Abstract
Background
Dengue virus (DENV) infection is widespread and its disease burden has increased in past decades. However, little is known about the epidemiology of dengue in the Middle East and North Africa (MENA).
Methodology / Principal Findings
Following Cochrane Collaboration guidelines and reporting our findings following PRISMA guidelines, we systematically reviewed available records across MENA describing dengue occurrence in humans (prevalence studies, incidence studies, and outbreak reports), occurrence of suitable vectors (Aedes aegypti and Aedes albopictus), and DENV vector infection rates. We identified 105 human prevalence measures in 13 of 24 MENA countries; 81 outbreaks reported from 9 countries from 1941–2015; and reports of Ae. aegypti and/or Ae. albopictus occurrence in 15 countries. The majority of seroprevalence studies were reported from the Red Sea region and Pakistan, with multiple studies indicating >20% DENV seroprevalence in general populations (median 25%, range 0–62%) in these subregions. Fifty percent of these studies were conducted prior to 1990. Multiple studies utilized assays susceptible to serologic cross-reactions and 5% of seroprevalence studies utilized viral neutralization testing. There was considerable heterogeneity in study design and outbreak reporting, as well as variability in subregional study coverage, study populations, and laboratory methods used for diagnosis.
Conclusions / Significance
DENV seroprevalence in the MENA is high among some populations in the Red Sea region and Pakistan, while recent outbreaks in these subregions suggest increasing incidence of DENV which may be driven by a variety of ecologic and social factors. However, there is insufficient study coverage to draw conclusions about Aedes or DENV presence in multiple MENA countries. These findings illustrate the epidemiology of DENV in the MENA while revealing priorities for DENV surveillance and Aedes control.
Author Summary
Dengue is a mosquito-transmitted flavivirus whose global distribution and disease incidence has increased in recent decades. In the Middle East and North Africa, the epidemiology of dengue remains poorly characterized despite increasing reports of outbreaks and transmission in new areas. In order to understand the evidence supporting the epidemiology of this virus in the region and the areas in need of further research, we conducted a systematic review of studies reporting human prevalence, incidence, and infection rates in the virus’ main mosquito vectors, Aedes aegypti and Aedes albopictus. Among the studies identified, the Red Sea subregion and Pakistan reported the highest seroprevalence estimates for dengue. However, we encountered substantial heterogeneity in the distribution, quality, and quantity of published studies. These findings inform future research and surveillance priorities for DENV in the MENA region.
Introduction
Dengue virus (DENV) is a globally distributed flavivirus with nearly 400 million estimated annual infections and a growing geographic distribution and disease burden [1–3]. DENV has a historic presence in the Middle East and North Africa (MENA), with outbreaks of dengue and dengue-like disease reported across much of the Eastern Mediterranean region in the 19th and early 20th centuries [4, 5]. Today, DENV may be resurging in the MENA [6, 7], with recent outbreaks of unprecedented or previously unrecognized magnitude occurring in the Arabian Peninsula and Pakistan [8, 9], and a 2015 outbreak in Egypt that occurred following a decades-long absence of reported cases from that country [10]. Still, despite increasing global concern about the threat of Aedes-transmitted arboviruses, the epidemiology of DENV in the MENA region remains largely uncharacterized.
Understanding the epidemiology of DENV in the MENA represents an ongoing challenge for multiple reasons [11]. Inadequate human and vector surveillance, non-reporting of illness syndromes, and poor diagnostic capacity limit DENV detection in many countries, resulting in delays in outbreak recognition and sparse data with which to estimate disease burden and infection rates [12–14]. Case series, outbreak reports, and national notification reports, which contribute much to the epidemiologic knowledge of DENV, may also contain bias in reflecting only those areas with sufficient capacity to detect and report DENV when it occurs [1]. Moreover, clinical diagnosis of DENV infection in the absence of laboratory confirmation is often unreliable [12, 15–18]. Cross-sectional serologic surveys for DENV exposure have the potential to shed light on the broader population burden of DENV without these biases. However, serologic cross-reactions among antibody-based assays for flaviviruses can limit the reliability of such studies in the absence of confirmatory testing, though the latter is difficult to perform and often unavailable [19, 20].
To further the knowledge of the epidemiology of DENV in the MENA, we undertook a comprehensive summary and appraisal of published DENV prevalence, incidence, vector infection rates, reported outbreaks, and Aedes occurrence reports in the MENA region. This report aims to enhance the understanding of the epidemiology of DENV in the MENA while informing priorities for future research.
Materials and Methods
Objectives
The objective of this study was to characterize the epidemiology of DENV in the MENA region through a systematic review of human prevalence and incidence studies and infection rates in Aedes mosquitoes. We also aimed to summarize reported human outbreaks and Ae. aegypti and Ae. albopictus occurrence in the region. The original search was last updated on December 9, 2015.
Eligibility criteria
Table 1 displays the eligibility criteria. In brief, studies containing primary prevalence, incidence, and vector infection rates for DENV in the MENA region were considered eligible for the systematic review. Publication year was not considered an inclusion criterion, as we reasoned that the historic distribution of DENV could be useful in understanding its current epidemiology by depicting ecologically viable regions in which DENV transmission continues to occur or could re-emerge. For incidence studies, those that reported the number of acute infections or seroconversions over any time interval were eligible. Vector infection rate studies were included if they contained a measure of the estimated proportion of infected Ae. aegypti or Ae. albopictus at a given time and setting in the MENA region.
Table 1. Criteria for study inclusion or exclusion.
| Study type | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Human prevalence/incidence | ||
| publication characteristics | Full article or abstract published in any year, language, setting, or population in the MENA region; any seroconversion interval for incidence studies | Case reports, case series, editorials, letters to editors, reviews, commentaries, qualitative studies, basic science research studies, studies from countries outside the MENA region |
| study design | Any randomized or non-randomized design | Non-empirical research/modelled data |
| outcomes | DENV seroprevalence or prevalence of laboratory-confirmed infection; DENV incidence (by any laboratory method) | No human prevalence or incidence measure reported |
| Vector infection rate | Reported Ae. aegypti or Ae. albopictus infection rates by any laboratory method | Basic science research studies, infection rates in other mosquito species or non-MENA country |
Outcomes
For the systematic review, the primary outcomes were DENV human prevalence, incidence, and vector infection rates in the MENA region. Secondary outcomes were reports of dengue outbreaks and vector occurrence.
Data sources and search strategy
We conducted a systematic search for DENV in the MENA following Cochrane Collaboration guidelines [21] and reported our findings using the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines [22]. The PRISMA checklist is found in S1 Fig and our search criteria in S2 Fig. Briefly, we searched PubMed, Embase, the World Health Organization (WHO) Index Medicus for the Eastern Mediterranean Region and WHO African Index Medicus without publication date or language restrictions, using text and MeSH/Emtree terms exploded to include all subheadings. Our review covered the 23 countries included in the MENA definitions of the WHO/EMRO, World Bank, and the Joint United Nations Programme on HIV/AIDS (UNAIDS) for consistency with earlier regional analyses of various infectious diseases including HIV [23].
Study selection
For each search, titles and abstracts were imported into Endnote (Thompson Reuters, Philadelphia, PA, USA), duplicates were removed, and were screened by one author (JH) with potential eligibility determined by consensus with a second author (NC) when eligibility was unclear. Full texts of potentially relevant records were retrieved and assessed for eligibility, contacting the author of the report as necessary. Reference lists of all potentially eligible articles and reviews were also searched. In this study, ‘report’ refers to the document (paper, abstract, or public health record) containing an outcome measure of interest, while ‘study’ refers to the outcome measure(s) within that report. Hence, reports could contribute more than one study, though multiple reports of the same study were counted only once.
Data Extraction and Synthesis
Data were extracted by one of the authors (JH) using a pre-piloted data extraction form and entered into a database created in Microsoft Access. Data from reports in English were extracted from the full texts, while reports in French (n = 6), Turkish (n = 3), Dutch (n = 1), and German (n = 1) were extracted from the abstracts and full texts with the help of online language software and French, Turkish, and German language speakers [24]. There were no records in other languages. Studies were compiled by country and organized by year, using separate tables for human prevalence, incidence, and vector infection rates. Prevalence studies were further stratified as follows: 1) general prevalence studies measuring the prevalence of anti-DENV antibodies among populations without acute infection (e.g. DENV exposure); and 2) acute DENV infection studies assessing the prevalence of laboratory-confirmed DENV infection in those with a) undifferentiated acute febrile illness (AFI) and b) suspected DENV infection (Table 2). These stratifications were made because of the different study aims and probabilities of having laboratory evidence of DENV infection in each of these populations. Finally, the geographic distribution of all included prevalence studies were mapped according to the first-level administrative division (e.g. state, province) in which each study was conducted (Tableau Software, Seattle, WA, USA).
Table 2. Definitions of human prevalence study populations identified through the systematic review.
| Study Population | Definition |
|---|---|
| General prevalence | Seroprevalence studies reporting anti-DENV IgG prevalence measures among individuals not suspected to have acute dengue infection, including community members, blood donors, military, students, and hospitalized patients and outpatients receiving care for non-febrile illnesses. |
| Acute DENV infection | Undifferentiated acute febrile illness (AFI): studies for which acute dengue infection is not differentiated by clinical grounds alone; IgG prevalence measures obtained during the acute phase of illness is these studies are presumed to reflect secondary infection. |
| Suspected dengue infection: studies in which defined or undefined clinical criteria for probable dengue infection is stated as an inclusion criterion in the study. |
Risk of bias assessment
In order to gain a better understanding of the quality of prevalence studies identified through the systematic review, the risk of bias (ROB) was assessed for each study based on the Cochrane approach [25] and by evaluating the precision of the reported measures. The methodology for this assessment is similar to that which we have previously developed for reviews of HIV and hepatitis C prevalence in the MENA region [26–28]. Each DENV prevalence measure was considered to have a low, high, or unclear ROB in three domains: sampling methodology, DENV infection ascertainment, and response rate. The latter was defined as the number of tested individuals divided by the number of persons invited to participate in the study [29]. ROB was considered low if (1) sampling was probability-based (i.e. using some form of random selection), (2) DENV prevalence measures included viral neutralization testing (VNT) for general prevalence studies or biological assays (i.e. cell culture, PCR, and NS1 ELISA) for acute infection studies, and (3) response rate was ≥80%. Studies with missing information for any of the domains were classified as having unclear ROB for that specific domain. Sampling strategy was not evaluated for acute infection studies because these studies enrolled individuals presenting to a health facility with acute infection, hence, no population-based sampling is needed to capture this population. Studies were considered to have high precision if the number of individuals tested was ≥ 100. We considered this to be a reasonably sensitive cutoff for precision given the heterogeneous epidemiology of DENV across the region (e.g. a prevalence of 1% entails a 95% CI of 0–3%).
DENV outbreaks and Aedes distribution
To supplement the epidemiologic data gathered through the systematic search, reported outbreaks and Ae. aegypti or Ae. albopictus occurrence in the MENA were also sought from the articles retrieved through the search databases as well as through ProMED-MENA and Google Scholar. Given that the characteristics and definitions of dengue outbreaks in the literature are implicitly variable and that there is currently no consensus on how to define such events [30], we broadly included any outbreak report if the author of the report defined the event as an outbreak. Multiple reports of the same outbreak were recorded only once. We manually marked the location of reported outbreaks on the map as well, designating one mark per each first-level administrative division in which one or more outbreaks were identified. In a separate map, we mapped the country-level occurrence of Ae. aegypti and Ae. albopictus in order to further inform the existing or potential epidemiology of DENV in the MENA.
Results
Search results
The selection process based on PRISMA guidelines is illustrated in Fig 1 [22]. Briefly, the DENV search yielded 1,258 citations, 91 of which were ultimately eligible for inclusion in the study following the addition of 4 reports identified from the bibliographies of relevant reports and reviews. Four studies from the 1970-80s were excluded that contained DENV seroprevalence of 0–11% in wild and domestic animals in Pakistan, Tunisia, and Turkey, though these may have represented cross-reactions with other flaviviruses [31–34].
Fig 1. PRISMA flow diagram of article selection.
Flow diagram for dengue prevalence, incidence, and vector infection rates in the Middle East and North Africa.
Characteristics of included studies
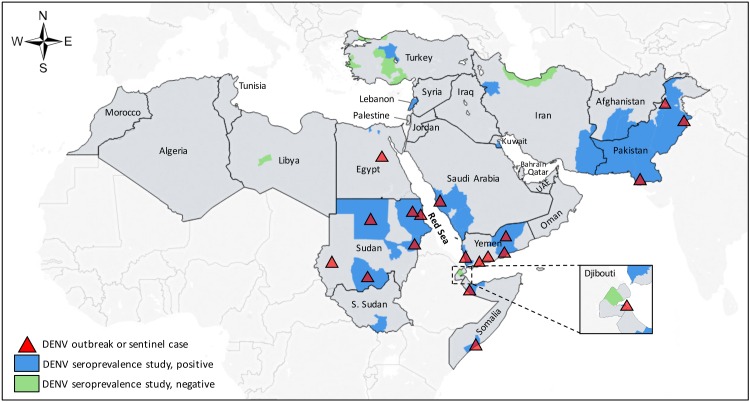
A total of 105 human prevalence studies for DENV were identified from eligible reports (Table 3). These studies covered 13 of 24 MENA countries and were conducted from 1962–2015. The geographic distribution of these studies is illustrated in Fig 2, and Table 4 contains a frequency summary of these studies. Anti-DENV antibodies were detected in 12 of 13 countries in which studies were reported with a single 1973 study from Libya reporting 0% seroprevalence [35]. The highest number of studies were reported from Pakistan (n = 32) and Sudan (n = 16), most of which targeted populations with acute DENV infection (undifferentiated AFI or suspected dengue infection). Among general population studies, IgG prevalence measures ranged from 0% to 61% and were reported from Djibouti (n = 4, 0–21%), Egypt (n = 4, 0–7%), Iran (n = 3, 0–7%), Kuwait (n = 3, 0–56%), Lebanon (n = 3, 0–61%), Pakistan (n = 3, 9–28%), Saudi Arabia (n = 4, 0–33%), and Sudan (n = 5, 9–49%). ELISAs were the most commonly used diagnostic method for all study types and the majority studies from the MENA used in-house assays (Table 4). VNT results were reported in 3% (n = 3) of all studies while observed or potential serologic cross-reactions with other flaviviruses were present in multiple studies [36–38] (Tables 3 and 4). Three human incidence measures for DENV were identified (Table 5): the first reported an ELISA IgM incidence of 35 cases per 10,000 people living in urban homes in Port Sudan City, Sudan where DENV-carrying mosquitoes were identified over an 11-month period [39]; the second reported an ELISA IgM incidence of 94 cases per 10,000 people in a general population in Port Sudan, Sudan over a 17-week period in 2010 [40]; the third reported an ELISA IgM, NS1 antigen, or PCR incidence of 185 cases per 100,000 febrile children in an urban slum in Karachi, Pakistan from 1999–2001 [41]. Three vector infection rate studies for Ae. aegypti and Ae. albopictus were identified from Pakistan and Yemen [42, 43] (Table 6).
Table 3. Human prevalence studies for dengue virus in the Middle East and North Africa (n = 105).
| Country, Ref. | Year(s) of study* | City or governorate | Setting; population (age range, years) | Sampling | Assay type | Assay make† | Target Protein | Assay serotype | Sample size | Prevalence | Additional testing& Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Afghanistan (n = 1) | |||||||||||
| Elyan [44] | 2008–10 | Uruzgon, Helmand, Kandahar | Hospital; AFI patients (20–59) | Conv. | ELISA | PanBio | Env | 1–4 | 913 | 19.2%** | 2.6% (8/312) were IgM+; observed cross-reaction to WNV, TBEV |
| Djibouti (n = 6) | |||||||||||
| Salah [45] | 1987 | Djibouti City | Military; healthy soldiers | Conv. | IIFA | In-house | wv | 2 | 50 | 0% | |
| Randa | Rural community; general pop. | Conv. | IIFA | In-house | wv | 2 | 69 | 0% | |||
| Djibouti City | Hospital; AFI patients | Conv. | IIFA | In-house | wv | 2 | 41 | 0% | |||
| Rodier [37] | 1991 | Djibouti City | Clinical setting; AFI patients (1–55) | Conv. | ELISA IgM | In-house | wv | 1 | 91 | 7.7%** | 3.7% (1/27) were VNT+; multiple observed cross-reactions |
| Conv. | ELISA IgM | In-house | wv | 2 | same | 25.2%** | 11.1% (3/27) were VNT+; multiple observed cross-reactions | ||||
| Conv. | ELISA IgM | In-house | wv | 3 | same | 16.4%** | multiple observed cross-reactions | ||||
| Conv. | ELISA IgM | In-house | wv | 4 | same | 18.7%** | multiple observed cross-reactions | ||||
| Fauld [46] | 2011 | Djibouti City | Animal quarantine station; workers | Conv. | IIFA | EuroImmun | wv | 1–4 | 10 | 10.0%** | not cross-reactive to WNV |
| Andayi [47] | 2010–11 | Djibouti City | Community; general pop. (<1–100) | SRS | ELISA | PanBio | Env | 1–4 | 911 | 21.8% | |
| Egypt (n = 5) | |||||||||||
| Mohammed [48] | 1966 | Abyss | rural community; general pop. | Conv. | HI | In-house | wv | 1 | 29 | 7.0%** | possible cross-reaction to WNV |
| HI | In-house | wv | 4 | same | 3.0%** | possible cross-reaction to WNV | |||||
| Alexandria | urban community; general pop. | Conv. | HI | In-house | wv | 1 | 55 | 4.0%** | possible cross-reaction to WNV | ||
| HI | In-house | wv | 4 | same | 5.0%** | possible cross-reaction to WNV | |||||
| Mohammed [49] | 1968 | Alexandria | Hospital; AFI patients (3–13) | Conv. | HI, CF | In-house | wv | 1 | 120 | 0%** | 0% (0/48) were convalescent + |
| Alexandria | Clinical setting; adults | Conv. | HI | In-house | wv | 1 | 78 | 0% | |||
| Darwish [50] | 1969 | Multiple | University; students | Conv. | HI | In-house | wv | 1 | 1133 | 0.3% | |
| Iran (n = 4) | |||||||||||
| Saidi [51] | 1970 | Multiple | n/s | n/s | HI | In-house | wv | 1,2,3 | 394 | 6.0%** | possible cross-reaction to WNV |
| Saidi [52] | 1970–71 | Caspian region | Community; children (1–6) | Conv. | HI | In-house | wv | 2 | 100 | 0% | |
| Chinikar [53] | 2000–12 | Countrywide | Clinical setting; AFI patients | Conv. | ELISA | Vircell | wv | 1,2 | 300 | 3.3% | 3.3% (10/300) were IgM+; DEN-1,2 were positive by PCR |
| Aghaie [14] | 2014 | Sistan-Baluchestan | blood donor center; general pop. | Conv. | ELISA | PanBio | Env | 1–4 | 540 | 7.6% | 78% (32/41) ELISA+ were IFA+ |
| Kuwait (n = 8) | |||||||||||
| Ibrahim [54] | 1966–68 | Multiple | Multiple settings; blood donors, non-AFI patients, children (1–60) | Conv. | HI | In-house | wv | 1 | 627 | 6.5%** | not cross-reactive to DEN-2 or WNV |
| HI | In-house | wv | 2 | same | 8.1%** | not cross-reactive to DEN-1 or WNV | |||||
| Al-Nakib [55] | 1979–82 | Jabriya | Hospital; non-AFI patients (0–60+) | SRS | HI | In-house | wv | 1 | 502 | 3.2%** | not cross-reactive to DEN-2 or WNV |
| HI | In-house | wv | 2 | same | 8.4%** | all were cross-reactive to DEN-1, WNV, or TBEV | |||||
| Pacsa [56] | 2002* | Multiple | n/s; Kuwaiti nationals | n/s | ELISA and IgG blot | CDC and Genlab | wv | 1–4 | 425 | 13.9% | only DENV 1–3 were positive |
| n/s; Kuwait Bedouins | n/s | ELISA and IgG blot | CDC and Genlab | wv | 1–4 | 47 | 0% | ||||
| n/s; expatriates from South Asia | n/s | ELISA and IgG blot | CDC and Genlab | wv | 1–4 | 266 | 37% | only DENV 1–3 were positive | |||
| n/s; expatriates from Southeast Asia | n/s | ELISA and IgG blot | CDC and Genlab | wv | 1–4 | 31 | 56.6% | only DENV 1–3 were positive | |||
| n/s; expatriates from Middle East | n/s | ELISA and IgG blot | CDC and Genlab | wv | 1–4 | 140 | 25% | only DENV 1–3 were positive | |||
| Hospital; returned travelers with dengue-like illness | n/s | ELISA IgM | PanBio | Env | 1–4 | 210 | 9.0% | only DENV 1–3 were positive; 10%(2/19) IgM+ were PCR+ | |||
| Lebanon (n = 3) | |||||||||||
| Garabedian [5] | 1962–65 | Multiple | Community; general pop. (0–41+) | SRS | HI | In-house | wv | 2 | 113 | 61.9%** | observed cross-reaction with WNV, YFV |
| Multiple | Community; general pop. (0–41+) | SRS | HI | In-house | wv | 1 | 171 | 49.1%** | observed cross-reaction with WNV, YFV | ||
| Hatem [57] | 1969 | Beirut | n/s | n/s | HI | In-house | wv | 2 | 126 | 0% | |
| n/s | n/s | HI | In-house | wv | 1 | same | 4.0%** | observed cross-reaction with WNV | |||
| Libya (n = 1) | |||||||||||
| Darwish [35] | 1973 | Sebha | community and clinic; children, non-AFI patients | Conv. | HI | In-house | wv | 1 | 148 | 0% | |
| Pakistan (n = 32) | |||||||||||
| Darwish [31] | 1983* | Karachi | Hospital; patients | Conv. | CF | In-house | wv | 1 | 43 | 9.3% | |
| Akram [58] | 1994 | Karachi | Hospital; AFI patients (<1–12) | Conv. | ELISA IgM | In-house | wv | 1 | 92 | 9.8%** | 12% (3/25) additional convalescent sera were +; observed cross-reaction to WNV |
| Conv. | ELISA IgM | In-house | wv | 2 | Same | 14.6%** | 24% (6/25) additional convalescent sera were +; observed cross-reaction to WNV | ||||
| Siddiqui [41] | 1999–2001 | Karachi | Community; AFI patients (<16) | Conv. | ELISA IgM | Diag. Auto. | wv | 1–4 | 341 | 15.8% | |
| Tariq [59] | 2003 | Mangla, Mirpur | Community; suspected dengue | Conv. | ELISA IgM | In-house | n/s | n/s | 52 | 73% | |
| Jamil [60] | 2005 | Karachi | Hospitals; suspected dengue | Conv. | ELISA IgM | Chemicon | n/s | n/s | 106 | 36.8% | |
| Khan [61] | 2006 | Karachi | Hospital; suspected dengue (2–72) | Conv. | ELISA IgM | PanBio | Env | 1–4 | 83 | 83.6% | |
| Conv | ELISA IgM | Calbiotech | PA | 1–4 | same | 50.7% | 87.8% (73/83) were PCR+ for DEN-2,3 only | ||||
| Khan [62] | 2006 | Karachi | Hospital; suspected dengue | Conv. | ELISA | PanBio | Env | 1–4 | 250 | 23.2% | 53.6% (134/250) were IgM+; 74% (185/250) were PCR+ for DEN-2 or 3 |
| Koo [63] | 2006–11 | Multiple | Clinic settings; suspected dengue | Conv. | PCR | In-house | 2,3 | 200 | 47% | none were DEN-1 positive | |
| Khan [64] | 2006–07 | Hyderabad | Hospital; suspected dengue (13–70) | Conv. | ELISA IgM | In-house | n/s | n/s | 50 | 40% | |
| Khan [65] | 2006–07 | Multiple | Hospital; suspected dengue | Conv. | ELISA IgM | Calbiotech | PA | 1–4 | 15,040 | 26.3% | |
| Abbasi [66] | 2007–08 | Karachi | Hospital; suspected dengue | Conv. | ELISA IgM | Commercial | n/s | n/s | 114 | 69.6% | |
| Tahir [67] | 2008 | Lahore | Hospital; suspected dengue | Conv. | ICT (IgM) | In-house | n/s | n/s | 3215 | 54.9% | |
| Murad [68] | 2008 | Shangla | Community; suspected dengue (1–80) | Conv. | ELISA IgM | n/s | n/s | n/s | 70 | 17.1% | |
| Mahmood [69] | 2008 | Lahore | Hospital; suspected dengue secondary infection (age 1–80) | Conv. | ELISA | NovaLisa | Env | 1–4 | 200 | 39.5% | |
| Hospital; suspected dengue primary infection (age 1–80) | Conv. | ELISA IgM | DRG | n/s | 2 | 341 | 48.7% | ||||
| Kidwai [70] | 2008–09 | Karachi | Hospital; suspected dengue (>13) | Conv. | ICT (IgG) | In-house | wv | 1–4 | 599 | 83.2% | 41.9% (251/599) were IgM+ |
| Zafar [71] | 2009 | Rawalpindi | rural communities; adults without history of flavivirus vaccination (>18) | StRS | ELISA | Omega | PA (DEN-2) | 1–4 | 96 | 19.8% | |
| Zafar [72] | 2009 | Rawalpindi | Community; general pop. | Conv. | ELISA | Omega,Vircell | PA (DEN-2) | 1–4 | 244 | 28.8% | |
| Qureshi [73] | 2010–12 | Karachi | Hospital; suspected dengue | Conv. | ICT (IgM) | In-house | n/s | n/s | 162 | 9.9% | |
| Khan [74] | 2010 | Punjab | Hospital; suspected dengue (4–60) | Conv. | ELISA IgM | n/s | n/s | n/s | 125 | 54.4% | |
| Hasan [75] | 2010 | Karachi | Hospital; suspected dengue (>12) | Conv. | ELISA IgM | n/s | n/s | n/s | 259 | 34.8% | |
| Umar [76] | 2010 | Rawalpindi | Hospital; suspected dengue | Conv. | ELISA IgM | n/s | n/s | n/s | 500 | 6.8% | |
| Jameel [77] | 2010 | Lahore | Hospital; suspected dengue | Conv. | ELISA IgM | In-house | n/s | n/s | 341 | 48.7% | |
| Naeem [78] | 2011 | Lahore | Hospital; suspected dengue (1–10+) | Conv. | ELISA IgM | n/s | n/s | n/s | 79 | 25.3% | |
| Ahmed [79] | 2011 | Lahore | Hospital; suspected dengue (13–81) | Conv. | ELISA IgM | n/s | n/s | n/s | 640 | 43.9% | |
| Ijaz [80] | 2011 | Lahore | Hospital; suspected dengue (<15–60+) | Conv. | ELISA | n/s | n/s | 1–4 | 5,274 | 49% | |
| Rashid [81] | 2011 | Lahore | Hospital; suspected dengue (<18) | Conv. | ELISA | n/s | n/s | n/s | 254 | 36.6% | 53.9% (137/254) were IgM+ |
| Khan [82] | 2011 | Lahore | Hospital; suspected dengue (5–50+) | Conv. | ELISA | In-house | wv | 1–4 | 50 | 72% | 30% (30/50) were IgM+; 66% (33/50) were PCR+ for DEN-1,2; 60% (30/50) were cell culture+ |
| Hasan [83] | 2007–13 | Multiple | Hospitals; suspected Crimean-Congo Hemorrhagic Fever | Conv. | ELISA IgM | PanBio | Env | 1–4 | 168 | 33.9% | 2.3% (4/168) were PCR+ |
| Ali [84] | 2011 | Khyber Pakhtunkhwa | Clinical settings; suspected dengue (<10 to >51) | Conv. | ELISA | Diag. Auto. | wv | 1–4 | 612 | 20.2% | 31.9% (195/612) were IgM+ |
| Hisam [85] | 2012 | Rawalpindi | Military Hospital; AFI patients | PS | ELISA IgM | n/s | n/s | n/s | 500 | 3.2% | |
| Assir [86] | 2012 | Lahore | Hospital; suspected dengue (12–90) | Conv. | ELISA IgM | GmbH | wv | 1–4 | 85 | 43.5% | 20% (3/15) were PCR + for DEN-2 |
| Saudi Arabia (n = 11) | |||||||||||
| Fakeeh [87] | 1994–99 | Jeddah | Hospitals; suspected dengue (1->50) | Conv. | IIFA, HI | In-house | wv | 1,2 | 985 | 31.9% | 16.2% (160/985) were ELISA IgM+; 21% (207/985) were PCR+ (DEN-1,2,3) |
| Fakeeh [88] | 1994–2002 | Jeddah | Hospitals; suspected dengue | Conv. | IFA, HI | In-house | wv | 1,2,3 | 1020 | 50.5% | 10.8% (110/1020) were ELISA IgM+; 20.5% (209/1020) were PCR+ (DEN-1,2,3) |
| Khan [89] | 2004 | Makkah | Hospital; suspected dengue (6–94) | ELISA | PanBio | Env | 1–4 | 136 | 32.4% | 58.8% (80/136) were IgM+; 28.1% (27/96) were PCR + (DEN-2,3) | |
| Ayyub [90] | 2004–05 | Jeddah | Hospital; suspected dengue (2–60) | Conv. | ELISA IgM | n/s | n/s | n/s | 80 | 48.8% | |
| Shahin [91] | 2006–08 | Makkah | Hospital; suspected dengue | Conv. | ELISA IgM and/or PCR | n/s | n/s | n/s | 159 | 100% | |
| Said [92] | 2006 | Jeddah | Hospital; suspected dengue (2–71) | Conv. | ELISA IgM | In-house | n/s | n/s | 525 | 19.2% | % includes paired serum sample |
| Memish [93] | 2010 | Multiple | Military; adults | Conv. | ELISA | PanBio | Env | 1–4 | 1024 | 0.1% | 0% of IgG+ were IgM+ |
| Gamil [94] | 2010–11 | Jeddah | Hospitals; suspected dengue (3–56) | Conv. | n/s | n/s | n/s | n/s | 553 | 47.7% | |
| Al-Azraqi [95] | 2013 | Jizan | Clinics; clinic attendants (1–60+) | SRS | ELISA | Focus | wv | 1–4 | 268 | 26.5% | |
| Aseer | Clinics; clinic attendants (1–60+) | SRS | ELISA | Focus | wv | 1–4 | 697 | 33.7% | |||
| Ashshi [96] | 2014 | Mecca | blood donation center; adults | Conv. | ELISA | PanBio | Env | 1–4 | 100 | 7% | 6% (6/100) were IgM+;1% (1/100) were NS1+ |
| Somalia (n = 7) | |||||||||||
| Botros [97] | 1987 | Hargeysa | Refugee camp; AFI patients | Conv. | ELISA | In-house | wv | 2 | 38 | 60.7% | acute and convalescent samples; 39.4% (15/38) were IFA+; 37.9% (11/29) were HI+; 14.2% (4/28) were ELISA IgM+ |
| Kanesa-thasan [98] | 1993 | n/s | Military base; AFI soldiers | Conv. | ELISA IgM and/or HI | n/s | n/s | n/s | 84 | 17.8% | 93% (14/15) were cell culture + (DEN-2 and 3 only) |
| Sharp [99] | 1992–93 | Mogadishu | Military Hospital; AFI patients (soldiers) | Conv. | ELISA IgM | In-house | wv | 1–4 | 129 | 34.9% | 40.6% (39/96) were cell culture positive for DEN-2; 2% (2/96) were cell culture positive for DEN-3 |
| Baardera | Military; adults (19–25) | Conv. | ELISA IgM | In-house | wv | 1–4 | 494 | 7.7%** | observed cross-reaction with WNV | ||
| Nur [100] | 1995 | Mogadishu | Hospital; children (<1 to > 2 years of age) | CC. | ELISA IgM | Progen | wv | 2 | 23 | 0% | |
| Hospital; AFI patients with / without rash (<1 to > 2 years of age) | CC. | ELISA IgM | Progen | wv | 2 | 46 | 0% | ||||
| Kyobe Bosa [101] | 2011 | Mogadishu | Hospitals; AFI patients (20–49) | Conv. | ELISA IgM | n/s | n/s | 1,2,3 | 134 | 80% | 62% (83/134) were PCR+ |
| Sudan (n = 16) | |||||||||||
| Omer [36] | 1976 | Gezira | Rural community; general pop. (5–40+) | Conv. | HI | In-house | wv | 2 | 109 | 27.5% | 17.4% (19/109) were VNT+ |
| Hyams [102] | 1984 | Port Sudan | Hospital; AFI patients (12–70) | Conv. | HI | In-house | wv | n/s | 100 | 3% | 14.8% (8/54) were convalescent +; 1% (1/100) DEN-1 cell culture +; 17% (17/100) DEN-2 cell culture + |
| Woodruff [103] | 1986 | Juba | Hospital; patients with history of fever within past 6 months and AFI patients (1–85) | Conv. | HI | In-house | n/s | n/s | 130 | 40.0%** | represents single virus activity not cross-reactive to multiple flaviviruses tested |
| McCarthy [104] | 1988 | Khartoum | Clinical setting; non-AFI patients | CC | ELISA | In-house | wv | 2 | 100 | 49% | 0% were IgM+ |
| Clinical setting; AFI patients (1–89) | CC | ELISA | In-house | wv | 2 | 196 | 48%** | 0% were IgM+; possible cross-reaction to WNV | |||
| Watts [18] | 1989 | Northern Province | Clinical setting; AFI patients (11–70) | Conv. | ELISA | In-house | n/s | 2 | 185 | 24.0%** | possible cross-reactions to multiple flaviviruses |
| Ibrahim [105] | 1997–99 | Khartoum | Clinical setting: suspected measles | Conv. | ELISA IgM | MRL Diag. | n/s | n/s | 188 | 3.2% | |
| Malik [106] | 2004–05 | Port Sudan | Hospitals; suspected dengue (<1–15) | Conv. | ELISA IgM | PanBio | Env | 1–4 | 40 | 90.0% | 39% (9/23) were PCR+ (DEN-3) |
| Gould [107] | 2005 | South Kordofan | Clinical setting; suspected YF patients (n = 3), severe illness (n = 8), AFI patients (n = 7), healthy (n = 16) | Conv. | ELISA IgM | In-house | wv | n/s | 34 | 5.9%** | observed cross-reaction with YFV, WNV |
| Farnon [38] | 2005 | Kortalla | Community; general pop., YF vaccinated (0–44+) | SSCS | ELISA | In-house | wv | 1–4 | 87 | 1.1%** | observed cross-reaction in YF vaccine recipient; 0% were IgM+; 52% (45/87) were VNT+ for DENV and YFV |
| Seidahmed [39] | 2008–09 | Port Sudan City | Urban community; individuals from houses with DENV-carrying mosquitoes (<1–80) | RSS | ELISA IgM | PanBio | Env | 1–4 | 791 | 5.2% | |
| Adam [108] | 2008–09 | Port Sudan City | Hospitals; pregnant women with deliveries | Ret. cohort | ELISA IgM | n/s | n/s | 1–4 | 10,820 | 0.7% | |
| Himatt [109] | 2011 | Kassala state | Community; general pop. (5–75+) | MSCS | ELISA | PanBio | Env | 1–4 | 489 | 9.4% | 0.6% (3/489) were IgM+ |
| Abdalla [110] | 2012 | Kassala State | Hospital; AFI patients with suspected measles (2–65) | Conv. | ELISA | PanBio | Env | 1–4 | 60 | 11.7% | |
| Elduma [15] | 2012 | Port Sudan | Hospital; pregnant women with AFI | Conv. | ELISA | Commercial | n/s | n/s | 39 | 12.8% | 2.6% (1/39) were IgM+ and PCR+ |
| Soghaier [111] | 2014 | South Kordofan | Urban and rural communities; general pop. (15–60) | MSCS | ELISA | PanBio | Env | 1–4 | 600 | 27.7% | 77% of study population were YFV vaccinated |
| Turkey (n = 6) | |||||||||||
| Ari [34] | 1971 | Izmir | Community and clinic; general pop. | Conv. | HI | In-house | wv | 2 | 270 | 0% | |
| Radda [112] | 1973* | Izmir | n/s; general pop. | Conv. | HI | In-house | wv | 2 | 270 | 0.3%** | observed cross-reaction with WNV |
| Istanbul | n/s; general pop. | Conv. | HI | In-house | wv | 2 | 90 | 0% | |||
| Ankara | n/s; general pop. | Conv. | HI | In-house | wv | 2 | 95 | 0% | |||
| Ergunay [113] | 2010 | Ankara, Konya, Eskisehir, Zonguldak | blood donation center; blood donors | Conv. | ELISA | EuroImmun | wv | 1–4 | 2435 | 0.9% | 14.2% (3/21) of IgG+ were IIFT+ for DEN-2; 9.5% (2/21) of IgG+ were IgM+ |
| Tezcan [114] | 2010–11 | Mersin | blood donation center; blood donors | Conv. | ELISA | Vircell | wv | 1–4 | 920 | 16.6% | 0.9% (8/920) were IgM+; 0% were NS1+ |
| Yemen (n = 5) | |||||||||||
| Bin Ghouth [115] | 2011 | Hadramout | Hospital; suspected dengue (<5 to 55+) | Conv. | ELISA | PanBio | Env | 1–4 | 982 | 50.6% | 64.1% (630/982) IgM+; 86.2% (163/189) PCR+ for DEN-3 |
| Malik [116] | 2010–11 | Al-Hudaydah | Clinical setting; AFI patients (0–45+) | Conv. | ELISA | PanBio | Env | 1–4 | 136 | 87.5% | 8.1% (11/136) were IgM+ |
| Madani [117] | 2010 | Hadramout | Clinical settings; suspected viral hemorrhagic fever (3–75) | Conv. | ELISA | PanBio | Env | 1–4 | 207 | 48.3% | 78.7% (163/207) IgM+; 46.9% (97/207) NS1+; 0.09% (2/207) PCR+ for DEN-1,2 |
| Rezza [118] | 2012 | Al Hudaydah | Hospitals; AFI patients with dengue-like illness (1–60) | CS | ELISA | NovaLisa | Env | 1–4 | 400 | 72.5% | 18% (72/400) IgM+; 13.8% (55/400) PCR+ for DEN-1,2 |
| Qassem [119] | 2013 | Hadramout | Clinical setting; suspected dengue and/or west nile infection | Conv. | ELISA IgM | n/s | n/s | n/s | 42 | 19.0%** | observed cross-reaction with WNV |
* Indicates year of publication when year(s) of data collection not available in report.
† All serologic assays were IgG unless otherwise stated.
**Indicates documented occurrence or suspicion of false-positives due to cross-reactions with other same family viruses or low serologic titers.
Abbreviations: AFI, acute febrile illness patients; Ag, antigen; CF, complement fixation; Conv, convenience; ELISA, enzyme-linked immunosorbent assay; HI, hemagglutinin inhibition; ICT, immunochromatography test; IIFA, indirect immunofluorescence antibody test; MSCS, multi-stage cluster sampling; n/s, not specified; NS1, NS1 antigen test; PA, purified antigen; PCR, polymerase chain reaction; pop., population; PS, purposive sampling; RSS, random stratified sampling; SRS, simple random sampling; SSCS, single stage cluster sampling; VNT, viral neutralization test
Assay Abbreviation: CDC (Centers for Disease Control and Prevention, USA); Chemicon (Chemicon, Temecula, CA, USA); Diag. Auto. (Diagnostic Automation, CA, USA); DRG (DRG International Inc); Euroimmun (Lubeck, Germany); Focus (Focus Diagnostics, Cypress CA, USA); Genlab (Genlab Diagnostics, Singapore); GmbH (Human GmbH, Wiesbaden, Germany); MRL Diagnostics (Cypress CA, USA); NovaLisa (Dietzenbach, Germany); Omega (Omega Diagnostics, Scotland, UK); PanBio (Brisbane, Australia); Progen (Heidelberg, Germany); SD Bioline (Standard Diagnostics, Korea); Vircell (Vircell Microbiologists, Granada, Spain)
Fig 2. Geographic distribution of human prevalence studies and reported outbreaks of dengue in the Middle East and North Africa.
Table 4. Summary of human prevalence studies for dengue virus in the Middle East and North Africa (n = 103).*.
| Study characteristics | General population (n = 42) n (%) | Acute febrile illness (n = 23) n (%) | Suspected dengue (n = 38) n (%) |
|---|---|---|---|
| Total sample size | 24,377 | 4,065 | 33,955 |
| Median DENV % prevalence (range %) | 25% (0–61.9) | 15.2% (0–87.5) | 47.4% (6.8–100) |
| Year of study | |||
| before 1990 | 21 (50%) | 7 (30%) | 0 |
| 1990 to 2015 | 21 (50%) | 16 (70%) | 38 (100%) |
| Study setting | |||
| community | 31 (74%)† | 2 (9%) | 2 (5%) |
| clinic or hospital | 11 (26%) | 21 (91%) | 36 (95%) |
| Assay | |||
| ELISA IgG | 19 (45%) | 8 (35%) | 10 (26%) |
| ELISA IgM | 11 (26%) | 17 (74%) | 31 (82%) |
| immunofluorescence antibody | 5 (12%) | 2 (9%) | 2 (5%) |
| hemagglutination inhibition | 15 (36%) | 5 (22%) | 2 (5%) |
| complement fixation | 1 (2%) | 1 (4%) | 0 |
| viral neutralization | 2 (5%) | 1 (4%) | 0 |
| PCR | 0 | 4 (17%) | 14 (37%) |
| cell culture | 0 | 3 (13%) | 1 (3%) |
| NS1 antigen | 2 (5%) | 0 | 1 (3%) |
| Assay make | |||
| in-house | 21 (50%) | 10 (43%) | 11 (29%) |
| commercial | 20 (48%) | 10 (43%) | 15 (39%) |
| not specified | 1 (2%) | 3 (13%) | 12 (32%) |
| Target protein** | |||
| whole virus | 32 (76%) | 12 (52%) | 6 (16%) |
| envelope | 7 (17%) | 4 (17%) | 9 (24%) |
| not specified | 3 (7%) | 7 (30%) | 21 (55%) |
| Risk of bias summary | |||
| Assay | |||
| low risk of bias | 2 (5%) | 8 (35%) | 14 (37%) |
| high risk of bias | 40 (95%) | 15 (65%) | 24 (63%) |
| unclear risk of bias | 0 | 0 | 1 (3%) |
| Sampling methodology | |||
| low risk of bias | 15 (36%) | n/a | n/a |
| high risk of bias | 17 (40%) | n/a | n/a |
| unclear risk of bias | 10 (24%) | n/a | n/a |
| Response rate | |||
| low risk of bias | 6 (14%) | 11 (48%) | 22 (58%) |
| high risk of bias | 1 (3%) | 0 | 0 |
| unclear risk of bias | 35 (83%) | 12 (52%) | 16 (42%) |
| Precision | |||
| High | 28 (67%) | 15 (65%) | 28 (74%) |
| Low | 14 (33%) | 8 (35%) | 10 (26%) |
* N = 103 because the study type (i.e. general prevalence, acute febrile illness, or suspected dengue) was not specified in two studies [51, 57].
†Community study settings also include animal quarantine station (n = 1), blood donation center (n = 5), military (n = 3), and university (n = 1).
** Indicates the target protein for the initial screening assay for studies in which multiple diagnostic assays were utilized.
Table 5. Summary of human incidence studies for dengue virus in the Middle East and North Africa (n = 3).
| Country, Ref. | Year(s) of study | Duration of follow-up | City or governorate | Setting; population(age range, years) | Study design | Sampling | Assay type | Assay make+ | Assay Target | Serotype tested | Sample size | Incidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pakistan (n = 1) | ||||||||||||
| Siddiqui [41] | 1999–2001 | 1999–2001 | Karachi | Urban slum; children <16 years of age with undifferentiated febrile illness | CS | Active surveillance | ELISA IgM | Diag. Auto. | wv | 1–4 | 1,248 | 185/100,000 |
| Sudan (n = 2) | ||||||||||||
| Seidahmed [39] | 2008–09 | 12 months | Port Sudan City | Urban community; general pop. living in houses where DENV-carrying mosquitoes were present (<1–80) | Pros. coh | RSS | ELISA IgM | PanBio | Env | 1–4 | 791 | 35/10,000 |
| Seidahmed [40] | 2010 | 17 weeks | Port Sudan | Urban community; general pop. | CS | Conv. | ELISA IgM, NS1, PCR | n/s | n/s | n/s | 3,765‡ | 94/10,000 |
‡ Reported cases
Abbreviations: CS, cross-sectional; ELISA, enzyme-linked immunosorbent assay; Env, envelope; PCR, polymerase chain reaction; Pros. coh, prospective cohort; RSS, random stratified sampling
Assay Abbreviation: Diag. Auto. (Diagnostic Automation, CA, USA); PanBio (Brisbane, Australia)
Table 6. Summary of vector infection rate studies for dengue virus in the Middle East and North Africa (n = 3).
| Author, Ref. | Year(s) of data collection | City or governorate | Setting | Mosquito species | Assay type | Sample size | Infection rate | Comments | |
|---|---|---|---|---|---|---|---|---|---|
| Pakistan | |||||||||
| Jahan [42] | 2011 | Lahore | Urban areas | Ae. aegypti | Ag-capture ELISA | 114 pools (n = 570 mosquitoes) | 27.2% | ||
| Ae. albopictus | Ag-capture ELISA | 4 pools (n = 20 mosquitoes) | 25% | ||||||
| Yemen | |||||||||
| Zayed [43] | 2010–11 | Al Hodayda | houses of CHIKV cases at Eritrean refugee camp | Ae. aegypti | RT-PCR | 11 pools (n = 30 mosquitoes) | 0% | 17 Culex spp. mosquitoes were also negative for DENV RNA. | |
Abbreviations: RT-PCR, reverse transcription-polymerase chain reaction
Risk of bias assessment results
The quality assessment for each study is found in S1 Table and a summary of the precision and risk of bias assessment is found in Table 4. In brief, most studies (≥65%) contained high precision as defined by a sample size of ≥100 participants. A minority (36%) of general population seroprevalence studies utilized some form of random sampling, and response rates were either <80% or not reported in 86% of general population studies. VNT or a biologic confirmatory assay (i.e. cell culture, PCR, and NS1 ELISA) was performed in 5% and 36% of general population seroprevalence and acute DENV infection studies, respectively, entailing low ROB for the assays used.
Dengue outbreaks and Aedes occurrence
Reported outbreaks of DENV in the region were gathered through citations collected from the search databases (S2 Table) and mapped along with the geographic distribution of prevalence studies in Fig 2. For DENV, 81 outbreaks were reported from 9 countries in the region from 1941–2015, including sentinel reports of autochthonous transmission in Egypt (2010) and Yemen (1983). Reports contained variable descriptions of outbreaks including ‘estimated’, ‘suspected’, ‘reported’, and/or laboratory ‘confirmed’ cases (S2 Table). The definition that qualified each event as an outbreak was unclear in most instances. Outbreaks of DENV serotypes 1–3 were reported from countries surrounding the Red Sea and DENV-4 was only reported from Pakistan [120, 121]. Although, in general, DENV serotypes were not reported consistently.
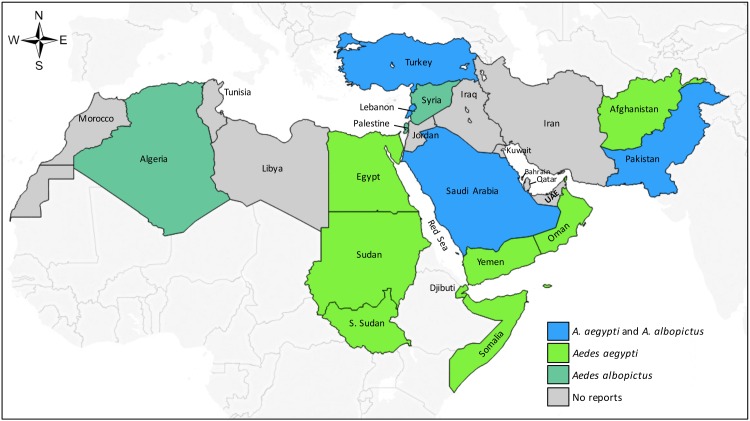
Published reports of Ae. aegypti and Ae. albopictus occurrence are recorded in S3 Table and mapped by country in Fig 3. Ae. aegypti occurrence was reported in 11 MENA countries and historically (i.e. prior to 1960) in Algeria, Libya, Morocco, Syria, and Tunisia. Ae. albopictus was reported in seven MENA countries, including Algeria, Palestine, and Syria, countries where Ae. aegypti is not currently reported. No published reports of Ae. aegypti or Ae. albopictus occurrence (or DENV outbreaks) were identified in seven MENA countries: Bahrain, Iran, Iraq, Jordan, Kuwait, Qatar, and United Arab Emirates. Since 2005, Ae. aegypti and/or Ae. albopictus occurrence has been documented in Afghanistan, Algeria, Lebanon, Oman, Palestine, Syria, and Turkey, though autochthonous transmission of DENV has not yet been reported from these countries.
Fig 3. Country-level distribution of Aedes aegypti and Aedes albopictus occurrence in the Middle East and North Africa.
Discussion
Our study offers an assessment of published prevalence, incidence, and outbreak reports pertaining to the epidemiology of dengue in the MENA region. Based on the study results, the MENA contains two apparent subregions known to harbor DENV: 1) Pakistan, and 2) the Red Sea countries (Djibouti, Egypt, Saudi Arabia, Somalia, Sudan, and Yemen). No seroprevalence or outbreak data was identified across broad areas of the MENA, however, including some Aedes endemic areas. There was also a paucity of reports estimating human incidence and vector infection rates. These findings suggest priorities for future research. However, they also challenge efforts to synthesize and compare the inter- and intra-country epidemiology of DENV in the region.
Dengue seroprevalence in the MENA
In our review, Pakistan reported the highest number of prevalence studies and the broadest study coverage among MENA countries. Multiple studies reported >20% prevalence in both general population and those with undifferentiated AFI [1, 106, 122–124]. DENV serotypes 1–4 are known to circulate in Pakistan, unlike other MENA countries [82]. Pakistan also reported the largest number of confirmed cases among all DENV outbreaks in the MENA, with 21,580 cases reported during the 2011 DENV-2 outbreak [79, 120] (S2 Table).
In the Red Sea region, multiple general population and AFI population IgG seroprevalence measures exceeding 20% were published from in Djibouti, Saudi Arabia, Somalia, Sudan, and Yemen within the past decade (Table 3) along with multiple confirmed outbreaks of DENV serotypes 1–3 since the 1980s (S2 Table). DENV-4 has not yet been identified in this subregion to our knowledge. Although reported outbreaks and cases often localize along the Red Sea coastline in these countries [1], seroprevalence studies suggest a broader distribution of DENV infections that are likely underdetected (Fig 2). This is illustrated by the sentinel report of a DENV-infected traveler returning from Yemen in 1983 [125], despite the first outbreaks of DENV in Yemen and Saudi Arabia not being reported until 1994 [126–128]. Our search also identified no published prevalence studies or outbreaks in Egypt after 1969 until a dengue outbreak was reported in November 2015 [10]. However, DENV transmission was suggested years prior by a report of two travelers diagnosed with dengue after returning from southern Egypt in 2011 [129] and the identification of Ae. aegypti in southern Egypt that same year [130]. It is plausible that undetected DENV transmission had been occurring in Egypt prior to this outbreak. However, it is not clear whether this and other recent outbreaks represent increasing incidence, increasing detection, or both, amidst the heterogeneity in study coverage and reporting in the MENA.
Clinical and methodological diversity among studies
An important finding in our study was the clinical and methodological diversity among DENV prevalence studies. This diversity represents a challenge to synthesizing the epidemiologic literature for DENV in the MENA. Clinically, studies represented a diversity of human populations of different ages and demographics, in different years, and different locations and transmission contexts. Ninety-six percent of studies from Afghanistan, Pakistan, Saudi Arabia, Somalia, and Yemen were conducted during or prior to 1990. However, 53% of studies in other MENA countries were conducted prior to 1990, when study methods and DENV epidemiology may have been different. Methodologically, most studies utilized convenience samples without reporting response rates, entailing high risk of bias and uncertainty in the representativeness of reported measures (Table 4). These findings, along with the high variability in regional study coverage, precluded meta-analyses of the available data.
Flavivirus cross-reactivity
Serologic cross-reactions remain a challenge to seroepidemiologic studies for DENV and other flaviviruses. Viral neutralization tests, considered the gold standard serologic assay for DENV, were performed in only 5% of general population seroprevalence studies in our review. Compared to ELISAs, seroprevalence measures were 22–86% lower by secondary/confirmatory testing with immunofluorescence or VNT in our review [14, 36, 37, 113]. This illustrates the potential uncertainty surrounding the reliability of ELISAs in DENV serologic studies, particularly in areas where the prevalence of antigenically similar viruses is broad or unknown. West Nile virus (WNV), for example, is thought to be distributed across the MENA on account of its ubiquitous Culex spp. vector and migratory bird flyways [131–133]. With up to 80% of WNV infections occurring subclinically, the potential for serologic cross-reactions with DENV antibody assays must be considered. Yellow fever vaccine-derived and natural antibodies may also cross-react with anti-DENV antibodies, especially relevant in YFV endemic regions such as Sudan [38, 107, 111]. As the emergence of zika virus in the Western Hemisphere or the re-emergence of YFV has shown, serologic assays with low specificity are inadequate to tackle the epidemiologic challenges of emerging arboviral diseases [134].
Heterogeneity in dengue outbreak reports
Our review identified DENV outbreaks in over a third of MENA countries, with most outbreaks reported from Pakistan, Sudan, and Saudi Arabia (S2 Table). Outbreaks varied widely across time and space in the MENA: reported cases varied from <10 to >100,000 over a span of months to years, reported from the village level to the level of the province and region. This presents a challenge to epidemiologic monitoring and policy planning for DENV, as use of different outbreak definitions results in differences in early detection and response [30]. There is currently no consensus on how to define DENV outbreaks, and adopting a common definition for the MENA is challenging given the region’s heterogeneous infection pressures, multiple DENV serotypes, and variable surveillance and detection capacity. At present, assessing whether a reported transmission event in the MENA significantly deviates from baseline transmission, and thus constitutes an outbreak, is often unclear.
Risk factors and research priorities
Our study did not identify confirmed DENV transmission in any of the MENA countries west of Egypt and east of Saudi Arabia until Pakistan (Fig 2). However, the paucity of published data in these sub-regions does not preclude the possibility of unrecognized transmission in some areas or the risk of emergence in others. Indeed, modeling studies suggest ecologic niches for Aedes along the coastal Mediterranean Basin of North Africa [1, 123, 135], and Ae. albopictus and/or Ae. aegypti has been recently reported in Algeria, Lebanon, Palestine, Syria, and Turkey [5, 136–140] (Fig 3 and S3 Table). In contrast, Ae. albopictus has been identified along the Mediterranean coast of Europe for decades along with local transmission of DENV and chikungunya since 2007 [141]. Near the Pakistan border, serologic evidence suggests possible DENV transmission in Iran [14, 51, 53] and Afghanistan [44], though local transmission has not been confirmed to our knowledge [53]. The presence of Aedes or DENV transmission in these areas should not be ruled out [53].
Several ecologic and social factors in the MENA may promote the spread of Aedes-borne viruses like DENV. Urbanization [142] may increase the risk of outbreaks and use of open water storage containers that promote Ae. aegypti breeding [1, 39, 43, 55, 95, 111, 123, 135]. Heavy rainfall has been implicated in DENV outbreaks in Sudan, Djibouti, and Yemen [12, 47, 143, 144], which may become increasingly unpredictable through climate change [145]. Armed conflicts and economic turmoil in Iraq, Syria, and Yemen may render these areas vulnerable to vector-borne diseases while diminishing surveillance and response [146]. Inter-regional migration poses risk for imported DENV, as millions of migrants travel from DENV-endemic countries to the Arabian Peninsula [111, 126, 146–149] and to Mecca, Saudi Arabia to attend Umra and Hajj [126]. Intra-regionally, heavy commerce in the Red Sea region likely drives DENV serotype mixing and spread [116, 148], as evidenced by multiple DENV outbreaks occurring at port cities in Djibouti [37, 45], Saudi Arabia [126], Sudan [39], and Yemen [116, 148]. Contiguous spread of DENV from Yemen to Oman [150], or from Pakistan to Iran or Afghanistan [14], may also pose risk.
A number of research priorities emerge concerning the epidemiology of DENV in the MENA. First, broader seroepidemiologic coverage in the region is needed. Such studies are efficient means of characterizing infection pressures in populations lacking surveillance and diagnostic capacity. Multiplexed diagnostics are increasingly available and are well-suited for concurrently exploring the distribution other undercharacterized arboviruses in the region (e.g. Alkhumra, Chikungunya, Crimean-Congo Hemorrhagic Fever, O’Nyong-nyong, Rift Valley Fever, Sandfly Fever virus complex, Usutu, and West Nile viruses). Second, serologic studies should include methods to minimize cross-reactions, particularly for flaviviruses [151]. Third, seroepidemiologic studies should incorporate uniformity in study design and enrollment criteria to minimize confounding, such as standard case definitions for studies of ‘suspected’ dengue [126]. Ideally this could include population-based sampling that provides baseline data to benchmark the regional impact of these pathogens over the coming years. Fourth, studies should incorporate vector surveillance and infection rates. Such studies are important for understanding transmission dynamics that inform vector control strategies and predict future transmission and disease risk [123, 135, 141]. Guidelines and tools for calculating vector infection rates are available [141, 152]. Finally, attaining a meaningful definition of DENV outbreaks in the MENA countries will require a thorough assessment of baseline surveillance, control, and treatment capacities in endemic regions [30].
Study limitations
Our study was limited by its reliance on select databases of peer-reviewed literature screened by one investigator with the exclusion of grey literature which may have provided additional data. Reviewing other Aedes-transmitted pathogens or studies reporting Aedes distribution in the MENA may also have provided further insights regarding the potential geographic distribution of DENV. Due to the limitations in the content and distribution of studies, we did not perform a meta-analysis nor did we explore bias in overall outcome measures through a funnel plot or Egger test. Non-publication of studies with small or zero effect size or studies targeted to known dengue-endemic areas may have biased the distribution and quantity of DENV studies. The prevalence measures themselves may have been biased through serologic-cross reactions, targeting of older study populations (with higher seroprevalence), and lack of convalescent titers for acute DENV infection studies (possibly underestimating seroprevalence).
Conclusions
DENV seroprevalence in the MENA is high among some populations in the Red Sea region and Pakistan, while recent outbreaks in these subregions suggest increasing DENV incidence driven by ecologic and social factors. Published prevalence and incidence, vector occurrence, and vector infection rates are lacking in broad areas of the MENA and available studies contain methodological limitations. These findings illustrate the need to strengthen programs for surveillance, reporting, and control of DENV and Aedes in the MENA, both to define DENV and Aedes epidemiology and to mitigate the risk of emerging Aedes-transmitted pathogens in the future.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Mary Charlson and Carol Mancuso (Weill Cornell Graduate School of Medical Sciences, New York, USA) for their contributions to the study planning and organization, and Ghina Mumtaz, Hiam Chamaitelly, and Karima Chaabna (Weill Cornell Medical College in Qatar) for their assistance with the study methodology.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This publication was made possible by support provided by the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core at the Weill Cornell Medical College in Qatar. JMH received support from NIH Research Training Grant T32 AI007613. The statements made herein are solely the responsibility of the authors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. 10.2147/CLEP.S34440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler DJ. The global resurgence of arboviral diseases. Trans R Soc Trop Med Hyg. 1996;90(5):449–51. [DOI] [PubMed] [Google Scholar]
- 4.H.R. R. The role of vectors in emerging and re-emerging diseases in the Eastern Mediterranean Region. East Mediterr Health J. 1996;2(1):61–7. [Google Scholar]
- 5.Garabedian GA, Matossian RM, Musalli MN. Serologic evidence of arbovirus infection in Lebanon. Le Journal medical libanais. 1971;The Lebanese medical journal. 24(4):339–50. [PubMed] [Google Scholar]
- 6.World Health Organization. Growing threat of viral haemorrhagic fevers in the Eastern Mediterranean Region: a call for action Regional Office for the Eastern Mediterranean. Cairo, Egypt. Available from: http://applications.emro.who.int/docs/em_rc54_r4_en.pdf?ua=1. Accessed 24 July 2014.
- 7.Hotez PJ, Savioli L, Fenwick A. Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2012;6(2):e1475 10.1371/journal.pntd.0001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai MA. Epidemic: Control of dengue fever in Pakistan. Nature. 2011;479(7371):41. [DOI] [PubMed] [Google Scholar]
- 9.Arya SC, Agarwal N. Apropos: An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Parasites and Vectors. 2014;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Dengue Fever—Egypt 2015. Available from: http://www.who.int/csr/don/12-november-2015-dengue/en/. Accessed 12 Dec 2015.
- 11.World Health Organization. Neglected tropical diseases: an emerging public health problem in the Eastern Mediterranean Region. 54th Regional Committe meeting, Eastern Mediterranean Region. Cairo, Egypt. 2006. Available from http://applications.emro.who.int/docs/EM_RC54_10e_en.pdf. Accessed 12 Dec 2015.
- 12.Malik MR, Mnzava A, Mohareb E, Zayed A, Al Kohlani A, Thabet AA, et al. Chikungunya outbreak in Al-Hudaydah, Yemen, 2011: epidemiological characterization and key lessons learned for early detection and control. J Epidemiol Glob Health. 2014;4(3):203–11. 10.1016/j.jegh.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan EH, Brewer TF, Madoff LC, Pollack MP, Sonricker AL, Keller M, et al. Global capacity for emerging infectious disease detection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21701–6. 10.1073/pnas.1006219107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghaie A, Aaskov J, Chinikar S, Niedrig M, Banazadeh S, Mohammadpour HK. Frequency of dengue virus infection in blood donors in Sistan and Baluchestan province in Iran. Transfusion and Apheresis Science. 2014;50(1):59–62. 10.1016/j.transci.2013.07.034 [DOI] [PubMed] [Google Scholar]
- 15.Elduma AH, Osman WM. Dengue and hepatitis E virus infection in pregnant women in Eastern Sudan, a challenge for diagnosis in an endemic area. Pan Afr Med J. 2014;19:391 10.11604/pamj.2014.19.391.5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379(9816):662–71. 10.1016/S0140-6736(11)60281-X [DOI] [PubMed] [Google Scholar]
- 17.Ali F, Saleem T, Khalid U, Mehmood SF, Jamil B. Crimean-congo hemorrhagic fever in a dengue-endemic region: Lessons for the future. Journal of Infection in Developing Countries. 2010;4(7):459–63. [DOI] [PubMed] [Google Scholar]
- 18.Watts DM, El-Tigani A, Botros BAM, Salib AW, Olson JG, McCarthy M, et al. Arthropod-borne viral infections associated with a fever outbreak in the Northern Province of Sudan. Journal of Tropical Medicine and Hygiene. 1994;97(4):228–30. [PubMed] [Google Scholar]
- 19.Bargaoui R, Lecollinet S, Lancelot R. Mapping the Serological Prevalence Rate of West Nile fever in Equids, Tunisia. Transbound Emerg Dis. 2013. [DOI] [PubMed] [Google Scholar]
- 20.Ben Hassine T, De Massis F, Calistri P, Savini G, BelHaj Mohamed B, Ranen A, et al. First Detection of Co-circulation of West Nile and Usutu Viruses in Equids in the South-west of Tunisia. Transbound Emerg Dis. 2014;61(5):385–9. 10.1111/tbed.12259 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: Wiley Online Library; 2008. [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFarland W, Abu-Raddad LJ, Mahfoud Z, DeJong J, Riedner G, Forsyth A, et al. HIV/AIDS in the Middle East and North Africa: new study methods, results, and implications for prevention and care. AIDS. 2010;24 Suppl 2:S1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Google Translate Mountain View, California, USA. Available from: http://www.translate.google.com/. Accessed 30 Sept 2015.
- 25.The Cochrane collaboration. Cochrane handbook for systematic reviews of interventions Hoboken (New Jersey): Wiley-Blackweill; 2008. [Google Scholar]
- 26.Chemaitelly H, Chaabna K, Abu-Raddad LJ. The Epidemiology of Hepatitis C Virus in the Fertile Crescent: Systematic Review and Meta-Analysis. PloS one. 2015;10(8):e0135281 10.1371/journal.pone.0135281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumtaz GR, Weiss HA, Thomas SL, Riome S, Setayesh H, Riedner G, et al. HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS medicine. 2014;11(6):e1001663 10.1371/journal.pmed.1001663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaabna K, Mohamoud YA, Chemaitelly H, Mumtaz GR, Abu-Raddad LJ. Protocol for a systematic review and meta-analysis of hepatitis C virus (HCV) prevalence and incidence in the Horn of Africa sub-region of the Middle East and North Africa. Syst Rev. 2014;3:146 PubMed Central PMCID: PMCPMC4274704. 10.1186/2046-4053-3-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–53. 10.1016/j.annepidem.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 30.Brady OJ, Smith DL, Scott TW, Hay SI. Dengue disease outbreak definitions are implicitly variable. Epidemics. 2015;11:92–102. 10.1016/j.epidem.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77(4):442–5. [DOI] [PubMed] [Google Scholar]
- 32.Chastel C, Rogues G, Beaucournu-Saguez F. A mixed sero-epidemiologic survey on arbovirus-arenavirus in small mammalians in Tunisia. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1977;70(5):471–9. [PubMed] [Google Scholar]
- 33.Chastel C, Bach Hamba D, Launay H. New serosurvey on arbovirus infections in small wild mammals of Tunisia. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1983;76(1):21–33. [PubMed] [Google Scholar]
- 34.Ari A. The prevalence and ecology of arboviruses in Turkey. Turk hijiyen ve tecrubi biyoloji dergisi. 1972;32(2):134–43. [PubMed] [Google Scholar]
- 35.Darwish MA, Ibrahim AH. A serological survey on group A and B arbovirus antibodies in Libya. Journal of the Egyptian Public Health Association. 1974;49(1):20–6. [PubMed] [Google Scholar]
- 36.Omer AHS, McLaren ML, Johnson BK. A seroepidemiological survey in the Gezira, Sudan, with special reference to arboviruses. Journal of Tropical Medicine and Hygiene. 1981;84(2):63–6. [PubMed] [Google Scholar]
- 37.Rodier GR, Gubler DJ, Cope SE, Cropp CB, Soliman AK, Polycarpe D, et al. Epidemic dengue 2 in the city of Djibouti 1991–1992. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90(3):237–40. [DOI] [PubMed] [Google Scholar]
- 38.Farnon EC, Gould LH, Griffith KS, Osman MS, El Kholy A, Brair ME, et al. Household-Based Sero-Epidemiologic Survey after a Yellow Fever Epidemic, Sudan, 2005. American Journal of Tropical Medicine and Hygiene. 2010;82(6):1146–52. 10.4269/ajtmh.2010.09-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seidahmed OME, Hassan SA, Soghaier MA, Siam HAM, Ahmed FTA, Elkarsany MM, et al. Spatial and Temporal Patterns of Dengue Transmission along a Red Sea Coastline: A Longitudinal Entomological and Serological Survey in Port Sudan City. PLoS Neglected Tropical Diseases. 2012;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidahmed OM, Siam HA, Soghaier MA, Abubakr M, Osman HA, Abd Elrhman LS, et al. Dengue vector control and surveillance during a major outbreak in a coastal Red Sea area in Sudan. East Mediterr Health J. 2012;18(12):1217–24. [PubMed] [Google Scholar]
- 41.Siddiqui FJ, Haider SR, Bhutta ZA. Endemic Dengue Fever: A seldom recognized hazard for Pakistani children. Journal of Infection in Developing Countries. 2009;3(4):306–12. [DOI] [PubMed] [Google Scholar]
- 42.Jahan N. Detection of dengue viruses in aedes mosquitoes from different localities of Lahore, Pakistan. American Journal of Tropical Medicine and Hygiene. 2013;1):35. [Google Scholar]
- 43.Zayed A, Awash AA, Esmail MA, Al-Mohamadi HA, Al-Salwai M, Al-Jasari A, et al. Detection of Chikungunya virus in Aedes aegypti during 2011 outbreak in Al Hodayda, Yemen. Acta Tropica. 2012;123(1):62–6. 10.1016/j.actatropica.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 44.Elyan DS, Moustafa L, Noormal B, Jacobs JS, Aziz MA, Hassan KS, et al. Serological evidence of Flaviviruses infection among acute febrile illness patients in Afghanistan. J Infect Dev Ctries. 2014;8(9):1176–80. 10.3855/jidc.4183 [DOI] [PubMed] [Google Scholar]
- 45.Salah S, Fox E, Abbatte EA, Constantine NT, Asselin P, Soliman AK. A negative human serosurvey of haemorrhagic fever viruses in Djibouti. Annales de l'Institut Pasteur Virology. 1988;139(4):439–42. [DOI] [PubMed] [Google Scholar]
- 46.Faulde MK, Spiesberger M, Abbas B. Sentinel site-enhanced near-real time surveillance documenting West Nile virus circulation in two Culex mosquito species indicating different transmission characteristics, Djibouti City, Djibouti. Journal of the Egyptian Society of Parasitology. 2012;42(2):461–74. [DOI] [PubMed] [Google Scholar]
- 47.Andayi F, Charrel RN, Kieffer A, Richet H, Pastorino B, Leparc-Goffart I, et al. A sero-epidemiological study of arboviral fevers in Djibouti, horn of Africa. PLoS Negl Trop Dis. 2014;8(12):e3299 10.1371/journal.pntd.0003299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohammed YS, Sekeyova M, Gresikova M, el-Dawala K. Studies on arboviruses in Egypt. I. Hemagglutination-inhibition antibodies against arboviruses in human population of Alexandria and Abyss areas. The Indian journal of medical research. 1968;56(4):381–5. [PubMed] [Google Scholar]
- 49.Mohammed YS, Gresikova M, Adamyova K, Ragib AHe-DK. Studies on arboviruses in Egypt. II. Contribution of arboviruses to the aetiology of undiagnosed fever among children. The Journal of hygiene. 1970;68(3):491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darwish MA, Ibrahim AH. Prevalence of antibodies to arboviruses in Egypt. Results of a serologic survey among 1,113 university students. American Journal of Tropical Medicine and Hygiene. 1975;24(6 I):981–5. [DOI] [PubMed] [Google Scholar]
- 51.Saidi S. Survey of antibodies to arboviruses in human population of Iran. Pahlavi Medical Journal. 1971;2(3):485–90. [Google Scholar]
- 52.Saidi S. Viral antibodies in preschool children from the Caspian area, Iran. Iranian Journal of Public Health. 1974;3(2):83–91. [Google Scholar]
- 53.Chinikar S, Ghiasi SM, Shah-Hosseini N, Mostafavi E, Moradi M, Khakifirouz S, et al. Preliminary study of dengue virus infection in Iran. Travel Medicine and Infectious Disease. 2013;11(3):166–9. 10.1016/j.tmaid.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 54.Ibrahim SH, Darwish MA, Wahdan MH, El Ghoroury AAA. Survey for antibodies against group B arboviruses in man in Kuwait. Journal of the Egyptian Public Health Association. 1974;49(2):77–95. [PubMed] [Google Scholar]
- 55.Al-Nakib W, Lloyd G, El-Mekki A, Platt G, Beeson A, Southee T. Preliminary report on arbovirus-antibody prevalence among patients in Kuwait: evidence of Congo/Crimean virus infection. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1984;78(4):474–6. [DOI] [PubMed] [Google Scholar]
- 56.Pacsa A, Mustafa AS, Chaturvedi UC. Study of dengue virus infection in Kuwait. Dengue Bulletin. 2002;26(pp 113–117). [Google Scholar]
- 57.Hatem J. The role of the laboratory in the surveillance of viral diseases in Lebanon. Journal Medical Libanais. 1972;25(3):151–65. [PubMed] [Google Scholar]
- 58.Akram DS, Igarashi A, Takasu T. Dengue virus infection among children with undifferentiated fever in Karachi. Indian journal of pediatrics. 1998;65(5):735–40. [DOI] [PubMed] [Google Scholar]
- 59.Tariq W KS, Hussain A, Bhani E. Outbreak of dengue fever in Mangla and Mirpur area. Pak J Pathol. 2006;17(3):122. [Google Scholar]
- 60.Jamil B, Hasan R, Zafar A, Bewley K, Chamberlain J, Mioulet V, et al. Dengue virus serotype 3, Karachi, Pakistan. Emerg Infect Dis. 2007;13(1):182–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan E, Mehraj V, Nasir A, Khan NA, Billoo B, Moatter T, et al. Evaluation of two ELISA Assay Kits against RT-PCR for diagnosis of Dengue Virus Infection in a Hospital Setting in Karachi, Pakistan. Journal of the Pakistan Medical Association. 2009;59(6):390–4. [PubMed] [Google Scholar]
- 62.Khan E, Hasan R, Mehraj V, Nasir A, Siddiqui J, Hewson R. Co-circulations of two genotypes of dengue virus in 2006 out-break of dengue hemorrhagic fever in Karachi, Pakistan. Journal of Clinical Virology. 2008;43(2):176–9. 10.1016/j.jcv.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 63.Koo C, Nasir A, Hapuarachchi HC, Lee KS, Hasan Z, Ng LC, et al. Evolution and heterogeneity of multiple serotypes of Dengue virus in Pakistan, 2006–2011. Virology Journal. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan AH, Hayat AS, Masood N, Solangi NM, Shaikh TZ. Frequency and clinical presentation of dengue fever at tertiary care hospital of Hyderabad/Jamshoro. Journal of the Liaquat University of Medical and Health Sciences. 2010;9(2):88–94. [Google Scholar]
- 65.Khan E, Kisat M, Khan N, Nasir A, Ayub S, Hasan R. Demographic and clinical features of dengue fever in Pakistan from 2003–2007: A retrospective cross- sectional study. PLoS ONE. 2010;5(9):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbasi A, Butt N, Sheikh QH, Bhutto AR, Munir SM, Ahmed SM. Clinical features, diagnostic techniques and management of dual dengue and Malaria infection. Journal of the College of Physicians and Surgeons Pakistan. 2009;19(1):25–9. [PubMed] [Google Scholar]
- 67.Tahir Z HS, Chaudhry A. Spatial and seasonal varation of dengue fever in Lahore 2008. Biomedica. 2010;26(Jul-Dec .):166. [Google Scholar]
- 68.Murad H, Asahar RJ, Zaheen M, Shawali R. Outbreak investigation of Dengue fever in Sundia, Chakaiser, Shangla, Pakistan-2008. Journal of Ayub Medical College, Abbottabad: JAMC. 2014;26(4):571–6. [PubMed] [Google Scholar]
- 69.Mahmood K AH, Tahir M. Incidence of dengue haemorrhagic fever in local population of Lahore, Pakistan. Biomedica. 2009;25(Jul.-Dec.):93–6. [Google Scholar]
- 70.Kidwai AA, Jamal Q, Saher, Mehrunnisa, Farooqi FU, Saleem U. Serodiagnosis of dengue infection using rapid immunochromatography test in patients with probable dengue infection. J Pak Med Assoc. 2010;60(11):936–9. [PubMed] [Google Scholar]
- 71.Zafar H, Hayyat A, Akhtar N, Rizwan SF. Prevalence of undifferentiated fever in adults of Rawalpindi having primary dengue fever. JPMA The Journal of the Pakistan Medical Association. 2013;63(6):770–1. [PubMed] [Google Scholar]
- 72.Zafar H, Hayyat A, Akhtar N. Incidence of primary dengue viral infection in healthy adults of Rawalpindi, Pakistan. Journal of the Pakistan Medical Association. 2011;61(10):1030–1. [PubMed] [Google Scholar]
- 73.Qureshi KA LA, Samoo AH. Screening for dengue virus infection at GMMMC hospital, Sukkur. Medical Forum Monthly. 2013;24(4):6–8. [Google Scholar]
- 74.Khan H KQ, Khan BA, Arif M, Raza AAH. Retrospective analysis of 68 cases of dengue fever. Pak J Med Res. 2012;51(1):18. [Google Scholar]
- 75.Hasan SR, Riaz M, Jafri FA. Characteristics and outcome of dengue infection; clinical perspective from a secondary care hospital of Karachi. Pakistan Journal of Medical Sciences. 2013;29(1):115–8. 10.12669/pjms.291.2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Umar S, Ashraf O, Umar M. Characteristics of febrile thrombocytopenia during dengue epidemic 2010 in Rawalpindi, Pakistan. International Journal of Infectious Diseases. 2011;15:S114–S115. [Google Scholar]
- 77.Jameel T MK, Ghulam Choudhry N, Afzal NP, Paul RF. Changing hematologic parameters in dengue viral infections. J Ayub med Coll-Abbotabad-Pak. 2012;24(1):3. [PubMed] [Google Scholar]
- 78.Naeem M SA, Batool S, Rubab S, Saba T, Riaz T, Mahmood A. Dengue fever; a clinical experience Professional Med J. 2014;23(2):243–46. [Google Scholar]
- 79.Ahmed S, Mohammad WW, Hamid F, Akhter A, Afzal RK, Mahmood A. The 2011 dengue haemorrhagic fever outbreak in lahore—an account of clinical parameters and pattern of haemorrhagic complications. Journal of the College of Physicians and Surgeons Pakistan. 2013;23(7):463–7. [PubMed] [Google Scholar]
- 80.Ijaz T, Ijaz S, Aslam S, Ahmad BM, Raja SA. A laboratory based study of dengue epidemic in the city of Lahore during year 2011. International Journal of Infectious Diseases. 2014;21:136. [Google Scholar]
- 81.Rashid A KH, Nadeem UR. Dengue Hemorrhagic Fever / dengue shock syndrome. Professional Med J. 2012;19(5):661. [Google Scholar]
- 82.Khan MA, Ellis EM, Tissera HA, Alvi MY, Rahman FF, Masud F, et al. Emergence and Diversification of Dengue 2 Cosmopolitan Genotype in Pakistan, 2011. PLoS ONE. 2013;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasan Z, Atkinson B, Jamil B, Samreen A, Altaf L, Hewson R. Short report: Diagnostic testing for hemorrhagic fevers in Pakistan: 2007–2013. American Journal of Tropical Medicine and Hygiene. 1243;91(6):1243–6. 10.4269/ajtmh.14-0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali A, Rehman HU, Nisar M, Rafique S, Ali S, Hussain A, et al. Seroepidemiology of dengue fever in Khyber Pakhtunkhawa, Pakistan. International Journal of Infectious Diseases. 2013;17(7):e518–e23. 10.1016/j.ijid.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 85.Hisam A, Mahmood ur R, Khan MB, Kadir E, Azam N. Frequency of co-existence of dengue and malaria in patients presenting with acute febrile illness. JPMA The Journal of the Pakistan Medical Association. 2014;64(3):247–51. [PubMed] [Google Scholar]
- 86.Assir MZK, Masood MA, Ahmad HI. Concurrent dengue and malaria infection in Lahore, Pakistan during the 2012 dengue outbreak. International Journal of Infectious Diseases. 2014;18(1):41–6. [DOI] [PubMed] [Google Scholar]
- 87.Fakeeh M, Zaki AM. Virologic and serologic surveillance for dengue fever in Jeddah, Saudi Arabia, 1994–1999. American Journal of Tropical Medicine and Hygiene. 2001;65(6):764–7. [DOI] [PubMed] [Google Scholar]
- 88.Fakeeh M, Zaki AM . Dengue in Jeddah, Saudi Arabia, 1994–2002. Dengue Bulletin. 2003;27(pp 13–18). [Google Scholar]
- 89.Khan NA, Azhar EI, El-Fiky S, Madani HH, Abuljadial MA, Ashshi AM, et al. Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Tropica. 2008;105(1):39–44. 10.1016/j.actatropica.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 90.Ayyub M, Khazindar AM, Lubbad EH, Barlas S, Alfi AY, Al-Ukayli S. Characteristics of dengue fever in a large public hospital, Jeddah, Saudi Arabia. Journal of Ayub Medical College, Abbottabad: JAMC. 2006;18(2):9–13. [PubMed] [Google Scholar]
- 91.Shahin W, Nassar A, Kalkattawi M, Bokhari H. Dengue fever in a tertiary hospital in Makkah, Saudi Arabia. Dengue Bulletin. 2009;33(1):34–44. [Google Scholar]
- 92.Said SM EK, Alyan Z. Benign acute myositis in association with acute dengue viruses' infections. Egypt J Neurol Psychiatry Neurosurg. 2008;45(1):193. [Google Scholar]
- 93.Memish ZA, Albarrak A, Almazroa MA, Al-Omar I, Alhakeem R, Assiri A, et al. Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerging Infectious Diseases. 2011;17(12):2316–8. 10.3201/eid1712.110658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gamil MA, Eisa ZM, Eifan SA, Al-Sum BA. Prevalence of dengue fever in Jizan area, Saudi Arabia. Journal of Pure and Applied Microbiology. 2014;8(1):225–31. [Google Scholar]
- 95.Al-Azraqi TA, El Mekki AA, Mahfouzc AA. Seroprevalence of dengue virus infection in Aseer and Jizan regions, Southwestern Saudi Arabia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2013;107(6):368–71. 10.1093/trstmh/trt022 [DOI] [PubMed] [Google Scholar]
- 96.Ashshi AM. Serodetection of Dengue virus and its antibodies among blood donors in the western region of Saudi Arabia: a preliminary study. Blood Transfus. 2015;13(1):135–8. 10.2450/2014.0134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Botros BAM, Watts DM, Soliman AK, Salib AW, Moussa MI, Mursal H, et al. Serological evidence of dengue fever among refugees, Hargeysa, Somalia. Journal of Medical Virology. 1989;29(2):79–81. [DOI] [PubMed] [Google Scholar]
- 98.Kanesa-thasan N, Iacono-Connors L, Magill A, Smoak B, Vaughn D, Dubois D, et al. Dengue serotypes 2 and 3 in US forces in Somalia. Lancet. 1994;343(8898):678. [DOI] [PubMed] [Google Scholar]
- 99.Sharp TW, Wallace MR, Hayes CG, Sanchez JL, DeFraites RF, Arthur RR, et al. Dengue fever in U.S. troops during Operation Restore Hope, Somalia, 1992–1993. Am J Trop Med Hyg. 1995;53(1):89–94. [PubMed] [Google Scholar]
- 100.Nur YA, Groen J, Yusuf MA, Osterhaus ADME. IgM antibodies in hospitalized children with febrile illness during an inter-epidemic period of measles, in Somalia. Journal of Clinical Virology. 1999;12(1):21–5. [DOI] [PubMed] [Google Scholar]
- 101.Kyobe Bosa H, Montgomery JM, Kimuli I, Lutwama JJ. Dengue fever outbreak in Mogadishu, Somalia 2011: Co-circulation of three dengue virus serotypes. International Journal of Infectious Diseases. 2014;21:3. [Google Scholar]
- 102.Hyams KC, Oldfield EC, McNair Scott R. Evaluation of febrile patients in Port Sudan, Sudan: Isolation of dengue virus. American Journal of Tropical Medicine and Hygiene. 1986;35(4):860–5. [DOI] [PubMed] [Google Scholar]
- 103.Woodruff PWR, Morrill JC, Burans JP, Hyams KC, Woody JN. A study of viral and rickettsial exposure and causes of fever in Juba, southern Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82(5):761–6. [DOI] [PubMed] [Google Scholar]
- 104.McCarthy MC, Haberberger RL, Salib AW, Soliman BA, El-Tigani A, Khalid IO, et al. Evaluation of arthropod-borne viruses and other infectious disease pathogens as the causes of febrile illnesses in the Khartoum province of Sudan. Journal of Medical Virology. 1996;48(2):141–6. [DOI] [PubMed] [Google Scholar]
- 105.Ibrahim SA, Mustafa OM, Mukhtar MM, Saleh EA, El Mubarak HS, Abdallah A, et al. Measles in suburban Khartoum: An epidemiological and clinical study. Tropical Medicine and International Health. 2002;7(5):442–9. [DOI] [PubMed] [Google Scholar]
- 106.Malik A, Earhart K, Mohareb E, Saad M, Saeed M, Ageep A, et al. Dengue hemorrhagic fever outbreak in children in Port Sudan. Journal of Infection and Public Health. 2011;4(1):1–6. 10.1016/j.jiph.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 107.Gould LH, Osman MS, Farnon EC, Griffith KS, Godsey MS, Karch S, et al. An outbreak of yellow fever with concurrent chikungunya virus transmission in South Kordofan, Sudan, 2005. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(12):1247–54. 10.1016/j.trstmh.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 108.Adam I, Jumaa AM, Elbashir HM, Karsany MS. Maternal and perinatal outcomes of dengue in PortSudan, Eastern Sudan. Virology Journal. 2010;7(153). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Himatt S, Osman KE, Okoued SI, Seidahmed OE, Beatty ME, Soghaier MA, et al. Sero-prevalence of dengue infections in the Kassala state in the eastern part of the Sudan in 2011. J Infect Public Health. 2015. [DOI] [PubMed] [Google Scholar]
- 110.Abdalla TM, Karsany MS, Ali AA. Correlation of measles and dengue infection in Kassala, Eastern Sudan. J Med Virol. 2014. [DOI] [PubMed] [Google Scholar]
- 111.Soghaier MA, Mahmood SF, Pasha O, Azam SI, Karsani MM, Elmangory MM, et al. Factors associated with dengue fever IgG sero-prevalence in South Kordofan State, Sudan, in 2012: Reporting prevalence ratios. Journal of Infection and Public Health. 2014;7(1):54–61. 10.1016/j.jiph.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 112.Radda A. Studies on the activity and ecology of arboviruses in Turkey. ZblBaktReihe A. 1973;225(1):19–26. [PubMed] [Google Scholar]
- 113.Ergunay K, Saygan MB, Aydogan S, Litzba N, Niedrig M, Pinar A, et al. Investigation of dengue virus and yellow fever virus seropositivities in blood donors from central/Northern Anatolia, Turkey. Mikrobiyoloji Bulteni. 2010;44(3):415–24. [PubMed] [Google Scholar]
- 114.Tezcan S, Kizildamar S, Ulger M, Aslan G, Tiftik N, Ozkul A, et al. Flavivirus seroepidemiology in blood donors in Mersin province, Turkey. Mikrobiyoloji bulteni. 2014;48(4):606–17. [DOI] [PubMed] [Google Scholar]
- 115.Bin Ghouth AS, Amarasinghe A, Letson GW. Dengue outbreak in Hadramout, Yemen, 2010: an epidemiological perspective. Am J Trop Med Hyg. 2012;86(6):1072–6. 10.4269/ajtmh.2012.11-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malik MR, Mnzava A, Mohareb E, Zayed A, Al Kohlani A, Thabet AAK, et al. Chikungunya outbreak in Al-Hudaydah, Yemen, 2011: Epidemiological characterization and key lessons learned for early detection and control. Journal of Epidemiology and Global Health. 2014;4(3):203–11. 10.1016/j.jegh.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Madani TA, Abuelzein ETME, Al-Bar HMS, Azhar EI, Kao M, Alshoeb HO, et al. Outbreak of viral hemorrhagic fever caused by dengue virus type 3 in Al-Mukalla, Yemen. BMC Infectious Diseases. 2013;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rezza G, El-Sawaf G, Faggioni G, Vescio F, Al Ameri R, De Santis R, et al. Co-circulation of Dengue and Chikungunya Viruses, Al Hudaydah, Yemen, 2012. Emerg Infect Dis. 2014;20(8):1351–4. 10.3201/eid2008.131615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qassem MAM, Jaawal AAT. Dengue fever or West Nile virus outbreak? Yemen 2013. International Journal of Infectious Diseases. 2014;21:457. [Google Scholar]
- 120.World Health Organizationj Regional Office for the Eastern Mediterranean. Dengue in Pakistan. Weekly Epidemiological Monitor. 2013;6(52). Available www.emro.who.int/surveillance-forecasting-response/weekly-epidemiological-monitor/. Accessed 17 Jan 2016. [Google Scholar]
- 121.Haider Z, Ahmad FZ, Mahmood A, Waseem T, Shafiq I, Raza T, et al. Dengue fever in Pakistan: A paradigm shift; Changing epidemiology and clinical patterns. Perspectives in Public Health. 2015;135(6):294–8. 10.1177/1757913915599019 [DOI] [PubMed] [Google Scholar]
- 122.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372(13):1231–9. 10.1056/NEJMra1406035 [DOI] [PubMed] [Google Scholar]
- 123.Nsoesie EO, Kraemer MU, Golding N, Pigott DM, Brady OJ, Moyes CL, et al. Global Distribution and Environmental Suitability for Chikungunya Virus, 1952–2015. Euro Surveillance. 2016;21(20):pii = 30234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koo C, Nasir A, Hapuarachchi HC, Lee KS, Hasan Z, Ng LC, et al. Evolution and heterogeneity of multiple serotypes of Dengue virus in Pakistan, 2006–2011. Virol J. 2013;10:275 10.1186/1743-422X-10-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jimenez-Lucho VE, Fisher EJ, Saravolatz LD. Dengue with hemorrhagic manifestations: An imported case from the Middle East. American Journal of Tropical Medicine and Hygiene. 1984;33(4):650–3. [DOI] [PubMed] [Google Scholar]
- 126.World Health Organization. Dengue: Guidelines for Diagnosis, Treatement, Prevention and Control. Geneva, Switzerland. 2009. Available at: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf Accessed 1 September 2016.
- 127.World Health Organization Regional Office for the Eastern Mediterranean. Multiple outbreaks from DF/DHF in the EMR. 2008;1(30). Available www.emro.who.int/surveillance-forecasting-response/weekly-epidemiological-monitor/. Accessed 17 Jan 2016. [Google Scholar]
- 128.Ghouth ASB, Amarasinghe A, Letson GW. Dengue outbreak in Hadramout, Yemen, 2010: An epidemiological perspective. American Journal of Tropical Medicine and Hygiene. 1072;86(6):1072–6. 10.4269/ajtmh.2012.11-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burdino E, Milia MG, Sergi G, Gregori G, Allice T, Cazzato ML, et al. Diagnosis of dengue fever in North West Italy in travelers from endemic areas: a retrospective study. J Clin Virol. 2011;51(4):259–63. 10.1016/j.jcv.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 130.Heikal OM, El-Bahnasawy MM, Morsy AT, Khalil HH. Aedes aegypti re-emerging in Egypt: a review and what should be done? Journal of the Egyptian Society of Parasitology. 2011;41(3):801–14. [PubMed] [Google Scholar]
- 131.Ahmadnejad F, Otarod V, Fallah MH, Lowenski S, Sedighi-Moghaddam R, Zavareh A, et al. Spread of West Nile virus in Iran: A cross-sectional serosurvey in equines, 2008–2009. Epidemiology and Infection. 2011;139(10):1587–93. 10.1017/S0950268811000173 [DOI] [PubMed] [Google Scholar]
- 132.Conley AK, Fuller DO, Haddad N, Hassan AN, Gad AM, Beier JC. Modeling the distribution of the West Nile and Rift Valley Fever vector Culex pipiens in arid and semi-arid regions of the Middle East and North Africa. Parasites & vectors. 2014;7:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–45. 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, et al. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. MMWR Morb Mortal Wkly Rep. 2016;65(21):543–6. 10.15585/mmwr.mm6521e1 [DOI] [PubMed] [Google Scholar]
- 135.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Izri A, Bitam I, Charrel RN. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clinical Microbiology and Infection. 1116;17(7):1116–8. 10.1111/j.1469-0691.2010.03443.x [DOI] [PubMed] [Google Scholar]
- 137.Haddad N, Harbach RE, Chamat S, Bouharoun-Tayoun H. Presence of Aedes albopictus in Lebanon and Syria. J Am Mosq Control Assoc. 2007;23(2):226–8. 10.2987/8756-971X(2007)23[226:POAAIL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 138.Adawi SHAA. Presence of Aedes albopictus in Palestine–West Bank. International Journal of Tropical Disease and Health. 2012;2(4):301–10. [Google Scholar]
- 139.Leshem E, Bin H, Shalom U, Perkin M, Schwartz E. Risk for emergence of dengue and chikungunya virus in Israel. Emerging Infectious Diseases. 2012;18(2):345–7. 10.3201/eid1802.111648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oter K, Gunay F, Tuzer E, Linton YM, Bellini R, Alten B. First record of Stegomyia albopicta in Turkey determined by active ovitrap surveillance and DNA barcoding. Vector Borne Zoonotic Dis. 2013;13(10):753–61. 10.1089/vbz.2012.1093 [DOI] [PubMed] [Google Scholar]
- 141.European Centre for Disease Prevention and Control. Guidelines for the surveillance of invasive mosquitoes in Europe Stockholm: ECDC; 2012. Available at: http://ecdc.europa.eu/en/publications/publications/ter-mosquito-surveillance-guidelines.pdf. Accessed 15 Jan 2016. [Google Scholar]
- 142.The World Bank Group. The Middle East and North Africa: Urban Development 2015. Available from: http://go.worldbank.org/Y88FI6V7R0. Accessed 8 Feb 2016.
- 143.Soghaier MA, Hagar A, Abbas MA, Elmangory MM, Eltahir KM, Sall AA. Yellow fever outbreak in Darfur, Sudan in october 2012; the initial outbreak investigation report. Journal of Infection and Public Health. 2013;6(5):370–6. 10.1016/j.jiph.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 144.Seidahmed O, Siam HA, Hassan SA, Mohamed SA, Abd Elrhman LS, Osman HA, et al. Vector control and surveillance during a major outbreak of dengue in a coastal Red Sea area: Port Sudan City. American Journal of Tropical Medicine and Hygiene. 2011;1):92. [Google Scholar]
- 145.Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009;103(2):109–21. 10.1016/j.trstmh.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Amarasinghe A, Letson GW. Dengue in the Middle East: A neglected, emerging disease of importance. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2012;106(1):1–2. 10.1016/j.trstmh.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 147.Fahmy NT, Klena JD, Mohamed AS, Zayed A, Villinski JT. Complete Genome Sequence of Chikungunya Virus Isolated from an Aedes aegypti Mosquito during an Outbreak in Yemen, 2011. Genome announcements. 2015;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ciccozzi M, Lo Presti A, Cella E, Giovanetti M, Lai A, El-Sawaf G, et al. Phylogeny of Dengue and Chikungunya viruses in Al Hudayda governorate, Yemen. Infection, genetics and evolution: Journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2014;27C:395–401. [DOI] [PubMed] [Google Scholar]
- 149.Rezza G, El-Sawaf G, Faggioni G, Vescio F, Al Ameri R, De Santis R, et al. Co-circulation of dengue and chikungunya viruses, Al Hudaydah, Yemen, 2012. Emerging Infectious Diseases. 2014;20(8):1351–4. 10.3201/eid2008.131615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Awaidy ST, Obeidani IA, Bawikar S, Mahrouqi SA, Busaidy SS, Baqlani SA, et al. Dengue epidemiological trend in Oman: a 13-year national surveillance and strategic proposition of imported cases. Trop Doct. 2014. [DOI] [PubMed] [Google Scholar]
- 151.Cleton NB, Godeke GJ, Reimerink J, Beersma MF, Doorn HR, Franco L, et al. Spot the difference-development of a syndrome based protein microarray for specific serological detection of multiple flavivirus infections in travelers. PLoS Negl Trop Dis. 2015;9(3):e0003580 10.1371/journal.pntd.0003580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Centers for Disease Control and Prevention. Mosquito Surveillance Software. Atlanta, GA, USA 2015. Available from: http://www.cdc.gov/westnile/resourcepages/mosqsurvsoft.html. Accessed 8 Feb 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.