Renal artery stenosis (RAS) is an important cause of chronic kidney disease. The underlying mechanisms are not well understood. Our study indicates that CXCL16 plays an important role in the pathogenesis of RAS-induced renal injury and fibrosis through regulation of myeloid fibroblast accumulation and inflammatory cell infiltration.
Keywords: renal artery stenosis, renal fibrosis, inflammation, chemokine
Abstract
Recent studies have shown that inflammation plays a critical role in the initiation and progression of hypertensive kidney disease, including renal artery stenosis. However, the signaling mechanisms underlying the induction of inflammation are poorly understood. We found that CXCL16 was induced in the kidney in a murine model of renal artery stenosis. To determine whether CXCL16 is involved in renal injury and fibrosis, wild-type and CXCL16 knockout mice were subjected to renal artery stenosis induced by placing a cuff on the left renal artery. Wild-type and CXCL16 knockout mice had comparable blood pressure at baseline. Renal artery stenosis caused an increase in blood pressure that was similar between wild-type and CXCL16 knockout mice. CXCL16 knockout mice were protected from RAS-induced renal injury and fibrosis. CXCL16 deficiency suppressed bone marrow-derived fibroblast accumulation and myofibroblast formation in the stenotic kidneys, which was associated with less expression of extracellular matrix proteins. Furthermore, CXCL16 deficiency inhibited infiltration of F4/80+ macrophages and CD3+ T cells in the stenotic kidneys compared with those of wild-type mice. Taken together, our results indicate that CXCL16 plays a pivotal role in the pathogenesis of renal artery stenosis-induced renal injury and fibrosis through regulation of bone marrow-derived fibroblast accumulation and macrophage and T-cell infiltration.
NEW & NOTEWORTHY
Renal artery stenosis (RAS) is an important cause of chronic kidney disease. The underlying mechanisms are not well understood. Our study indicates that CXCL16 plays an important role in the pathogenesis of RAS-induced renal injury and fibrosis through regulation of myeloid fibroblast accumulation and inflammatory cell infiltration.
renal artery stenosis (RAS) is an important cause of chronic kidney disease (CKD). Atherosclerosis is the most common etiology for the development of RAS, which affected 6.8% of people over 65 yr of age and in almost 40% of patients with a history of coronary or peripheral vascular disease (12, 34). Patients with renovascular disease are at high risk for cardiovascular death (15). A prominent pathological feature in patients with renovascular disease is inflammation, tubular atrophy, and fibrosis. The degree of renal fibrosis correlates well with the rapidity of CKD prognosis (23). Renal interstitial fibrosis is characterized by extensive fibroblast activation and excessive production and deposition of extracellular matrix (ECM), which leads to destruction of renal parenchyma and progressive loss of kidney function. The current therapeutic options in the clinical setting are limited and often ineffective, except for dialysis or kidney transplantation (2). Despite improvement in the knowledge of various aspects of CKD, the initial molecular events leading to chronic renal failure remain elusive. The Angioplasty and Stenting for Renal Artery Lesion Study has demonstrated that percutaneous transluminal renal angioplasty fails to improve renal function, blood pressure, renal or cardiovascular events, or mortality (1). This study indicates that other factors, in addition to renal perfusion, affect outcome in patient with renovascular disease. Therefore, a better understanding of the cellular and molecular mechanisms underlying the initiation and progression of renovascular disease is essential for developing effective strategies to treat this disorder and prevent its progression.
Recent evidence indicates that inflammatory and immune cell infiltration is characteristic of hypertensive kidney disease (6, 20). The infiltration of circulating cells into sites of injury is mediated by locally produced chemokines through interaction with their respective receptors (9). Chemokines are divided into four subfamilies, CXC, CC, C, and CX3C, according to the number and spacing of conserved cysteine residues in their sequences (9). CXCL16 is a cytokine belonging to the CXC chemokine subfamily (21). There are two forms of CXCL16. The transmembrane form of CXCL16 functions as an adhesion molecule for CXCR6-expressing cells, while the soluble form of CXCL16 mediates infiltration of circulating cells into sites of injury (10, 44). In this study, we investigated the role of CXCL16 in renal injury and fibrosis in an experimental model of RAS using CXCL16 knockout (KO) mice.
MATERIALS AND METHODS
Animals.
Wild-type (WT) C57BL/6 mice were purchased from the Jackson Laboratory, and CXCL16 KO mice on a C57BL/6 background were a generous gift from Dr. Shuhua Han at Baylor College of Medicine, as described (5, 38). Mice were bred and maintained in the animal care facility of Baylor College of Medicine and had access to food and water ad libitum. All animal procedures were in accordance with national and international animal care and ethical guidelines and have been approved by the Institutional Animal Care and Use Committee.
Surgical procedure.
Male 8- to 10-wk-old mice were anesthetized by intraperitoneal injection of a cocktail (80 mg/kg of ketamine, 10 mg/kg of xylazine, and 3 mg/kg of acepromazine). After skin preparation, the left kidney was exposed through a small flank incision, the renal artery was isolated, and a small segment was dissected free of the renal vein. A 0.5-mm length of 0.36 mm (outer diameter) × 0.2 mm (inner diameter) polytetrafluoroethylene tubing (Braintree Scientific, Braintree, MA) was surgically placed around the renal artery and held in place with nylon circumferential suture as described (36). Sham surgeries consisted of a flank incision and mobilization of the renal artery without placement of a cuff. Left kidneys were harvested 4 wk after surgery.
Blood pressure and heart rate.
Systolic blood pressure (SBP) and heart rate were measured in conscious mice using a tail-cuff system (Visitech Systems), as reported (38, 39, 41).
Histopathological analysis.
At the end of experiments, mice were perfused with PBS to remove the blood. A portion of kidney tissue was fixed in 10% buffered formalin, embedded in paraffin, and cut at 5-μm thickness. After deparaffinization and rehydration, sections were stained with hematoxylin and eosin and Sirius red, as described (14, 38, 39). To assess monocyte/macrophage and T-lymphocyte infiltration into the kidneys, sections were stained with antibodies against F4/80 (Serotec) and CD3 (Abcam), respectively. Interstitial infiltrating F4/80-positive macrophages and CD3-positive T cells were counted in the cortex under ×400 magnification, observing 10 consecutive non-overlapping fields per animal (38, 39).
Immunofluorescence.
Kidney tissues were embedded in OCT compound, cut at 5-μm thickness, and mounted. After fixation, nonspecific binding was blocked with serum-free protein block (Dako, Carpinteria, CA). Kidney sections were then incubated with rabbit anti-CXCL16 antibody (Bioss, Woburn, MA), followed by Alexa-488 conjugated donkey anti-rabbit antibody (Invitrogen, Carlsbad, CA); rabbit anti-collagen I antibody (Rockland Immunochemicals, Gilbertsville, PA), followed by Alexa-488 conjugated donkey anti-rabbit antibody (Invitrogen); rabbit anti-fibronectin antibody (Sigma-Aldrich, St. Louis, MO), followed by Alexa-488 conjugated donkey anti-rabbit antibody (Invitrogen); or rabbit anti-α-smooth muscle actin (α-SMA) antibody (Abcam, Cambridge, MA), followed by Alexa-488 conjugated donkey anti-rabbit antibody (Invitrogen). For double immunofluorescence, kidney sections were fixed and stained with rat anti-CD45 (BD Biosciences) and rabbit anti-platelet-derived growth factor receptor-β (PDGFR-β) (Santa Cruz Biotechnology), followed by appropriate secondary antibodies sequentially. Slides were mounted with medium containing 4,6-diamidino-2-phenylindole. Fluorescence intensity was visualized using a microscope equipped with a digital camera (Nikon Instruments, Melville, NY). Quantitative evaluation of sections stained with antibodies to α-SMA, collagen I, and fibronectin was performed using NIS-Elements Br 3.0 software. The fluorescence positive area was calculated as a percentage of the total area (5, 38, 39, 42).
Quantitative real-time RT-PCR.
Total RNA was extracted from kidney tissues with TRIzol reagent (Invitrogen). Aliquots of total RNA were reverse transcribed. Real-time PCR was performed using IQ SYBR green supermix reagents (Bio-Rad, Hercules, CA) with a Bio-Rad real-time PCR machine, according to the manufacturer's instructions. The specificity of real-time PCR was confirmed via melting-curve analysis. The comparative Ct method (ΔΔCt) was used to quantify gene expression, and the relative quantification was calculated as 2−ΔΔCt. The expression levels of CXCL16 were normalized to GAPDH level in each sample (5, 38). The primer sequences were as follows: CXCL16, forward 5′-ACCCTTGTCTCTTGCGTTCTTCCT-3′ and reverse 5′-ATGTGATCCAAAGTACCCTGCGGT-3′; GAPDH, forward 5′-CCAATGTGTCCGTCGCGTGGATCT-3′ and reverse 5′-GTTGAAGTCGCAGGAGACAACC-3′.
Western blot analysis.
Protein was extracted using the RIPA buffer containing a cocktail of proteinase inhibitors (Thermo Fisher Scientific, Rockford, IL) and quantified with Bio-Rad protein assay. Equal amounts of protein were separated on SDS-polyacrylamide gels in a tris/glycine buffer system, transferred onto nitrocellulose membranes, and blotted according to standard procedures with primary antibodies (collagen I, fibronectin, and α-SMA), followed by appropriate secondary antibodies, as described (42). Membranes were reblotted with anti-GAPDH antibody (Millipore, Billerica, CA). The specific bands of target proteins were analyzed using an Odyssey IR scanner (LI-COR Bioscience, Lincoln, NE), and band intensities were quantified using National Institutes of Health Image/J.
Statistical analysis.
All data are expressed as means ± SE. Two group comparisons were performed by Student's t-test. Multiple group comparisons were performed by ANOVA, followed by the Bonferroni procedure for comparison of means. A P value < 0.05 was considered statistically significant.
RESULTS
RAS induces CXCL16 expression.
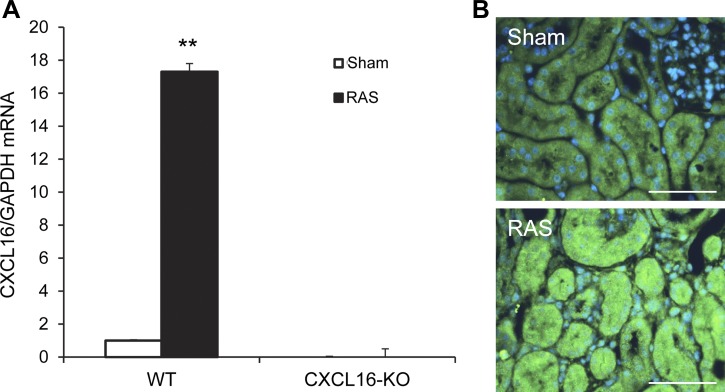
To determine whether CXCL16 was induced in the kidney following RAS, quantitative real-time RT-PCR was performed. The results showed that mRNA levels of CXCL16 were upregulated in the kidneys with RAS compared with those of control kidneys (Fig. 1A). To identify the cell type responsible for CXCL16 production in the kidney, kidney sections were stained with an anti-CXCL16 antibody. The results revealed that CXCL16 protein was mainly detected in tubular epithelial cells of kidneys with RAS (Fig. 1B).
Fig. 1.
CXCL16 is induced in the kidney with RAS. A: RAS induces CXCL16 mRNA in the kidneys. Values are means ± SE; n = 6 per group. **P < 0.01 vs. sham controls. B: representative photomicrographs of kidney sections stained for CXCL16 (green) and counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 50 μm.
CXCL16 does not regulate blood pressure.
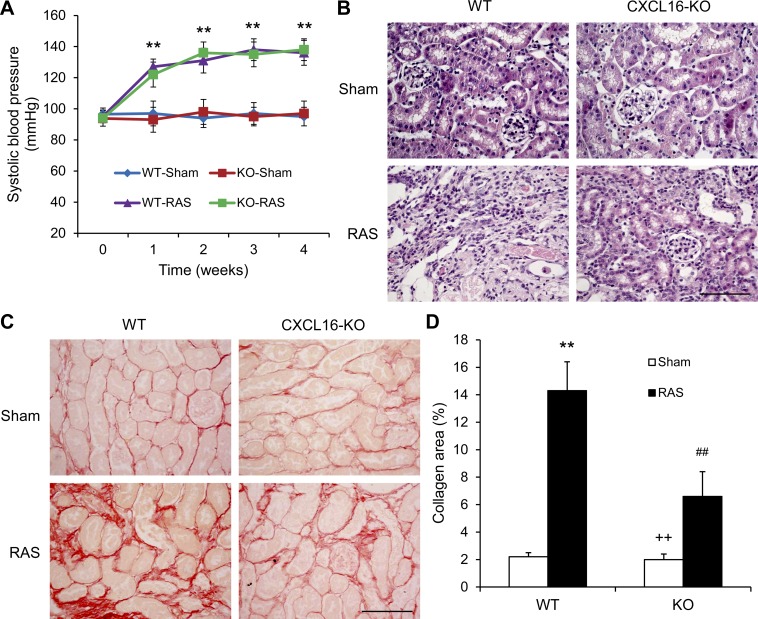
To determine the functional significance of CXCL16 in the pathogenesis of renovascular disease, WT and CXCL16 KO mice were subjected to sham or RAS surgical operation. There were no significant differences in SBP among the four groups at baseline. RAS led to an increase in SBP in both WT and CXCL16 KO mice that was comparable between the two treatment groups (Fig. 2A).
Fig. 2.
CXCL16 deficiency does not affect blood pressure, but suppresses renal injury and fibrosis. A: CXCL16 deficiency has no effect on RAS-induced elevation of blood pressure. Values are means ± SE; n = 6 per group. **P < 0.01 between RAS groups and sham control groups. B: representative photomicrographs of hematoxylin- and eosin-stained sections showing RAS-induced kidney damage in WT and CXCL16 KO mice. Scale bar, 50 μm. C: representative photomicrographs of kidney sections from WT and CXCL16 KO mice 4 wk after sham or RAS stained with Sirius red for assessment of total collagen deposition. Scale bar, 50 μm. D: quantitative analysis of interstitial collagen content in the kidneys of WT and CXCL16 KO mice. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT controls. ++P < 0.01 vs. KO RAS. ##P < 0.01 vs. WT RAS.
CXCL16 deficiency reduces kidney injury and fibrosis.
To assess the role of CXCL16 in RAS-induced hypertensive kidney damage, kidney sections were stained with hematoxylin and eosin. The sham-operated groups had minimal kidney damage. WT mice with RAS exhibited a remarkable renal injury as reflected by tubular atrophy, thickening of basement membrane, and interstitial inflammatory infiltrates, which was substantially reduced in CXCL16 KO mice (Fig. 2B). Sirius red staining showed that WT mice developed significant collagen deposiftion in the stenotic kidneys compared with those of sham-operated WT mice (Fig. 2, C and D). These fibrotic responses were significantly reduced in the stenotic kidneys of CXCL16 KO mice (Fig. 2, C and D).
CXCL16 deficiency attenuates ECM protein expression.
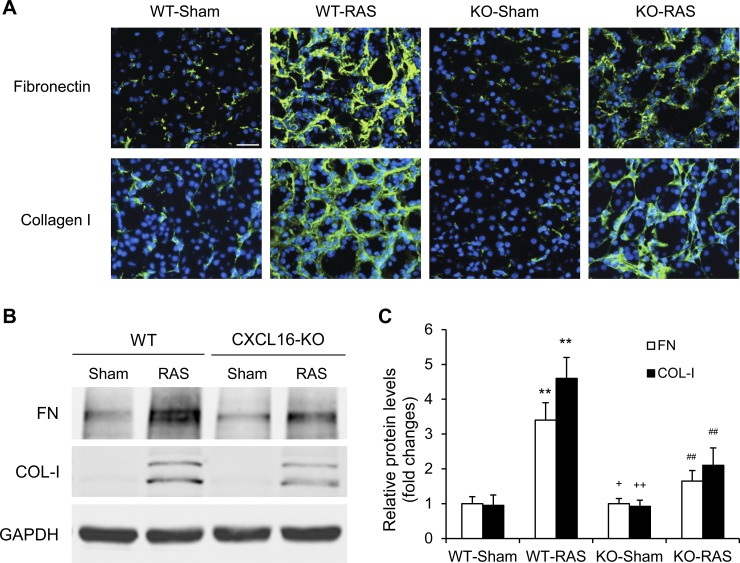
We next evaluated the effect of CXCL16 deficiency on the expression and accumulation of collagen I and fibronectin, two major ECM proteins. Immunofluorescence and Western blot analysis demonstrated that CXCL16 deficiency attenuated the upregulation of collagen I and fibronectin in the stenotic kidneys (Fig. 3). These data indicate that CXCL16 deficiency inhibits RAS-induced ECM protein expression.
Fig. 3.
CXCL16 deficiency reduces fibronectin and collagen I expression. A: representative photomicrographs of immunofluorescence staining for fibronectin and collagen in the kidneys of WT and CXCL16 KO mice 4 wk after sham or RAS. Scale bar, 25 μm. B: representative Western blots show the protein levels of fibronectin and collagen I in the kidneys of WT and CXCL16 KO mice 4 wk after sham or RAS. C: quantitative analysis of fibronectin and collagen I protein expression in the kidneys of WT and CXCL16 KO mice. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT controls. ++P < 0.01 vs. KO RAS. +P < 0.05 vs. KO RAS. ##P < 0.01 vs. WT RAS.
CXCL16 deficiency suppresses myeloid fibroblast accumulation.
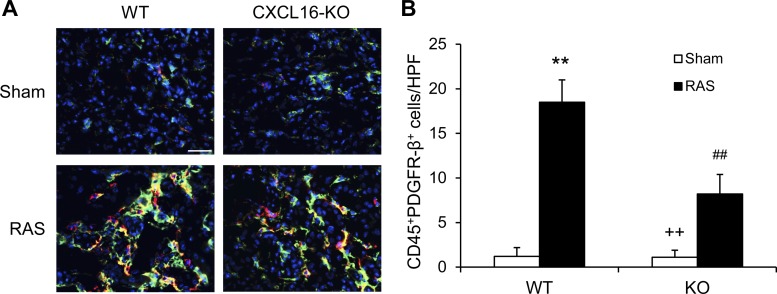
Recent studies have shown that myeloid fibroblasts contribute significantly to the development of renal fibrosis (3, 5, 11, 17, 18, 31, 38, 39, 42, 43). We then examined the role of CXCL16 in myeloid fibroblast accumulation. Kidney sections were stained for CD45 and PDGFR-β and examined with a fluorescence microscope. The results showed that the number of bone marrow-derived fibroblasts dual-positive for CD45 and PDGFR-β was significantly reduced in the stenotic kidneys of CXCL16 KO mice with RAS compared with WT mice (Fig. 4). These data indicate that CXCL16 plays a critical role in recruiting bone marrow-derived fibroblasts into the kidneys with RAS.
Fig. 4.
CXCL16 deficiency suppresses bone marrow-derived fibroblast accumulation. A: representative photomicrographs of kidney sections from WT and CXCL16 KO mice 2 wk after sham or RAS stained for CD45 (red), procollagen I (green), and DAPI (blue). Scale bar, 25 μm. B: quantitative analysis of CD45+ and procollagen I+ fibroblasts in kidneys of WT and CXCL16 KO mice 2 wk after sham or RAS. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT controls. ++P < 0.01 vs. KO RAS. ##P < 0.01 vs. WT RAS.
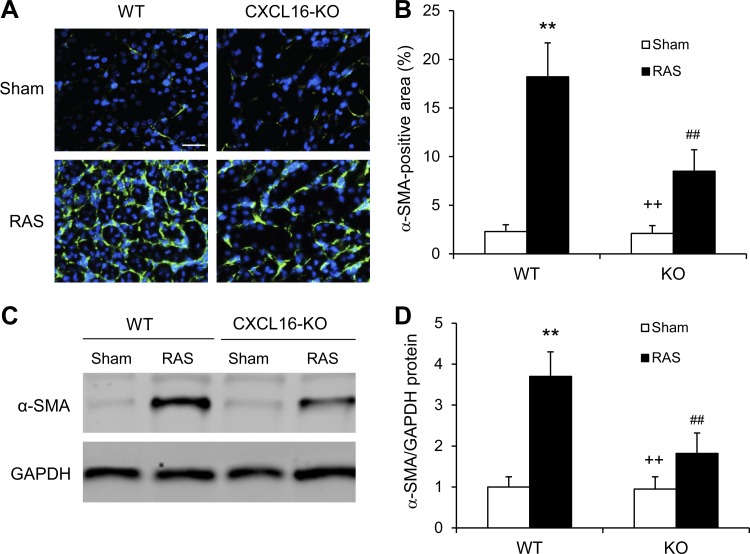
CXCL16 deficiency inhibits myofibroblasts formation.
To determine whether CXCL16 deficiency influences myofibroblast population, kidney sections were stained for α-SMA, a myofibroblast marker, and examined with a fluorescence microscope. CXCL16 KO mice with RAS exhibited a significant reduction in the number of α-SMA+ myofibroblasts in the kidneys compared with WT mice (Fig. 5, A and B). Consistent with these findings, Western blot analysis showed that CXCL16 deficiency significantly reduced the protein expression levels of α-SMA in the stenotic kidneys compared with WT mice (Fig. 5, C and D). These results indicate that CXCL16 deficiency suppresses myofibroblast transformation in the kidney.
Fig. 5.
CXCL16 deficiency inhibits myofibroblast formation in the kidney. A: representative photomicrographs of kidney sections stained for α-SMA. Scale bar, 25 μm. B: quantitative analysis of α-SMA positive area in kidneys of WT and CXCL16 KO mice. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT controls. ++P < 0.01 vs. KO RAS. ##P < 0.01 vs. WT RAS. C: representative Western blots show the levels of α-SMA protein expression in the kidneys of WT and CXCL16 KO mice 4 wk after sham or RAS. D: quantitative analysis of α-SMA protein expression in the kidneys of WT and CXCL16 KO mice. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT controls. ++P < 0.01 vs. KO RAS. ##P < 0.01 vs. WT RAS.
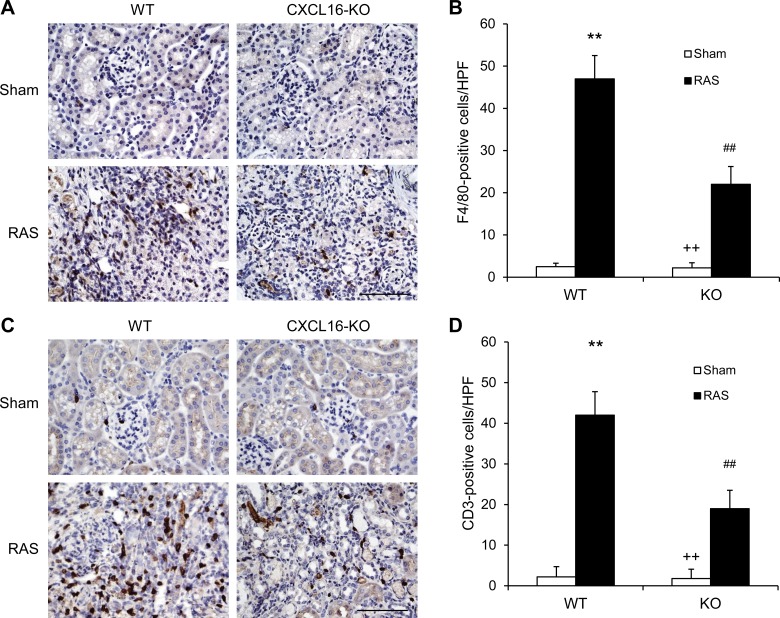
CXCL16 deficiency suppresses macrophage and T-cell infiltration.
To examine if CXCL16 is involved in the regulation of inflammatory cell infiltration into the kidney, kidney sections were stained for F4/80 and CD3. Significant infiltration of macrophages and T cells was observed in the kidneys of WT mice with RAS compared with sham-operated control group (Fig. 6). In comparison, CXCL16 deficiency significantly inhibited macrophage and T-cell infiltration into the stenotic kidneys (Fig. 6). These results indicate that CXCL16 plays a pivotal role in recruiting inflammatory cell into the kidney in renovascular disease.
Fig. 6.
CXCL16 deficiency reduces macrophage and T-cell infiltration. A: representative photomicrographs of kidney sections stained for F4/80 (a macrophage marker; brown) and counterstained with hematoxylin (blue) in WT and CXCL16 KO mice 4 wk after sham or RAS. Scale bar, 50 μm. B: quantitative analysis of F4/80+ macrophages in the kidneys of WT and CXCL16 KO mice. Values are means ± SE; n = 6 in each group. **P < 0.01 vs. WT controls. ##P < 0.01 vs. WT RAS. ++P < 0.01 vs. KO RAS. C: representative photomicrographs of kidney sections stained for CD3 (a T-lymphocyte marker; brown) and counterstained with hematoxylin (blue) in WT and CXCL16 KO mice 4 wk after sham or RAS. Scale bar, 50 μm. D: quantitative analysis of CD3+ T cells in the kidneys of WT and CXCL16 KO mice. Values are means ± SE; n = 6 in each group. **P < 0.01 vs. WT controls. ##P < 0.01 vs. WT RAS. ++P < 0.01 vs. KO RAS.
DISCUSSION
In this study, we demonstrate that RAS induces CXCL16 gene expression in kidney, and genetic deletion of CXCL16 led to renal protection in an experimental model of chronic progressive kidney disease induced by RAS. In the RAS-induced hypertension model, genetic deletion of CXCL16 reduces renal injury and fibrosis. It is well known that the development of hypertension is dependent on activation of renin-angiotensin system in RAS. In the present study, we show that blood pressure is similarly elevated between WT and CXCL16 KO mice with RAS. These results suggest that the effect of CXCL16 deficiency on renal protection is independent of renin-angiotensin system activation.
CXCL16 is a chemokine that belongs to the CXC chemokine subfamily (21). There are two forms of CXCL16. The transmembrane form of CXCL16 functions as an adhesion molecule for CXCR6-expressing (the only known receptor for CXCL16) cells. The soluble form of CXCL16 resulting from cleavage by ADAM10 at the cell surface functions as a chemoattractant to promote migration of CXCR6-expressing cells (5, 16, 32, 39, 40). CXCL16 has been reported to be expressed in the kidney (5, 10, 26, 38). Our laboratory has recently shown that CXCL16 plays an important role in the pathogenesis of kidney injury in angiotensin II-induced hypertension and deoxycorticosterone acetate-salt hypertension (19, 38). However, its role in the pathogenesis of renovascular disease is not known. In the present study, we demonstrate that genetic disruption of CXCL16 protects the kidney from kidney injury in RAS. These data indicate that CXCL16 plays an important role in the pathogenesis of renovascular disease.
Renal interstitial fibrosis is a hallmark of CKD, and the degree of interstitial fibrosis strongly predicts the prognosis of CKD (23). Renal interstitial fibrosis is characterized by extensive fibroblast activation and excessive production and deposition of ECM, which leads to the destruction of renal parenchyma and progressive loss of kidney function. Because activated fibroblasts are the principal cells responsible for ECM production in the fibrotic kidney, their activation is regarded as a key event in the pathogenesis of renal fibrosis (24, 33). They are traditionally thought to arise from resident renal fibroblasts (27–29). Recent evidence suggests they may originate from bone marrow-derived cells (3, 5, 11, 13, 18, 31, 37–40, 42). Bone marrow-derived fibroblast precursors, termed fibrocytes, are derived from circulating mononuclear cells (4, 7, 8, 22, 30). These cells express hematopoietic markers, such as CD45 and CD11b, and mesenchymal markers, such as collagen I, vimentin, and PDGFR-β. Our laboratory and others have shown that these cells migrate into the kidney in response to renal injury and contribute significantly to the development of renal fibrosis (5, 25, 31, 37–40, 42). In the present study, we have showed that CXCL16 deficiency inhibits bone marrow-derived fibroblast accumulation and myofibroblast formation in the kidney and the development of renal fibrosis in an experimental model of RAS. These data indicate that CXCL16 signaling plays a key role in the recruitment of bone marrow-derived fibroblasts into the kidney and the development of renal fibrosis in renovascular disease.
Recent studies have shown that macrophages and T cells play an important role in the development of hypertensive kidney disease, including renovascular disease (6, 20, 35). Chemokines interacting with their receptors mediate the infiltration of inflammatory and immune cells into the kidney (37, 39, 45). In the present study, our results demonstrate that macrophages and T-cell infiltration into the kidney is significantly reduced in CXCL16 KO mice with RAS. These data indicate that CXCL16 is involved in recruiting macrophages and T cells into the kidney in RAS.
In summary, our study identifies CXCL16 as an important chemokine that regulates renal injury and fibrosis in RAS. In response to RAS, CXCL16 recruits macrophages, T cells, and myeloid fibroblasts into the kidney, leading to renal injury and fibrosis. These results suggest that inhibition of CXCL16 could constitute a novel therapeutic strategy for renovascular disease.
GRANTS
This work was supported by National Institutes of Health grants (K08HL92958, R01DK95835), the US Department of Veterans Affairs grant (I01BX02650), and an American Heart Association grant (11BGIA7840054) to Y. Wang, and National Natural Science Foundation of China grant (81373615) to L. He.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.M., L.H., and Y.W. conception and design of research; Z.M. and X.J. performed experiments; Z.M. and X.J. analyzed data; Z.M., X.J., L.H., and Y.W. interpreted results of experiments; Z.M., X.J., and Y.W. prepared figures; Z.M. and Y.W. drafted manuscript; Z.M., X.J., L.H., and Y.W. approved final version of manuscript; L.H. and Y.W. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. William E. Mitch for helpful discussion and Dr. Shuhua Han at Baylor College of Medicine for providing CXCL16 knockout mice.
REFERENCES
- 1.ASTRAL Investigators, Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization vs. medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Broekema M, Harmsen MC, van Luyn MJ, Koerts JA, Petersen AH, van Kooten TG, van Goor H, Navis G, Popa ER. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol 18: 165–175, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1: 71–81, 1994. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Lin SC, Chen J, He L, Dong F, Xu J, Han S, Du J, Entman ML, Wang Y. CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J Am Soc Nephrol 22: 1876–1886, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 297: F1055–F1068, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 94: 6307–6312, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesney J, Bucala R. Peripheral blood fibrocytes: novel fibroblast-like cells that present antigen and mediate tissue repair. Biochem Soc Trans 25: 520–524, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol 42: 469–499, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Garcia GE, Truong LD, Li P, Zhang P, Johnson RJ, Wilson CB, Feng L. Inhibition of CXCL16 attenuates inflammatory and progressive phases of anti-glomerular basement membrane antibody-associated glomerulonephritis. Am J Pathol 170: 1485–1496, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm PC, Nickerson P, Jeffery J, Savani RC, Gough J, McKenna RM, Stern E, Rush DN. Neointimal and tubulointerstitial infiltration by recipient mesenchymal cells in chronic renal-allograft rejection. N Engl J Med 345: 93–97, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 36: 443–451, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin X, Chen J, Hu Z, Chan L, Wang Y. Genetic deficiency of adiponectin protects against acute kidney injury. Kidney Int 83: 604–614, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 68: 293–301, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, Greenberg HB, Butcher EC. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest 107: 595–601, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Deane JA, Campanale NV, Bertram JF, Ricardo SD. The contribution of bone marrow-derived cells to the development of renal interstitial fibrosis. Stem Cells 25: 697–706, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Liang H, Ma Z, Peng H, He L, Hu Z, Wang Y. CXCL16 deficiency attenuates renal injury and fibrosis in salt-sensitive hypertension. Sci Rep 6: 28715, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luft FC, Dechend R, Muller DN. Immune mechanisms in angiotensin II-induced target-organ damage. Ann Med 44, Suppl 1: S49–S54, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol 1: 298–304, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci 60: 1342–1350, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nath KA. The tubulointerstitium in progressive renal disease. Kidney Int 54: 992–994, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Neilson EG. Mechanisms of disease: fibroblasts–a new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Gobel N, Talke Y, Schweda F, Mack M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A 106: 17892–17897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol 293: F575–F585, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol 130: 141–155, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277: C1–C9, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Qi W, Chen X, Poronnik P, Pollock CA. The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol 38: 1–5, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 36: 598–606, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A 103: 14098–14103, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, Butcher EC. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol 174: 277–283, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Strutz F, Muller GA. Renal fibrosis and the origin of the renal fibroblast. Nephrol Dial Transplant 21: 3368–3370, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens 23: 1159–1169, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Textor SC, Lerman LO. Paradigm shifts in atherosclerotic renovascular disease: where are we now? J Am Soc Nephrol 26: 2074–2080, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol 302: F1455–F1464, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Y, Entman ML, Wang Y. CCR2 regulates the uptake of bone marrow-derived fibroblasts in renal fibrosis. PLoS One 8: e77493, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Y, Entman ML, Wang Y. Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension 62: 1129–1137, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Y, Jin X, Yan J, Entman ML, Wang Y. CXCR6 plays a critical role in angiotensin ii-induced renal injury and fibrosis. Arterioscler Thromb Vasc Biol 34: 1422–1428, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y, Yan J, Jin X, Entman ML, Wang Y. The chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors in renal fibrosis. Kidney Int 86: 327–337, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, Wang Y. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol 301: H538–H547, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Chen J, Yan J, Zhang L, Chen G, He L, Wang Y. Effect of interleukin 6 deficiency on renal interstitial fibrosis. PLoS One 7: e52415, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Lin SC, Chen G, He L, Hu Z, Chan L, Trial J, Entman ML, Wang Y. Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J Am Soc Nephrol 24: 1644–1659, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Ran L, Garcia GE, Wang XH, Han S, Du J, Mitch WE. Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am J Pathol 175: 2518–2527, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, Chen K, Lei H, Sun Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26: 121–132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]