Abstract
Entamoeba histolytica trophozoites adhere to human colonic mucins and epithelial cells by a cell surface galactose-specific lectin. This lectin, which is composed of two subunits linked by disulfide bonds, has been shown to be a protective antigen in an animal model of amebiasis. We have determined the sequence of the mature form of the 170-kDa heavy subunit from cDNA clones and PCR-amplified fragments. The heavy subunit sequence consisted of a putative extracellular domain containing 1209 amino acids with 16 potential sites for N-linked glycosylation, a 26-amino acid hydrophobic region, and a 41-amino acid cytoplasmic tail. The presence of N-linked oligosaccharides was confirmed by culturing amebae with tunicamycin, which resulted in a decrease in the heavy subunit molecular mass to 160 kDa and a loss of lectin activity. The extracellular domain was remarkable for an extensive cysteine-rich domain that shared identify with similar regions of several other cell surface proteins and appeared to confer protease resistance to the subunit.
Full text
PDF

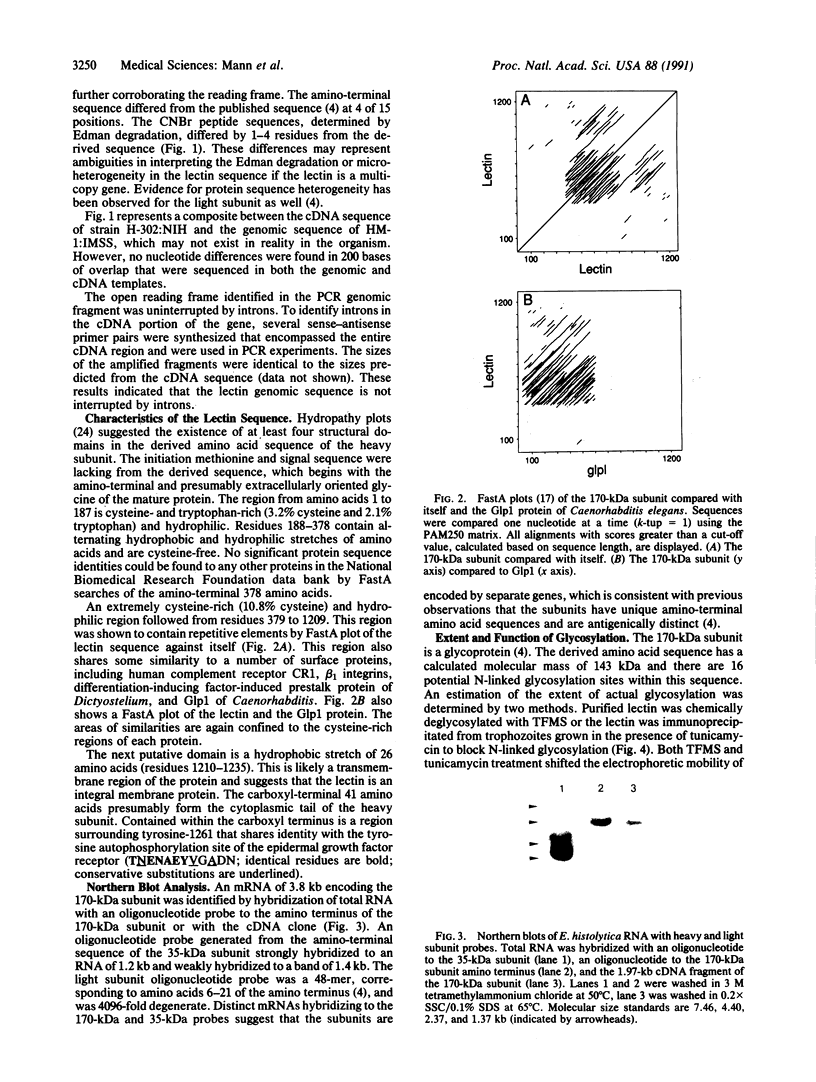


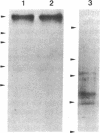
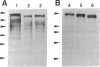
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Bailey G. B., Nudelman E. D., Day D. B., Harper C. F., Gilmour J. R. Specificity of glycosphingolipid recognition by Entamoeba histolytica trophozoites. Infect Immun. 1990 Jan;58(1):43–47. doi: 10.1128/iai.58.1.43-47.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Braiterman L. T., Hubbard A. L. Biochemical characterization of domain-specific glycoproteins of the rat hepatocyte plasma membrane. J Biol Chem. 1985 Oct 15;260(23):12792–12802. [PubMed] [Google Scholar]
- Bracha R., Mirelman D. Adherence and ingestion of Escherichia coli serotype 055 by trophozoites of Entamoeba histolytica. Infect Immun. 1983 Jun;40(3):882–887. doi: 10.1128/iai.40.3.882-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Petri W. A., Jr, Innes D. J., Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Edman U., Meza I., Agabian N. Genomic and cDNA actin sequences from a virulent strain of Entamoeba histolytica. Proc Natl Acad Sci U S A. 1987 May;84(9):3024–3028. doi: 10.1073/pnas.84.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Hagblom P., Harwood J., Aley S. B., Reiner D. S., McCaffery M., So M., Guiney D. G. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Huber M., Garfinkel L., Gitler C., Mirelman D., Revel M., Rozenblatt S. Entamoeba histolytica: cloning and characterization of actin cDNA. Mol Biochem Parasitol. 1987 Jul;24(3):227–235. doi: 10.1016/0166-6851(87)90154-x. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusukawa N., Uemori T., Asada K., Kato I. Rapid and reliable protocol for direct sequencing of material amplified by the polymerase chain reaction. Biotechniques. 1990 Jul;9(1):66-8, 70, 72. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Broman J., Healy G., Quinn T., Ravdin J. I. Antigenic stability and immunodominance of the Gal/GalNAc adherence lectin of Entamoeba histolytica. Am J Med Sci. 1989 Mar;297(3):163–165. doi: 10.1097/00000441-198903000-00006. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Chapman M. D., Snodgrass T., Mann B. J., Broman J., Ravdin J. I. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J Biol Chem. 1989 Feb 15;264(5):3007–3012. [PubMed] [Google Scholar]
- Petri W. A., Jr, Jackson T. F., Gathiram V., Kress K., Saffer L. D., Snodgrass T. L., Chapman M. D., Keren Z., Mirelman D. Pathogenic and nonpathogenic strains of Entamoeba histolytica can be differentiated by monoclonal antibodies to the galactose-specific adherence lectin. Infect Immun. 1990 Jun;58(6):1802–1806. doi: 10.1128/iai.58.6.1802-1806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Ravdin J. I. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect Immun. 1991 Jan;59(1):97–101. doi: 10.1128/iai.59.1.97-101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Snodgrass T. L., Jackson T. F., Gathiram V., Simjee A. E., Chadee K., Chapman M. D. Monoclonal antibodies directed against the galactose-binding lectin of Entamoeba histolytica enhance adherence. J Immunol. 1990 Jun 15;144(12):4803–4809. [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981 Nov;68(5):1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Jackson T. F., Petri W. A., Jr, Murphy C. F., Ungar B. L., Gathiram V., Skilogiannis J., Simjee A. E. Association of serum antibodies to adherence lectin with invasive amebiasis and asymptomatic infection with pathogenic Entamoeba histolytica. J Infect Dis. 1990 Sep;162(3):768–772. doi: 10.1093/infdis/162.3.768. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Flores B. M., Stroeher V. L., Hagen F. S., Stamm W. E. cDNA sequence analysis of a 29-kDa cysteine-rich surface antigen of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6358–6362. doi: 10.1073/pnas.87.16.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Mottet G., Kolakofsky D., Roux L. Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J Virol. 1989 Feb;63(2):892–900. doi: 10.1128/jvi.63.2.892-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986 Mar-Apr;8(2):228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]