Abstract
In the gastrointestinal (GI) tract, abnormalities in secretion, absorption, motility, and sensation have been implicated in functional gastrointestinal disorders (FGIDs). Ion channels play important roles in all these GI functions. Disruptions of ion channels' ability to conduct ions can lead to diseases called ion channelopathies. Channelopathies can result from changes in ion channel biophysical function or expression due to mutations, posttranslational modification, and accessory protein malfunction. Channelopathies are strongly established in the fields of cardiology and neurology, but ion channelopathies are only beginning to be recognized in gastroenterology. In this review, we describe the state of the emerging field of GI ion channelopathies. Several recent discoveries show that channelopathies result in alterations in GI motility, secretion, and sensation. In the epithelium, mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) or CFTR-associating proteins result in channelopathies with constipation or diarrhea as phenotypes. In the muscle, mutations in the SCN5A-encoded voltage-gated sodium channel NaV1.5 are associated with irritable bowel syndrome. In the sensory nerves, channelopathies of voltage-gated sodium channels NaV1.7 and NaV1.9 (encoded by SCN9A, SCN11A, respectively) manifest by either GI hyper- or hyposensation. Recent advances in structural biology and ion channel biophysics, coupled with personalized medicine, have fueled rapid discoveries of novel channelopathies and direct drug targeting of specific channelopathies. In summary, the emerging field of GI ion channelopathies has significant implications for functional GI disease stratification, diagnosis, and treatment.
Keywords: ion channel, mutation, channelopathy, gastrointestinal
ion channels, encoded by more than 400 genes, are transmembrane proteins that coordinate passage of ions across a semipermeable lipid bilayer. Given the importance of ionic gradients for most cellular functions, ion channels are expressed in all cells. Ion channels are also critical for many cells' specialized functions. In the gastrointestinal (GI) epithelium, for example, chloride movement through ion channels is vital for normal electrolyte and fluid secretion and absorption. In enteroendocrine cells, which make up the body's largest endocrine organ, ion channels are vital for the depolarization and calcium influx required for hormone secretion. In neurons, ion channels generate propagating action potentials, indispensable for normal motility and sensation. In cells of the GI contractile apparatus, including interstitial cells of Cajal (pacemakers), fibroblast-like cells, and smooth muscle cells, ion channels produce and organize the cyclical or stimulated electrical discharges that lead to contractions.
Channelopathies are diseases that develop due to ion channel defects, caused by either genetic (congenital) or acquired factors, such as autoimmune disorders or drugs (24). Most often, channelopathies are caused by defects in channel function or expression. Channelopathies can result from a wide range of changes in the genome or in transcription, translation, and posttranslational modifications, such as regulation by noncoding RNAs, phosphorylation, ubiquitination, glycosylation, and palmitoylation (31). Furthermore, ion channel pore-forming subunits commonly reside within large multiprotein complexes, and their function is modified by associating proteins (10). Ion channel function can be modulated by multiple signaling molecules such as cyclic nucleotides and lipids. Abnormalities in the associating proteins and signaling molecules can also lead to channelopathies.
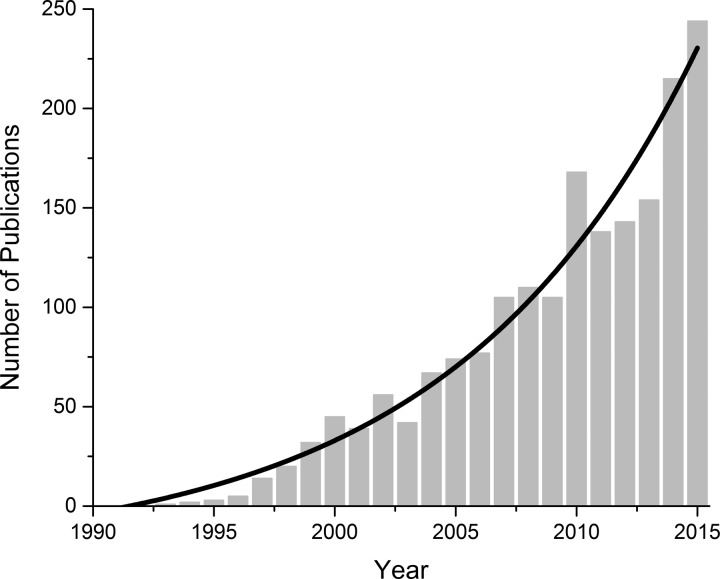
Ion channel gene mutations that impair channel function are the most commonly recognized cause of channelopathies (26). The number of recognized ion channelopathies has grown exponentially with recent advances in human genome sequencing and “big data” analysis capabilities (Fig. 1). Importantly, functional data are now being paired with the growing number of ion channel structures to further refine the understanding of channel functions and drug-protein interactions that allow for intelligent drug design (36). This has allowed new therapeutic approaches to focus on targeting mutation-specific defects (2). As personalized medicine comes into sharper focus, we expect that the number of ion channelopathies and channelopathy-specific therapies will markedly increase.
Fig. 1.
NBCI publications on “channelopathies” since the first publication in 1991 (Accessed on June 8th, 2016). Solid black line is an exponential fit of the data.
Ion channelopathies are well established in cardiology and neurology. In both, contributions of specific ion channels to electrical and mechanical functions are relatively well understood. Functional abnormalities of these ion channels are assayed by electrophysiology, and channelopathies are then attributed to specific ion channels. Cardiac arrhythmias are a flagship example, having been subdivided into groups based not only on specific channels involved, but also by the type of dysfunction (e.g., loss- or gain-of-function) (27). Interestingly, recent studies show that even some forms of cardiac mechanical dysfunction, like dilated cardiomyopathy, may be due to channelopathies. In neurology, similar advances have been made in the fields of epilepsy and pain-related neuropathies (40). In both cardiology and neurology, ion channelopathies have uncovered physiology, pathophysiology, and, in some cases, personalized therapies (3).
Ion Channelopathies in Functional GI Diseases
Functional GI diseases (FGIDs) are complex multisystem pathologies that involve abnormalities in GI secretion, motility, sensation, and central perception. One particular difficulty in diagnosing FGIDs is that they do not have a signature histological phenotype, raising the possibility that the pathophysiology may be at the molecular level. In support of this hypothesis, studies show that FGIDs have a heritable, genetic component (15, 42). This suggests that alterations in protein sequences (mutations and polymorphisms) can lead to abnormalities in molecular function and contribute to FGID pathophysiology (15). Ion channelopathies may contribute to FGIDs since ion channels play major roles in all phases of GI function, including secretion, absorption, motility, and sensation (5).
GI epithelium ion channelopathies.
In the gut mucosa, ion channels are important in the physiological processes of secretion and absorption. Epithelial cells use ion channels to move ions that drive fluid secretion, and this epithelial secretion is tightly controlled by the many ion channels types on the submucosal nerves and enterochromaffin cells (47). Secretion abnormalities are strongly implicated in FGIDs (50).
The recent drugs for functional constipation and constipation-predominant inflammatory bowel syndrome (IBS-C) target ion transport pathways to increase epithelial secretion (39). Lubiprostone activates a chloride ion channel, CLCN2-encoded chloride channel protein 2 (ClC2), by activation of a prostaglandin E1-derived fatty acid. Another drug, linaclotide, stimulates cystic fibrosis transmembrane conductance regulator (CFTR) via an increase in cGMP secondary to activation of guanylate 2C (encoded by GUCY2C) (39). A recent report described a multigeneration Norwegian family in which 32 members suffered from severe secretory diarrhea. Using linkage analysis, the authors first identified a candidate region on chromosome 12, then found a GUCY2C missense mutation (c.2519G→T) in all affected members. When the mutated guanylate cyclase 2C receptor was functionally profiled, the authors discovered a gain-of-function phenotype with a significantly increased production of cGMP. Secretory diarrhea in the individuals with this mutation is due presumably to an increase in cGMP and CFTR activation that leads to hypersecretion (17). While this example is not strictly a channelopathy, given that the mutation is in a protein upstream of ion channel activation, it demonstrates how mutations in ion channel-associating or -modulating proteins affect ion channel function. These studies raise an additional possibility that CFTR mutations and polymorphisms may directly contribute to secretory disturbances in FGIDs (25).
It is now well established that the serotonergic system is involved in functional GI diseases such as irritable bowel syndrome (IBS). Serotonin released from the enterochromaffin cells in response to chemicals (nutrients and toxins) and mechanical forces (49) is an important regulator of GI secretion, motility, and sensation (30). A testament to the importance of the serotonergic system is the fact that some of the most effective drugs for treating IBS have targeted serotonin receptors 5HT3R and 5HT4R. In a recent study in female patients with diarrhea-predominant IBS (IBS-D), a noncoding polymorphism in an ionotropic 5-HT receptor subunit HTR3E was found to alter mRNA binding to miR-510, resulting in an elevated expression (23). Both of the molecules (miR-510 and 5HT3RE) were localized in the myenteric plexus and epithelium, presumably in enterochromaffin cells.
Smooth muscle ion channelopathies.
Normal function of the GI smooth muscle is critical for GI motility. In electromechanical tissues that require coordinated contractions (smooth, cardiac, and striated muscle), electrical depolarizations underpin normal electromechanical coupling. In these tissues, the coordination of electrical activity is so well tuned that even small perturbations can lead to functional abnormalities. This is most notable in the GI tract, where efficient motor function means coordination of motility over many feet of intestine.
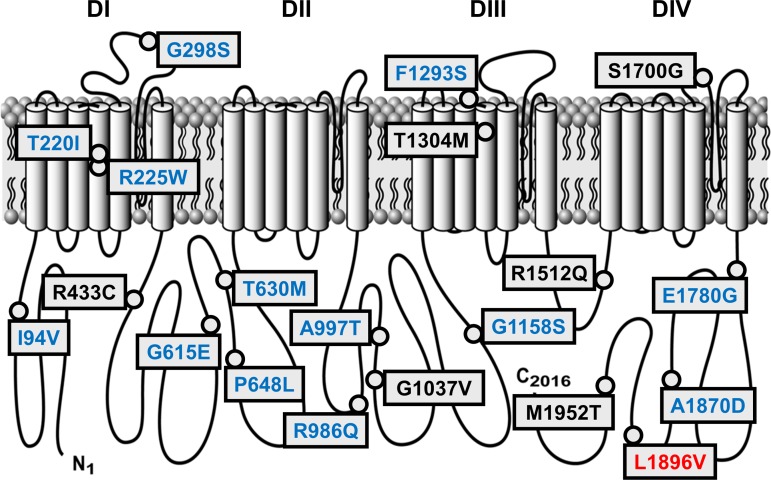
The voltage-gated sodium-selective ion channel NaV1.5, whose pore-forming α subunit is encoded by SCN5A, is important for cardiac function and is also expressed in the human GI tract (20, 35, 46). In smooth muscle cells (20) and interstitial cells of Cajal (46), NaV1.5 contributes to electrical (46) and mechanical (33) smooth muscle functions. SCN5A mutations are well known to cause the cardiac conduction disorders long QT and Brugada. Patients with SCN5A channelopathies and known cardiac arrhythmias had an increased prevalence of functional GI disease. Furthermore, these SCN5A mutations were associated with a significant increase in GI symptoms associated with IBS and functional dyspepsia (29). Importantly, this was not the case for patients with cardiac arrhythmias due to mutations in a different ion channel gene, KCNH2, which is also expressed in the GI tract (29). First, in a pilot study (43) and then in a large cohort (7), it was found that that 2–3% of IBS patients have SCN5A channelopathies (Fig. 2). Recent work also identified SCN5A channelopathies in a small cohort of Rome III-defined functional dyspepsia patients, but the finding did not reach statistical significance owing to a small sample size (22).
Fig. 2.
IBS-associated SCN5A mutations. Black, no functional abnormalities (6/20, 30%); blue, loss-of-function (13/20, 65%); red, gain-of-function (1/20, 5%).
When the IBS-associated SCN5A mutations were heterologously expressed and examined by whole cell voltage-clamp electrophysiology, ∼70% were functionally abnormal. The majority of these were loss-of-function and associated with IBS-C; in one patient with IBS-C, symptoms were improved by restoring NaV1.5 function (7). Interestingly, recent data show that, in addition to the channelopathies causing changes to voltage-dependent behavior, other NaV1.5 functional modes, such as mechanosensitivity, are also affected (43, 45). Several SCN5A polymorphisms were also found to be more common in IBS patients than in controls. Elsewhere, ion channel polymorphisms often contribute to phenotypes (41), but it is unknown whether IBS-associated SCN5A polymorphisms contribute to GI functional abnormalities. The finding of SCN5A mutations in IBS patients is the first set of ion channelopathies in functional GI disease. Future work will need to clarify the impact of SCN5A mutations on tissue physiology and associate patients' symptoms and GI functional abnormalities with specific NaV1.5 gating disruptions (6).
SCN5A-encoded NaV1.5 is a part of a large multimolecular complex, and its accessory proteins alter NaV1.5 function (1). Intriguingly, a patient with GI pseudobstruction had a mutation in telethonin, encoded by the gene TCAP (32). Telethonin is expressed both in the heart and gut, and it is an SCN5A-associating protein, since it colocalized and coprecipitated with NaV1.5 (32). Expression of this telethonin mutation (R76C) significantly altered the steady-state activation kinetics of NaV1.5 and doubled the size of the window current (32). Computational modeling showed that R76C is predicted to decrease slow wave duration and depolarize the resting potential in ICC and SMC, respectively. These changes would conspire to alter smooth muscle contractility (37).
In summary, the evidence suggests that the SCN5A-encoded channel NaV1.5 is involved in GI motility, and NaV1.5 channelopathies make up a cohort of functional GI diseases such as IBS and pseudobstruction (reviewed in Ref. 48).
Sensory nerve ion channelopathies.
Abdominal pain is a prominent feature of FGIDs. Ion channels are important for the generation and propagation of signals related to visceral sensation (8). As outlined above, channelopathies are well established in sensory neurology, but relatively little is known about visceral sensation channelopathies (40). Point mutations in the “pain” voltage-gated sodium channels — NaV1.7 (SCN9A), NaV1.8 (SCN10A), and NaV1.9 (SCN11A) — lead to pain sensation channelopathies. Several of these are relevant for visceral sensation in the GI tract.
Familial rectal pain disorder, recently renamed paroxysmal extreme pain disorder, is an autosomal dominant disorder (14). Starting early in childhood, these patients have defecation- and anal probing-associated attacks of severe rectal pain that spread to the lower half of the body and typically associate with flushing (14). Symptoms may be induced by ingestion of cold drinks and acidic or spicy foods. The familial rectal pain disorder is linked to several mutations in SCN9A-encoded NaV1.7, which is specifically expressed in dorsal root ganglion (peripheral) nociceptive neurons (9, 16). Functional analysis of NaV1.7 mutations involved in this disorder revealed a gain-of-function phenotype. The channels had impaired inactivation that resulted in persistent current (16), and sometimes had accelerated recovery from inactivation, resulting in resurgent currents (12). These changes were predicted to extend action potentials and allow for repetitive firing in response to stretch and cold (12, 16). Interestingly, some SCN9A polymorphisms that have differences in voltage-dependent slow inactivation are associated with setting the pain threshold in the general population, suggesting that a range of pain phenotypes may be involved in other painful conditions such as functional GI diseases (41).
The voltage-gated sodium channel NaV1.9 (encoded by SCN11A) is found in nociceptive small-diameter neurons of the peripheral nervous system (13). Compared with other sodium channels, NaV1.9 has unique slow kinetics that allow it produce a persistent current. While its slow activation kinetics preclude NaV1.9 from contributing significantly toward action potentials, it is important for regulating excitability by setting the firing threshold (38). Indeed, a NaV1.9 knockout significantly attenuated the colonic afferent fiber response to inflammatory mediators in rodents (18). SCN11A mutations that result in NaV1.9 channelopathies are implicated in pain, including visceral pain disorders (reviewed in Ref. 19). Two recently discovered mutations in NaV1.9 (I381T, L158P) resulted in gain-of-function phenotypes due to hyperpolarizing shifts in the half-points of voltage-dependent activation, leading to resting membrane potential depolarization and a hyperexcitable cell membrane. Interestingly, the patients with these missense mutations developed symptoms late in life (>50 yr old). These symptoms included pain and numbness in lower limbs as well as several autonomic system symptoms, including diarrhea (21). In another study, a different missense mutation (L811P) was found in two patients, one with congenital insensitivity to pain and the other with early-onset severe sensory loss. Both patients had a significant GI dysfunction that required temporary parenteral nutrition. One of these patients had alternating episodes of diarrhea and constipation with reduced peristaltic waves and a grossly enlarged colon but histologically normal small intestine (28). As with mutations I381L and l158P, the I811L mutation resulted in a hyperpolarizing shift in the half-point of activation of NaV1.9 and a depolarization of the resting membrane potential of dorsal root ganglion neurons. However, this mutation also caused hypoexcitability, potentially due to the inactivation of other sodium channels (28), but this hypothesis remains controversial (21). The results of these studies show that NaV1.9 channelopathies are important in visceral sensitivity. Still, further work on novel mutations is required, as phenotypes of mutations vary in functional significance and therefore analysis of novel mutations requires functional analysis to understand the link between channelopathies and pathophysiology.
In summary, the studies of pain-related NaV channelopathies provide rich information about both GI physiology and functional GI disorder pathophysiology. Much remains to be discovered in this field. For example, a recent study in rodents showed for the first time that another voltage-gated sodium channel, Scn1a-encoded Nav1.1, is important for mechanically stimulated visceral pain and that NaV1.1 may be specifically involved in IBS (34).
Therapeutic Targeting of Channelopathies in Functional GI Diseases
Ion channels are attractive drug targets due to their importance for many cell functions and their accessibility at the cell surface. Indeed, about 15% of all drugs target ion channels (4). Ion channels are important for GI function, so not only are they viable functional GI disease drug targets (5), but novel FGID therapies target ion channels (44). The studies of channelopathies expand the knowledge of the ion channel roles in functional GI diseases. Since ion channel mutations often alter ion channel gating or density, there is an exciting possibility that drugs in this era of personalized medicine will allow targeting of the specific functional abnormalities in well-defined patient cohorts. The consequence of this approach is that increased drug specificity may allow us to transcend treatment of symptoms and deliver disease-specific treatments.
Some examples are already appearing. A particular NaV1.5 mutation (p.A997T) in a patient with IBS-C decreased NaV1.5 function by more than 90%. The cardiac medication mexiletine restored channel function in vitro and normalized GI function in vivo in this patient with lifelong IBS-C symptoms (7). In the case of CFTR, the most transformative recent medication is ivacaftor, which was developed to target the specific CFTR p.G551D mutation that is responsible for cystic fibrosis in 4–5% of patients. This particular mutation results in defective channel gating; ivacaftor was specifically designed to facilitate CFTR opening and therefore it increased chloride secretion. Ivacaftor had dramatically positive effects in these cystic fibrosis patients (2). Ivacaftor is opening the door to the possibility that channelopathy-specific treatments are on the horizon.
Conclusions and Future Directions
Ion channels are important for normal GI function, and studies show that ion channel abnormalities are linked to GI pathologies. Most patients with FGIDs likely have complex interactions of a susceptible genetic background with epigenetic and other environmental factors (11). However, within the very large cohort of FGID patients, there are smaller cohorts of patients with highly penetrant monogenetic abnormalities, such as recently demonstrated with SCN5A-encoded voltage-gated sodium channel NaV1.5 involvement in IBS (7), familial rectal pain disorder (16), and cystic fibrosis (25).
Further work is required on many fronts. First, the structure-function relationship at the molecular level requires deep understanding of the ion channel structures and correlation with functional data. Second, on the functional level, our understanding is limited by experimental protocols, which in some cases require novel protocols (e.g., to test mechanosensitivity) and advances in computational and cell biology to understand the consequences of channelopathies for cell function. Third, it is now well understood that the ion channel does not exist in isolation and that associating proteins are critical for channel function. Therefore, future studies require use of primary cell systems, further development of animal models, and evaluation of family genealogy for FGID patients. Fourth, while the advent of high-throughput sequencing has resulted in large databases of whole exomes, FGIDs continue to be difficult to study. Some large studies focus specifically on FGIDs (15). However, given the molecular complexities of FGIDs and their high prevalence in the population (up to 20%), FGID cohorts have to be compared with large cohorts specifically negative for FGID (11). Also, there are important differences between sexes when it comes to channelopathies. For example, with mutations in the same sodium channel, males are almost 10-fold more likely to develop cardiac conduction disorder, while females are severalfold more likely to develop IBS. Unfortunately, the vast majority of the current databases do not take functional GI diseases into account, making large-scale sex-sensitive comparison between genomes of healthy and FGID patients very difficult.
In conclusion, the recent scientific advances have uncovered important roles of ion channels and ion channel mutations (channelopathies) in FGIDs. These discoveries are significant because they shed light on physiology and pathophysiology, promise significant advances in the understanding of FGIDs, and bring the potential of personalized treatments.
GRANTS
This work was supported by NIH R01 to G. Farrugia (DK52766) and by NIH K08 (DK106456) and American Gastroenterological Association Research Scholar Award (AGA RSA) to A. Beyder.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.B. and G.F. conception and design of research; A.B. and G.F. analyzed data; A.B. and G.F. interpreted results of experiments; A.B. prepared figures; A.B. and G.F. drafted manuscript; A.B. and G.F. edited and revised manuscript; A.B. and G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kristy Zodrow for administrative assistance and Peter Strege for assistance with figures and manuscript editing.
REFERENCES
- 1.Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: physiology and pathophysiology. J Mol Cell Cardiol 48: 2–11, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordonez CL, Campbell PW, Ashlock MA, Ramsey BW. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 363: 1991–2003, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft FM. From molecule to malady. Nature 440: 440–447, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE, Stevens EB, Storer RI, Swain NA. Ion channels as therapeutic targets: a drug discovery perspective. J Med Chem 56: 593–624, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Beyder A, Farrugia G. Targeting ion channels for the treatment of gastrointestinal motility disorders. Therap Adv Gastroenterol 5: 5–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyder A, Gibbons SJ, Mazzone AM, Strege PR, Saravanaperumal SA, Sha L, Higgins S, Eisenman ST, Bernard CE, Geurts AM, Kline CF, Mohler PJ, Farrugia G. Expression and function of the Scn5a-encoded voltage-gated sodium channel NaV1.5 in the rat jejunum. Neurogastroenterol Motil 28: 64–73, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, Enders FT, Ek WE, Schmidt PT, Dlugosz A, Lindberg G, Karling P, Ohlsson B, Gazouli M, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Portincasa P, Bellini M, Barbara G, Camilleri M, Locke GR 3rd, Talley NJ, D'Amato M, Ackerman MJ, Farrugia G. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology 146: 1659–1668, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brierley SM. Molecular basis of mechanosensitivity. Auton Neurosci 153: 58–68, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Choi JS, Boralevi F, Brissaud O, Sanchez-Martin J, Te Morsche RH, Dib-Hajj SD, Drenth JP, Waxman SG. Paroxysmal extreme pain disorder: a molecular lesion of peripheral neurons. Nat Rev Neurol 7: 51–55, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Curran J, Mohler PJ. Alternative paradigms for ion channelopathies: disorders of ion channel membrane trafficking and posttranslational modification. Annu Rev Physiol 77: 505–524, 2015. [DOI] [PubMed] [Google Scholar]
- 11.D'Amato M. Genes and functional GI disorders: from casual to causal relationship. Neurogastroenterol Motil 25: 638–649, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Dib-Hajj SD, Estacion M, Jarecki BW, Tyrrell L, Fischer TZ, Lawden M, Cummins TR, Waxman SG. Paroxysmal extreme pain disorder M1627K mutation in human Nav1.7 renders DRG neurons hyperexcitable. Mol Pain 4: 37, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA 95: 8963–8968, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drenth JP, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest 117: 3603–3609, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ek WE, Reznichenko A, Ripke S, Niesler B, Zucchelli M, Rivera NV, Schmidt PT, Pedersen NL, Magnusson P, Talley NJ, Holliday EG, Houghton L, Gazouli M, Karamanolis G, Rappold G, Burwinkel B, Surowy H, Rafter J, Assadi G, Li L, Papadaki E, Gambaccini D, Marchi S, Colucci R, Blandizzi C, Barbaro R, Karling P, Walter S, Ohlsson B, Tornblom H, Bresso F, Andreasson A, Dlugosz A, Simren M, Agreus L, Lindberg G, Boeckxstaens G, Bellini M, Stanghellini V, Barbara G, Daly MJ, Camilleri M, Wouters MM, D'Amato M. Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts. Gut 64: 1774–1782, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM, Rees M. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52: 767–774, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, Havik B, Tonder SL, Levy SE, Brackman D, Boman H, Biswas KH, Apold J, Hovdenak N, Visweswariah SS, Knappskog PM. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med 366: 1586–1595, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Hockley JR, Boundouki G, Cibert-Goton V, McGuire C, Yip PK, Chan C, Tranter M, Wood JN, Nassar MA, Blackshaw LA, Aziz Q, Michael GJ, Baker MD, Winchester WJ, Knowles CH, Bulmer DC. Multiple roles for NaV1.9 in the activation of visceral afferents by noxious inflammatory, mechanical, and human disease-derived stimuli. Pain 155: 1962–1975, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hockley JR, Winchester WJ, Bulmer DC. The voltage-gated sodium channel Na 1.9 in visceral pain. Neurogastroenterol Motil 28: 316–326, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Holm AN, Rich A, Miller SM, Strege P, Ou Y, Gibbons S, Sarr MG, Szurszewski JH, Rae JL, Farrugia G. Sodium current in human jejunal circular smooth muscle cells. Gastroenterology 122: 178–187, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Han C, Estacion M, Vasylyev D, Hoeijmakers JG, Gerrits MM, Tyrrell L, Lauria G, Faber CG, Dib-Hajj SD, Merkies IS, Waxman SG. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 137: 1627–1642, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Jung KT, Park H, Kim JH, Shin DJ, Joung BY, Lee MH, Jang YS. The relationship between gastric myoelectric activity and SCN5A mutation suggesting sodium channelopathy in patients with brugada syndrome and functional dyspepsia — A pilot study. J Neurogastroenterol Motil 18: 58–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapeller J, Houghton LA, Monnikes H, Walstab J, Moller D, Bonisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Buchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet 17: 2967–2977, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Kass RS. The channelopathies: novel insights into molecular and genetic mechanisms of human disease. J Clin Invest 115: 1986–1989, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly T, Buxbaum J. Gastrointestinal manifestations of cystic fibrosis. Dig Dis Sci 60: 1903–1913, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Kim JB. Channelopathies. Korean J Pediatr 57: 1–18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehnart SE, Ackerman MJ, Benson DW Jr, Brugada R, Clancy CE, Donahue JK, George AL Jr, Grant AO, Groft SC, January CT, Lathrop DA, Lederer WJ, Makielski JC, Mohler PJ, Moss A, Nerbonne JM, Olson TM, Przywara DA, Towbin JA, Wang LH, Marks AR. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation 116: 2325–2345, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Leipold E, Liebmann L, Korenke GC, Heinrich T, Giesselmann S, Baets J, Ebbinghaus M, Goral RO, Stodberg T, Hennings JC, Bergmann M, Altmuller J, Thiele H, Wetzel A, Nurnberg P, Timmerman V, De Jonghe P, Blum R, Schaible HG, Weis J, Heinemann SH, Hubner CA, Kurth I. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet 45: 1399–1404, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Locke GR 3rd, Ackerman MJ, Zinsmeister AR, Thapa P, Farrugia G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol 101: 1299–1304, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ordog T, Gibbons SJ, Farrugia G. Altered expression of ANO1 variants in human diabetic gastroparesis. J Biol Chem 286: 13393–13403, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, De Giorgio R, Makielski JC, Stanghellini V, Gibbons SJ, Ackerman MJ, Farrugia G. A mutation in telethonin alters Nav1.5 function. J Biol Chem 283: 16537–16544, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neshatian L, Strege PR, Rhee PL, Kraichely RE, Mazzone AM, Bernard CE, Cima RR, Larson DW, Dozois EJ, Kline CF, Mohler PJ, Beyder A, Farrugia G. Ranolazine inhibits voltage-gated mechanosensitive sodium channels in human colon circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 309: G506–G512, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osteen JD, Herzig V, Gilchrist J, Emrick JJ, Zhang C, Wang X, Castro J, Garcia-Caraballo S, Grundy L, Rychkov GY, Weyer AD, Dekan Z, Undheim EA, Alewood P, Stucky CL, Brierley SM, Basbaum AI, Bosmans F, King GF, Julius D. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 534: 494–499, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou Y, Gibbons SJ, Miller SM, Strege PR, Rich A, Distad MA, Ackerman MJ, Rae JL, Szurszewski JH, Farrugia G. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil 14: 477–486, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Pless SA, Galpin JD, Frankel A, Ahern CA. Molecular basis for class Ib anti-arrhythmic inhibition of cardiac sodium channels. Nat Commun 2: 351, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Poh YC, Beyder A, Strege PR, Farrugia G, Buist ML. Quantification of gastrointestinal sodium channelopathy. J Theor Biol 293C: 41–48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priest BT, Murphy BA, Lindia JA, Diaz C, Abbadie C, Ritter AM, Liberator P, Iyer LM, Kash SF, Kohler MG, Kaczorowski GJ, MacIntyre DE, Martin WJ. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior. Proc Natl Acad Sci USA 102: 9382–9387, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol 13: 295–305, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Raouf R, Quick K, Wood JN. Pain as a channelopathy. J Clin Invest 120: 3745–3752, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, Drenth JP, Dai F, Wheeler J, Sanders F, Wood L, Wu TX, Karppinen J, Nikolajsen L, Mannikko M, Max MB, Kiselycznyk C, Poddar M, Te Morsche RH, Smith S, Gibson D, Kelempisioti A, Maixner W, Gribble FM, Woods CG. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci USA 107: 5148–5153, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology 138: 1276–1285, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito YA, Strege PR, Tester DJ, Locke GR 3rd, Talley NJ, Bernard CE, Rae JL, Makielski JC, Ackerman MJ, Farrugia G. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol 296: G211–G218, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanger GJ, Alpers DH. Development of drugs for gastrointestinal motor disorders: translating science to clinical need. Neurogastroenterol Motil 20: 177–184, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Neshatian L, Strege PR, Beyder A, Bernard CE, Mazzone A, Gibbons SJ, Tester DJ, Will ML, Mayer EA, Chang L, Ackerman MJ, Farrugia G. 677 IBS patients have SCN5A mutations that result in decreased NaV1.5 current mechanosensitivity. Gastroenterology 148: S-130–S-131, 2015. [Google Scholar]
- 46.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 285: G1111–G1121, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Vanner S, Macnaughton WK. Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil 16, Suppl 1: 39–43, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Verstraelen TE, Ter Bekke RM, Volders PG, Masclee AA, Kruimel JW. The role of the SCN5A-encoded channelopathy in irritable bowel syndrome and other gastrointestinal disorders. Neurogastroenterol Motil 37: 906–913, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap PK, Grover M, Oeckler R, Gottlieb PA, Li HJ, Leiter AB, Farrugia G, Beyder A. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol. 2016 Jul 9. doi: 10.1113/JP272718 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut 45, Suppl 2: II6–II16, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]