Abstract
Recent studies have suggested that in humans and animals with significant skeletal pain, changes in the mechanical hypersensitivity of the skin can be detected. However, whether measuring changes in skin hypersensitivity can be a reliable surrogate for measuring skeletal pain itself remains unclear. To explore this question we generated skeletal pain by injecting and confining GFP-transfected NCTC 2472 osteosarcoma cells unilaterally to the femur of C3H male mice. Beginning at day 7 post-tumor injection, animals were administered vehicle, an antibody to the P2X3 receptor (anti-P2X3) or anti-NGF antibody. Pain and analgesic efficacy was then measured on days 21, 28 and 35 post-tumor injection using a battery of skeletal pain-related behaviors and von Frey assessment of mechanical hypersensitivity on the plantar surface of the hindpaw. Animals with bone cancer pain treated with anti-P2X3 showed a reduction in skin hypersensitivity but no attenuation of skeletal pain behaviors. Whereas animals with bone cancer pain treated with anti-NGF showed a reduction in both skin hypersensitivity and skeletal pain behaviors. These results suggest that while bone cancer can induce significant skeletal pain-related behaviors and hypersensitivity of the skin, relief of hypersensitivity of the skin is not always accompanied by attenuation of skeletal pain. Understanding the relationship between skeletal and skin pain may provide insight into how pain is processed and integrated and help define the preclinical measures of skeletal pain that are predictive endpoints for clinical trials.
INTRODUCTION
Skeletal pain is one of the leading causes of chronic pain and long-term disability. Thus, skeletal pain arising from osteoarthritis, osteoporosis, age-related bone fracture, and bone cancer frequently result in a decline in functional status with an increase in both morbidity and mortality [37; 44; 51]. Unfortunately, the incidence of skeletal pain frequently increases with age. Without new treatments that more effectively relieve skeletal pain, this burden is projected to rapidly grow with the increasing global life expectancy [10; 13; 15; 45].
There are several reasons why skeletal pain remains a significant and largely untreated worldwide health issue. First, there are relatively few analgesic therapies that can effectively relieve skeletal pain without significant unwanted side effects. For example, non-steroidal anti-inflammatory drugs and opiates, the most commonly used therapies to treat skeletal pain, are accompanied by significant renal, hepatic, respiratory, dependency and/or functional status issues when these therapies are used long term [2; 61; 66]. Secondly there is a limited understanding of the underlying mechanisms that drive skeletal pain. This is, in large part, due to the paucity of preclinical skeletal pain models that closely mirror the severity and chronicity of human skeletal pain. Lastly, the available surrogates for skeletal pain in preclinical models (i.e. limb use, weight bearing, nocifensive behaviors, or day/night activity) are time and labor intensive compared to measuring skin hypersensitivity. These factors make it difficult to rapidly screen promising new pharmacological therapies to attenuate skeletal pain.
Recently, hypersensitivity of the skin has been detected in human patients and preclinical animal models of osteoarthritis, low back pain, and bone cancer pain [4; 33; 43; 56; 58; 59; 72]. Given that skin hypersensitivity can be measured much more quickly and easily than skeletal pain-related behaviors, a major question is whether skeletal pain-induced hypersensitivity of the skin is an appropriate and reliable surrogate for assessing skeletal pain.
In the present study we explore this question by measuring hypersensitivity of the skin and skeletal pain-related behaviors in a murine model of cancer-induced bone pain (CIBP) in combination with therapeutic administration of either anti-P2X3 or anti-NGF monoclonal antibodies. Using this approach we found that anti-P2X3 significantly reduced hypersensitivity of skin, but had no significant effect on attenuating skeletal pain-related behaviors. In contrast, anti-NGF, which has been shown to reduce skeletal pain in both humans and animals models [16; 21; 28; 30; 35; 36; 38; 42; 46; 54; 57], attenuated both mechanical hypersensitivity of the skin and skeletal pain-related behaviors. These results suggest that therapies that attenuate skeletal pain-induced skin hypersensitivity may not always predict therapies that also attenuate the underlying skeletal pain.
METHODS
Experiments were performed on adult, male C3H/HeJ mice (n= 49, numbers represent two independent experiments) (Jackson Laboratories, Bar Harbor, ME, USA), approximately 8–9 weeks old, weighing 25–30 g at the time of tumor cell injection. This strain was chosen for its histocompatibility with the NCTC clone 2472 sarcoma tumor line (American Type Culture Collection, Manassas, VA, USA), which has been previously shown to form osteolytic lesions in bone following intramedullary injection [11; 12]. The mice were housed in accordance with National Institutes of Health Guidelines and kept in a vivarium maintained at 22°C with a 12h alternating light–dark cycle and provided food and water ad libitum. All procedures adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [74] and were approved by the Institutional Animal Care and Use Committee at the University of Arizona (Tucson, AZ, USA).
Surgery and implantation of cancer cells
Surgery was performed as previously described in [46]. In brief, an arthrotomy was performed following induction of general anesthesia with ketamine/xylazine (100 mg/kg ketamine and 10 mg/kg xylazine; s.c.). A hole was drilled, using a pneumatic dental high-speed hand piece, in the patellar groove of the femur. A needle was inserted into the intramedullary canal to core a pathway for the sarcoma cells. Mice were injected with Hanks buffered saline (HBSS) (5 μL, Sigma, St. Louis, MO, USA) or 5 × 104 osteolytic murine sarcoma cells (NCTC 2472, American Type Culture Collection, Manassas, VA, USA) transfected with green fluorescent protein (GFP) and suspended in HBSS. The injection site was sealed with a dental amalgam plug (Dentsply, Milford, DE), to confine the cells within the intramedullary canal, followed by irrigation with sterile saline. To prevent the patella from becoming displaced post-arthrotomy, muscles were secured back in position using a horizontal mattress suture. In addition, an incision closure was achieved using wound clips. After surgery, animals were individually housed and allowed to recover for one week before being handled for behavioral and radiological assessment.
Anti-NGF Treatment
The anti-NGF antibody (mAb911) was kindly provided by Drs. Kris Poulsen and David Shelton (Rinat/Pfizer, San Francisco, CA, USA). How this antibody was made and its physical properties have been previously described [23]. This antibody does not effectively cross the blood brain barrier. We have shown that anti-NGF effectively reduces the skeletal pain associated with malignant bone cancer [46]. A dose of 10 mg/kg delivered i.p. was used and was administered on the same schedule as the anti-P2X3 (12D4) antibody.
Anti-P2X3 Treatment
The anti-P2X3 sequestering antibody (12D4) was kindly provided by Drs. Kris Poulsen and David Shelton (Rinat/Pfizer, San Francisco, CA, USA). This antibody was made similarly to the anti-NGF, has similar pharmacokinetics and pharmacodynamics, and does not effectively cross the blood brain barrier. A dose of 30 mg/kg was delivered by intra-peritoneal injection (i.p.) starting at day 7 post-cancer cell injection, and every five days thereafter.
Exclusion Criteria
Animals were observed daily, and criteria for exclusion from the study (and euthanasia) included: rapid weight loss (>20% in one week), patella displacement, lack of intra-femoral cancer cell growth by the end of the study, prolonged digestive abnormalities (e.g., diarrhea or vomiting for over 3 days), severe ulcerative dermatitis or infected tumors, and paralysis. A total of 6 animals were excluded in accordance with the above criteria.
Behavioral Measures of Cancer Induced Bone Pain (CIBP)
Mice were acclimatized to the testing environment for 30 minutes on four consecutive days one week prior to naïve baseline behavioral testing. All behavioral testing was performed in the morning and completed before noon. Testing was performed at baseline (pre-sarcoma inoculation) and 21, 28, and 35 days post-sarcoma inoculation. Mice were assessed for cutaneous stimulus evoked-pain, spontaneous nocifensive behavioral indicators of pain, weight born by the ipsilateral hindlimb, and rearing activity. Each individual behavioral measure was performed by the same experimenter for the duration of the experiment. These individuals were blinded to the treatments animals received.
Cutaneous stimulus evoked-pain (von Frey test)
Mechanical withdrawal thresholds were measured using calibrated von Frey monofilaments (Stoelting, Wood Dale, IL, USA). Animals were placed in plastic cages with a wire-mesh floor. The von Frey filaments were applied in ascending order, beginning with the filament of 0.6 g, to the mid-plantar surface of the ipsilateral hindpaw through the mesh floor. Each probe was applied to the foot until it bent. The time between filament applications was at least 5 seconds. An algorithm was used to compute the 50% withdrawal threshold based on sequentially increasing and decreasing the strength of the filament stimulus applied incrementally [9]. Both ipsilateral and contralateral responses were recorded.
Tail Flick Assay
Animals had approximately two thirds of their tails immersed in a 48°C water bath. The time it took for the mice to remove their tail completely out of the water was recorded three times per mouse. All investigators were blinded to the groups. The average tail flick latency was plotted.
Spontaneous nocifensive behaviors
Briefly, mice were placed in a large Plexiglass box raised 20 inches above the surface of the bench and allowed to acclimate for approximately 15 minutes. Each animal’s movements were recorded from below using Sony Handycam DCR-SR68 cameras. Time spent in nocifensive behavior was assessed by a blinded observer over a 300 second observation period after a 15 minute habituation period. Nocifensive behaviors were defined as any of the following: (a) full guarding (lifting the affected limb and holding it against its body), (b) reduced weight-bearing (affected limb is held in such a way that the foot, or the side of the foot, is merely resting on the floor), (c) tending to the affected limb (abnormal grooming behavior directed solely to affected limb, specifically licking lower limb and foot), (d) flinching the affected limb and (e) sporadic hopping (intermittent jumps without utilizing affected limb). The cumulative duration of pain-related behaviors during the 300-second testing period were plotted. This method is the same as that reported previously [46].
Dynamic weight bearing (DWB)
The percentage of weight borne by each limb of a freely moving animal was measured using a floor-instrumented dynamic weight bearing system (DWB, BioSeb EB Instruments, Pinellas Park, FL). Mice were placed in a small Plexiglass cage (11 × 11 × 22 cm) and a camera was placed on top of the enclosure. Weight of all tested mice was recorded, as it is a required entry for the data acquisition. The animal was allowed to move freely within the apparatus for 5 minutes while the pressure data and live recording were transmitted to a laptop computer via a USB interface. Raw pressure data was automatically synchronized with images from the video camera and the averaged values were recorded on a computer. Using the BioSeb software v1.3, the operator manually “validated” each test period, ensuring each print corresponded to the appropriate paw using the synchronized video feed as a reference. A zone is considered valid when the following parameters were detected: ≥4 g on one captor with a minimum of 2 adjacent captors recording ≥1 g. For each time segment where the weight distribution is stable for more than 0.5 sec, zones that met the minimal criteria are then validated and assigned as either right or left hind paw or front paw by the experimenter according to the video and the scaled map of activated captors. Data is presented as percent weight borne by ipsilateral hindlimb over the total weight borne by both hindlimbs. Dynamic weight bearing was performed as previously described [42; 53; 62].
Rearing Activity
The number of times an animal reared (simultaneously lifted both front paws up from the floor) over a 300 second period was recorded as a measure of voluntary activity. Rearing was assessed during the time the animal was freely moving in the DWB apparatus (see above).
Immunohistochemistry
At day 28 post-sarcoma inoculation, three sham mice and five CIBP mice inoculated vehicle-treated mice were deeply anesthetized with ketamine/xylazine (100 mg/kg ketamine, and 10 mg/kg xylazine, s.c.) and perfused intracardially with 20 mL of 0.1 M phosphate buffered saline (PBS, pH = 7.4 at 4°C) followed by 20 mL of 4% formaldehyde/12.5% picric acid solution in 0.1M PBS (pH 6.9 at 4°C). After perfusion, the ipsilateral and contralateral dorsal root ganglia (DRG) corresponding to lumbar regions L2 were removed and placed in 30% sucrose overnight at 4°C. Ganglia were frozen with a small amount of OCT (Optimal Cutting Temperature) embedding medium (Sakura Finetek USA Inc., Torrance, CA) and placed on the mounting block surface. The block was then inserted into the cryostat (Bright OTF5000, Hacker Industries Inc., Winnsboro, SC) and sections were cut 16 μm thick and stored at −20°C until use. Sections were removed from −20°C and allowed to reach room temperature and then washed in PBS for 30 min. Sections were blocked 1 h at room temperature in 3% normal donkey serum (NDS; Jackson ImmunoResearch, Cat# 017-11-121, West Grove, PA, USA) diluted in PBS with 0.3% Triton-X 100 (Sigma Chemical Co., Cat# X100). The sections were then incubated overnight at room temperature with primary antibodies made in 1% NDS and 0.1% Triton-X 100 in 0.1M PBS. The primary antibodies used were: anti-P2X3 (1:1,000 dilution; Alomone Labs APR-016; lot APR016AN0925, Jerusalem, Israel) and anti-TrkA (1:1,000; R&D Systems AF1056; lot VFA0111011, Minneapolis, MN, USA). To control for nonspecific staining, the primary antibodies were omitted which resulted in the abolishment of specific signal for all antibodies used (data not shown). After three washes in PBS, sections were incubated for 3 h at room temperature with a mixture of secondary fluorescent antibodies: Donkey anti-rabbit Cy3 (1:600; Jackson ImmunoResearch, lot 120534, West Grove, PA, USA) and Donkey anti-goat Alexa Fluor 488 (1:400; Jackson ImmunoResearch, lot 114772). Both mixtures were incubated in 1% NDS in 0.1% Triton-X 100 in PBS. Preparations were then washed three times for 10 min each in PBS and dehydrated through an alcohol gradient (2 min each, 70, 80, 90 and 100%), cleared in xylene (twice for 2 min), and coverslipped with Eukitt mounting media (Sigma Chemical Co., St. Louis, MO, USA). Preparations were allowed to dry at room temperature for at least 12 hours before imaging.
Image acquisition and analysis
Confocal images were acquired with an Olympus Fluoview FV1200 system (Olympus, Melville, NY, USA) equipped with LD (405, 440, 473, 559, 635 nm), Multiline Argon (457, 488, 515 nm), and HeNe(G) (534 nm) lasers. Sequential acquisition mode was used to reduce bleed-through from fluorophores. Images were obtained using 20x air and 60x oil objectives. The average dimensions of the images collected were 211.7 μm × 211.7 μm × 20 μm with each Z-axis slice being 1 μm/slice. All images displayed as a Z projection.
Number of neuronal cell bodies expressing P2X3 alone, TrkA alone, or co-expressing P2X3 and TrkA were counted in the image processing software, FIJI [55]. To quantify the overlap between TrkA+ and P2X3+ neuronal cell bodies in the DRG, digital images were acquired and analyzed on a minimum of three ipsilateral and contralateral DRG sections per animal. The percentage of total neurons that are TrkA+ and P2X3+ was calculated by dividing TrkA+ or P2X3+ neuronal cell bodies by total number of neuronal cell bodies. Images used for illustrative purposes in this publication were globally brightened in FIJI. These images were not used for quantification of TrkA/P2X3 overlap.
Statistical analysis
All statistical analyses were calculated in GraphPad Prism software (GraphPad, La Jolla, CA, USA). One way-ANOVA was used to compare pain-related behavioral changes and TrkA and P2X3 changes between experimental groups, followed by a Tukeys’ post-hoc test comparing each group to each other at each time point. Significance level was set at P < 0.05.
Results
Sustained blockade of P2X3 reduces skin mechanical hypersensitivity in CIBP mice
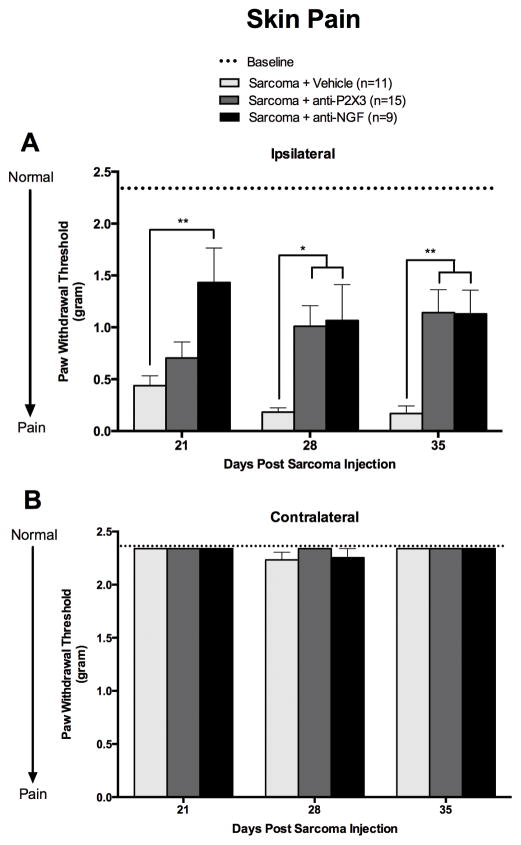
Our mouse model of sarcoma bone metastasis served as a platform to test whether or not skin pain serves as an effective surrogate for skeletal pain. In order to accomplish this we evaluated the effect of two antibody treatments that bind either P2X3 or NGF on a battery of skeletal pain-related behaviors and on cancer-induced mechanical hypersensitivity of the skin. Animals injected with sarcoma cells and treated with vehicle developed significant mechanical hypersensitivity of the hind paw skin by day 21-post sarcoma cell injection and paw withdrawal thresholds continued to decline for the duration of the study. This hypersensitivity did not extend to the tail, as tail flick latencies were not statistically different between any of the tested groups (Supplemental Figure 1). Mechanical hypersensitivity was relieved when sarcoma injected animals were treated with either anti-P2X3 (on days 28 and 35) or anti-NGF (on days 21, 28, and 35) (Figure 1). Throughout the study, sham mice and the contralateral limb of sarcoma injected mice exhibited paw withdrawal thresholds that did not significantly deviate from baseline values (2.34 g) (Figure 1B and data not shown).
Figure 1. Mechanical hypersensitivity of the hindpaw occurs in mice with cancer-induced bone pain (CIBP) and is attenuated by treatment with anti-P2X3 or anti-NGF.
Animals with CIBP, induced by injecting and confining tumor cells to one femur, treated with vehicle (PBS; light gray bars), developed mechanical hypersensitivity of the plantar surface of the hindpaw. This hypersensitivity of the skin increased with time and was present in the ipsilateral (A) but not contralateral plantar surface of the hindpaw (B). Sustained treatment with anti-P2X3 (30 mg/kg, i.p.; dark gray bars) significantly relieved mechanical hypersensitivity of the skin in sarcoma-injected mice at days 28, and 35 when compared to sarcoma + vehicle-treated animals. Anti-NGF treatment (10 mg/kg, i.p.; black bars) significantly reversed mechanical hypersensitivity of the skin at days 21, 28, and 35 when compared to vehicle-treated bone cancer bearing mice. Dashed line represents the baseline (naïve, pre-surgery) values. Error bars represent S.E.M. * P≤0.05 vs. vehicle, ** P≤0.01 vs vehicle.
Skeletal pain behaviors in CIBP mice were alleviated by anti-NGF but not anti-P2X3 treatment
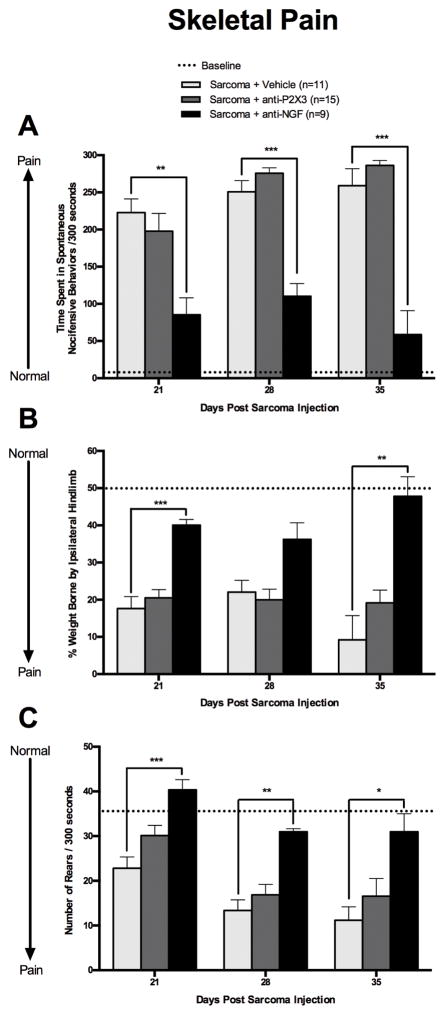
The analgesic efficacy of anti-P2X3 and anti-NGF were tested in CIBP mice using a battery of skeletal pain-related behaviors. These behaviors include spontaneous nocifensive behaviors, weight born by the ipsilateral hind limb, and rearing activity of sarcoma-injected mice (Figs. 2A, B, and C). Sarcoma injected animals treated with vehicle exhibited increased spontaneous nocifensive behaviors on day 21 that continued for the duration of the experiment (Figure 2A, light gray bars). Anti-P2X3 administration in CIBP mice (Figure 2A, dark gray bars) resulted in no difference in spontaneous nocifensive behaviors when compared to tumor-bearing mice treated with vehicle (Figure 2A). In contrast, sarcoma bearing mice treated with anti-NGF (black bars) showed a significant decrease in spontaneous nocifensive behaviors that was significantly different from sarcoma + vehicle (Figure 2A).
Figure 2. Skeletal pain related behaviors in animals with CIBP are reduced by treatment with anti-NGF but not anti-P2X3.
Animals with CIBP treated with vehicle (PBS; light grey bars) exhibited several skeletal pain related behaviors including; increased spontaneous nocifensive behaviors (A), a decline in their ability to place weight on the affected limb (B), and a decline in their ability to rear on their hindlimbs for exploration purposes (C) was significant by day 21 post-tumor injection and these skeletal pain related behaviors increased with disease progression. Sustained anti-P2X3 treatment (30 mg/kg, i.p.; dark grey bars) was ineffective at reducing bone cancer-induced spontaneous nocifensive, changes in hindlimb weight bearing, and increasing rearing activity at all time points. However, animals treated with anti-NGF (10 mg/kg, i.p.; black bars) showed a significant reduction in time spent in spontaneous nocifensive behaviors (at all time points), an increased ability of animals to bear weight on the disease-burdened limb (at days 21 and 35), and an increase in rearing activity (at all time points) when compared to CIBP vehicle treated mice. Dashed line represents the baseline (naïve, pre-surgery) values. Error bars represent S.E.M. * P≤0.05, ** P≤0.01 vs. vehicle, *** P≤0.001 vs vehicle.
The inability of anti-P2X3 treatment to reduce sarcoma-induced skeletal pain-related behaviors was also apparent in the weight bearing of the affected limb (Figure 2B). Sarcoma + vehicle (light gray bars) and sarcoma + anti-P2X3 treated (dark gray bars) mice showed a significant decrease in the amount of weight borne on the tumor-bearing limb over time and were not statistically different from each other at any time point. By day 35 post-sarcoma cell injection, the tumor-bearing hind limb of anti-P2X3 treated animals only bore approximately 20% of the weight borne by the hindlimbs (Figure 2B, dark gray bars). In contrast, sarcoma + anti-NGF treated animals (black bars) restored their ability to bear weight on the ipsilateral limb and was statistically different from sarcoma + vehicle (day 21 and 35, Figure 2B). Similarly, rearing activity in sarcoma + vehicle (light gray bars) and sarcoma + anti-P2X3 (dark gray bars) treated mice was not statistically different from each other (Figure 2C). However, sarcoma + anti-NGF treated animals (black bars) had significantly increased rearing activity compared to sarcoma + vehicle at day 21, 28 and 35 post sarcoma implantation (Figure 2C).
Minimal colocalization of P2X3 and TrkA in neurons of the dorsal root ganglia
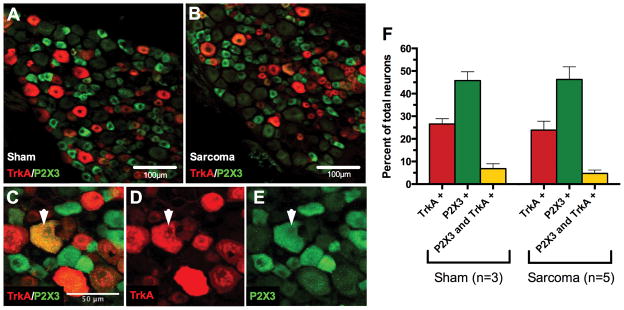
To explore why there was an effect of anti-P2X3 treatment on skin pain but not skeletal pain, we analyzed the distribution of neurons in femur innervating ganglia that expressed TrkA, P2X3, and both TrkA and P2X3. We compared ipsilateral sham to ipsilateral sarcoma L2 dorsal root ganglia from day 28-post sarcoma implantation and quantified the number of neurons that were immunopositive for either receptor by confocal microscopy (Figure 3). Neurons that express TrkA (red bars, sham 26.6%±2.3 of total neurons, sarcoma 23.9%±4.0, mean±SEM) and P2X3 (green bars, sham 45.8%±3.9 of total neurons, sarcoma 46.3%±5.6, mean±SEM) were not statistically different between the two groups, suggesting there is no change in either receptor expression due to sarcoma growth and its associated pain. Others have shown that intrathecal NGF administration increases P2X3 expression in a subset of neurons [52], however here we show that peripheral expression of NGF (by cancer or stromal cells) does not result in an up-regulation of P2X3 expression in neurons in the dorsal root ganglia that innervate the tumor bearing bone. Consistent with previous reports [31], these two receptors label largely separate populations of neurons with limited overlap (yellow bars, sham 6.8±2.2, sarcoma 4.7%±1.5, mean±SEM).
Figure 3. The expression and co-localization of TrkA and P2X3 in the cell bodies of sensory neurons in the L2 dorsal root ganglia that innervate the tumor bearing femur.
L2 dorsal root ganglia isolated from 28-day sham or post sarcoma-injected animals (A and B, respectively) were immunostained for expression of TrkA (red, D) and P2X3 (green, E). The number of neurons that express both P2X3 and TrkA (yellow, C) represent a very small population of neurons in the dorsal root ganglia (6.8% sham, 4.7% sarcoma, F) (these dual immunostained neurons do not necessarily correspond to neuronal cell body size). Note that there was no significant change in the percentage of sensory neurons expressing either P2X3, TrkA, or co-expressing P2X3 and TrkA in animals with CIBP vs. sham control animals (F). Mean±SEM plotted.
DISCUSSION
Potential mechanisms that drive skeletal pain-induced hypersensitivity of the skin
The present study confirms previous results obtained in the mouse [19; 20; 25; 34], rat [31; 40; 67; 73], and human [58] that bone cancer pain can induce a hypersensitivity of the nearby skin. Currently, the mechanism(s) by which skeletal pain drives skin hypersensitivity are not well understood. However, there are at least three possible mechanisms that may be driving this skin hypersensitivity. The first and probably most well investigated mechanism is that skeletal pain is highly efficacious in driving central sensitization and in turn may affect signaling to and from higher centers of the brain resulting in changes to descending modulation and ascending facilitation of pain signaling [18; 26; 63–65; 68].
The second possible mechanism is spinal sensitization, which has been shown to occur in several chronic skeletal pain states such as osteoarthritis and bone cancer pain. In preclinical studies using these models, significant glia, neuronal, and inflammatory changes have been observed in the superficial lamina of the spinal cord [7; 17; 20; 24; 68; 71]. As many of these neurochemical changes evolve and increase with the escalating skeletal dysfunction and accompanying pain, these changes may also affect how even normally non-noxious sensory information from the uninjured skin is processed and perceived by the organism.
Third, most dorsal root ganglia (DRG) contain both the cell bodies of sensory neurons that innervate the skeleton and skin [29; 47]. For example, the DRGs that house the sensory nerve fibers that innervate the tumor-bearing femur (lumbar ganglia) also house the cell bodies of sensory neurons that innervate the plantar surface of the skin [32; 50]. Interestingly, previous studies have shown that in animal models of chronic skeletal pain, the DRGs that innervate the damaged or diseased skeleton show remarkable infiltration by macrophages and changes in supporting cells [50]. Importantly, these changes in the DRG in terms of inflammatory and supporting cells are not confined to the area around sensory nerve fibers that innervate the bone but rather appear to be disseminated throughout the entire ganglia and are equally likely to occur around DRG neurons that innervate the bone or the skin.
Analgesic therapies that attenuate skeletal pain-induced skin hypersensitivity do not always attenuate skeletal pain-related behaviors
To explore the linkage and potential use of skeletal pain-induced skin hypersensitivity as a surrogate for measuring skeletal pain itself, we focused on two therapies in the same mouse model of bone cancer pain. Both therapies, anti-P2X3 and anti-NGF, are mouse monoclonal antibodies that do not readily cross the blood brain barrier (BBB) and thus most likely exert their effects outside the BBB [49]. It should also be noted that previous studies [22; 31; 39; 67; 73] examining whether P2X3 antagonists can relieve bone cancer pain and/or bone cancer pain induced skin hypersensitivity all employed small molecule P2X3 antagonists which unlike, anti-P2X3 or anti-NGF, probably readily cross the BBB.
In the present study, sarcoma + vehicle animals displayed significant skeletal pain behaviors and mechanical hypersensitivity of the skin when compared to sham animals and baseline values. Sarcoma + anti-P2X3 treated animals showed a reduction in skin hypersensitivity but no attenuation of skeletal pain behaviors when compared to the sarcoma + vehicle group. In marked contrast, sarcoma + anti-NGF treated animals showed a reduction in both skin hypersensitivity and skeletal pain behaviors when compared to the sarcoma + vehicle group. Interestingly, these results are in line with those observed by Hansen and colleagues who used the same mouse model of osteosarcoma [22] and a small molecule P2X3 receptor antagonist from which they concluded that while bone cancer can induce significant skeletal pain-related behaviors and hypersensitivity of the skin, relief of hypersensitivity of the skin is not always accompanied by attenuation of the underlying skeletal pain.
In light of the data presented above, a major question is what mechanisms might explain how anti-P2X3 could relieve bone cancer-induced hypersensitivity of the skin but not the underlying bone cancer pain itself? One possibility is that this difference may in part be explained by the unique populations of primary afferent sensory nerve fibers that innervate the skin vs. the skeleton. It has been shown that the number of P2X3 or TrkA expressing neurons in the DRG appears to be conserved between humans and rodents [70]. Previous studies have demonstrated that in the mouse, P2X3 receptors are expressed by sensory nerve fibers that also express Mas-related G protein-coupled receptor member D (MrgprD)+ and IsolectinB 4 (IB4)+ and that this population of P2X3 expressing sensory nerve fibers has been shown to densely innervate the skin but not the bone or joint [3; 14; 29; 41; 47; 69; 75]. In contrast, the great majority of sensory neurons that innervate the bone express TrkA [8; 60] (which is the cognate receptor for NGF) whereas only a minority of the sensory nerve fibers that innervate the skin express TrkA [5; 6; 27; 75]. One other possibility would be that NGF may induce an up-regulation of P2X3 receptors in TrkA+ DRG neurons. Indeed, Ramer et al [52] showed that intrathecal infusion of NGF induced an approximately 1.2 fold increase in the number of DRG neurons that express P2X3 receptor. In the present study we examined whether peripherally released NGF (whether it be by the tumor or stromal cells) also resulted in an up-regulation of P2X3 expression in neurons in the DRG that innervate the tumor bearing bone. However, in the present study there was no increase in the percentage of P2X3 or TrkA expressing neurons in the dorsal root ganglia in sarcoma vs. naïve animals. Together, the present results suggest that blockade of P2X3 only attenuates skin pain whereas sequestration of NGF attenuates both the skeletal pain itself and the skin pain induced by skeletal pain. While other mechanisms are probably involved in driving skeletal pain-induced hypersensitivity of the skin, the present data suggest that the neurochemistry and repertoire of nociceptors that transmit skin vs. skeletal pain may be different and that therapies that relieve skin pain may not always relieve skeletal pain and vice versa.
Is skeletal pain-induced hypersensitivity of the skin a major driver of skeletal pain-related behaviors?
A major question addressed in the present study was whether mechanical sensitivity of skin, that occurs with many skeletal pain states, could be a major player in driving “skeletal pain-related behaviors” such as dynamic weight bearing, limb use, and spontaneous guarding / flinching of the tumor bearing limb. Severe injury to skin (such as a severe burn on the plantar surface of the foot) would be expected to induce very significant changes in mechanical sensitivity of the foot and therefore affect the ability of one to place significant weight on the affected foot. This has been shown in other pain models including inflammatory pain models of the hindpaw [53; 62]. Given that animals with bone cancer exhibit increased mechanical sensitivity in the hindpaw skin, could this skin sensitivity be a driving force behind the “skeletal pain behaviors” measured here? At least in the present model the answer to this question appears to be no, as relief of skin pain by anti-P2X3 treatment did not affect any of the skeletal pain measures in the present study. In contrast, anti-NGF relieved skeletal pain-related behaviors in this model and has been shown to relieve skeletal pain in humans, dogs, rats and mice [16; 21; 28; 30; 36; 38; 54]. Thus, while skin hyperalgesia as assessed by quantitative sensory testing may be common in humans with moderate to severe skeletal pain, these patients rarely self-report skin hypersensitivity as their attention maybe focused on obtaining relief from the underlying skeletal pain. Indeed, it has been noted by clinicians that cancer patients rarely report skin pain even when they are reporting severe cancer pain [56].
Conclusions
The present results suggest that the relief of skin hypersensitivity may not always be a predictive surrogate for measuring the relief of underlying skeletal pain itself. Measuring skin hypersensitivity is much more straightforward, easier and less time intensive than measuring skeletal pain-related behaviors. However, as the repertoire of sensory nerve fibers that innervate the skeleton is quite different from the repertoire of sensory nerve fibers that innervate the skin, it would not be surprising if the set of peripheral factors that drive skeletal pain are not in some ways different from the set of peripheral factors that drive skin pain. Developing a better understanding of how skeletal pain drives skin hypersensitivity should provide interesting and unique insights into how pain from different tissues and regions of the body is processed and integrated. However, using attenuation of skin hypersensitivity as the sole or a pivotal surrogate for measuring the relief of skeletal pain may lead to false positives (or negatives) in preclinical and clinical studies.
Supplementary Material
Supplemental Figure 1. Skin hypersensitivity to heat that might be driven by CIBP does not appear to extend caudally to the skin of the tail. CIBP animals treated with vehicle (PBS; light grey bars) exhibited tail flick latency that was not statistically different from either naïve (black bars) or CIBP animals treated with anti-P2X3 (dark grey bars). Mean±SEM plotted.
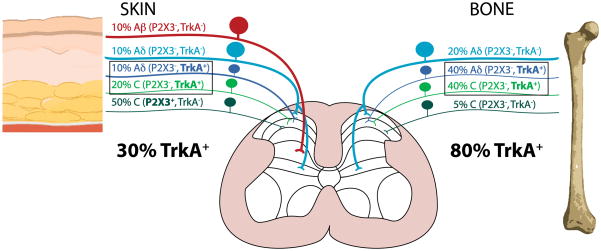
Figure 4. Schematic illustrating the approximate percentage and types of TrkA and P2X3 expressing sensory nerve fibers that innervate the skin vs. bone.
The skin is innervated by thickly myelinated A-beta fibers (TrkA−, P2X3−), thinly myelinated A-delta fibers (TrkA−, P2X3−), unmyelinated peptide-rich C fibers (TrkA+, P2X3−) and unmyelinated peptide-poor C fibers (TrkA+, P2X3+). In contrast, the bone appears to completely lack any A-beta sensory nerve fibers and to be predominantly innervated by thinly myelinated A-delta fibers (TrkA+, P2X3−) and unmyelinated C fibers (TrkA+, P2X3−). In skin and bone there is also a small proportion (5% of the total) of unmyelinated C fibers (TrkA−, P2X3−). The percentages and types of sensory nerve fibers innervating the skin were estimated using data from previous studies (Bennett et al., 1996; Lu et al., 2001; Ambalavanar et al., 2005; Zylka et al., 2005) and for bone from the present and previous studies (Nakajima et al., 2008; Sugiura et al., 2008; Jimenez-Andrade et al., 2010). Note that the skin is heavily innervated by P2X3+ but not TrkA+ nerve fibers, where as the majority of sensory nerve fibers in bone are TrkA+ but not P2X3+.
Acknowledgments
We would like to thank Alec Calac, Stephane Chartier, Natalie Hamdan, Logan Moore, and Sabrina Rivas for their help with image analysis. We would also like to thank Stefanie Mitchell for help with editing the manuscript. This research was funded by NIH grants CA154550, CA157449, and NS023970 to Patrick Mantyh. The antibodies used in this study were a kind gift from Drs. Kris Poulsen and David Shelton (Rinat/Pfizer, San Francisco, CA, USA). Dr. Mantyh has served as a consultant and/or received research grants from Abbott (Abbott Park, IL), Adolor (Exton, PA), Array Biopharma (Boulder, CO), Johnson and Johnson (New Brunswick, NJ), Merck (White Plains, New York), Pfizer (New York, NY), Plexxikon (Berkeley, CA), Rinat (South San Francisco, CA), and Roche (Palo Alto, CA).
Footnotes
All other authors report no conflict of interest.
References
- 1.Abdelaziz DM, Abdullah S, Magnussen C, Ribeiro-da-Silva A, Komarova SV, Rauch F, Stone LS. Behavioral signs of pain and functional impairment in a mouse model of osteogenesis imperfecta. Bone. 2015;81:400–406. doi: 10.1016/j.bone.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, Knaggs R, Martin D, Sampson L, Schofield P. Guidance on the management of pain in older people. Age and ageing. 2013;42:i1–57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117(3):280–291. doi: 10.1016/j.pain.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Andresen T, Pfeiffer-Jensen M, Brock C, Drewes AM, Arendt-Nielsen L. A human experimental bone pain model. Basic & clinical pharmacology & toxicology. 2013;112(2):116–123. doi: 10.1111/bcpt.12002. [DOI] [PubMed] [Google Scholar]
- 5.Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30(13):1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neuroscience letters. 1996;206(1):33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 7.Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, Turner JM, Hathway GJ, Bennett AJ, Walsh DA, Kendall DA, Lichtman A, Chapman V. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PloS one. 2013;8(11):e80440. doi: 10.1371/journal.pone.0080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR, Mantyh PW. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196–207. doi: 10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 10.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clohisy DR, Ogilvie CM, Carpenter RJ, Ramnaraine ML. Localized, tumor-associated osteolysis involves the recruitment and activation of osteoclasts. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1996;14(1):2–6. doi: 10.1002/jor.1100140103. [DOI] [PubMed] [Google Scholar]
- 12.Clohisy DR, Ogilvie CM, Ramnaraine ML. Tumor osteolysis in osteopetrotic mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1995;13(6):892–897. doi: 10.1002/jor.1100130613. [DOI] [PubMed] [Google Scholar]
- 13.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Annals of the rheumatic diseases. 2014 doi: 10.1136/annrheumdis-2013-204763. annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 14.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106(5):619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 15.Gerland P, Raftery AE, Ševčíková H, Li N, Gu D, Spoorenberg T, Alkema L, Fosdick BK, Chunn J, Lalic N. World population stabilization unlikely this century. Science. 2014;346(6206):234–237. doi: 10.1126/science.1257469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Coughlin KA, Kaczmarska MJ, Castaneda-Corral G, Bloom AP, Kuskowski MA, Mantyh PW. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis and rheumatism. 2012;64(7):2223–2232. doi: 10.1002/art.34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu X, Zhang J, Ma Z, Wang J, Zhou X, Jin Y, Xia X, Gao Q, Mei F. The role of N-methyl-D-aspartate receptor subunit NR2B in spinal cord in cancer pain. European journal of pain. 2010;14(5):496–502. doi: 10.1016/j.ejpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Guan X, Fu Q, Xiong B, Song Z, Shu B, Bu H, Xu B, Manyande A, Cao F, Tian Y. Activation of PI3Kgamma/Akt pathway mediates bone cancer pain in rats. Journal of neurochemistry. 2015;134(3):590–600. doi: 10.1111/jnc.13139. [DOI] [PubMed] [Google Scholar]
- 19.Hald A, Hansen RR, Thomsen MW, Ding M, Croucher PI, Gallagher O, Ebetino FH, Kassem M, Heegaard AM. Cancer-induced bone loss and associated pain-related behavior is reduced by risedronate but not its phosphonocarboxylate analog NE-10790. International journal of cancer Journal international du cancer. 2009;125(5):1177–1185. doi: 10.1002/ijc.24436. [DOI] [PubMed] [Google Scholar]
- 20.Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. European journal of pain. 2009;13(2):138–145. doi: 10.1016/j.ejpain.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer research. 2005;65(20):9426–9435. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 22.Hansen RR, Nasser A, Falk S, Baldvinsson SB, Ohlsson PH, Bahl JM, Jarvis MF, Ding M, Heegaard AM. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. European journal of pharmacology. 2012;688(1–3):27–34. doi: 10.1016/j.ejphar.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Hongo JS, Laramee GR, Urfer R, Shelton DL, Restivo T, Sadick M, Galloway A, Chu H, Winslow JW. Antibody binding regions on human nerve growth factor identified by homolog- and alanine-scanning mutagenesis. Hybridoma. 2000;19(3):215–227. doi: 10.1089/02724570050109611. [DOI] [PubMed] [Google Scholar]
- 24.Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, O’Keefe PF, Ramnaraine ML, Clohisy DR, Mantyh PW. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nature medicine. 2000;6(5):521–528. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 25.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98(3):585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 26.Hu XM, Liu YN, Zhang HL, Cao SB, Zhang T, Chen LP, Shen W. CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. Journal of neurochemistry. 2015;132(4):452–463. doi: 10.1111/jnc.12985. [DOI] [PubMed] [Google Scholar]
- 27.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nature reviews Neuroscience. 2001;2(2):83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152(11):2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone. 2010;46(2):306–313. doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, Barthold CM, Peters CM, Buus RJ, Ghilardi JR, Lewis JL, Kuskowski MA, Mantyh PW. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133(1–3):183–196. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain : a journal of neurology. 2010;133(9):2549–2564. doi: 10.1093/brain/awq194. [DOI] [PubMed] [Google Scholar]
- 32.Kawarai Y, Suzuki M, Yoshino K, Inoue G, Orita S, Yamauchi K, Aoki Y, Ishikawa T, Miyagi M, Kamoda H, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Sato J, Nakamura J, Takaso M, Toyone T, Takahashi K, Ohtori S. Transient receptor potential vanilloid 1-immunoreactive innervation increases in fractured rat femur. Yonsei medical journal. 2014;55(1):185–190. doi: 10.3349/ymj.2014.55.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JS, Ahmadinia K, Li X, Hamilton JL, Andrews S, Haralampus CA, Xiao G, Sohn HM, You JW, Seo YS. Development of an Experimental Animal Model for Lower Back Pain by Percutaneous Injury-Induced Lumbar Facet Joint Osteoarthritis. Journal of cellular physiology. 2015 doi: 10.1002/jcp.25015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, Vanderah TW, Lai J, Porreca F. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain. 2007;132(1–2):154–168. doi: 10.1016/j.pain.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, West CR, Verburg KM. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154(7):1009–1021. doi: 10.1016/j.pain.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Kras JV, Kartha S, Winkelstein BA. Intra-articular nerve growth factor regulates development, but not maintenance, of injury-induced facet joint pain & spinal neuronal hypersensitivity. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(11):1999–2008. doi: 10.1016/j.joca.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen P, Lund H, Laessoe U, Graven-Nielsen T, Rasmussen S. Restrictions in Quality of Life after Intramedullary Nailing of Tibial Shaft Fracture: A retrospective follow-up study of 223 cases. Journal of orthopaedic trauma. 2014;28(9):507–512. doi: 10.1097/BOT.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 38.Lascelles BD, Knazovicky D, Case B, Freire M, Innes JF, Drew AC, Gearing DP. A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC veterinary research. 2015;11:101. doi: 10.1186/s12917-015-0413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Zhang X. Epigallocatechin-3-gallate attenuates bone cancer pain involving decreasing spinal Tumor Necrosis Factor-alpha expression in a mouse model. International immunopharmacology. 2015 doi: 10.1016/j.intimp.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Liu M, Yang H, Fang D, Yang JJ, Cai J, Wan Y, Chui DH, Han JS, Xing GG. Upregulation of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in dorsal root ganglions contributes to the bone cancer pain in rats. Pain. 2013;154(9):1551–1568. doi: 10.1016/j.pain.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Lu J, Zhou XF, Rush RA. Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neuroscience research. 2001;41(4):355–363. doi: 10.1016/s0168-0102(01)00293-0. [DOI] [PubMed] [Google Scholar]
- 42.Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain. 2015;156(1):157–165. doi: 10.1016/j.pain.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malfait A-M, Little CB, McDougall JJ. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis and Cartilage. 2013;21(9):1316–1326. doi: 10.1016/j.joca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nature reviews Neuroscience. 2006;7(10):797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 45.Mathers CD, Stevens GA, Boerma T, White RA, Tobias MI. Causes of international increases in older age life expectancy. The Lancet. 2015;385(9967):540–548. doi: 10.1016/S0140-6736(14)60569-9. [DOI] [PubMed] [Google Scholar]
- 46.McCaffrey G, Thompson ML, Majuta L, Fealk MN, Chartier S, Longo G, Mantyh PW. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer research. 2014;74(23):7014–7023. doi: 10.1158/0008-5472.CAN-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakajima T, Ohtori S, Yamamoto S, Takahashi K, Harada Y. Differences in innervation and innervated neurons between hip and inguinal skin. Clinical orthopaedics and related research. 2008;466(10):2527–2532. doi: 10.1007/s11999-008-0432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogbonna AC, Clark AK, Gentry C, Hobbs C, Malcangio M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. European journal of pain. 2013;17(4):514–526. doi: 10.1002/j.1532-2149.2012.00223.x. [DOI] [PubMed] [Google Scholar]
- 49.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36(4):555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 50.Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, Mach DB, Schwei MJ, Sevcik MA, Mantyh PW. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Experimental neurology. 2005;193(1):85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 51.Rabenda V, Manette C, Lemmens R, Mariani A-M, Struvay N, Reginster J-Y. Prevalence and impact of osteoarthritis and osteoporosis on health-related quality of life among active subjects. Prevalence. 2015 doi: 10.1007/BF03325211. [DOI] [PubMed] [Google Scholar]
- 52.Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. Journal of neurochemistry. 2001;77(3):864–875. doi: 10.1046/j.1471-4159.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 53.Robinson I, Sargent B, Hatcher JP. Use of dynamic weight bearing as a novel end-point for the assessment of Freund’s Complete Adjuvant induced hypersensitivity in mice. Neuroscience letters. 2012;524(2):107–110. doi: 10.1016/j.neulet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Sanga P, Katz N, Polverejan E, Wang S, Kelly KM, Haeussler J, Thipphawong J. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain. 2013;154(10):1910–1919. doi: 10.1016/j.pain.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 55.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt BL. The neurobiology of cancer pain. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014;20(5):546–562. doi: 10.1177/1073858414525828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnitzer TJ, Ekman EF, Spierings EL, Greenberg HS, Smith MD, Brown MT, West CR, Verburg KM. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Annals of the rheumatic diseases. 2014 doi: 10.1136/annrheumdis-2013-204905. annrheumdis-2013-204905. [DOI] [PubMed] [Google Scholar]
- 58.Scott AC, McConnell S, Laird B, Colvin L, Fallon M. Quantitative Sensory Testing to assess the sensory characteristics of cancer-induced bone pain after radiotherapy and potential clinical biomarkers of response. European journal of pain. 2012;16(1):123–133. doi: 10.1016/j.ejpain.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Shechter R, Yang F, Xu Q, Cheong Y-K, He S-Q, Sdrulla A, Carteret AF, Wacnik PW, Dong X, Meyer RA. Conventional and kilohertz-frequency spinal cord stimulation produces intensity-and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology. 2013;119(2):422. doi: 10.1097/ALN.0b013e31829bd9e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiura A, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Norimoto M, Orita S, Eguchi Y, Takahashi Y, Watanabe TS, Ochiai N, Takaso M, Takahashi K. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine. 2008;33(19):2047–2051. doi: 10.1097/BRS.0b013e31817f8d58. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154(Suppl 1):S94–100. doi: 10.1016/j.pain.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tetreault P, Dansereau MA, Dore-Savard L, Beaudet N, Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiology & behavior. 2011;104(3):495–502. doi: 10.1016/j.physbeh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Urch CE, Donovan-Rodriguez T, Dickenson AH. Alterations in dorsal horn neurones in a rat model of cancer-induced bone pain. Pain. 2003;106(3):347–356. doi: 10.1016/j.pain.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. The Journal of physiology. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 66.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis care & research. 2014;66(10):1489–1495. doi: 10.1002/acr.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu JX, Xu MY, Miao XR, Lu ZJ, Yuan XM, Li XQ, Yu WF. Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. European journal of pain. 2012;16(10):1378–1388. doi: 10.1002/j.1532-2149.2012.00149.x. [DOI] [PubMed] [Google Scholar]
- 68.Yanagisawa Y, Furue H, Kawamata T, Uta D, Yamamoto J, Furuse S, Katafuchi T, Imoto K, Iwamoto Y, Yoshimura M. Bone cancer induces a unique central sensitization through synaptic changes in a wide area of the spinal cord. Molecular pain. 2010;6:38. doi: 10.1186/1744-8069-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye Y, Ono K, Bernabe DG, Viet CT, Pickering V, Dolan JC, Hardt M, Ford AP, Schmidt BL. Adenosine triphosphate drives head and neck cancer pain through P2X2/3 heterotrimers. Acta neuropathologica communications. 2014;2:62. doi: 10.1186/2051-5960-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yiangou Y, Facer P, Birch R, Sangameswaran L, Eglen R, Anand P. P2X3 receptor in injured human sensory neurons. Neuroreport. 2000;11(5):993–996. doi: 10.1097/00001756-200004070-00019. [DOI] [PubMed] [Google Scholar]
- 71.Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008;154(4):1533–1538. doi: 10.1016/j.neuroscience.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han J-S, Xing G-G. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. PAIN®. 2013;154(3):434–448. doi: 10.1016/j.pain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Zhou YL, Jiang GQ, Wei J, Zhang HH, Chen W, Zhu H, Hu S, Jiang X, Xu GY. Enhanced Binding Capability of Nuclear Factor-small ka, CyrillicB with Demethylated P2X3 receptor Gene Contributes to Cancer Pain in Rats. Pain. 2015 doi: 10.1097/j.pain.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 75.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45(1):17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Skin hypersensitivity to heat that might be driven by CIBP does not appear to extend caudally to the skin of the tail. CIBP animals treated with vehicle (PBS; light grey bars) exhibited tail flick latency that was not statistically different from either naïve (black bars) or CIBP animals treated with anti-P2X3 (dark grey bars). Mean±SEM plotted.