Abstract
Transactivation of EGF receptor (EGFR) by angiotensin II (Ang II) plays important roles in the initiation and progression of chronic kidney diseases. Studies suggest that heparin-binding EGF-like factor (HB-EGF) may be a critical mediator in this process, but its role in vivo has not been investigated. In the current study, we found that in response to Ang II infusion, kidneys from endothelial HB-EGF deletion mice had significantly reduced EGFR activation compared with controls. Meanwhile, deletion of endothelial HB-EGF expression decreased Ang II infusion related renal injury, as demonstrated by 1) less albuminuria; 2) less glomerulosclerosis; 3) preserved endothelial integrity and decreased podocyte injury, as shown by greater glomerular tuft area and WT1-positive cells, and fewer apoptotic cells measured by cleaved caspase 3 staining; 4) reduced inflammation in the perivascular area and interstitium measured by F4/80 and CD3 immunostaining; and 5) reduced renal fibrosis. In conclusion, our results suggest that shedding of HB-EGF from endothelium plays an important role in Ang II-induced renal injury by linking Ang II-AT1R with EGFR transactivation. Inhibition of HB-EGF shedding could be a potential therapeutic strategy for chronic kidney disease.
Keywords: heparin-binding epidermal growth factor-like factor, angiotensin II, chronic kidney disease
chronic kidney disease (CKD) caused by diabetes and hypertension is the leading cause of end-stage renal disease in the US, and the rate of CKD continues to rise (http://www.usrds.org) (16, 19, 54). Therefore, development of new approaches for prevention and early intervention and treatment is needed. Studies are focusing increasingly not only on the root causes of diabetes- and hypertension-mediated kidney injury but also on associated factors exacerbating these conditions. Elevated renal expression and action of angiotensin II (Ang II), the major bioactive peptide of the renin-angiotensin system, are major factors involved in the initiation and progression of CKD (27, 43). Reducing Ang II production with angiotensin-converting enzyme inhibitors or blocking Ang II action with Ang II type I receptor (AT1R) blockers has become a mainstay of therapy to attenuate progression of CKD (28, 29). However, the underlying mechanisms of how Ang II inhibition exerts its renoprotective effects remain incompletely understood.
Chronic Ang II activation of AT1R produces detrimental effects, including vasoconstriction, inflammation, and fibrosis, and therefore, it plays a critical role in the renal injury in CKD (43). In addition to direct intracellular signaling events, AT1R activation can lead to transactivation of the epidermal growth factor receptor (EGFR) by either an EGFR ligand-dependent or -independent process (8, 9), and EGFR activation has been implicated in the detrimental effects seen with chronic AT1R activation (13, 21). Compelling in vitro studies suggest that the EGFR ligand HB-EGF may be an important mediator in the EGFR transactivation by Ang II-AT1R signaling (9, 18, 30), but this role of HB-EGF in vivo has not been explored previously.
HB-EGF, like all members of the EGF family of growth factors, is synthesized as a transmembrane precursor protein (pro-HB-EGF) that can be cleaved by metalloproteinases such as ADAM17 (a desintegrin and metalloproeinase domain 17), also called TACE (tumor necrosis factor-α-converting enzyme), in the kidney and ADAM12 in the heart within the juxtamembrane domain on the cell surface to release a mature soluble HB-EGF (sHB-EGF) (21). Although in vitro studies demonstrated that pro-HB-EGF is functionally active, it probably plays a limited role in vivo due to the barrier or distance from its receptors (45). In this regard, shedding of sHB-EGF is more important for its function. In addition, sHB-EGF has the ability to bind to cell surface heparan sulfate proteoglycans (HSPG), which may increase the local growth factor concentration in the cellular microenvironment (42). HB-EGF has been implicated in a variety of physiological and pathological processes, including wound healing (44), atherosclerosis (35), blastocyte implantation (12), tumor progression (17), glomerulonephritis (4, 15), and diabetic nephropathy (36). Of interest is that the four components mediating Ang II transactivation of EGFR, AT1R, ADAM17, HB-EGF, and EGFR, are all widely expressed in the vasculature, and their expression levels increase in CKD (14, 24, 36, 46, 48).
In our previous studies, we found that specific deletion of HB-EGF expression in endothelium attenuated renal injury of diabetic nephropathy in eNOS-knockout diabetic mice (36), a condition in which elevated Ang II levels may also exist. In the current study, by applying chronic Ang II infusion, a well-documented method to induce experimental hypertension and hypertensive nephropathy, to mice with specific HB-EGF deletion in endothelium, we demonstrated that endothelial HB-EGF deletion significantly reduced Ang II-induced renal injury through reduction of endothelial injury, inflammation, and renal fibrosis, whereas no significant effects on hypertension were observed.
MATERIALS AND METHODS
Reagents and antibodies.
Tamoxifen, proteinase K, Sigmafast 3,3′-diaminobenzidine tablets, monoclonal antiactin, α-smooth muscle-Cy3 antibody, and goat anti-mouse EGFR antibody were purchased from Sigma-Aldrich (St. Louis, MO). Terminal deoxynucleotidyl transferase (TdT) was purchased from Thermo Fisher Scientific (Waltham, MA). Bio-14-dATP was from Invitrogen (Grand Island, NY). The avidin-biotin complex (ABC) kit was from Vector Laboratories (Burlingame, CA). Angiotensin II acetate salt was from Bachem Americas (Torrance, CA). X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) was from RPI Research Products International (Mt. Prospect, IL). The origins of other antibodies were phospho-EGF receptor antibody and WT1 (c-19) from Santa Cruz Biotechnology (Santa Cruz, CA), polyclonal rabbit anti-human Von Willebrand factor (vWF) antibody from Dako (Agilent Technologies, Carpinteria, CA), cleaved caspase 3 from Cell Signaling Technology (Danvers, MA), anti-TGF-β1 polyclonal antibody from Promega (Madison, WI), and anti-IL-6, anti-MCP1, anti-ADAM17, anti-iNOS (inducible nitric oxide synthase), and anti-liver arginase antibodies from Abcam (Cambridge, MA); anti-F4/80, anti-CD3, anti-CD4, and anti-CD8 antibodies were from AbD Serotec/Bio-Rad (Raleigh, NC).
Generation of endothelial HB-EGF deletion mice.
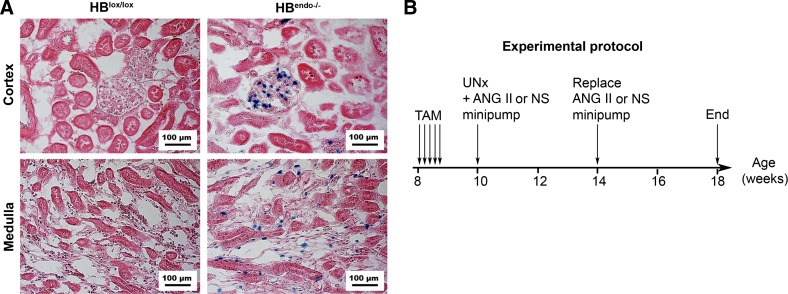
HB-EGF loxP and tamoxifen-inducible endothelial Cre [endothelial-SCL-Cre-ER (T)] mice on the C57BL/6 background were used as described previously (36). The targeting construct of HB-EGF loxP was described in detail by Iwamoto et al. (23). Briefly, a construct containing “loxP-mouse HB-EGF cDNA-Poly (A)-loxP-LacZ gene” was fused at Exon 1 of mouse Hb-egf gene. Cre-mediated recombination results in the deletion of the HB-EGF cDNA and the expression of the lacZ gene. Therefore, positive X-gal staining indicates that HB-EGF cDNA was present and was now deleted by Cre-recombinase. By cross-breeding, HB-EGFlox/lox/Cre (+) or HB-EGFlox/lox/Cre (−) mice were generated, and 8-wk-old male littermates were used in the studies. Tamoxifen (100 mg·kg−1·day−1) dissolved in corn oil was administrated daily through intraperitoneal (ip) injection for 5 consecutive days to activate Cre recombinase. As a result, mice with (HBendo−/−) or without specific endothelial HB-EGF deletion (HBlox/lox, serving as control) were generated. HB-EGF deletion was confirmed by X-gal staining (Fig. 1A). All animal experiments were performed under National Institutes of Health guidelines with the approval of the Institutional Animal Care and Use Committee at Vanderbilt University.
Fig. 1.
Induction and confirmation of heparin-binding (HB) epidermal growth factor (EGF)-like factor deletion. A: X-gal staining (blue) showed that HB-EGF gene was expressed and had been deleted in the endothelium of glomerular and peritubular capillaries in HBendo−/− mouse kidneys 2 wk after tamoxifen (TAM) injection. The kidney from HBlox/lox mice was used as a negative control. Counterstain: sirius red. B: experimental protocol. HB-EGF deletion was induced by TAM injection at the age of 8 wk. Uninephrectomy (UNx) was performed in all mice, and minipumps infusing angiotensin II (Ang II; n = 7) or normal saline (NS; n = 6) were implanted subcutaneously and replaced after 4 wk. Measurements and tissue sampling were performed before and after Ang II or normal saline infusion.
X-gal staining.
X-gal staining was performed as described previously, with some modification (6). Briefly, isolated kidneys were fixed in 4% paraformaldehyde (PFA) in PBS for 4 h at 4°C and then cryoprotected by precipitation in 15% sucrose and then 30% sucrose in PBS, followed by OCT embedding and storage at −80°C. The frozen blocks were sectioned at 10 μm, air-dried for 30 min, and equilibrated in detergent washing buffer (0.1 M phosphate buffer, pH 7.3, containing 2 mM MgCl2, 5 mM EGTA, 0.02% NP-40, and 0.01% sodium deoxycholate) for 15 min. X-gal staining was performed at 37°C for 4–6 h in detergent washing buffer containing 1 mg/ml X-gal, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide. Sections were counterstained with eosin, dehydrated, mounted, and photographed under a Zeiss Axioskop 40 light microscope with AxioCam MRc.
Chronic administration of Ang II.
Ten-week-old HBendo−/− or HBlox/lox mice were subjected to unilateral nephrectomy by removing the right kidney, followed by osmotic minipump-mediated infusion (Oztec, model 2004, replaced once after 4 wk) with saline or Ang II (1.4 mg·kg−1·day−1; n = 6–7 in each group) for 8 wk, as described previously (8, 30). Urine and blood samples were collected and body weight and systolic blood pressure (SBP) measured every 2 wk. At the end of the experiments, mice were euthanized and heart and kidney weighed and processed for tissue analysis. The experimental protocol was illustrated in Fig. 1B.
SBP measurement.
SBP was measured every 2 wk after implantation of Ang II or saline minipump using a tail-cuff monitor (BP-2000 BP Analysis system; Visitech System), as described previously (36). In another set of Ang II-infused mice (4 in each group), blood pressure was monitored by radiotelemetry as described previously, with minor modifications (32, 57). Under anesthesia, radiotelemetric catheters (PA-C10; Data Sciences International) were inserted into the left carotid artery, with the transmitter implanted subcutaneously. After recovery for 1 wk, heart rate and blood pressure were recorded at 10-s intervals for 1 wk and used as baseline. Ang II minipump was then implanted subcutaneously, and heart rate and blood pressure were recorded continuously for 4 more wk. Finally, the data were collected using the DataQuest ART version 4.0 developed by Data Sciences International and analyzed.
Urine albumin and creatinine.
Spot urine from individual mice was collected and centrifuged. Supernatants were stored in aliquot at −80°C until assay. Urinary albumin was determined using the Albuwell M Murine Microalbuminuria enzyme-linked immunosorbent assay kit (Exocell, Philadelphia, PA). Urinary creatinine was measured using the Creatinine Companion kit (Exocell).
Renal morphology.
For assessing morphological changes, 5-μm paraffin sections were stained with periodic acid-Schiff reagent and examined by light microscopy. Semiquantitative glomerular lesions were graded for glomerular score (0–4), as described previously (52). Briefly, at least 25 glomeruli were randomly selected from each specimen and graded from 0 to 4+ according the degree of mesangial cell and/or matrix expansion using the following criteria: 0 = normal glomerular cellularity with no significant mesangial expansion, whereas 1+, 2+, 3+, and 4+ = mesangial cell and/or matrix expansion involving <25%, 26–50%, 51–75%, and >75% of the glomerular area. All sections were examined without knowledge of the treatment protocol.
Immunohistochemistry staining and quantitative analysis.
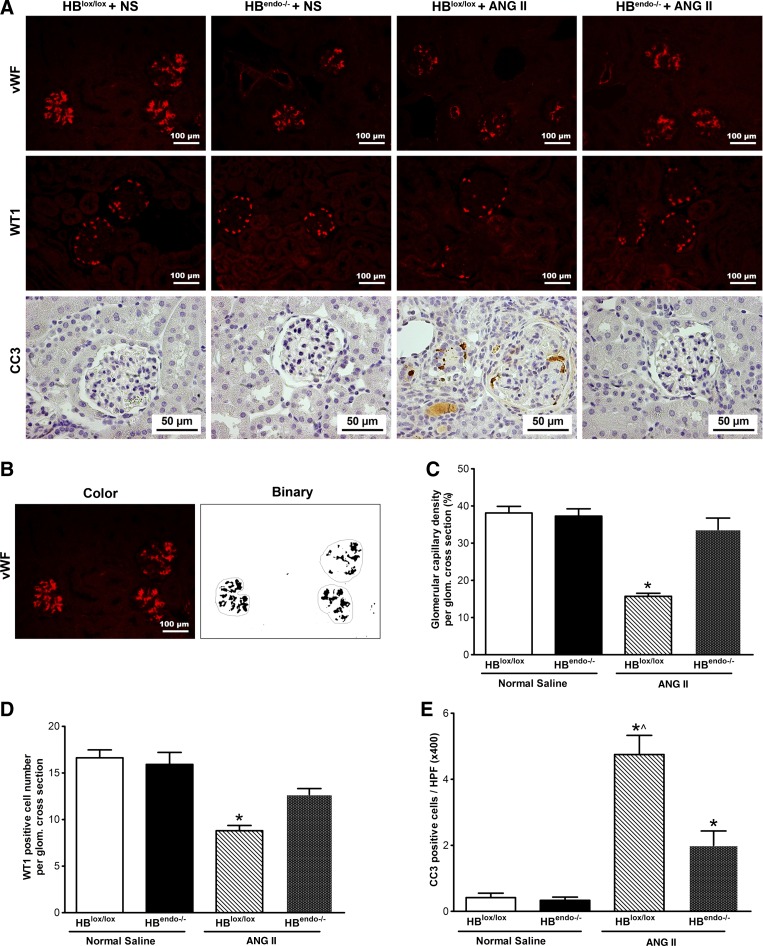
Glomerular endothelial injury was evaluated by immunofluorescence staining of von Willebrand factor (vWF) and analysis of glomerular capillary density according to a previously described method, with minor modifications (22). After deparaffinization and rehydration, 5-μm sections were treated with 0.6 U/ml proteinase K in TE-CaCl buffer (10 mM Tris Base, 1 mM EDTA, 5 mM CaCl2, and 0.5% Triton X-100, pH 8.0) for 15 min at room temperature, followed by immunostaining with polyclonal rabbit anti-human vWF (1:200) as the primary antibody, and DyLight 549 AffiniPure donkey anti-rabbit IgG (H + L) (1:200; Jackson ImmunoResearch Laboratories) was used as the secondary antibody. Sections were examined and photographed under a fluorescence microscope (Zeiss Imager A1 with AxioCam MRc5). Glomerular capillaries were identified by positive staining for vWF. All images were split into RGB channels using ImageJ (National Institutes of Health, Bethesda, MD), and the red channels were saved in grayscale as a tiff file, which was further converted to binary for glomerular capillary density measurement (Fig. 5B). Twnenty to 30 randomly selected glomeruli were measured for each sample.
Fig. 5.
Glomerular injury and apoptosis in the kidneys of saline- or Ang II-infused mice. A, top: glomerular capillary density shown by Von Willebrand factor (vWF) immunostaining; middle: podocytes shown by WT1 staining; bottom: apoptosis shown by cleaved caspase-3 (CC3) immunostaining. B: representative images of glomerular capillary tuft quantification by converting color image to binary image using ImageJ software, as described in the materials and methods. C: quantification of glomerular capillary density through vWF staining in A. *P < 0.05 compared with all of the other groups. D: quantification of WT1-positive cells. *P < 0.05 compared with all of the other groups. E: quantification of CC3-positive cells. *P < 0.05 compared with normal saline-infused groups; ^P < 0.05 compared with Ang II-infused HBendo−/− mouse kidneys. HPF, high-power field.
WT1 antibody (1:500) was used to stain podocytes on kidney sections. WT1-positive podocytes in each glomerular cross-section were enumerated in 15–20 glomeruli per mouse in a blinded fashion.
The other antibodies used for immunostaining were cleaved caspase 3 (1:50), F4/80 (1:50), VEGF (1:200), and α-smooth muscle-Cy3 (1:500). Antigen retrieval was performed with citrate buffer (pH 6.0) in a steamer for 15 min for the following antibodies: p-EGFR (1:50), CD3 (1:100), CD4 (1:100), CD8 (1:100), TGF-β (1:100), IL-6 (1:400), MCP-1 (1:500), arginase (1:500), and iNOS (1:500). Immunostaining was performed as described previously (56).
Immunoblot analysis.
A quarter of the kidney was snap-frozen and stored at −80°C until use. Frozen samples were homogenized and lysed in 0.5 ml ice-cold TGH lysis buffer (1% Triton X-100, 10% glycerol, 20 mM HEPES, pH 7.2, 100 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM Na3VO4). Western blots were conducted as described previously (56). Membranes were blotted using antibodies against EGFR (1:1,000), phospho-EGFR (1:100), ADAM17 (1:400), and β-actin (1:2,000).
Serum IL-6 and MCP-1 detection.
An ELISA kit (RayBiotech, Norcross, GA) was used to detect the levels of serum IL-6 and MCP-1, following the manufacturer's instructions. Results were expressed as picograms per milliliter.
Statistical analysis.
Data were evaluated using an unpaired t-test for two-group comparison or analysis of variance followed by a post hoc Bonferroni's multiple comparison test for three or more groups. Values were expressed as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
HB-EGF gene was widely expressed in renal endothelium.
By employing X-gal staining, we confirmed that the HB-EGF gene was widely expressed in the endothelium of blood vessels and was abundant in glomerular endothelium and peritubular capillaries in the inner cortex and outer medulla. The positive X-gal staining also validated successful HB-EGF deletion in the kidney of endothelial HB-EGF deletion (HBendo−/−) mice (Fig. 1A).
Levels of renal EGFR activation decreased in Ang II-infused mice with endothelial HB-EGF deletion.
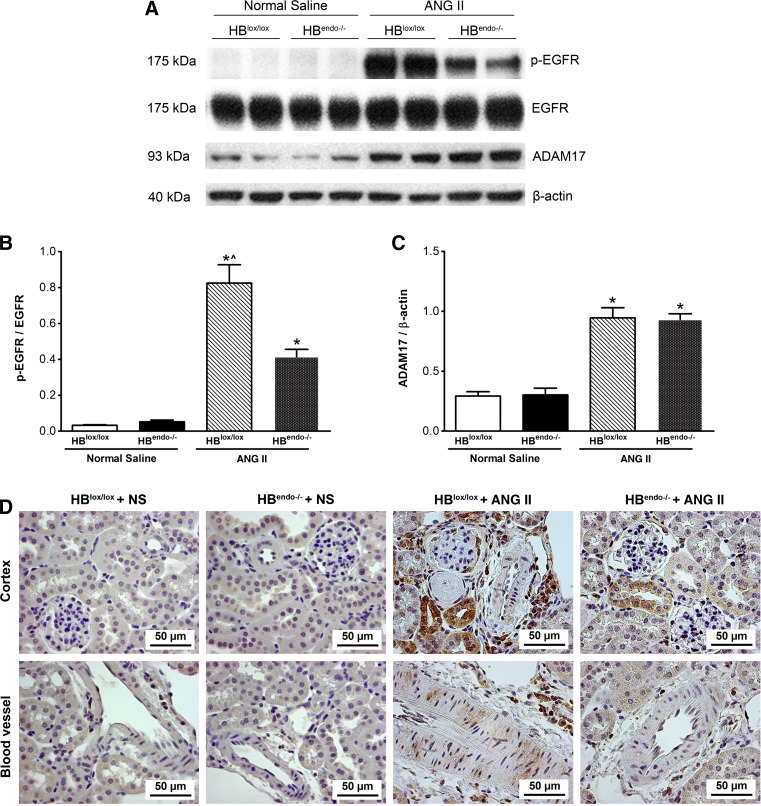
Chronic Ang II infusion can cause renal damage through AT1R-induced transactivation of EGFR signaling (8, 30). Therefore, levels of EGFR activation after saline or Ang II infusion were investigated. Increased EGFR phosphorylation was detected in kidneys from Ang II-infused mice compared with that from saline-infused kidneys. Endothelial HB-EGF deletion significantly reduced Ang II-induced phospho-EGFR levels compared with controls, whereas no changes were detected in the total EGFR expression levels (Fig. 2, A and B). Previous studies from our group have also indicated a role for ligand-independent Src-mediated transactivation of EGFR in proximal tubule by Ang II, which may account for the residual EGFR activation by Ang II in HB-EGF deletion mice (8). Increased levels of ADAM17, a metalloproteinase reported to mediate Ang II-mediated EGFR transactivation by cleavage of ligands for EGFR, including HB-EGF, were detected in both HB-EGF deletion and wild-type mice in AngII-infused groups compared with saline-infused mice (Fig. 2, A and C). Immunostaining indicated widespread EGFR activation localized to tubular epithelial cells, glomerular, periglomerular, and perivascular infiltrating cells, and blood vessel smooth muscle cells (SMCs) in the kidneys of chronic Ang II-infused HBlox/lox mice, whereas decreased EGFR activation was detected in Ang II-infused HBendo−/− kidneys, with localization primarily to the tubules. Phospho-EGFR immunoactivity was not detected in the kidneys from saline-infused mice (Fig. 2D).
Fig. 2.
EGFR activation and a disintegrin and metalloprotease 17 (ADAM17) augmentation by chronic Ang II infusion. A: compared with saline-infused mice, Ang II infusion increased kidney phospho-EGFR (p-EGFR) levels in both HBlox/lox and HBendo−/− mice, with the latter having much lower levels of p-EGFR. The total amount of EGFR was almost identical in all groups. Levels of ADAM17 were also increased in Ang II-infused kidneys compared with saline-infused groups. B and C: densitometric analysis of the Western blot bands in A expressed as p-EGFR/EGFR and ADAM17/β-actin. *P < 0.05 compared with normal saline infusions; ^P < 0.05 compared with Ang II-infused mice with endothelial HB-EGF deletion. Values represent the mean and SE of at least 3 independent experiments (n = 6–7 in each group). D: 8 wk after chronic Ang II infusion, increased p-EGFR immunostaining was seen in glomeruli, proximal tubules, perivascular infiltrating cells, and smooth muscle cells in the large blood vessels in the control HBlox/lox mice, with significantly decreased expression in HBendo−/− mouse kidneys. No p-EGFR immunostaining was detected in saline-infused groups.
Endothelial HB-EGF deletion did not prevent ang II-induced high blood pressure.
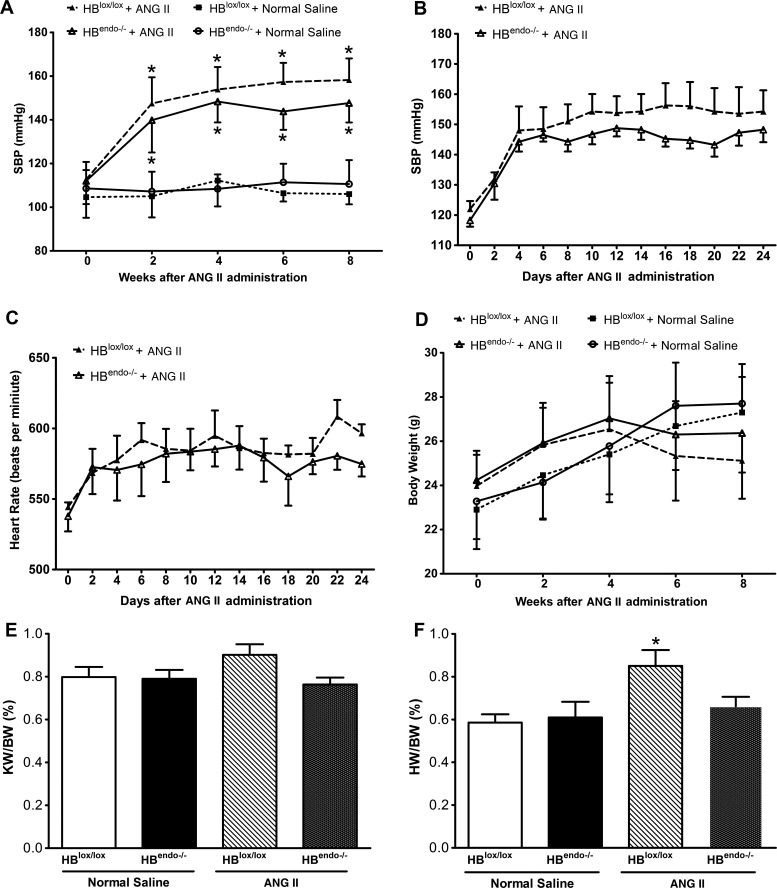
It is well known that chronic Ang II infusion results in hypertension in experimental animals (11). Compared with saline infusion, increased systolic blood pressure (SBP) was detected 2 wk after Ang II infusion, which peaked around 4 wk and remained elevated until the end of the experiments. Endothelial HB-EGF deletion did not significantly prevent the hypertension induced by Ang II infusion, although numerically but not statistically significant lower SBP levels were detected in HBendo−/− mice compared with HBlox/lox mice (Fig. 3A), which was further confirmed by telemetry studies (Fig. 3B). A similar trend was also noted in the rise (8–10% above baseline) of heart rate after Ang II infusion, with comparable changes found in both groups (Fig. 3C).
Fig. 3.
Characterization of physical parameters of Ang II- or saline-infused mice. A: compared with saline-infused mice, systolic blood pressure (SBP) was increased in Ang II-infused mice, with no significant differences between HBlox/lox and HBendo−/− mice. B: telemetry studies were performed on Ang II-infused mice (n = 4 in each group). There were no significance differences between Ang II-infused HBlox/lox and HBendo−/− mice. In both groups, increased SBP levels were detected 2 days after Ang II infusion peaked around 4 days and remained elevated until the end of the experiment. C: heart rate profiles recorded by telemetry. There is an 8–10% increase in heart rate after Ang II infusion, with comparable levels in both groups (P > 0.05). D: mice body weight (BW) was slightly decreased after 4-wk Ang II infusion, whereas no significant differences were detected compared with saline-infused mice. E: at the end of the study, kidney and heart were weighed. No significant differences were seen in kidney weight (KW)/BW ratios in either Ang II- or saline-infused mice. F: compared with Ang II-infused HBlox/lox mice, HBendo−/− mice with endothelial HB-EGF deletion significantly reduced cardiac hypertrophy, indicated by lower heart weight (HW)/BW ratios. All the results shown are means ± SE of 6–7 mice in each group. *P < 0.05.
There were also no significant differences in body weight between the two Ang II-treated groups (Fig. 3D). At the conclusion of the experiments, there were no significant changes detected in kidney weight/body weight (KW/BW) ratios between saline- and Ang II-infused groups (Fig. 3E). Cardiac hypertrophy was seen in Ang II-infused HBlox/lox mice, as indicated by a statistically significant increased heart weight/body weight (HW/BW) ratio, but was not seen in Ang II-infused HBendo−/− mice (Fig. 3F).
Endothelial HB-EGF deletion alleviated renal injury induced by chronic ang II infusion.
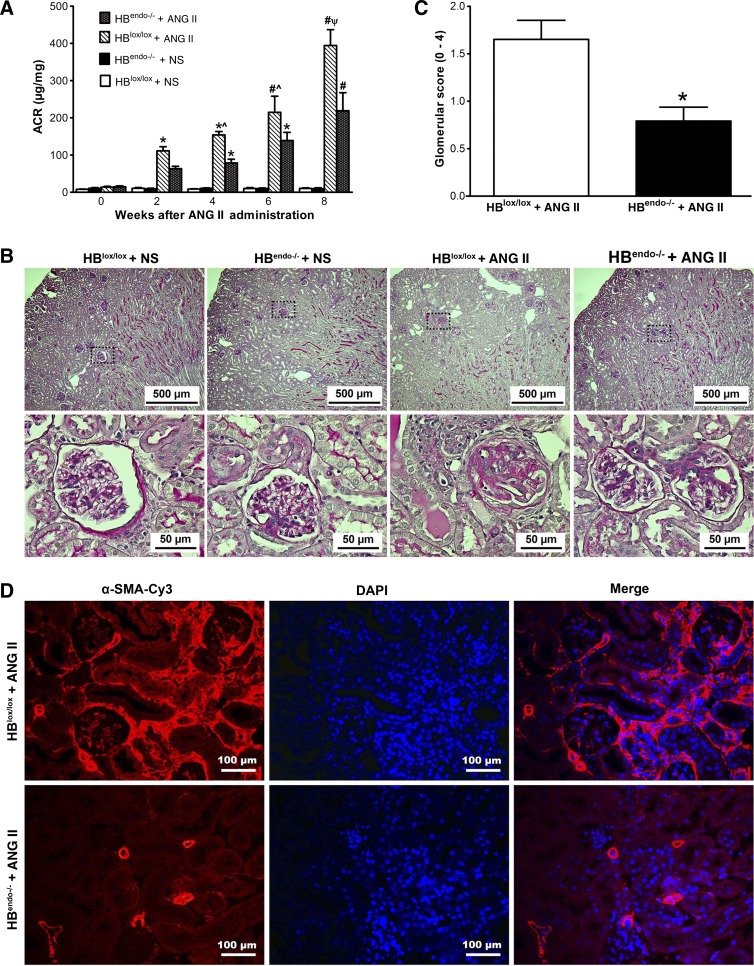
Chronic Ang II infusion can induce endothelial dysfunction and eventually renal damage. Therefore, urinary albumin excretion, a functional evaluation of the extent of kidney injury, was measured by albumin/creatinine ratios every 2 wk after saline or Ang II infusion to assess the degree of renal injury. Albuminuria levels were low and almost identical in saline-infused HBlox/lox and HBendo−/− mice during the observation period. However, despite the similar increase in blood pressure, compared with HBlox/lox mice, HBendo−/− mice had a slower progression of albuminuria, which was significantly different after 6 wk of Ang II infusion (Fig. 4A).
Fig. 4.
Endothelial HB-EGF deletion slowed down renal injury induced by chronic Ang II infusion. A: compared with saline-infused mice, albuminuria, as indicated by increased urinary albumin/creatinine ratios (ACR), was present after 2 wk of Ang II infusion and increased continuously with prolonged Ang II infusion. Compared with HBlox/lox controls, HBendo−/− mice had significantly lower ACR. (*P < 0.05 or #P < 0.001 compared with normal saline-infused mice; ^P < 0.05 or ΨP < 0.001 compared with Ang II-infused HBendo−/− mice). B: periodic acid-Schiff (PAS)-stained kidney sections from Ang II- or saline-infused mice. In Ang II-infused mouse kidneys, tubular casts and glomerulosclerosis were detected in HBlox/lox controls, whereas only mesangial expansion was seen in HBendo−/− mice. No obvious renal morphological changes were observed in the kidneys of saline-infused mice. C: glomerular sclerosis shown by PAS staining in B was assessed by glomerular score (0–4) and shown as means ± SE of 6–7 samples in each group. D: renal fibrosis shown by anti-α-smooth muscle actin-Cy3 (α-SMA-Cy3) immunofluorescence staining. Original magnification ×400. All images represent the results of 6–7 mice in each group.
After 8 wk of Ang II infusion, kidneys from HBlox/lox mice had tubular casts and glomerular injury, with a glomerular injury score of 1.652 ± 0.20, whereas only mesangial expansion was detected in the kidneys from HBendo−/− mice (glomerular injury score: 0.793 ± 0.14, P < 0.05; Fig. 4, B and C). No significant renal morphological changes were observed in either saline-infused group (Fig. 4B).
In the kidneys of Ang II-infused HBlox/lox mice, there was increased immunoreactivity for α-smooth muscke actin (α-SMA), a marker for activated mesangial cells in glomeruli (26), and for myofibroblast activation in the interstitium (5). By contrast, there was only minimal α-SMA immunoreactivity in Ang II-infused HBendo−/− mice (Fig. 4D).
Endothelial HB-EGF deletion alleviated endothelial and podocyte injury induced by chronic ang II infusion.
Glomerular endothelial damage induced by chronic Ang II infusion was evaluated by vWF immunostaining. Kidneys from saline-infused mice with or without endothelial HB-EGF deletion showed strong vWF immunoactivity, which was markedly reduced in Ang II-infused HBlox/lox kidneys but not in endothelial HB-EGF-deleted HBendo−/− mice (Fig. 5, A, top, and B). Similarly, glomerular capillary density was significantly reduced in Ang II-infused HBlox/lox kidneys but not in HBendo−/− mice (Fig. 5C).
WT1 immunostaining was used to assess the possible podocyte loss induced by Ang II infusion. No significant differences were detected in the saline-infused groups. In Ang II-infused groups, there was a significant decrease in WT1-positive cells in HBlox/lox kidneys, which was partially ameliorated in HBendo−/− kidneys (Fig. 5, A, middle, and D).
Cell apoptosis related to the glomerular injury was studied with cleaved caspase 3 immunostaining. In Ang II-infused HBlox/lox kidneys, apoptotic cells were widely detected in glomeruli, tubular, vascular endothelial cells, and interstitial cells, whereas only minimal positive immunoactivity was detectable in the tubules and interstitium in HBendo−/− kidneys (Fig. 5, A, bottom, and E).
Endothelial HB-EGF deletion partially inhibited the increased podocyte VEGF expression in response to Ang II infusion.
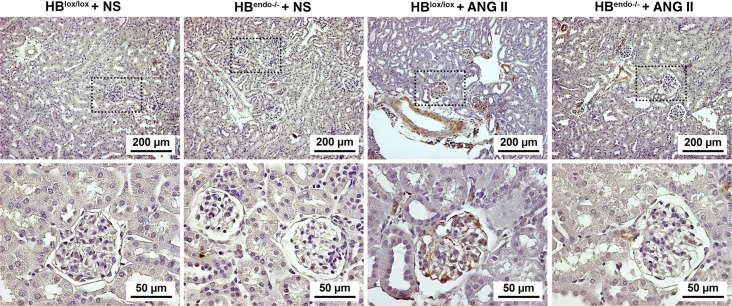
Ang II can activate VEGF signaling through HB-EGF-EGFR pathway, and high levels of VEGF can impose detrimental effects on podocytes (18, 53). Previous studies have indicated that VEGF and HB-EGF may interact in the vascular endothelium and smooth muscle cells (1, 38). After 8 wk of Ang II infusion, increased levels of VEGF were detected in both glomeruli and vasculature. Compared with HBlox/lox mice, the kidneys from HBendo−/− mice had markedly decreased VEGF immunoreactivity, especially in the glomeruli (Fig. 6).
Fig. 6.
Representative images of VEGF-A staining in kidney sections from saline- or Ang II-infused mice. No obvious VEGF-A staining was detected in saline-infused kidneys in either group. In the kidneys from Ang II-infused mice, strong VEGF-A staining was seen in the glomeruli and vasculature in HBlox/lox control, which was decreased dramatically in the HBendo−/− kidneys.
Endothelial HB-EGF deletion decreased Ang II-induced renal inflammation.
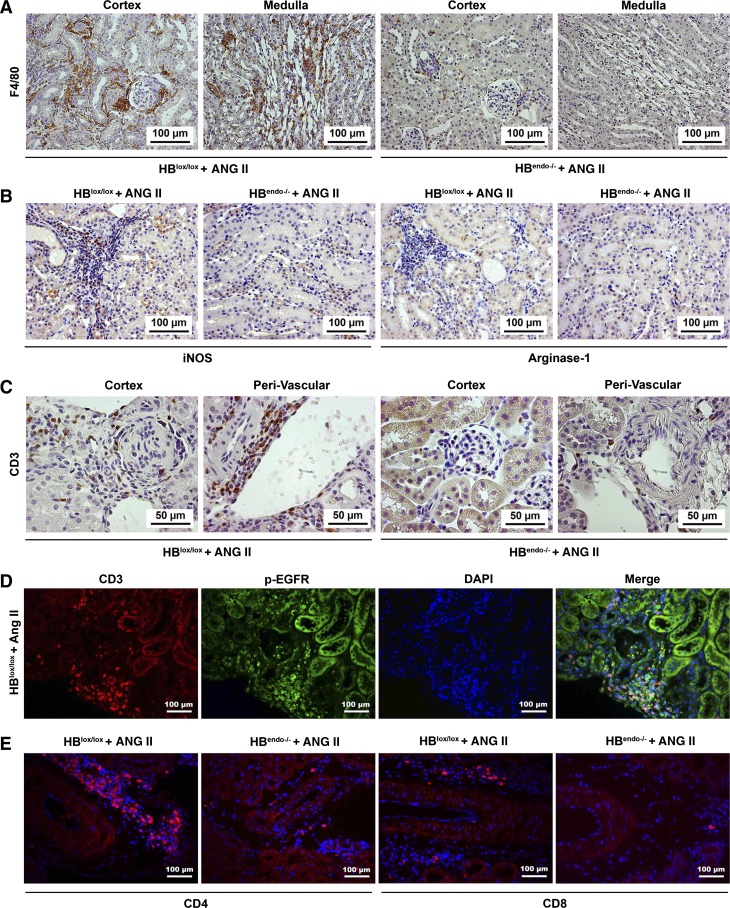
Renal inflammation was evaluated by immunostaining with both F4/80, a marker for macrophages, and CD3, a marker for T lymphocytes, in the kidneys of the Ang II-infused mice. Compared with HBendo−/− kidneys, HBlox/lox kidneys had markedly increased immunoreactivity for F4/80, which was abundant in the periglomerular and interstitial regions (Fig. 7A). Furthermore, in Ang II-infused HBlox/lox kidneys, M1 macrophage polarization was evident by strong immunostaining of iNOS (M1 marker) and minimal immunostaining of arginase 1 (M2 marker), whereas significantLY fewer immunoreactive cells for both markers were seen in HBendo−/− kidneys (Fig. 7B). CD3 immunoreactivity was detected predominantly at the perivascular area of the large blood vessels (Fig. 7C), where strong p-EGFR reactivity was also localized (Fig. 2D). Colocalization indicated that p-EGFR was expressed by some of the perivascular infiltrated CD3-positive cells in Ang II-infused HBlox/lox kidneys (Fig. 7D). Further studies showed that most of the perivascular CD3 lymphocytes are CD4 positive (Fig. 7E).
Fig. 7.
Endothelial HB-EGF deletion reduced Ang II-induced renal inflammation. A: F4/80 staining indicated macrophage infiltration in the HBlox/lox control kidneys after Ang II infusion, whereas only sparse F4/80-positive cells were detected in the HBendo−/− kidneys. B: compared with Ang II-infused kidneys from HBendo−/− mice, Ang II-infused HBlox/lox kidneys showed increased interstitial staining of iNOS, an M1 marker, and weak positivity for arginase I, an M2 marker. C: infiltration of CD3-positive lymphocytes was seen in the periglomerular and perivascular regions in Ang II-infused HBlox/lox control kidneys but was rarely detected in Ang II-infused HBendo−/− kidneys. D: some of the infiltrating CD3-positive lymphocytes also expressed p-EGFR. E: more CD4- vs. CD8-positive cells were detected in the perivascular infiltration areas in both Ang II-infused groups, with HBlox/lox control kidneys having significantly higher numbers of both CD4- and CD8-positive infiltrated cells.
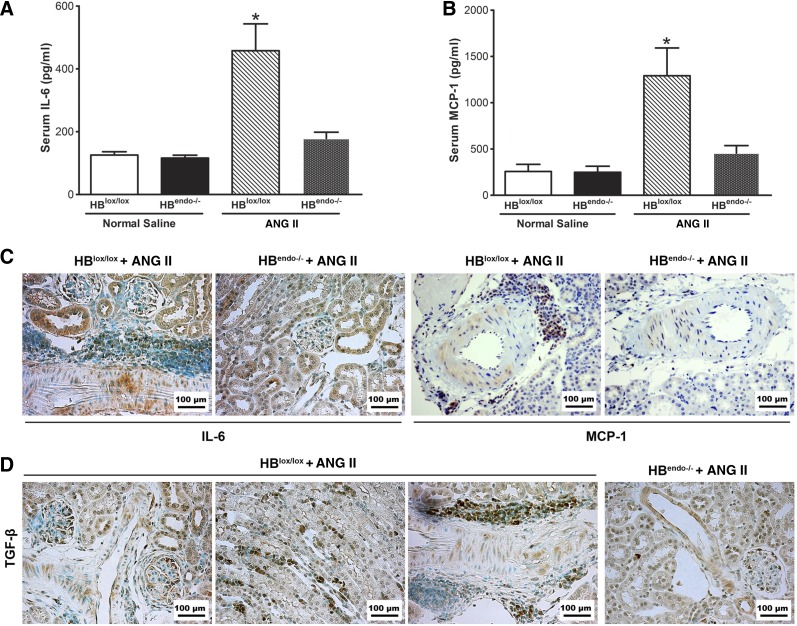
IL-6, TGF-β, and MCP-1 are known proinflammatory cytokines secreted by damaged endothelium. In saline-infused mouse serum, low levels of IL-6 and MCP-1 were detected. Ang II significantly increased their levels in the serum from Ang II-infused HBlox/lox mice but not in Ang II-infused HBendo−/− mice (Fig. 8, A and B). Immunoreactive IL-6, MCP-1, and TGF-β increased in Ang II-infused kidneys from HBlox/lox mice compared with the HBendo−/− mice, with immunoreactivity localized strongly in tubular epithelial cells (IL-6), interstitium and glomeruli (TGF-β), vascular SMCs, and perivascular infiltrating cells (IL-6, MCP-1, and TGF-β) (Fig. 8, C and D).
Fig. 8.
Endothelial HB-EGF deletion lessened Ang II-induced inflammation cytokine production. A and B: increased proinflammatory cytokines IL-6 and monocyte chemoattractant protein-1 (MCP-1) were detected by ELISA in serum of Ang II-infused HBlox/lox control mice, and their levels were significantly reduced in Ang II-infused HBendo−/− mice. *P < 0.05 compared with all other groups. Values represent the mean SE of 6–7 mice in each group. C: both IL-6 and MCP-1 immunoreactivity were strongly detected in smooth muscle cells and perivascular infiltrating cells, with IL-6 also expressed in tubular epithelial cells, after Ang II infusion in the HBlox/lox mice, which was markedly reduced in HBendo−/− mice. D: strongtransforming growth factor-β (TGF-β) immunostaining was seen in the glomeruli, interstitium, and perivascular infiltrating cells after Ang II infusion in HBlox/lox kidneys but was markedly reduced in HBendo−/− kidneys.
DISCUSSION
There is increasing evidence that EGFR activation plays an important role in the initiation and progression of CKD (7, 30). In the current study, we demonstrated that specific endothelial deletion of the EGFR ligand HB-EGF inhibited transactivation of renal EGFR receptors by angiotensin II (Ang II) and reduced Ang II-induced renal injury in mice. To our knowledge, this is the first in vivo study to clarify that HB-EGF expressed in the endothelium is a critical mediator in Ang II-AT1R transactivation of EGFR-induced renal injury.
Since its original discovery in the 1970s, the physiological and pathophysiological roles of EGFR have been studied extensively. EGFR activation has been implicated in blood pressure regulation, endothelial dysfunction, neointimal hyperplasia, and cardiac remodeling (33, 49). Seven known ligands can bind and activate EGFR, namely EGF, TGFα, HB-EGF, epiregulin, amphiregulin, β-cellulin, and epigen (55). Among them, increased expression of TGFα and HB-EGF has been associated with the progression of CKD (40, 47). Although both of these ligands are expressed in the endothelial cells and SMCs of the blood vessels, studies from transgenic null mice indicated that only HB-EGF plays a critical role in vasculature development, whereas TGFα is dispensable (23, 31). Unlike TGFα-null mice, which have no apparent developmental abnormalities, the majority of mice with either global or selective smooth muscle or endothelial deletion of HB-EGF die of heart failure in the first postnatal week with cardiac hypertrophy and defective valvulogenesis (23, 39), a phenotype that resembles those displayed by mice lacking EGFR (50), ErbB4 (for which HB-EGF can also serve as a ligand) (3, 56), or TACE/ADAM17, a major sheddase for HB-EGF (25). Those studies suggested that EGFR activation by TACE/ADAM17-derived soluble HB-EGF plays an important role in vasculature development and homeostasis. In the current studies, we utilized conditional deletion of endothelial HB-EGF in adult mice with a tamoxifen-inducible endothelial Cre [endothelial-SCL-Cre-ER (T)] to avoid any developmental abnormalities. Since the C57BL/6 mouse strain is relatively resistant to renal injury, uninephrectomy was performed in addition to Ang II infusion. We did not detect significant pathological changes in the contralateral kidneys by uninephrectomy alone in our experiments, which may be due to the resistance to injury of the C57BL/6 background.
A protective role for HB-EGF deletion has been reported in renal ischemia-reperfusion injury (37), diabetic nephropathy (36), and glomerulonephritis (4). In line with these findings, results from our current study demonstrated that endothelial deletion of HB-EGF reduced renal injury induced by chronic Ang II infusion, as indicated by retardation of albuminuria, less glomerulosclerosis, and reduced inflammation and renal fibrosis. Endothelial HB-EGF deletion reduced apoptosis in both endothelial cells and podocytes, which protected glomerular capillary integrity and reduced podocyte injury/loss. These effects may be partially related to the reduced levels of VEGF-A in the glomeruli in the setting of Ang II infusion that is consistent with the previous reports that increased VEGF-A expression in the podocytes contributes to the glomerular filtration barrier structural and functional abnormalities (51, 53).
Studies have shown that Ang II is also a proinflammatory cytokine that promotes renal inflammation. Consistent with previous studies, after 8 wk of Ang II infusion, increased infiltrating monocytes/macrophages and lymphocytes were detected in the kidneys of wild-type mice, whereas their number was dramatically reduced with endothelial HB-EGF deletion. F4/80 positive macrophages localized mainly in the periglomeruli and interstitial regions, especially the medullary interstitium. On the other side, most of the CD3-positive lymphocytes were located in the perivasculature region, particularly around the larger blood vessels. The different localization of those two major inflammatory cells may reflect their different roles in the inflammatory response. Of interest is that some CD3-positive T lymphocytes also express phospho-EGFR. Further studies will be required to determine the role of phospho-EGFR in the CD3 lymphocytes.
Ang II-infused wild-type mice also exhibited increased serum levels of proinflammatory cytokines such as IL-6 and MCP-1, which were decreased significantly in endothelial HB-EGF deletion mice. Increased IL-6 immunoreactivity was localized to the SMC of large blood vessels, and the adjacent perivascular area and may have contributed to the recruitment of monocytes and lymphocytes in the kidney (20). The results of the present study clearly show that upregulation of these proinflammatory factors caused by Ang II was blocked in the kidneys of endothelial HB-EGF deletion mice.
Although there have been reports that EGFR inactivation following treatment with EGFR antisense nucleotides or EGFR tyrosine kinase inhibitors or in Waved-2 mice can lower blood pressure (34), in the current study, endothelial HB-EGF did not significantly affect Ang II induced hypertension. This may be due to the following reasons: 1) Ang II-mediated vasoconstriction being not dependent only upon EGFR transactivation; 2) HB-EGF being a critical but not the sole ligand for EGFR; 3) the existence of ligand-independent transactivation of EGFR, such as through a GPCR-Src pathway; 4) low expression levels of HB-EGF in larger muscular blood vessels that can mediate alterations in blood pressure (in the current studies, HB-EGF was detected predominantly in the glomeruli and peritubular capillaries in the kidney); and 5) in addition to vasoconstriction, Ang II-induced hypertension being associated with sodium retention caused by tubule sodium reabsorption (10, 11, 41).
Of interest is that endothelial HB-EGF deletion reduced cardiac hypertrophy without significantly lowering blood pressure. Studies have shown that HB-EGF shedding can play a major role in cardiac hypertrophy (2, 42). Specific blockade of ADAM12, a sheddase for HB-EGF in the heart, inhibited the transactivation of EGFR and attenuated the development of cardiac hypertrophy in mice (2). HB-EGF is expressed in the coronary artery; therefore, endothelial HB-EGF deletion may lessen Ang II-induced cardiac injury similarly to what we have shown in the kidney.
In summary, our data clearly demonstrate that HB-EGF plays a crucial role in Ang II-induced renal injury, and endothelial HB-EGF deletion reduces renal injury through inhibiting EGFR activation and consequently endothelial dysfunction, renal inflammation, and fibrosis. These results suggest that HB-EGF could be a potential therapeutic target for Ang II-mediated renal damage as well as other etiologies of CKD that are associated with elevated renal Ang II production, including diabetic nephropathy.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney DiseasesGrants DK-051265, DK-062794, DK-079341, and DK-095785 (R. C. Harris), funds from the Department of Veterans Affairs BX000320 (R. C. Harris), by National Heart, Lung, and Blood Institute P01-HL-056693 (A. Diedrich).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.Z., A.D., and R.C.H. conception and design of research; F.Z., L.A.K., and C.F. performed experiments; F.Z. and A.D. analyzed data; F.Z. interpreted results of experiments; F.Z. prepared figures; F.Z. drafted manuscript; F.Z. and R.C.H. edited and revised manuscript; F.Z. and R.C.H. approved final version of manuscript.
REFERENCES
- 1.Arkonac BM, Foster LC, Sibinga NE, Patterson C, Lai K, Tsai JC, Lee ME, Perrella MA, Haber E. Vascular endothelial growth factor induces heparin-binding epidermal growth factor-like growth factor in vascular endothelial cells. J Biol Chem 273: 4400–4405, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, Asanuma H, Sanada S, Matsumura Y, Takeda H, Beppu S, Tada M, Hori M, Higashiyama S. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med 8: 35–40, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Beerli RR, Hynes NE. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem 271: 6071–6076, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casal I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17: 1242–1250, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boukhalfa G, Desmouliere A, Rondeau E, Gabbiani G, Sraer JD. Relationship between alpha-smooth muscle actin expression and fibrotic changes in human kidney. Exp Nephrol 4: 241–247, 1996. [PubMed] [Google Scholar]
- 6.Boyle S, Shioda T, Perantoni AO, de Caestecker M. Cited1 and Cited2 are differentially expressed in the developing kidney but are not required for nephrogenesis. Dev Dyn 236: 2321–2330, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Chen JK, Harris RC. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol 32: 981–991, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Chen JK, Neilson EG, Harris RC. Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. J Am Soc Nephrol 17: 1615–1623, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension 15: 451–458, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120: 1071–1083, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis 186: 38–53, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of ADAM-mediated shedding in vascular biology. Eur J Cell Biol 91: 472–485, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Feng L, Garcia GE, Yang Y, Xia Y, Gabbai FB, Peterson OW, Abraham JA, Blantz RC, Wilson CB. Heparin-binding EGF-like growth factor contributes to reduced glomerular filtration rate during glomerulonephritis in rats. J Clin Invest 105: 341–350, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Fu S, Bottoli I, Goller M, Vogt PK. Heparin-binding epidermal growth factor-like growth factor, a v-Jun target gene, induces oncogenic transformation. Proc Natl Acad Sci USA 96: 5716–5721, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiyama S, Matsubara H, Nozawa Y, Maruyama K, Mori Y, Tsutsumi Y, Masaki H, Uchiyama Y, Koyama Y, Nose A, Iba O, Tateishi E, Ogata N, Jyo N, Higashiyama S, Iwasaka T. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res 88: 22–29, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev 4: 28–33, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomolak JR, Didion SP. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front Physiol 5: 396, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 284: 2–13, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Iakovlev VV, Gabril M, Dubinski W, Scorilas A, Youssef YM, Faragalla H, Kovacs K, Rotondo F, Metias S, Arsanious A, Plotkin A, Girgis AH, Streutker CJ, Yousef GM. Microvascular density as an independent predictor of clinical outcome in renal cell carcinoma: an automated image analysis study. Lab Invest 92: 46–56, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, Nanba D, Higashiyama S, Hori M, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA 100: 3221–3226, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izuagie IA, Pelham CJ, Agrawal DK. Synergistic effect of angiotensin II on vascular endothelial growth factor-A-mediated differentiation of bone marrow-derived mesenchymal stem cells into endothelial cells. Stem Cell Res Ther 6: 4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J 22: 2704–2716, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 87: 847–858, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiyama M, Urushihara M, Morikawa T, Konishi Y, Imanishi M, Nishiyama A, Kobori H. Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int J Mol Sci 14: 23045–23062, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori H, Mori H, Masaki T, Nishiyama A. Angiotensin II blockade and renal protection. Curr Pharm Des 19: 3033–3042, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolesnyk I, Struijk DG, Dekker FW, Krediet RT. Effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with chronic kidney disease. Neth J Med 68: 15–23, 2010. [PubMed] [Google Scholar]
- 30.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73: 263–278, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Mai TH, Wu J, Diedrich A, Garland EM, Robertson D. Calcitonin gene-related peptide (CGRP) in autonomic cardiovascular regulation and vascular structure. J Am Soc Hypertens 8: 286–296, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makki N, Thiel KW, Miller FJ. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int J Mol Sci 14: 20597–20613, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K, Eguchi S, van Goor H. Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension 52: 987–993, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Miyagawa J, Higashiyama S, Kawata S, Inui Y, Tamura S, Yamamoto K, Nishida M, Nakamura T, Yamashita S, Matsuzawa Y, et al. Localization of heparin-binding EGF-like growth factor in the smooth muscle cells and macrophages of human atherosclerotic plaques. J Clin Invest 95: 404–411, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyazawa T, Zeng F, Wang S, Fan X, Cheng H, Yang H, Bian A, Fogo AB, Harris RC. Low nitric oxide bioavailability upregulates renal heparin binding EGF-like growth factor expression. Kidney Int 84: 1176–1188, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulder GM, Nijboer WN, Seelen MA, Sandovici M, Bos EM, Melenhorst WB, Trzpis M, Kloosterhuis NJ, Visser L, Henning RH, Leuvenink HG, Ploeg RJ, Sunnarborg SW, van Goor H. Heparin binding epidermal growth factor in renal ischaemia/reperfusion injury. J Pathol 221: 183–192, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Nakai K, Yoneda K, Moriue T, Igarashi J, Kosaka H, Kubota Y. HB-EGF-induced VEGF production and eNOS activation depend on both PI3 kinase and MAP kinase in HaCaT cells. J Dermatol Sci 55: 170–178, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Nanba D, Kinugasa Y, Morimoto C, Koizumi M, Yamamura H, Takahashi K, Takakura N, Mekada E, Hashimoto K, Higashiyama S. Loss of HB-EGF in smooth muscle or endothelial cell lineages causes heart malformation. Biochem Biophys Res Commun 350: 315–321, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Nemo R, Murcia N, Dell KM. Transforming growth factor alpha (TGF-alpha) and other targets of tumor necrosis factor-alpha converting enzyme (TACE) in murine polycystic kidney disease. Pediatr Res 57: 732–737, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 13: 1131–1135, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 21: 16–20, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, Higashiyama S, Hashimoto K. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 118: 2363–2370, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Singh AB, Tsukada T, Zent R, Harris RC. Membrane-associated HB-EGF modulates HGF-induced cellular responses in MDCK cells. J Cell Sci 117: 1365–1379, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Stanic B, Pandey D, Fulton DJ, Miller FJ Jr. Increased epidermal growth factor-like ligands are associated with elevated vascular nicotinamide adenine dinucleotide phosphate oxidase in a primate model of atherosclerosis. Arterioscler Thromb Vasc Biol 32: 2452–2460, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staruschenko A, Palygin O, Ilatovskaya DV, Pavlov TS. Epidermal growth factors in the kidney and relationship to hypertension. Am J Physiol Renal Physiol 305: F12–F20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swaminathan N, Vincent M, Sassard J, Sambhi MP. Elevated epidermal growth factor receptor levels in hypertensive Lyon rat kidney and aorta. Clin Exp Pharmacol Physiol 23: 793–796, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225–234, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269: 230–234, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Tufro A, Veron D. VEGF and podocytes in diabetic nephropathy. Semin Nephrol 32: 385–393, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchimura H, Marumo T, Takase O, Kawachi H, Shimizu F, Hayashi M, Saruta T, Hishikawa K, Fujita T. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol 16: 997–1004, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989–999, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Walker WG. Hypertension-related renal injury: a major contributor to end-stage renal disease. Am J Kidney Dis 22: 164–173, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng F, Zhang MZ, Singh AB, Zent R, Harris RC. ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis. Mol Biol Cell 18: 4446–4456, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]