synthase catalytic subunit (IRX3) | CW | 16.2 | AUXRETGA2GMGH3 | Y (19775) |
| At4g12280 | Copper amine oxidase-like | SM | 14.0 | CACGCAATGMGH3, CATATGGMSAUR | Y (3743, 26716, 26717, 19772) |
| At2g36220 | Unknown protein | UN | 13.7 | AUXRETGA1GMGH3 | Y (3749, 26715, 26716) |
| At4g30270 | Xyloglucan endo-1,4-beta-d-glucanase | CW | 13.4 | ARFAT, AUXRETGA1GMGH3 | Y (27308, 26714, 26716, 26717) |
| At1g09440 | Putative ser/thr protein kinase | ST | 10.2 | AUXRETGA1GMGH3 | ND |
| At1g71030 | Similar to myb-related transcription factor | RT | 5.9 | AUXREPSIAA4, GGTCCCATGMSAUR | Y (3749, 3743, 26715, 7228) |
| At2g38860 | Unknown protein | UN | 5.4 | AUXREPSIAA4, CATATGGMSAUR, GGTCCCATGMSAUR | Y (3749, 7229, 7206, 27308) |
| At2g29450 | Glutathione S-transferase | SM | 5.0 | AUXRETGA1GMGH3, CATATGGMSAUR | Y (7229) |
| At2g29500 | Putative small heat shock protein | DF | 5.0 | AUXRETGA1GMGH3 | Y (7229, 26716, 26717, 7228, 26715, 7206) |
| At1g30640 | Putative protein kinase | ST | 4.8 | ARFAT, CATATGGMSAUR, DR5GMGH3 | ND |
| At4g37470 | Putative beta-ketoadipate enol-lactone hydrolase | DF | 4.7 | AUXRETGA2GMGH3 | Y (3743, 3749) |
| At4g16370 | isp4 like protein, oligopeptide transporter | TF | 4.4 | ARFAT, 2 SGBFGMGMAUX28 | ND |
| At2g46830 | MYB-related transcription factor (CCA1) | RT | 4.3 | AUXRETGA2GMGH3, CATATGGMSAUR | N |
| At1g17250 | Putative receptor protein kinase | DF | 4.3 | ARFAT, AUXRETGA1GMGH3 | ND |
| At5g42800 | Dihydroflavonol 4-reductase | SM | 4.1 | ARFAT, AUXREPSIAA4 | Y (26716, 26717, 27308) |
| At4g04830 | Similar to transcriptional regulator | RT | 4.1 | AUXREPSIAA4, CATATGGMSAUR | Y (26715, 26717) |
| At3g50770 | Calmodulin-like protein | ST | 3.9 | ARFAT, AUXREPSIAA4, CATATGGMSAUR | ND |
| At1g19670 | Unknown protein | UN | 3.5 | AUXRETGA2GMGH3 | Y (26716, 26717, 19772) |
| At2g24180 | Putative cytochrome P450 | SM | 3.4 | ARFAT, DR5GMGH3 | N |
| At4g12020 | AtWRKY19 | RT | 3.4 | 2 AUXRETGA1GMGH3 | ND |
| At2g15390 | Unknown protein | UN | 3.3 | AUXRETGA2GMGH3, CATATGGMSAUR | Y (3749, 26716, 26717) |
| At2g35940 | Putative homeodomain transcription factor | RT | 3.2 | ARFAT, AUXREPSIAA4 | Y (7202, 7229, 7206, 6338) |
| At4g20840 | Reticuline oxidase-like | SM | 3.2 | ARFAT, AUXRETGA2GMGH3 | Y (3743) |
| At4g15440 | Hydroperoxide lyase (HPOL) like protein | PM | 3.1 | CACGCAATGMGH3 | Y (7229, 26715, 26716, 26717, 3743) |
Arabidopsis Gene Index (AGI) number.
Functional classification was based on MIPS (http://mips.gsf.de/proj/thal/db/tables/tables_func_frame.html); RT, Regulation of transcription ; ST, signal Transduction; CW, cell wall; TF, transport facilitation; DF, defense; PM, primary metabolism; SM, secondary metabolism; UN, unclassified.
Fold changes of gene expression signals over immature stem; (mature/immature).
AuxREs searched in the PLACE (http://www.dna.affrc.go.jp/htdocs/PLACE/).
Analyzed using Microarray data from Stanford Microarray database (http://genome-www5.stanford.edu/MicroArray/SMD/); Y, at least 2-fold changes of gene expression; N, less than 2-fold change; ND, no data available. Each experiment ID is shown in the parenthesis.
DISCUSSION
Despite trees' prominent role in the economy and environment, the molecular biology of tree growth and development is surprisingly understudied. In recent years, a genomics approach has been successfully used to examine global gene expression patterns in developing xylem tissues of pine (Allona et al., 1998; Lorenz and Dean, 2002) and poplar (Sterky et al., 1998; Hertzberg et al., 2001). Although the information derived from those studies undoubtedly provided novel insights into the molecular mechanisms of this important differentiation pathway, it is still insufficient to account for the complete process of wood formation. In this study, we demonstrate that the plant body weight triggers the transition from primary growth to secondary growth, and auxin mediates the weight signaling.
Wood Formation in Arabidopsis
Secondary xylem (i.e. wood) is derived from the vascular cambium that originates from the fascicular procambium and the interfascicular parenchyma between the strands of procambium (Lev-Yadun and Flaishman, 2001). This cambial activity is reduced by flowering (Wilton, 1938). Therefore, any treatments that stop flower development promote prolonged cambial activity. When it is kept from flowering by repeated removal of inflorescences (i.e. decapitation), Arabidopsis produces a significant quantity of secondary xylem (Lev-Yadun, 1994; Zhao et al., 2000). Another means that delays Arabidopsis from flowering is subjecting the facultative long-day plants to short-day (8 h light/16 h dark) conditions. Recently, Chaffey et al. (2002) reported wood formation in the hypocotyls of short-day grown Arabidopsis plants. However, the hypocotyl region of Arabidopsis is so short that it is difficult to apply experimental treatments, and the amount of secondary xylem that can be produced in hypocotyl region is very small. The system we described here can induce inflorescence stems to initiate secondary growth without using a decapitation treatment. It also allows researchers to have synchronized stems (same age and diameter) but different degrees of secondary xylem differentiation (from immature to mature wood forming stems), making it a suitable experiment system for the study of the entire secondary xylem development process.
What Triggers Wood Formation in Arabidopsis?
The development of secondary xylem was highly dependent on the height of the plant in the experiments with the synchronized plants. Secondary xylem development did not occur in stems shorter than 10 cm, regardless of stem thickness. Furthermore, the amount of secondary xylem tissue increased with the stem height. These observations led us to conclude that the weight carried by the stem is the cue for wood formation. This is an interesting observation in light of earlier studies, which showed that a physical (pressure) stimulus (e.g. compression forces) induced the differentiation of cambium from dedifferentiated cell mass (i.e. callus) both at graft union and in in vitro culture (Lintilhac and Vesecky, 1981). The meristematic status of cambial cells cannot be maintained in vitro because the cells dedifferentiate to callus on being removed from the stem. However, the application of mechanical force to the surface of the callus mass induced an ordering of cell division that resulted in the formation of cambium-like tissues (Barnett and Asante, 2000). In addition, it has been known that wood formation is positively correlated with the weight of the crown in trees and other physical stresses, such as touch, bending, wind, and wound (Higuchi, 1997). Early studies indicate that external pressure may be involved in the maintenance of the cambial state in normal woody stems (Brown and Sax, 1962). However, it is unclear whether their stimulatory effects are through the induction of cambium differentiation or stimulation of the growth of the existing secondary meristem.
Auxin Regulation of Wood Formation
In this report, we provide evidence to support the hypothesis that auxin is an integral part of the weight signaling that induces the transition from primary growth to secondary growth in Arabidopsis. This led us to examine the expression profiles of the genes encoding auxin carriers. Auxin has long been proposed to regulate vascular development, with polar transport being of particular importance (Sachs, 1981, 2000; Nelson and Dengler, 1997). Auxin, either endogenous or exogenously supplied, is an important regulator of cambial growth (Aloni, 1987; Ye, 2002) and a key signal for the production of xylem and phloem by the vascular cambium (Little and Sundberg, 1991; Little et al., 2002). Recent studies identified the genes encoding putative auxin influx and efflux carrier proteins represented by AUX and PIN gene families, respectively (Bennett et al., 1996; Palme and Galweiler, 1999). Down-regulation of the expression of auxin efflux carriers (PIN3 and PIN4) resulted in the dramatic reduction of auxin polar flow and consequently blocked vascular cambium activity at the basal parts of the inflorescence stems (Zhong and Ye, 2001). The AtPIN3 protein has been suggested to be involved in lateral auxin transport (Friml et al., 2002), which facilitates the exchange of auxin between the main basipetal stream in vasculature and peripheral regions of shoot, where control of elongation occurs (Epel et al., 1992). In our experiments, the expression of four auxin efflux carrier genes (AtPIN1, AtPIN3, AtPIN4, and AtPIN7) was increased at the intermediate stem and decreased at the mature stem, while two (AtPIN5 and AtPIN6) did not change with stem development (Fig. 7). Differential gene expression was also observed with auxin-influx carriers at the three different development stages. These differential expressions of auxin influx and efflux carriers may support the hypothesis that fine regulation mechanisms of auxin distribution exist, with regard to secondary xylem formation.
For decades, it has been known that the plant hormone auxin IAA can regulate gene expression (Key, 1989; Theologis, 1989). The Aux/IAA genes are themselves auxin-induced (Abel et al., 1995), while there is no evidence for auxin-dependent changes in transcription of the ARF genes (Ulmasov et al., 1999). Milioni et al. (2002) were able to identity 68 transcripts that were expressed within only 30 min of tracheary element formation induction. Interestingly, these include those homologous to known components of the auxin-signaling pathway such as the Aux/IAA genes. The mutations in different Aux/IAA genes have contrasting effects on the same auxin response. The gain-of-function mutant phenotypes also suggest some of the complexity permitted by the large number of Aux/IAA genes in Arabidopsis (Liscum and Reed, 2002). Thus, it appears that different Aux/IAA genes have opposing functions. Aux/IAA genes in hybrid aspen, referred to as PttIAA genes, form a multigene family comprised of at least 8 members that were differentially expressed in the tissues of the cambial region (Moyle et al., 2002). Therefore, differentially expressed Aux/IAA genes in wood-forming stems of Arabidopsis might have their own roles in xylogenesis. In our study, there were 23 Aux/IAA genes expressed in the stems. Of those, 7 were up-regulated in mature stems, 3 had the highest expression in the intermediate stems, and 13 had no change in their expression level. Eighteen ARF genes were differentially regulated during wood formation. Interestingly, the well-characterized ARFs such as ARF3/Ett (Sessions and Zambryski, 1995), ARF5/Mp (Berleth and Jurgens, 1993), and ARF7/nph4/tir5/msg1 (Watahiki and Yamamoto, 1997) had no significant changes in their expression levels. However, we found that 3 ARFs (ARF2, ARF4, and ARF12) had the most dramatic expression changes in wood forming stems, suggesting their putative role in wood formation. A protein kinase, PINOID, has been suggested to mediate auxin-mediated induction of wood formation (Christensen et al., 2000). Overexpression of the protein kinase increases polar auxin transport in Arabidopsis (Benjamins et al., 2001). PINOID was up-regulated (>2-fold), specifically at the intermediate stem, although the signal intensity of this gene was very low.
Transcriptional Regulators Involved in the Initiation of Secondary Growth
Our experiments showed that the weight carried by the plant body could be a trigger for the initiation of secondary growth. In addition, secondary xylem development began to appear at the intermediate stems. From these observations, it was prudent to think that we could identify the regulatory genes involved in the initiation step of wood formation by comparing the transcription profile of weight-treated stems with that of intermediate stem. In terms of genetic regulation of wood formation, it is notable that Arabidopsis is an herbaceous nonwoody plant and does not undergo secondary growth when grown in high density. However, it can be induced to produce secondary xylem. Furthermore, a recent comparative analysis of a large number of poplar expressed sequence tags with Arabidopsis sequences showed that transcription regulation-related genes are the most divergent between the two species (S. Park, S. Oh, K.-H. Han, unpublished data). Based on these observations, we postulate that the major differences (e.g. secondary growth, perennial growth habit) between poplar and Arabidopsis may be in transcriptional control rather than in structural genes. Therefore, it is logical to expect that any transcription regulation-related genes with differential expression in the wood-forming stems are likely to be the key players responsible for the transition from primary growth to secondary growth.
The Arabidopsis genome encodes at least 125 R2R3-type MYB transcription factors. Among them, 4 MYB transcription factors were identified as candidate regulatory genes for wood formation. The R2R3-type MYB factors have been categorized into 22 subgroups based on the conserved amino acid sequence motifs present carboxy-terminal to the MYB domain (Kranz et al., 1998). The genes categorized into the same subgroup are often functionally conserved. For example, AtMYB91/AS1 and AmMYBPHAN, and AtMYB66/WER and AtMYB0/GL1 have been shown to functionally complement each other (Stracke et al., 2001). Interestingly, 3 of the 4 candidate MYB genes (AtMYB77, AtMYB73, and AtMYB44) were classified into the same subgroup number 22, suggesting those genes may have similar functions in stem development. Previously, AtMYB77 and AtMYB44 were reported to show up-regulation in the late stages of embryogenesis (Kirik et al., 1998).
The formation and maintenance of the shoot apical meristem (SAM) is very important for vegetative growth as well as reproduction. Homeobox genes such as WUSCHEL (WUS) and SHOOT MERISTEMLESS (STM) are known as key regulators of SAM differentiation in Arabidopsis (Long et al., 1996; Mayer et al., 1998). NAC family genes such as NAM and CUC also are involved in SAM formation. Formation of vascular tissue within the shoot is closely associated with the activity of the SAM. However, the relationship between SAM and vascular cambium development remains unclear. Since wood is formed from vascular cambium, the differentiation and development of vascular cambium is essential in secondary growth. We have found that two NAC genes (At2g17040 and At3g49530) and two homeobox genes (At4g34160 and At1g05710) were up-regulated in both weight-treated and intermediate stage stems. The functions of these genes are currently unknown. However, AtHB-8 (At4g32880), a homeobox gene, was previously reported to be positively regulated by auxin (Baima et al., 1995). Moreover, ectopic expression of AtHB-8 increased the production of xylem tissue and promoted vascular cell differentiation in Arabidopsis, although the formation of vascular system was not affected in athb8 mutants (Baima et al., 2001). The expression levels of AtHB-1, AtHB-6, AtHB-7, AtHB-8, and AtHB-12 were also increased significantly in mature stem.
Resolving the dilemma of achieving greater environmental protection of forest ecosystems while meeting the increasing demand for forest utilization necessitates gaining a fundamental understanding of the biochemical processes involved in tree growth and development. Here, we show that the plant body weight triggers the transition from primary growth to secondary growth and auxin mediates the weight signaling. The candidate genes in the signaling and transcriptional regulation identified here will assist future investigations aimed at unraveling the molecular mechanisms that regulate the induction and growth of the vascular cambium.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (ecotype Columbia) was grown in a growth chamber under a short-day condition (8 h light/16 h dark) at 23°C for 7 weeks. Each Arabidopsis plant was grown on individual pots (12 cm diameter). To induce inflorescence stem, the short-day grown plants were moved to long-day condition (16 h light/8 h dark) for 5 to 10 d according to the experimental design. After the long-day treatment, the plants were moved back to short-day condition and grown for about 2 weeks. For weight treatment, aluminum foil-wrapped cap tube (2.5 g) was placed on the top of 5 cm tall (immature) Arabidopsis plant for 3 d. For agar block tests, a cap tube containing 2% agarose with or without 5 μm NAA (total weight is 2.5 g) was placed on top of the decapitated inflorescence stem. Decapitation was done by removing the shoot apex (0.5–1.0 cm in length). Plants with same age and height were used in the experiments with or without auxin. All of the experiments included 4 to 6 plants in each treatment group and were repeated at least 3 times with similar results.
Histological Analysis
The stem area located immediately above the rosette (basal level) was cross-sectioned by hand and stained with 2% phloroglucinol-HCl or 0.05% toluidine blue for 1 min to visualize secondary xylem tissues. For confocal laser scanning microscopy, the same basal level stems were cross-sectioned by hand and stained with 0.1% acridine orange for 15 min. The images were recorded using a Zeiss (Jena, Germany) PASCAL confocal laser scanning microscope with a 488-nm excitation mirror, a 560-nm emission filter, and a 505 to 530-nm emission filter. Image analysis was performed using Laser scanning microscope PASCAL LSM version 3.0 SP3 software.
Measurement of Auxin Transport
To test the effects of weight application on the measurement of auxin movement in the stem, a stem section (10 cm long) of intermediate stage plant was cut by basal level and placed upside down in a 15-mL conical tube containing 50 μL of one-half-strength Murashige and Skoog liquid media with [14C]IAA (0.1mCi; 57 mCi/mmol; 1 Ci = 37 GBq). Weight application was carried out by placing aluminum foil wrapped cap tube (2.5 g) on the basal region of the upside down stem. Control experiments were performed exactly the same way except the weight treatment. After 12 h of incubation at room temperature in dark, the stem was sectioned into 1.5-cm segments from top to bottom and measured the transported [14C]IAA in the liquid scintillation counter (Tri-Carb, Packard, Meriden, CT).
RNA Extraction and Northern-Blot Analysis
For total RNA isolation, main stems (harvesting the segment 3 cm above the rosette level) of 20 to 30 individual Arabidopsis plants for each stage (immature [5 cm height], intermediate [10–15 cm] and mature stem [>25 cm]; 2.5-g weight treatment and control [same as immature stem]) sample were pooled from several batches to eliminate much of the variation in gene expression patterns caused by subtle differences in environmental conditions and among individuals. All samples were harvested around 4 pm. Total RNA was extracted using the method of Trizol reagent (Gibco-BRL, Gaithersburg, MD). For northern-blot analysis, 15 μg of total RNA of each sample was denatured and separated by 1% agarose formaldehyde gel. RNA was transferred onto Hybond N+ membrane (Stratagene, La Jolla, CA) by capillary techniques. Gene specific probes were prepared by PCR and labeled with [γ32P]-dCTP using a Prime-it II Random Primer Labeling kit (Stratagene). Hybridization was carried out according to the manufacturer's instructions and then exposed to Kodak Biomax film (Sigma, St. Louis). Ethidium bromide-stained ribosomal RNA was used as a loading control.
Gene Expression Analysis Using Affymetrix GeneChip
Sample preparation and total RNA extraction was described above. Poly(A)+ RNA was isolated from total RNA using a Poly(A) Purist mRNA purification kit (Ambion, Austin, TX). In order to confirm reproducibility, all experiments were duplicated. All methods for the preparation of cRNA from mRNA, as well as the subsequent steps leading to hybridization and scanning of the U95 GeneChip Arrays, were provided by the manufacturer (Affymetrix). Briefly, first-stranded cDNAs were synthesized from 1 μg of mRNAs from the samples (3 stages of immature, intermediate and mature; 2.5-g weight treatment and control) with a special oligo(dT)24 primer containing a T7 RNA polymerase promoter at its 5′ end. After second-strand synthesis, biotin-labeled cRNA was generated from the cDNA sample by an in vitro transcription reaction using a BioArray RNA Transcript Labeling Kit (Enzo Diagnostics, New York) with biotin-labeled CTP and UTP. cRNA (20 μg) was fragmented by heating at 94°C for 35 min in fragmentation buffer (40 mm Tris-acetate, pH 8.1, 125 mm potassium acetate, and 30 mm magnesium acetate). An aliquot of fragmented and unfragmented cRNA was analyzed by formaldehyde/agarose gel electrophoresis to ensure appropriate size distribution (average size, 700 bp of unfragmented cRNA and 100 bp after fragmentation). The control cRNA mixture was composed of a second set of four biotinylated in vitro antisense transcripts of cDNAs encoding the Escherichia coli biotin synthesis genes bioB, bioC, and bioD and the P1 bacteriophage cre recombinase gene. Probes corresponding to these bacterial transcripts are also represented on all Affymetrix GeneChips (including test chips). Each synthetic transcript was quantified and represented at the copy numbers of 2 × 108 to 2 × 1010, approximately corresponding to the expected dynamic range of detection for the GeneChip. This set of control cRNAs allows for the monitoring of hybridization, washing, and staining conditions and also provides a second set of reference samples for normalizing between experiments. The cRNA hybridization mixes were hybridized GeneChip arrays at 45°C for 16 h in a rotisserie oven set at 60 rpm (GeneChip Hybridization Oven 640, Affymetrix). Then, the arrays were washed with sodium chloride/sodium phosphate/EDTA, stained with streptavidin-phycoerythrin (Molecular Probes, Eugene, OR), and washed again (GeneChip Fluidics Station 400, Affymetrix). Finally, the chip was scanned using a GeneArray Scanner (Hewlett-Packard, Palo Alto, CA, and Affymetrix). The average difference and expression call, for each of the duplicated samples, was computed using Affymetrix GeneChip Analysis Suite version 5.0 with default parameters. The resulting hybridization intensity values reflect the abundance of a given mRNA relative to the total mRNA population and were used in all subsequent analyses. Normalization and K-Mean clustering was done by GeneSpring 4.2.1 software (Silicon Genetics, Redwood City, CA). The 50th percentile of all measurements was used as a positive control for each sample; each gene measurement was divided by this synthetic positive control, assuming that this was at least 10. The bottom tenth percentile was used as a test for correct background subtraction. Each gene was normalized to itself by making a synthetic positive control for that gene, and dividing all measurements for that gene by this positive control, assuming it was at least 0.01. This synthetic control was the median of the gene's expression values over all of the samples.
Promoter Sequence Analysis
The promoter sequences (1 kb upstream) of the selected genes were obtained from the PlantsT web site (http://plantst.sdsc.edu/plantst/affy_promoter.shtml; Ghassemian et al., 2001) and were searched against the Plant Cis-acting Regulatory DNA Elements database (http://www.dna.affrc.go.jp/htdocs/PLACE/; Higo et al., 1999).
Supplementary Material
Acknowledgments
We thank Kenneth Keegstra, Michael Thomashow, and Daniel Keathley for their critical reading of a draft manuscript and discussion and Merilyn Ruthig for her technical assistance.
This work was supported by the U.S. Department of Agriculture grants to the Eastern Hardwood Utilization Program at Michigan State University (nos. 98–34158–5995, 00–34158–9236, and 01–34158–11222).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.038844.
References
- Abel S, Nguyen MD, Theologis A (1995) The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251: 533–549 [DOI] [PubMed] [Google Scholar]
- Allona I, Quinn M, Shoop E, Swope K, Cyr SS, Carlis J, Riedl J, Retzel E, Campbell MM, Sederoff R, et al. (1998) Analysis of xylem formation in pine by cDNA sequencing. Proc Natl Acad Sci USA 95: 9693–9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Biol 38: 179–204 [Google Scholar]
- Aloni R (1995) The induction of vascular tissues by auxin and cytokinin. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 531–546
- Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, Morelli G (1995) The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121: 4171–4182 [DOI] [PubMed] [Google Scholar]
- Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, Ruberti I, Morelli GS (2001) The arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol 126: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JR, Asante AK (2000) The formation of cambium from callus in grafts of woody species. In RA Savidge, JR Barnett, R Napier, eds, Cell and Molecular Biology of Wood Formation. BIOS Scientific Publishers, Oxford, pp 155–167
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Berleth T, Jurgens G (1993) The role of the monopterous gene in organising the basal body region of the Arabidopsis embryo. Development 118: 575–587 [Google Scholar]
- Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4: 57–62 [DOI] [PubMed] [Google Scholar]
- Braam J (1992) Regulated expression of the calmodulin-related TCH genes in cultured Arabidopsis cells: induction by calcium and heat shock. Proc Natl Acad Sci USA 89: 3213–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Sax K (1962) The influence of pressure on the differentiation of secondary tissues. Am J Bot 49: 683–691 [Google Scholar]
- Chaffey N (1999) Cambium: old challenges-new opportunities. Trees (Berl) 13: 138–151 [Google Scholar]
- Chaffey N, Cholewa E, Regan S, Sundberg B (2002) Secondary xylem development in Arabidopsis: a model for wood formation. Physiol Plant 114: 594–600 [DOI] [PubMed] [Google Scholar]
- Che P, Gingerich DJ, Lall S, Howell SH (2002) Global and hormone-induced gene expression changes during shoot development in Arabidopsis. Plant Cell 14: 2771–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100: 469–478 [DOI] [PubMed] [Google Scholar]
- Dengler N, Kang J (2001) Vascular patterning and leaf shape. Curr Opin Plant Biol 4: 50–56 [DOI] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51: 21–37 [DOI] [PubMed] [Google Scholar]
- Edwards BF, Gray SW (1977) Chronic acceleration in plants. Life Sci Space Res 15: 273–278 [PubMed] [Google Scholar]
- Epel BL, Warmbrodt RP, Bandurski RS (1992) Studies on the longitudinal and lateral transport of IAA in the shoots of etiolated corn seedlings. J Plant Physiol 140: 310–318 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5: 199–206 [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fukuda H (1997. a) Tracheary element differentiation. Plant Cell 9: 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H (1997. b) Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol 47: 299–325 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Waner D, Tchieu J, Gribskov M, Schroeder JI (2001) An integrated Arabidopsis annotation database for Affymetrix Genechip data analysis, and tools for regulatory motif searches. Trends Plant Sci 6: 448–449 [DOI] [PubMed] [Google Scholar]
- Guilfoyle T, Hagen G, Ulmasov T, Murfett J (1998. a) How does auxin turn on genes? Plant Physiol 118: 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle TJ, Ulmasov T, Hagen G (1998. b) The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell Mol Life Sci 54: 619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49: 373–385 [PubMed] [Google Scholar]
- Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 2: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290: 2110–2113 [DOI] [PubMed] [Google Scholar]
- Hertzberg M, Aspeborg H, Schrader J, Andersson A, Erlandsson R, Blomqvist K, Bhalerao R, Uhlén M, Teeri T, Lundberg J, et al. (2001) A transcriptional roadmap to wood formation. Proc Natl Acad Sci USA 98: 14732–14737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T (1997) Biochemistry and molecular biology of wood. In Formation and Development of Wood Tissues. Springer-Verlag, New York, pp 263–290
- Jacobs WP (1952) The role of auxin in differentiation of xylem around a wound. Am J Bot 39: 301–309 [Google Scholar]
- Key JL (1989) Modulation of gene expression by auxin. Bioessays 11: 52–58 [DOI] [PubMed] [Google Scholar]
- Kirik V, Kolle K, Misera S, Baumlein H (1998) Two novel MYB homologues with changed expression in late embryogenesis-defective Arabidopsis mutants. Plant Mol Biol 37: 819–827 [DOI] [PubMed] [Google Scholar]
- Kirst M, Johnson AF, Baucom C, Ulrich E, Hubbard K, Staggs R, Paule C, Retzel E, Whetten R, Sederoff R (2003) Apparent homology of expressed genes from wood-forming tissues of loblolly pine (Pinus taeda L.) with Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 7383–7388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL (1987) The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev 1: 86–96 [Google Scholar]
- Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C, et al. (1998) Towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J 16: 263–276 [DOI] [PubMed] [Google Scholar]
- Lev-Yadun S (1994) Induction of sclereid differentiation in the pith of Arabidopsis thaliana (L) Heynh. J Exp Bot 45: 1845–1849 [Google Scholar]
- Lev-Yadun S, Flaishman MA (2001) The effect of submergence on ontogeny of cambium and secondary xylem and on fiber lignification in inflorescence stems of Arabidopsis. IAWA J 22: 159–169 [Google Scholar]
- Lintilhac PM, Vesecky TB (1981) Mechanical-stress and cell-wall orientation in plants. 2. The application of controlled directional stress to growing plants with a discussion on the nature of the wound reaction. Am J Bot 68: 1222–1230 [Google Scholar]
- Liscum E, Reed JW (2002) Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol 49: 387–400 [PubMed] [Google Scholar]
- Little CHA, MacDonald JE, Olsson O (2002) Involvement of indole-3-acetic acid in fascicular and interfascicular cambial growth and interfascicular extraxylary fiber differentiation in Arabidopsis thaliana inflorescence stems. Intl J Plant Sci 163: 519–529 [Google Scholar]
- Little CHA, Sundberg B (1991) Tracheid production in response to indole-3-acetic acid varies with internode age in Pinus sylvestris stems. Trees (Berl) 5: 101–106 [Google Scholar]
- Lockhart DJ, Winzeler EA (2000) Genomics, gene expression and DNA arrays. Nature 405: 827–836 [DOI] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69 [DOI] [PubMed] [Google Scholar]
- Lorenz WW, Dean JF (2002) SAGE profiling and demonstration of differential gene expression along the axial developmental gradient of lignifying xylem in loblolly pine (Pinus taeda). Tree Physiol 22: 301–310 [DOI] [PubMed] [Google Scholar]
- Mauseth J (1998) Botany: An Introduction to Plant Biology. Jones and Bartlett Publishers, Sudbury, MA
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815 [DOI] [PubMed] [Google Scholar]
- Milioni D, Sado PE, Stacey NJ, Roberts K, McCann MC (2002) Early gene expression associated with the commitment and differentiation of a plant tracheary element is revealed by cdna-amplified fragment length polymorphism analysis. Plant Cell 14: 2813–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Oka M, Yamamoto R, Masuda Y, Hoson T, Kamisaka S, Ueda J (1999) Auxin polar transport in Arabidopsis under simulated microgravity conditions: relevance to growth and development. Adv Space Res 23: 2033–2036 [DOI] [PubMed] [Google Scholar]
- Moyle R, Schrader J, Stenberg A, Olsson O, Saxena S, Sandberg G, Bhalerao RP (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid aspen. Plant J 31: 675–685 [DOI] [PubMed] [Google Scholar]
- Nelson T, Dengler N (1997) Leaf vascular pattern formation. Plant Cell 9: 1121–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Park S, Han K-H (2003) Transcriptional regulation of secondary growth in Arabidopsis thaliana. J Exp Bot 54: 2709–2722 [DOI] [PubMed] [Google Scholar]
- Palme K, Galweiler L (1999) PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol 2: 375–381 [DOI] [PubMed] [Google Scholar]
- Plomion C, Leprovost G, Stokes A (2001) Wood formation in trees. Plant Physiol 127: 1513–1523 [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Meyerowitz EM (1998) The AP2/EREBP family of plant transcription factors. Biol Chem 379: 633–646 [DOI] [PubMed] [Google Scholar]
- Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9: 152–262 [Google Scholar]
- Sachs T (2000) Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol 41: 649–656 [DOI] [PubMed] [Google Scholar]
- Sessions RA, Zambryski PC (1995) Arabidopsis gynoecium structure in the wild and in ettin mutants. Development 121: 1519–1532 [DOI] [PubMed] [Google Scholar]
- Sterky F, Regan S, Karlsson J, Hertzberg M, Rohde A, Holmberg A, Amini B, Bhalerao R, Larsson M, Villarroel R, et al. (1998) Gene discovery in the wood-forming tissues of poplar: analysis of 5,692 expressed sequence tags. Proc Natl Acad Sci USA 95: 13330–13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH (2001) Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc Natl Acad Sci USA 98: 9437–9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A (1989) Auxin-regulated gene expression in plants. Biotechnology 12: 229–243 [DOI] [PubMed] [Google Scholar]
- Turner S, Somerville CR (1997) Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9: 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz EJ, Sundberg B (1998) Indole-3-acetic acid controls cambial growth in scots pine by positional signaling. Plant Physiol 117: 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B (1996) Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA 93: 9282–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1999) Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci USA 96: 5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Estelle M (1998) Molecular mechanisms of auxin action. Curr Opin Plant Biol 1: 434–439 [DOI] [PubMed] [Google Scholar]
- Watahiki MK, Yamamoto KT (1997) The massugu1 mutation of Arabidopsis identified with failure of auxin-induced growth curvature of hypocotyl confers auxin insensitivity to hypocotyl and leaf. Plant Physiol 115: 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton OC (1938) Correlation of cambial activity with flowering and regeneration. Bot Gaz 99: 854–864 [Google Scholar]
- Yang J, Park S, Kamdem DP, Keathley DE, Retzel E, Paule C, Kapur V, Han K-H (2003) Novel gene expression profiles define the metabolic and physiological processes characteristic of wood and its extractive formation in a hardwood tree species, Robinia pseudoacacia. Plant Mol Biol 52: 935–956 [DOI] [PubMed] [Google Scholar]
- Ye Z-H (2002) Vascular tissue differentiation and pattern formation in plants. Annu Rev Plant Biol 53: 183–202 [DOI] [PubMed] [Google Scholar]
- Zhao C, Johnson BJ, Kositsup B, Beers EP (2000) Exploiting secondary growth in Arabidopsis. Construction of xylem and bark cDNA libraries and cloning of three xylem endopeptidases. Plant Physiol 123: 1185–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye Z-H (2001) Alteration of auxin polar transport in the Arabidopsis ifl1 mutants. Plant Physiol 126: 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.