Abstract
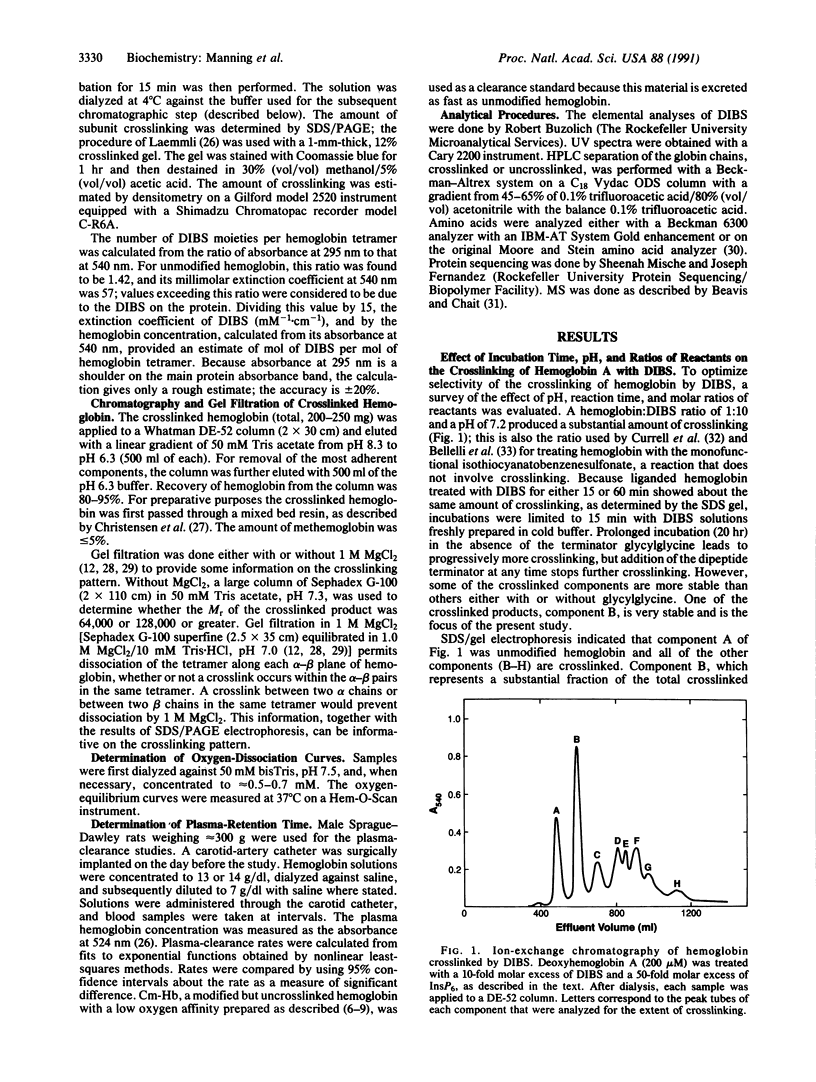
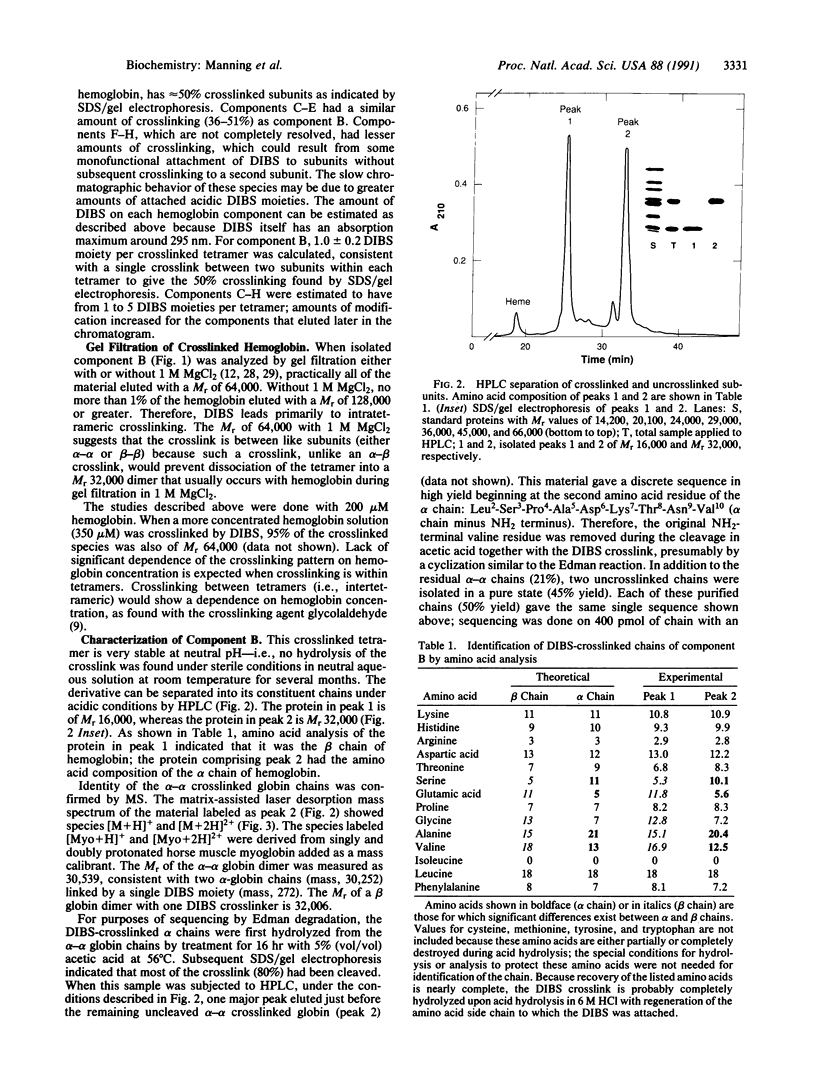
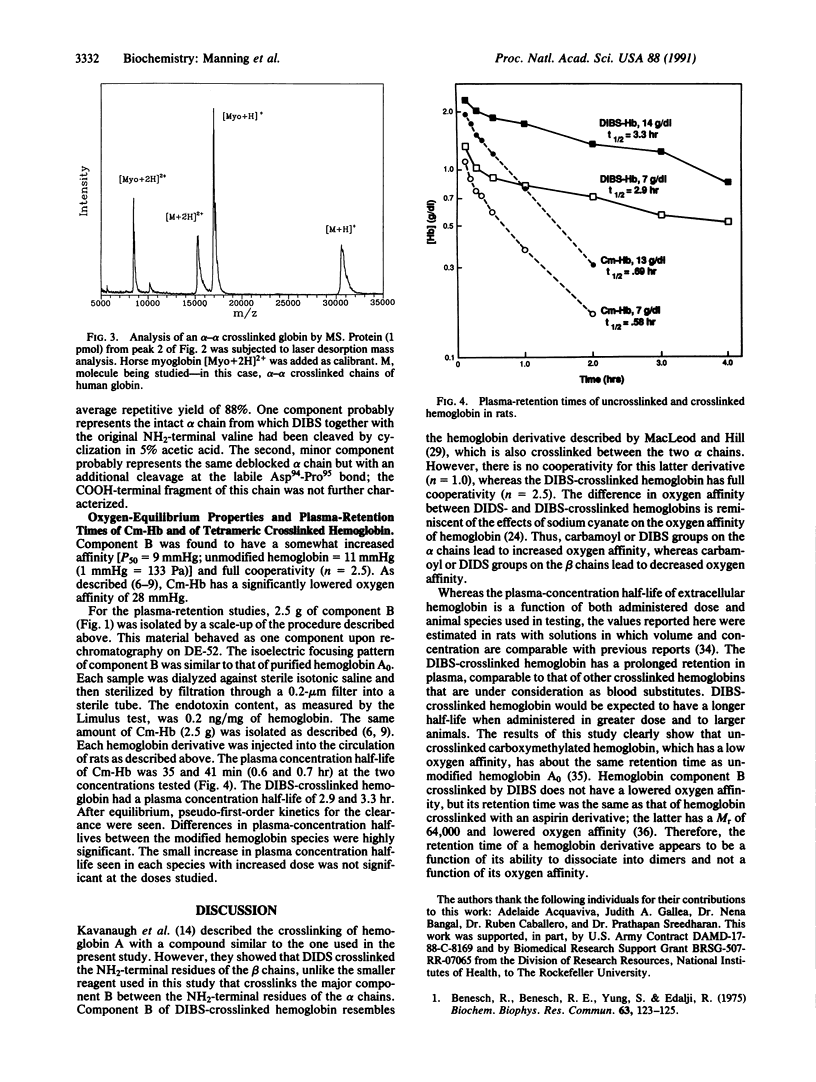
Human hemoglobin A has been crosslinked by diisothiocyanatobenzenesulfonate to give a limited number of products in a yield of approximately 70%. The predominant product was crosslinked between subunits within a tetramer and had a Mr of 64,000; no higher Mr species were formed. This product had one crosslink per tetramer located between the NH2 termini of its alpha chains, as established by HPLC analysis, amino acid analysis, Edman degradation, and mass spectrometry. This crosslinked derivative had a slightly increased oxygen affinity [P50 = 9 mmHg (1 mmHg = 133 Pa); P50 for unmodified hemoglobin = 11 mmHg], and the retention time of this derivative in the circulation of rats was 2.9 and 3.3 hr at two hemoglobin concentrations (7 g/dl and 14 g/dl, respectively). The half-life of an uncrosslinked carboxymethylated derivative, which has a low oxygen affinity (P50 = 28 mmHg), was 0.6 and 0.7 hr under the same conditions. Therefore, prolongation of the plasma-retention time of infused hemoglobin is dependent on the crosslinking of the tetramer but independent of the oxygen affinity of the derivative.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajisaka K., Iwashita Y. Modification of human hemoglobin with polyethylene glycol : a new candidate for blood substitute. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1076–1081. doi: 10.1016/0006-291x(80)91485-0. [DOI] [PubMed] [Google Scholar]
- Beavis R. C., Chait B. T. High-accuracy molecular mass determination of proteins using matrix-assisted laser desorption mass spectrometry. Anal Chem. 1990 Sep 1;62(17):1836–1840. doi: 10.1021/ac00216a020. [DOI] [PubMed] [Google Scholar]
- Bellelli A., Ippoliti R., Currell D., Condò S. G., Giardina B., Brunori M. On the oxygen-linked anion-binding sites in human hemoglobin. Functional properties of human hemoglobin reacted with 4-isothiocyanatobenzenesulphonic acid and its hybrids. Eur J Biochem. 1986 Dec 1;161(2):329–333. doi: 10.1111/j.1432-1033.1986.tb10451.x. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Renthal R. D., Maeda N. Affinity labeling of the polyphosphate binding site of hemoglobin. Biochemistry. 1972 Sep 12;11(19):3576–3582. doi: 10.1021/bi00769a013. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Kwong S. Bis-pyridoxal polyphosphates: a new class of specific intramolecular crosslinking agents for hemoglobin. Biochem Biophys Res Commun. 1988 Oct 14;156(1):9–14. doi: 10.1016/s0006-291x(88)80798-8. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Yung S., Suzuki T., Bauer C., Benesch R. Pyridoxal compounds as specific reagents for the alpha and beta N-termini of hemoglobin. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2595–2599. doi: 10.1073/pnas.70.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Kwong S., Acharya A. S., Manning J. M. Labeling of hemoglobin with pyridoxal phosphate. J Biol Chem. 1982 Feb 10;257(3):1320–1324. [PubMed] [Google Scholar]
- Bleeker W. K., van der Plas J., Agterberg J., Rigter G., Bakker J. C. Prolonged vascular retention of a hemoglobin solution modified by cross-linking with 2-nor-2-formylpyridoxal 5'-phosphate. J Lab Clin Med. 1986 Nov;108(5):448–455. [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. The renal handling of hemoglobin. Trans Assoc Am Physicians. 1968;81:147–152. [PubMed] [Google Scholar]
- Chang T. M., Varma R. Pyridoxalated heterogenous and homologous polyhemoglobin and hemoglobin: systemic effects of replacement transfusion in rats previously received immunizing doses. Biomater Artif Cells Artif Organs. 1987;15(2):443–451. doi: 10.3109/10731198709118538. [DOI] [PubMed] [Google Scholar]
- Chatterjee R., Walder R. Y., Arnone A., Walder J. A. Mechanism for the increase in solubility of deoxyhemoglobin S due to cross-linking the beta chains between lysine-82 beta 1 and lysine-82 beta 2. Biochemistry. 1982 Nov 9;21(23):5901–5909. doi: 10.1021/bi00266a027. [DOI] [PubMed] [Google Scholar]
- Chatterjee R., Welty E. V., Walder R. Y., Pruitt S. L., Rogers P. H., Arnone A., Walder J. A. Isolation and characterization of a new hemoglobin derivative cross-linked between the alpha chains (lysine 99 alpha 1----lysine 99 alpha 2). J Biol Chem. 1986 Jul 25;261(21):9929–9937. [PubMed] [Google Scholar]
- Christensen S. M., Medina F., Winslow R. W., Snell S. M., Zegna A., Marini M. A. Preparation of human hemoglobin Ao for possible use as a blood substitute. J Biochem Biophys Methods. 1988 Oct;17(2):143–154. doi: 10.1016/0165-022x(88)90045-0. [DOI] [PubMed] [Google Scholar]
- Currell D. L., Law B., Stevens M., Murata P., Ioppolo C., Martini F. The effect of 4-isothiocyanatobenzene sulfonic acid on the oxygen affinity of human hemoglobin. Biochem Biophys Res Commun. 1981 Sep 16;102(1):348–354. doi: 10.1016/0006-291x(81)91528-x. [DOI] [PubMed] [Google Scholar]
- DeVenuto F., Zegna A. Preparation and evaluation of pyridoxalated-polymerized human hemoglobin. J Surg Res. 1983 Mar;34(3):205–212. doi: 10.1016/0022-4804(83)90061-6. [DOI] [PubMed] [Google Scholar]
- DiDonato A., Fantl W. J., Acharya A. S., Manning J. M. Selective carboxymethylation of the alpha-amino groups of hemoglobin. Effect on functional properties. J Biol Chem. 1983 Oct 10;258(19):11890–11895. [PubMed] [Google Scholar]
- Fantl W. J., Di Donato A., Manning J. M., Rogers P. H., Arnone A. Specifically carboxymethylated hemoglobin as an analogue of carbamino hemoglobin. Solution and X-ray studies of carboxymethylated hemoglobin and X-ray studies of carbamino hemoglobin. J Biol Chem. 1987 Sep 15;262(26):12700–12713. [PubMed] [Google Scholar]
- Fantl W. J., Manning L. R., Ueno H., Di Donato A., Manning J. M. Properties of carboxymethylated cross-linked hemoglobin A. Biochemistry. 1987 Sep 8;26(18):5755–5761. doi: 10.1021/bi00392a026. [DOI] [PubMed] [Google Scholar]
- Fronticelli C., Bucci E., Razynska A. Modulation of oxygen affinity in hemoglobin by solvent components. Interaction of bovine hemoglobin with 2,3-diphosphoglycerate and monatomic anions. J Mol Biol. 1988 Jul 20;202(2):343–348. doi: 10.1016/0022-2836(88)90463-9. [DOI] [PubMed] [Google Scholar]
- Hess J. R., Fadare S. O., Tolentino L. S., Bangal N. R., Winslow R. M. The intravascular persistence of crosslinked human hemoglobin. Prog Clin Biol Res. 1989;319:351–360. [PubMed] [Google Scholar]
- KIRSHNER A. G., TANFORD C. THE DISSOCIATION OF HEMOGLOBIN BY INORGANIC SALTS. Biochemistry. 1964 Mar;3:291–296. doi: 10.1021/bi00891a002. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Shih D. T., Jones R. T. Affinity labeling of hemoglobin with 4,4'-diisothiocyanostilbene-2,2'-disulfonate: covalent cross-linking in the 2,3-diphosphoglycerate binding site. Biochemistry. 1988 Mar 8;27(5):1804–1808. doi: 10.1021/bi00405a062. [DOI] [PubMed] [Google Scholar]
- Keipert P. E., Adeniran A. J., Kwong S., Benesch R. E. Functional properties of a new crosslinked hemoglobin designed for use as a red cell substitute. Transfusion. 1989 Nov-Dec;29(9):768–773. doi: 10.1046/j.1537-2995.1989.29990070179.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macleod R. M., Hill R. J. Reaction of human CO hemoglobin with p,p'-difluoro-m,m'-dinitrodiphenylsulfone. J Biol Chem. 1970 Oct 10;245(19):4875–4879. [PubMed] [Google Scholar]
- Manning J. M. Preparation of hemoglobin carbamylated at specific NH2-terminal residues. Methods Enzymol. 1981;76:159–167. doi: 10.1016/0076-6879(81)76124-x. [DOI] [PubMed] [Google Scholar]
- Manning L. R., Manning J. M. Influence of ligation state and concentration of hemoglobin A on its cross-linking by glycolaldehyde: functional properties of cross-linked, carboxymethylated hemoglobin. Biochemistry. 1988 Aug 23;27(17):6640–6644. doi: 10.1021/bi00417a064. [DOI] [PubMed] [Google Scholar]
- Nigen A. M., Njikam N., Lee C. K., Manning J. M. Studies on the mechanism of action of cyanate in sickle cell disease. Oxygen affinity and gelling properties of hemoglobin S carbamylated on specific chains. J Biol Chem. 1974 Oct 25;249(20):6611–6616. [PubMed] [Google Scholar]
- Sehgal L. R., Gould S. A., Rosen A. L., Sehgal H. L., Moss G. S. Polymerized pyridoxylated hemoglobin: a red cell substitute with normal oxygen capacity. Surgery. 1984 Apr;95(4):433–438. [PubMed] [Google Scholar]
- Tam S. C., Blumenstein J., Wong J. T. Soluble dextran-hemoglobin complex as a potential blood substitute. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2128–2131. doi: 10.1073/pnas.73.6.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Plas J., de Vries-van Rossen A., Bleeker W. K., Bakker J. C. Effect of coupling of 2-nor-2-formylpyridoxal 5'-phosphate to stroma-free hemoglobin on oxygen affinity and tissue oxygenation. Studies in the isolated perfused rat liver under conditions of normoxia and stagnant hypoxia. J Lab Clin Med. 1986 Sep;108(3):253–260. [PubMed] [Google Scholar]
- Vandegriff K. D., Medina F., Marini M. A., Winslow R. M. Equilibrium oxygen binding to human hemoglobin cross-linked between the alpha chains by bis(3,5-dibromosalicyl) fumarate. J Biol Chem. 1989 Oct 25;264(30):17824–17833. [PubMed] [Google Scholar]