Abstract
The chicken anemia virus protein Apoptin induces apoptosis in the absence of p53 by a mechanism that remains to be elucidated. Here we show that in transformed cells, Apoptin is associated with APC1, a subunit of the anaphase-promoting complex/cyclosome (APC/C). We demonstrate that Apoptin expression, or depletion of APC1 by RNA interference, inhibits APC/C function in p53 null cells, resulting in G2/M arrest and apoptosis. Our results explain the ability of Apoptin to induce apoptosis in the absence of p53 and suggest that the APC/C is an attractive target for anticancer drug development.
Keywords: Apoptin, apoptosis, G2/M arrest, anaphase-promoting complex/cyclosome, p53-independent, RNA interference
Apoptosis is a physiological form of cell death that is required during normal development and plays a key role in controlling disease by mediating the elimination of cancerous or virus-infected cells. Many animal viruses have been found to regulate apoptosis (for review, see Teodoro and Branton 1997; Roulston et al. 1999). Inhibition of apoptosis can maximize viral replication efficiency and help evade an immune response. Conversely, induction of apoptosis near the end of virus replication can facilitate viral egress.
Development of novel and effective cancer therapies depends upon the discovery of agents that selectively destroy tumor cells while leaving normal cells intact. Several viruses have such selective intrinsic oncolytic activity or have been engineered to become oncolytic (for review, see Kirn et al. 2001). The chicken anemia virus protein Apoptin can induce apoptosis in a variety of human malignant cell lines (Zhuang et al. 1995). Two properties of Apoptin-induced cell death are particularly intriguing: First, Apoptin does not induce apoptosis in normal (untransformed) cells; and second, Apoptin-induced cell death is not dependent upon the p53 tumor suppressor (Danen-Van Oorschot et al. 1997, 1999). Thus, Apoptin represents a potential agent for the treatment of tumors that have lost their p53 status and are therefore refractory to many cancer therapies. Apoptin has shown efficacy in treating human xenografted tumors in mice and is currently being evaluated as a gene therapy agent to selectively destroy cancer cells (van der Eb et al. 2002).
The molecular mechanism by which Apoptin induces apoptosis is largely unknown. Several studies have demonstrated that nuclear localization of Apoptin is required for induction of apoptosis (Danen-Van Oorschot et al. 2003; Guelen et al. 2004), suggesting that the cellular target of Apoptin is a nuclear protein. Here we show that in transformed cells, Apoptin is associated with subunit 1 of the anaphase-promoting complex/cyclosome (APC/C), which results in G2/M cell-cycle arrest and induction of p53-independent apoptosis.
Results and Discussion
Apoptin coimmunoprecipitates with subunit 1 of the anaphase-promoting complex in transformed cells
To optimize expression of Apoptin for proteomic studies, we constructed an adenovirus (Ad) expressing Flag-tagged Apoptin (Ad-Apwt). Apoptin protein expression was confirmed by immunoblot using an α-Flag monoclonal antibody (Fig. 1A, inset). Figure 1A shows that following infection with Ad-Apwt, p53 null Saos-2 osteosarcoma cells underwent pronounced apoptosis after 24 h, and most cells were dead by 72 h. Little or no cell death was observed in cells infected with a control adenovirus expressing LacZ (Ad-LacZ). Similar results were obtained in H1299 cells, a p53-negative non-small cell lung carcinoma cell line.
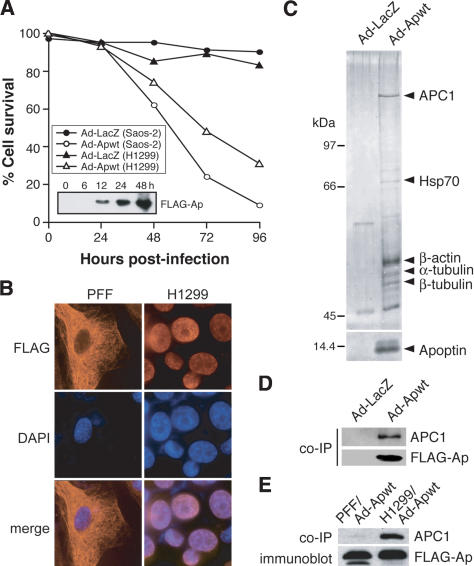
Figure 1.
Apoptin is associated in vivo with APC1. (A) Saos-2 and H1299 cells were infected with adenovirus expressing Flag-Apoptin (Ad-Apwt) or LacZ (Ad-LacZ), and cell death was monitored for 96 h postinfection by trypan blue exclusion. (Inset) Immunoblot analysis using an α-Flag antibody to monitor Apoptin protein expression in Saos-2 cells. (B) Immunofluorescence of PFF and H1299 cells 24 h postinfection with Ad-Apwt. Cells were stained with an α-Flag antibody (left) or DAPI (middle); merged images (right). Magnification, 1000×. (C) Affinity purification of Apoptin-associated proteins from H1299 cells infected with Ad-Apwt. Proteins were separated by SDS-PAGE and visualized by silver staining. Microsequenced bands are indicated. (D) Apoptin was immunoprecipitated from Ad-Apwt-and Ad-LacZ-infected H1299 cells using an α-Flag antibody, and the immunoprecipitates were analyzed for APC1 by immunoblotting with a polyclonal α-APC1 antibody. (E) Apoptin was immunoprecipitated from Ad-Apwt-infected PFF or H1299 cells using an α-Flag antibody, and the immunoprecipitates were analyzed for APC1 by immunoblotting. Immunoblotting for Flag-Apoptin was performed on whole-cell extracts.
Previous studies have shown that Apoptin is nuclear-localized in transformed cells and cytoplasmic in untransformed or primary cells (Danen-Van Oorschot et al. 1997). To determine the intracellular localization of Flag-tagged Apoptin, immunocytochemistry was performed using an α-Flag monoclonal antibody. Figure 1B shows that in primary foreskin fibroblasts (PFFs), Apoptin stained with a characteristic cytoskeletal-like pattern with nuclear exclusion, whereas in transformed H1299 cells the protein was completely nuclear. These results suggest that the protein may be associated with cytoskeletal components. In summary, Flag-tagged Apoptin induces apoptosis and exhibits the characteristic differential localization in transformed and primary cells described previously (Danen-Van Oorschot et al. 1997).
To identify Apoptin-associated cellular proteins, we prepared extracts from H1299 cells infected with Ad-Apwt, and purified Apoptin and associated proteins on an α-Flag affinity resin. Polypeptides bound to the affinity column were separated by SDS-PAGE and visualized by silver staining. Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectroscopic analysis revealed that a major Apoptin-associated polypeptide was APC1, the largest subunit of the APC/C and an essential component of the mitotic checkpoint apparatus (Fig. 1C). Apoptin also coimmunoprecipitated with HSP-70, which is known to associate with overexpressed proteins, as well as α-tubulin, β-tubulin and β-actin, suggesting an association with filamentous networks, consistent with the immunocytochemistry results of Figure 1B.
To confirm the association with APC1, Apoptin was immunoprecipitated from Ad-Apwt-infected H1299 cells with an α-Flag antibody, and the immunoprecipitate was analyzed for APC1 by immunoblotting. Figure 1D shows that APC1 was present in the immunoprecipitate from Apoptin but not in that of a control LacZ protein. In contrast, Figure 1E shows that APC1 did not coimmunoprecipitate with Apoptin in PFFs, suggesting that the Apoptin-APC1 association is specific to transformed cells.
Apoptin expression in transformed cells induces G2/M cell-cycle arrest by inhibition of APC/C function
The association of Apoptin with APC1 raised the possibility that Apoptin expression could affect cell-cycle progression. To address this issue, H1299 and PFF cells were infected with Ad-Apwt or Ad-LacZ and analyzed by fluorescence-activated cell sorting (FACS). Figure 2A and B shows that following infection with Ad-Apwt, H1299 cells began to accumulate in G2/M after 12 h, whereas the cell-cycle profile of PFF cells was unaffected. Although FACS analysis of Apoptin-expressing H1299 cells clearly showed a 4N DNA content, morphological examination did not reveal a classical mitotic-arrested appearance but rather a condensed nuclear morphology that is a hallmark of apoptosis (see Supplementary Fig. 1). The onset of apoptosis was rapid, as evidenced by the appearance of a prominent sub-G1 peak concomitant with G2/M accumulation.
Figure 2.
Apoptin expression induces G2/M arrest by inhibition of APC/C function. (A) Cell-cycle analysis of H1299 cells infected with either Ad-LacZ or Ad-Apwt at 0, 12, 24, and 36 h postinfection. (B) Percent of H1299 (top) or PFF (bottom) cells in G2/M were quantitated. (C) Immunoblot analysis of APC/C substrates cyclin B1 and Polo-like kinase (Plk), and a non-APC/C substrate, cyclin E, in H1299 whole-cell lysates 24 h following infection with Ad-Apwt or Ad-LacZ, or in cells treated with nocodozole (noc) for 12 h. Tubulin levels were monitored as a loading control. (D) Gel filtration analysis of the APC/C complex in mock- or Ad-Apwt-infected H1299 cells. Fractions from the gel filtration column were analyzed by immunoblotting for Flag-Apoptin, APC1, or another APC/C subunit, Cdc27. Positions of molecular-weight markers are indicated.
To verify that Apoptin inhibited APC/C function in H1299 cells, we used an independent assay to measure APC/C activity. The APC/C catalyzes the ubiquitination of several substrates including cyclin B1 and Polo-like kinase (Plk), whose degradation by the proteasome results in anaphase progression and mitotic exit. If APC/C function is inhibited, these substrates are not degraded. Thus, stabilization of APC/C substrates is indicative of cyclosome dysfunction (Wirth et al. 2004). To test whether Apoptin expression resulted in stabilization of APC/C substrates, H1299 cells were infected with Ad-Apwt or Ad-LacZ, and 24 h later levels of mitotic APC/C substrates were determined by immunoblotting. As a positive control, cells were arrested in G2/M by nocodazole treatment. Figure 2C shows that the protein levels of cyclin B1 and Plk were stabilized in Ad-Apwt-infected and nocodozole-treated cells compared to Ad-LacZ-infected cells. In contrast, protein levels of cyclin E, a G1 cyclin that is not an APC/C substrate, were slightly decreased by Apoptin expression or nocodazole treatment. RT-PCR analysis confirmed that Apoptin did not affect cyclin B1 or Plk mRNA levels (Supplementary Fig. 2). We conclude that Apoptin inhibits cyclosome function leading to G2/M arrest and apoptosis.
To gain insight into how Apoptin might inhibit APC/C activity, we performed gel filtration analysis of the APC/C complex in mock- or Ad-Apwt-infected H1299 cells. Fractions from the gel filtration column were analyzed by immunoblotting for APC1 or another APC/C subunit, Cdc27 (APC3). The results of Figure 2D reveal two dramatic effects of Apoptin expression on the APC/C complex. First, following Apoptin expression, both APC1 and Cdc27 migrated at significantly lower native molecular weights, strongly suggesting disruption of the APC/C complex. In fact, in Apoptin-expressing cells some APC1 migrated at the size expected for a free subunit (∼200 kDa). Second, in Apoptin-expressing cells, Cdc27 degradation products appeared in the lower molecular weight range. Collectively, these results suggest a model in which Apoptin binds to APC1, leading to disruption of the APC/C complex and the resultant degradation of some APC/C subunits. Finally, we note that despite its small size (13.6 kDa), essentially all Apoptin migrated at a native molecular weight of ∼200 kDa to >1 Mda, consistent with the possibility that Apoptin is associated with one or more large multisubunit complexes.
The C-terminal domain of Apoptin is required for association with APC1 and apoptosis
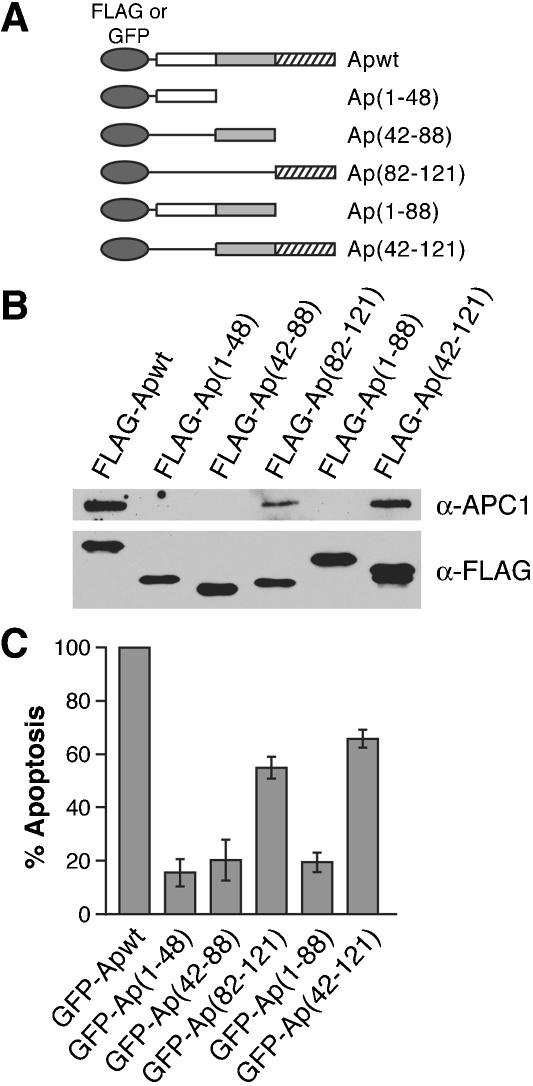
To delineate the region of Apoptin required for association with APC1, a panel of Flag-tagged Apoptin deletion mutants (Fig. 3A) was transiently expressed in H1299 cells. Forty-eight h following transfection, Apoptin was immunoprecipitated with an α-Flag antibody, and the immunoprecipitate was analyzed for APC1 by immunoblotting. Figure 3B shows that APC1 was present only in immunoprecipitates of Apoptin derivatives that contained the C-terminal domain (amino acids 82-121).
Figure 3.
The C-terminal domain of Apoptin is required for association with APC1 and apoptosis. (A) Schematic representations of N-terminal Flag- or GFP-tagged Apoptin deletion mutants. (B) H1299 cells were transfected with Flag-Apoptin deletion constructs and 48 h later, Apoptin was immunoprecipitated with an α-Flag antibody and the immunoprecipitate analyzed for APC1 and Apoptin by immunoblotting. (C) H1299 cells were transfected with GFPApoptin deletion constructs and 4 d later fixed and stained with DAPI and analyzed by fluorescence microscopy for GFP expression associated with apoptotic morphology. The percent apoptosis in cells expressing GFP alone was taken as background and subtracted from all samples. All mutant samples are shown as percent apoptosis of Apwt.
To investigate whether association with APC1 is required for Apoptin-mediated cell death, green fluorescent protein (GFP)-derivatives of the Apoptin deletion mutants were transiently expressed in H1299 cells. Four days after transfection, cells were fixed, stained with DAPI, and analyzed by fluorescence microscopy for apoptotic morphology. Figure 3C shows that the ability of mutants lacking the C-terminal domain to induce cell death was significantly reduced. In contrast, Apoptin mutants Ap(82-121) and Ap(42-121) retained 55% and 67% of wild-type killing activity, respectively. Collectively, the results of Figure 3B and C indicate that the C-terminal domain of Apoptin is necessary and sufficient for induction of apoptosis, and they suggest that association with APC1 is responsible for G2/M arrest and cell death.
Loss of APC1 induces G2/M arrest and apoptosis in the absence of p53
The results described above suggest a model whereby Apoptin inhibits APC/C function, resulting in G2/M arrest, followed by apoptosis. A prediction of this model is that inhibition of APC1 through other means should have an effect similar to that of Apoptin expression. To test this prediction, we analyzed the consequence of depleting APC1 by RNA interference (RNAi). Figure 4A demonstrates that transfection of a pool of short interfering RNAs (siRNAs) directed against APC1 resulted in specific and near complete reduction of APC1 protein levels. In contrast, a comparable pool of siRNAs directed against the control Lamin A/C had no significant effect on APC1 levels.
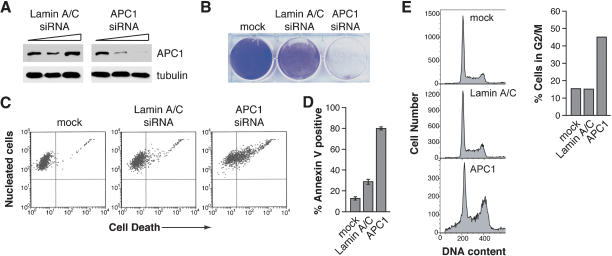
Figure 4.
Depletion of APC1 by RNA interference induces G2/M arrest and apoptosis in the absence of p53. (A) Immunoblot analysis of H1299 cells transfected with 0, 0.05, and 0.5 μg siRNAs directed against either Lamin A/C or APC1. Tubulin was monitored as a loading control. (B) Crystal violet staining of H1299 cells following two rounds of transfection with Lamin A/C or APC1 siRNAs, or mock-transfected. (C) Cell viability assays of H1299 cells 48 h following transfection of siRNAs, or mock-transfected. (D) Annexin V-FITC staining to monitor apoptosis in siRNA-transfected cells. (E) Cell-cycle analysis (left) and quantitation of the percentage of cells arrested in G2/M (right) of siRNA-transfected cells.
Transfection of the APC1 siRNA pool resulted in a dramatic reduction in cell density (Fig. 4B) and viability (Fig. 4C) compared to the Lamin A/C siRNA- and mock-transfected controls. Annexin V-FITC staining (Fig. 4D) and caspase-3 activation (Supplementary Fig. 3) confirmed, as expected, that cell death in APC1 siRNA-transfected cells was due to apoptosis, analogous to that observed following Apoptin expression (Danen-van Oorschot et al. 2000). Figure 4E shows that transfection of APC1 siRNAs, but not the control Lamin A/C siRNAs, resulted in G2/M accumulation and the appearance of cells in the sub-G1 region. Quantitation of the FACS data indicates that nearly 50% of APC1 siRNA-transfected cells were arrested in G2/M, compared to ∼15% for the Lamin A/C siRNA- or mock-transfected controls (Fig. 4E, right). Thus, RNAi-mediated depletion of APC1 has an effect analogous to that observed following Apoptin expression.
The majority of human cancers lack the tumor suppressor p53, which increases their resistance to conventional chemotherapeutic agents (Hussain and Harris 1998; Beroud and Soussi 2003). It is therefore critical to identify and characterize novel p53-independent apoptotic pathways. Our results suggest that Apoptin induces G2/M arrest and apoptosis in p53 null transformed cells through association with and inhibition of the APC/C.
Interestingly, the adenovirus E4orf4 protein also arrests cells in G2/M (Kornitzer et al. 2001) and induces apoptosis in the absence of p53 (Marcellus et al. 1998). Moreover, yeast genetics experiments suggest that E4orf4-induced cell-cycle arrest is mediated by the APC/C, most likely through its Cdc16 subunit (Kornitzer et al. 2001). Therefore, inhibition of the cyclosome complex may represent a convergently evolved viral cytopathic mechanism. Arresting cells in mitosis may facilitate viral replication. For example, mitotic cells become rounded and lift from the surrounding substrate, which may provide a means whereby infected cells become mobilized to uninfected areas and more efficiently disseminate virus.
APC1 is thought to be essential for assembly and regulation of the cyclosome complex (Kraft et al. 2003; Vodermaier et al. 2003). In our preparative immunoprecipitation/mass spectroscopic analysis we identified APC1 but not other APC/C subunits (Fig. 1C), suggesting that Apoptin associates with free APC1 and not the intact cyclosome complex. Furthermore, gel filtration analysis suggested that in the presence of Apoptin, APC1 may be dissociated from the APC/C, resulting in disruption of the complex and loss of APC/C activity. Interestingly, subunits of the APC/C, including APC1, have been shown to localize to the active centromere of dicentric chromosomes (Saffery et al. 2000). Thus, it is possible that the association of Apoptin with α- and β-tubulin observed in our proteomic analysis may result from interaction between Apoptin and the spindle complex.
Several previous observations are consistent with the notion that apoptotic signaling from the mitotic checkpoint apparatus functions independently of p53. For example, microtubule-stabilizing drugs that promote mitotic arrest and cell death, such as paclitaxel (Taxol), are generally unaffected by p53 status (Debernardis et al. 1997). In fact, cancer cells that are p53-negative are sensitized to paclitaxel-mediated killing (Wahl et al. 1996; Vikhanskaya et al. 1998). Other drugs that target terminal steps of the pathway, such as degradation of mitotic cyclins, have also been shown to be p53-independent. For example, the proteasome inhibitor PS-341 (Bortezomib) induces apoptosis in cells lacking functional p53 (Yu et al. 2003). The mechanism of Apoptin-induced cell death proposed here is therefore consistent with previous studies implicating the mitotic-check-point apparatus in p53-independent apoptosis.
Materials and methods
Cell culture
Saos-2, H1299, and primary foreskin fibroblast cells (PFFs) were obtained from ATCC and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum plus 10 μg/mL streptomycin and 10 U/mL penicillin (Sigma) at 37°C under 5% CO2 (95% air).
Adenovirus and plasmid construction
Apoptin adenovirus (Ad-Apwt) containing a single 5′ Flag epitope was generated using the AdEasy XL Adenoviral Vector System (Stratagene) according to the manufacturer's instructions. Viral supernatants were plaque purified, amplified, and titers determined by plaque assay. LacZ adenovirus (Ad-LacZ) was prepared as described (Bacchetti and Graham 1993). Cells were infected at ∼80% confluence at an MOI of 35. Flag-Apoptin deletion mutants were constructed by PCR amplification of truncated Apoptin sequences, which were directionally cloned into the vector p3XFlag-myc-CMV-26 (Sigma) to construct an in-frame N-terminal fusion to the Flag epitopes. Stop sequences were generated to exclude the C-terminal myc tag. N-terminal-tagged GFP-fusion deletion mutants were subcloned from the Flag-deletion library. All clones were confirmed by restriction digest analysis and DNA sequencing.
Immunofluorescence
PFF and H1299 cells were infected with Ad-Apwt, and 24 h later, cells were fixed in 4% paraformaldehyde (in PBS), permeabilized in 0.5% Triton X-100 (in PBS), and stained with α-Flag M5 monoclonal antibody (Sigma) followed by α-mouse Ig Texas Red conjugated secondary antibody (Jackson Laboratories). Cells were visualized with a Zeiss Axiophot2 fluorescence microscope using Axiovision 3.1 software.
Immunoprecipitations
For affinity purification, ∼8 × 107 cells were infected with Flag-tagged Ad-Apwt. After 24 h, cells were washed in PBS, harvested by scraping, and lysed in Buffer X (50 mM Tris at pH 8.5, 250 mM NaCl, 1 mM EDTA, 1% NP40, Complete Mini Tablet [Roche]) on ice for 20 min. Following centrifugation, lysates were precleared with Protein A/G-agarose for 1 h, followed by incubation with Flag-agarose at 4°C for 4 h. Beads were washed in Buffer X and eluted using Flag-peptide. Eluates were resuspended in 1× SDS sample buffer and boiled for 5 min. Proteins were resolved by SDS-PAGE and visualized by silver staining using the SilverQuest kit (Invitrogen). Bands were excised and submitted for MALDI-TOF mass spectrometry to the Proteomic Mass Spectrometry Lab at the University of Massachusetts Medical School.
For coimmunoprecipitations, ∼2 × 107 cells were infected with Ad-Apwt or Ad-LacZ, and harvested and lysed as described above. Following centrifugation, supernatants were incubated with 20 μL equilibrated EZview Red α-Flag M2 affinity beads (Sigma) at 4°C for 4 h. Beads were washed in Buffer X, and bound proteins were eluted in 1× SDS sample buffer by boiling for 5 min.
For APC1 association domain mapping, ∼1 × 107 cells were transiently transfected with 3XFlag-Apoptin truncation mutants using Effectene reagent (QIAGEN). After 48 h, cells were processed as described for the coimmunoprecipitation experiments.
Gel filtration chromatography
H1299 cells (∼2 × 107) were either mock- or Ad-Apwt-infected and after 24 h, cells were washed in PBS, harvested by scraping and lysed in Buffer A (20 mM Tris at pH 7.5, 100 mM NaCl, 20 mM b-glycerophosphate, 0.2% NP-40, 10% glycerol, 0.5 mM DTT, Complete Mini Tablet [Roche]) on ice for 30 min. Lysates were sonicated for ∼10 sec, centrifuged for 1 h at 100,000g, and ∼500 μg of total protein was injected into a Pharmacia FPLC apparatus and separated on a Superose 6 10/30 (Pharmacia) column. Fractions (500 μL) were collected and precipitated with 20% trichloroacetic acid (TCA) on ice for 1 h. Precipitates were then centrifuged at 13,000g for 15 min, washed with -20°C acetone, dried, resuspended in 1× SDS sample buffer, and boiled for 5 min.
Immunoblotting
Proteins were resolved by SDS-PAGE and transferred to nitrocellulose. Blots were blocked with 5% milk in TBS-T and probed with α-Flag M2 monoclonal (Sigma), α-APC1 polyclonal (affinity-purified α-peptide serum), α-Cdc27 monoclonal (Santa Cruz); α-cyclin B1 (Upstate Biotech); α-Plk (Zymed Laboratories), α-cyclin E (Upstate Biotech), or α-tubulin (Sigma) antibodies followed by appropriate HRP-conjugated α-Ig secondary antibody (Amersham Biosciences).
Apoptosis and cell cycle assays
To generate kill curves, Ad-Apwt- and Ad-LacZ-infected H1299 and Saos-2 cells were harvested by trypsinization, washed in PBS, and monitored for cell viability by trypan blue exclusion. Apoptotic assays using GFP-Apoptin deletion mutants were performed by transfecting the deletion panel into H1299 cells, and 4 d later, cells were fixed with 4% paraformaldehyde and stained with DAPI. Percent apoptosis was scored as the percent of 100 GFP-positive cells showing apoptotic morphology. Results were collected in a blind study by at least three individuals in two separate experiments. Data were graphed after subtraction of GFP background; all mutant samples are shown as percent apoptosis of wild type. For Annexin V analysis, cells were stained using the Annexin V-PE Apoptosis Detection Kit-I (BD Pharmingen) and analyzed using a Guava Personal flow cytometer (Guava Technologies). Data points were collected as percent Annexin V-positive cells per 5000 events. For cell-cycle analysis, cells were harvested by trypsinization, fixed and stained with propidium iodide, and analyzed by FACS.
RNA interference
Double-stranded RNAs (dsRNAs) were synthesized in vitro using a 500-bp PCR fragment as the template. Primer sequences, containing T7 priming sites, were as follows: APC1, 5′-GCGTAATACGACTCACTA TAGGGAGAAAAGGAGTAAGTGAAATTGG-3′ and 5′-GCGTAATA CGACTCACTATAGGGAGAGGAAAGGTGAAGTCACAGGG-3′; Lamin A/C, 5′-GCGTAATACGACTCACTATAGGGAGAGGCAGTCT GCTGAGAGGAAC-3′ and 5′-GCGTAATACGACTCACTATAGGG AGAAGGTGTTCTGTGCCTTCCAC-3′. In vitro transcription was performed using the T7 Megascript Kit (Ambion), and the dsRNA products were cleaved using the Dicer siRNA Generation Kit (Gene Therapy Systems). The pool of diced-siRNAs was then transfected into cells using Oligofectamine (Invitrogen) as described (Elbashir et al. 2001).
Acknowledgments
We thank F. Graham for the Ad-LacZ adenovirus vector. Thanks to members of the Green lab both past and present for useful discussions, and to S. Evans for editorial assistance. This work was supported in part by an NIH grant to M.R.G and postdoctoral fellowships from the NCI of Canada and the Medical Foundation Charles A. King Trust to J.G.T. M.R.G. is an investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1198404.
References
- Bacchetti S. and Graham, F.L. 1993. Inhibition of cell proliferation by an adenovirus vector expressing the human wild type p53 protein. Int. J. Onc. 3: 781-788. [DOI] [PubMed] [Google Scholar]
- Beroud C. and Soussi, T. 2003. The UMD-p53 database: New mutations and analysis tools. Hum. Mutat. 21: 176-181. [DOI] [PubMed] [Google Scholar]
- Danen-Van Oorschot A.A., Fischer, D.F., Grimbergen, J.M., Klein, B., Zhuang, S., Falkenburg, J.H., Backendorf, C., Quax, P.H., Van der Eb, A.J., and Noteborn, M.H. 1997. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc. Natl. Acad. Sci. 94: 5843-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen-Van Oorschot A.A., van der Eb, A.J., and Noteborn, M.H. 1999. BCL-2 stimulates Apoptin-induced apoptosis. Adv. Exp. Med. Biol. 457: 245-249. [DOI] [PubMed] [Google Scholar]
- Danen-van Oorschot A.A., van Der Eb, A.J., and Noteborn, M.H. 2000. The chicken anemia virus-derived protein apoptin requires activation of caspases for induction of apoptosis in human tumor cells. J. Virol. 74: 7072-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen-Van Oorschot A.A., Zhang, Y.H., Leliveld, S.R., Rohn, J.L., Seelen, M.C., Bolk, M.W., Van Zon, A., Erkeland, S.J., Abrahams, J.P., Mumberg, D., et al. 2003. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J. Biol. Chem. 278: 27729-27736. [DOI] [PubMed] [Google Scholar]
- Debernardis D., Sire, E.G., De Feudis, P., Vikhanskaya, F., Valenti, M., Russo, P., Parodi, S., D'Incalci, M., and Broggini, M. 1997. p53 status does not affect sensitivity of human ovarian cancer cell lines to paclitaxel. Cancer Res. 57: 870-874. [PubMed] [Google Scholar]
- Elbashir S.M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., and Tuschl, T. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494-498. [DOI] [PubMed] [Google Scholar]
- Guelen L., Paterson, H., Gaken, J., Meyers, M., Farzaneh, F., and Tavassoli, M. 2004. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene 23: 1153-1165. [DOI] [PubMed] [Google Scholar]
- Hussain S.P. and Harris, C.C. 1998. Molecular epidemiology of human cancer: Contribution of mutation spectra studies of tumor suppressor genes. Cancer Res. 58: 4023-4037. [PubMed] [Google Scholar]
- Kirn D., Martuza, R.L., and Zwiebel, J. 2001. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat. Med. 7: 781-787. [DOI] [PubMed] [Google Scholar]
- Kornitzer D., Sharf, R., and Kleinberger, T. 2001. Adenovirus E4orf4 protein induces PP2A-dependent growth arrest in Saccharomyces cerevisiae and interacts with the anaphase-promoting complex/cyclosome. J. Cell Biol. 154: 331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C., Herzog, F., Gieffers, C., Mechtler, K., Hagting, A., Pines, J., and Peters, J.M. 2003. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22: 6598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellus R.C., Lavoie, J.N., Boivin, D., Shore, G.C., Ketner, G., and Branton, P.E. 1998. The early region 4 orf4 protein of human adenovirus type 5 induces p53-independent cell death by apoptosis. J. Virol. 72: 7144-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulston A., Marcellus, R.C., and Branton, P.E. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53: 577-628. [DOI] [PubMed] [Google Scholar]
- Saffery R., Irvine, D.V., Griffiths, B., Kalitsis, P., and Choo, K.H. 2000. Components of the human spindle checkpoint control mechanism localize specifically to the active centromere on dicentric chromosomes. Hum. Genet. 107: 376-384. [DOI] [PubMed] [Google Scholar]
- Teodoro J.G. and Branton, P.E. 1997. Regulation of apoptosis by viral gene products. J. Virol. 71: 1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eb M.M., Pietersen, A.M., Speetjens, F.M., Kuppen, P.J., van de Velde, C.J., Noteborn, M.H., and Hoeben, R.C. 2002. Gene therapy with apoptin induces regression of xenografted human hepatomas. Cancer Gene Ther. 9: 53-61. [DOI] [PubMed] [Google Scholar]
- Vikhanskaya F., Vignati, S., Beccaglia, P., Ottoboni, C., Russo, P., D'Incalci, M., and Broggini, M. 1998. Inactivation of p53 in a human ovarian cancer cell line increases the sensitivity to paclitaxel by inducing G2/M arrest and apoptosis. Exp. Cell Res. 241: 96-101. [DOI] [PubMed] [Google Scholar]
- Vodermaier H.C., Gieffers, C., Maurer-Stroh, S., Eisenhaber, F., and Peters, J.M. 2003. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 13: 1459-1468. [DOI] [PubMed] [Google Scholar]
- Wahl A.F., Donaldson, K.L., Fairchild, C., Lee, F.Y., Foster, S.A., Demers, G.W., and Galloway, D.A. 1996. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat. Med. 2: 72-79. [DOI] [PubMed] [Google Scholar]
- Wirth K.G., Ricci, R., Gimenez-Abian, J.F., Taghybeeglu, S., Kudo, N.R., Jochum, W., Vasseur-Cognet, M., and Nasmyth, K. 2004. Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes & Dev. 18: 88-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tiwari, S., Steiner, P., and Zhang, L. 2003. Differential apoptotic response to the proteasome inhibitor Bortezomib (VELCADE™, PS-341) in Bax-deficient and p21-deficient colon cancer cells. Cancer Biol. Ther. 2: 694-699. [PubMed] [Google Scholar]
- Zhuang S.M., Shvarts, A., van Ormondt, H., Jochemsen, A.G., van der Eb, A.J., and Noteborn, M.H. 1995. Apoptin, a protein derived from chicken anemia virus, induces p53-independent apoptosis in human osteosarcoma cells. Cancer Res. 55: 486-489. [PubMed] [Google Scholar]