Abstract
Natural killer (NK) T cells with an invariant Vα14 rearrangement (Vα14i) are the largest population of lipid antigen-specific T lymphocytes identified in animals. They react to the glycolipid α-galactosyl ceramide (α-GalCer) presented by CD1d, and they may have important regulatory functions. It was previously shown that the Vα14i T cell antigen receptor (TCR) has a high affinity for the α-GalCer/CD1d complex, driven by a long half-life (t1/2). Although this result could have reflected the unique attributes of α-GalCer, using several related glycolipid compounds, we show here that the threshold for full activation of Vα14i NKT cells by these glycosphingolipids requires a relatively high-affinity TCR interaction with a long t1/2. Furthermore, our data are consistent with the view that the mechanism of recognition of these compounds presented by CD1d to the Vα14i NKT cell TCR is likely to fit a lock-and-key model. Overall, these findings emphasize the distinct properties of glycosphingolipid antigen recognition by Vα14i NKT cells.
Most T lymphocytes recognize antigenic peptides presented by MHC-encoded class I and class II molecules, although T cells responding to glycolipid antigens also have been characterized (1). Invariant Vα14 rearrangement (Vα14i) natural killer (NK) T cells constitute the most numerous glycolipid-reactive population identified in mice, and a homologous population of Vα24i NKT cells is present in humans (2, 3). Vα14i NKT cells produce large amounts of cytokines rapidly after stimulation (4), and this cytokine burst may have a widespread influence on immune responses, including protection against autoimmune diseases, the host response to parasites and bacteria, and antitumor responses (2, 3, 5–8).
Vα14i NKT cells bear a T cell antigen receptor (TCR) containing an invariant Vα14–Jα18 rearrangement paired preferentially with either Vβ8.2, Vβ7, or Vβ2 (9). Vα14i NKT cells are reactive to CD1d, an antigen-presenting molecule distantly related to the peptide-presenting MHC class I and class II molecules (10). The lack of diversity of their TCR α chains, as well as the absence of CD1d polymorphism, suggests that Vα14i NKT cells may have been selected to respond to a small set of related compounds.
The biochemical basis for the interaction of peptide-reactive TCRs with antigen–MHC complexes has been well studied (for review see refs. 11–13). Less is known, however, about the biochemical mechanisms that trigger the responses of Vα14i NKT cells. Although the natural antigen(s) that stimulate the majority of Vα14i NKT cells have not been unequivocally identified, Vα14i NKT cells are strongly reactive to a synthetic version of a marine sponge-derived glycolipid: α-galactosyl ceramide (α-GalCer) (14). We and others have shown previously that the TCR of Vα14i NKT cells exhibits a high-affinity binding to the α-GalCer/CD1d complex and a long half-life (t1/2) (15–18). α-GalCer is a very potent antigen, however, and it was not certain if those properties are linked to the peculiar nature of this antigen, or if they reflect the intrinsic requirements for the activation of Vα14i NKT cells. Here, using α-GalCer-related compounds, we demonstrated that the full activation of Vα14i NKT cells by glycolipids requires a stable and distinct type of TCR interaction.
Materials and Methods
Mice and Immunizations. C57BL/6J mice (The Jackson Laboratory) were used between 6 and 10 weeks of age. Glycosyl (Gal, Glc, and Man) ceramides were prepared from commercially available d-lyxose, and the complete synthesis will be reported elsewhere (S.D.M. and G.S.B., unpublished results). Structural analogues and intermediates were characterized by 1H and 13C-NMR NMR and electrospray MS. The syntheses of β-Gal-Cer and 3,4-dideoxy α-GalCer have been described (19). The synthesis of 4-deoxy α-GalCer will be reported elsewhere (S.K.R., R.M.N., and A.R.H., unpublished results). Glycolipids were dissolved in a vehicle comprised of 0.5% polysorbate 20 (Nikko Chemicals, Tokyo) in a 0.9% NaCl solution, and mice were immunized i.p. with either vehicle alone or 2 μg of compound dissolved in vehicle.
Protein Expression and Purification. Baculovirus-mediated mouse CD1d expression and purification (4, 20) and expression of a single chain (sc) Vα14i TCR in bacteria (15) have been described. The scVα14i TCR construct also was expressed by using baculovirus. The scTCR construct, which has the Vβ8.2 sequence from the 2C12 NKT cell hybridoma, was liberated from the pET28a(+) plasmid and subcloned into the NcoI and EcoRI sites of the pAcGP67-B plasmid (PharMingen), downstream of the GP67 secretion signal sequence. Recombinant baculoviruses were produced as described (4), and High Five cells were infected at a multiplicity of infection of 5–10. After 4–5 days, supernatants were harvested, and the scTCR was eluted from Ni-NTA agarose beads. The eluted material was immediately injected onto a Superdex 200 gel filtration column (Amersham Pharmacia Biotech) for size exclusion chromatography. Each preparation was checked by SDS/PAGE for purity and quantified by using the BCA protein assay kit (Pierce). Similar results were obtained with bacteria-derived or baculovirus-derived TCRs. Tetramers of mouse CD1d molecules were produced as described (4).
Cell Preparation. Single-cell suspensions were prepared from the liver of 6- to 10-week-old C57BL/6J mice as described (4, 21). Tetramer staining at equilibrium was performed according to a published protocol (15). Analysis was performed with a FACScalibur instrument (Becton Dickinson). flowjo (Tree Star, Ashland, OR) software was used for analysis.
Antigenic Stimulation by Glycolipid Antigens. The Vα14Vβ8.2 NKT cell hybridomas N38-2C12 (2C12), N38-3C3 (3C3), and DN3A4-1.2 (1.2) and the Vα14Vβ10 NKT cell hybridoma DN3A4-1.4 (1.4) have been described (22, 23). Stimulation of NKT cell hybridomas in an antigen-presenting cell-free assay and measurement of IL-2 by ELISA were performed according to published protocols (15).
Surface Plasmon Resonance. All real-time binding experiments were performed, at 25°C, on a BIAcore X biosensor system (BIAcore, Piscataway, NJ), as described for analysis of TCR binding to α-GalCer/CD1d complexes (15). Kinetic parameters and/or apparent equilibrium dissociation constants were obtained by fitting the specific sensorgrams with biaeval 3.1 software (BIAcore). The association rate is expressed by the relation ka × [immobilized glycolipid/CD1d complexes], but if the level of immobilization is high, the association rate becomes faster than the mass transport of the scTCR from the bulk of the solution to the interface. As a consequence, the interaction is partially under the control of mass transport, and it is then difficult to obtain an accurate value for ka. By contrast, the dissociation proceeds according to a zero-order reaction and therefore is independent of the quantity of glycolipid/CD1d complexes immobilized. An accurate value for kd therefore could be obtained by fitting the dissociation phase of the sensorgrams (Table 1). The equilibrium dissociation constants (KD) were obtained by fitting the plot of the response at equilibrium against the concentration of scTCR (see Fig. 5E). ka values were calculated by using the relation ka = kd/KD.
Table 1. Biophysical parameters of the interaction of the Vα14 Vβ8.2 scTCR with immobilized CD1d/glycolipid complexes.
| Interaction | KD†, μM | kd‡ × 103, s-1 | ka × 10-4, M-1·s-1 | t1/2, s | Eα°, kcal/mol | ΔG°, kcal/mol | ϕ |
|---|---|---|---|---|---|---|---|
| α-GalCer/wtCD1d | 0.35 | 3.87 | 1.10 | 179 | –5.51 | –8.81 | |
| 4-Deoxy/wtCD1d | 1.12 | 5.38 | 0.48 | 128 | –5.02 | –8.11 | 0.71 |
| α-GlcCer/wtCD1d | 3.8 | 12.11 | 0.32 | 57 | –4.78 | –7.39 | 0.52 |
| α-ManCer/wtCD1d | 13.23 | 40.21 | 0.30 | 17 | –4.74 | –6.65 | 0.35 |
| β-GalCer/wtCD1d | nd | nd | nd | nd | nd | nd | nd |
| 3,4-Dideoxy/wtCD1d | nd | nd | nd | nd | nd | nd | nd |
| α-GalCer/wtCD1d* | 0.21 | 3.96 | 1.36 | 175 | –5.64 | –9.11 | |
| α-GalCer/R79E* | 1.1 | 14.3 | 1.32 | 48 | –5.62 | –8.13 | 0.02 |
Data presented from one representative experiments of three, in which five different concentrations of scTCR have been used. nd, not detectable.
Values from ref. 15
KD values obtained by steady-state equilibrium fitting. (Fig. 6E)
kd values obtained by fitting the dissociation phase of the sensorgrams (Fig. 5 A–D)
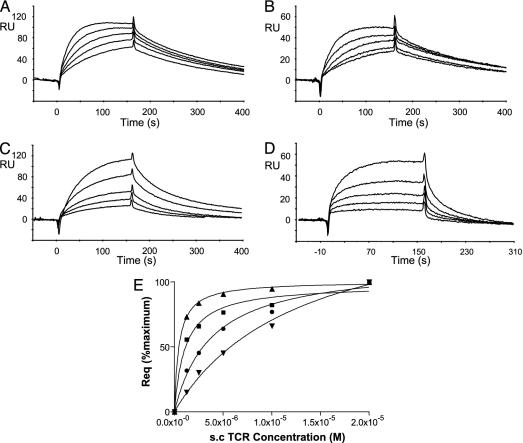
Fig. 5.
Binding of the Vα14 Vβ8.2 scTCR to glycolipid/CD1d complexes. (A–D) The scTCR was injected at 25°C at increasing concentrations (1.25, 2.5, 5, 10, or 20 μM) at a flow rate of 10 μl/min over Fc1 (control flow cell) and Fc2, where α-GalCer-loaded (A), 4-deoxy-loaded (B), α-GlcCer-loaded (C), or α-ManCer-loaded (D) CD1d molecules were immobilized. The sensorgrams plot the specific binding obtained from the subtracted RU values (Fc2 - Fc1). (E) Steady-state equilibrium fitting. Normalized RU at equilibrium (Req) values obtained from fitting of the sensograms (A–D) with the 1:1 Langmuir association, expressed as percentage of the maximum for each compound, were plotted against the scTCR concentration. α-GalCer, ▴; α-GlcCer, •; 4-deoxy, ▪; and α-ManCer, ▾. One representative experiment of three is shown.
The variation of the free energy standard of the system upon binding is 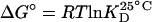 , where T is the absolute temperature in Kelvin, and R is the gas constant. The kinetic association constant, ka, at 25°C can be converted to an activation energy standard by the relation
, where T is the absolute temperature in Kelvin, and R is the gas constant. The kinetic association constant, ka, at 25°C can be converted to an activation energy standard by the relation 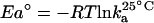 (24). Comparison of the activation energies for α-GalCer/CD1d binding to the TCR versus the binding of the TCR to complexes of α-GalCer-related glycolipids plus CD1d are given as φ values, with φ being calculated as ΔEa° normalized by ΔΔG° (25), and expressed by the relation:
(24). Comparison of the activation energies for α-GalCer/CD1d binding to the TCR versus the binding of the TCR to complexes of α-GalCer-related glycolipids plus CD1d are given as φ values, with φ being calculated as ΔEa° normalized by ΔΔG° (25), and expressed by the relation: 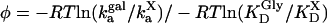 , where X refers to TCR binding to α-GalCer-related glycolipid/CD1d complexes and Gal to the same for α-GalCer-loaded complexes.
, where X refers to TCR binding to α-GalCer-related glycolipid/CD1d complexes and Gal to the same for α-GalCer-loaded complexes.
Results
α-GalCer Analogs Stimulate Cytokine Secretion by Vα14i NKT Cells. α-GalCer-related glycolipids can stimulate Vα14i NKT cells in vitro when they are incubated with antigen-presenting cells (14, 21, 26, 27). Here, we analyzed IL-2 production of Vα14i NKT cell hybridomas in response to soluble plate-bound CD1d molecules loaded with glycolipids (15, 28). This process allowed us to assess directly the effect of structural modifications of the antigen on the TCR-triggered response, in the absence of a contribution of accessory molecules. The five α-GalCer-related glycolipids are shown in Fig. 1. The compounds have subtle modifications of the hydrophilic head group, but with an almost identical ceramide moiety, and which therefore were likely to be equivalently loaded into CD1d. α-GalCer was the most potent antigen tested in this way, followed closely by 4-deoxy and then by α-glucosyl ceramide (α-GlcCer). α-Mannosyl ceramide (α-ManCer) triggered only a low level of cytokine release, whereas β-galactosyl ceramide (β-GalCer) and 3,4-dideoxy did not stimulate the T cell hybridomas (Fig. 2 and data not shown).
Fig. 1.
Structure of α-GalCer analogs. α-GlcCer is an α-d-glucopyranoside, in which the hydroxyl in position 4′ is in an equatorial position rather than the axial position for galactose. β-GalCer has a β glycosidic bound between the d-galactopyranoside unit and the ceramide. α-ManCer has an α-d-mannopyranoside unit, in which the hydroxyls in positions 2′ and 4′ of the carbohydrate are oriented differently when compared to d-galactopyranoside. The compound we named 4-deoxy, lacking the hydroxyl on the 4 carbon of the sphingosine, was referred to previously as AGL 514 (19). Similarly, 3,4-dideoxy was referred to as AGL 535 (19).
Fig. 2.
Stimulation of Vα14i NKT cell-derived hybridomas with glycolipid antigens bound to plate-bound CD1d. One microgram per well of CD1d protein was immobilized on 96-well plates, incubated with the indicated amounts of α-GalCer (▴), 4-deoxy (▪), α-GlcCer (•), α-ManCer (X), 3,4-dideoxy (♦), and β-GalCer (○). 3C3 hybridoma cells were then cultured in the wells for 16 h. IL-2 release was measured by ELISA. Shown is a representative experiment of five, carried out in triplicate. The specific response was obtained by subtracting the responses to wells coated with CD1d protein alone and to the α-GalCer analogs alone. Similar results were obtained with the 1.2 and 1.4 hybridomas.
α-GalCer Analogs Stimulate Cytokine Secretion in Vivo. To investigate the ability of the α-GalCer analogs to stimulate Vα14i NKT cells in vivo, we measured cytokines in the blood 2 and 14 h after i.p. injection (Fig. 3). IL-4 was detected in the serum of mice injected 2 h previously with α-GlcCer, but was present at higher levels in mice injected with 4-deoxy and α-GalCer (Fig. 3A). IL-4 remained detectable in the 4-deoxy- and α-GalCer-injected mice at 14 h. However, no IL-4 was detected in mice injected with α-ManCer (Fig. 3A). IFN-γ was also detected in the serum 2 h after mice were injected with 4-deoxy and α-GalCer (Fig. 3B). Fourteen hours after Vα14i NKT cell activation, a peak of IFN-γ is observed in the blood, which is mostly produced by NK cells that are activated by the stimulated Vα14i NKT cells (reviewed in ref. 8). IFN-γ was present in similar high levels in the serum of mice injected with 4-deoxy and α-GalCer, lower amounts were found with α-GlcCer, and it was notable that serum IFN-γ was also detected in some mice given α-ManCer 14 h earlier (Fig. 3B). Consistent with the differential ability of these compounds to activate Vα14i NKT cells in vivo, we found that α-GalCer and 4-deoxy induced the complete down-regulation of Vα14i TCR expression by tetramer-positive cells in the liver 14 h after antigen injection, α-GlcCer induced somewhat less down-regulation, and α-ManCer caused the least down-regulation (Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 3.
Cytokine secretion after stimulation of Vα14i NKT cells with glycolipid antigens in vivo. Two (♦) and 14 h (▪) after i.p. injection with 2 μg of antigen, serum was harvested and analyzed for IL-4 (A) and IFN-γ (B) by ELISA. Each point represents the cytokine measured in an individual mouse. The dashed line indicates the limit of detection of the ELISA. There were three mice in each group, and data are representative of two separate experiments.
Differential Staining of CD1d Tetramers Loaded with α-GalCer Analogs. Recently, we used site-directed mutants of CD1d to show that the intensity of tetramer staining at equilibrium is correlated with the affinity of the Vα14i TCR for the glycolipid/CD1d complex (15), and similar results have been reported elsewhere (18). To verify that structural modifications of α-GalCer affect TCR engagement on living cells, we produced CD1d tetramers loaded with the α-GalCer-related glycolipids. α-GalCer-loaded CD1d tetramers bound to the Vα14i NKT cell-derived hybridomas, with little reactivity of the unloaded CD1d tetramers (data not shown). To verify that the differences observed in the potency of the α-GalCer-related compounds are related to TCR affinity, 2C12 Vα14i NKT cell hybridoma cells were stained with a concentration range of CD1d tetramers loaded with different glycolipids (Fig. 4). The binding isotherms were then subjected to a Scatchard transformation to access the apparent equilibrium dissociation constant characteristic of the avidity (15). We found that the avidity is correlated with the ranking of the antigenic potencies of the α-GalCer analogs (α-GalCer > 4-deoxy > α-GlcCer). The α-GalCer-loaded CD1d tetramer had a KD of 0.55 ± 0.08 nM, followed by the 4-deoxy-loaded CD1d tetramer (KD = 1.45 ± 0.16 nM), and the α-GlcCer-loaded CD1d tetramer (KD = 3.78 ± 0.28 nM). We also detected a specific staining of intrahepatic lymphocytes from C57BL6/J mice by using 4-deoxy- and α-GlcCer-loaded CD1d tetramers. The intensity of staining was, however, too low to allow an accurate measurement of the KD, when dilutions of the tetramers were used. By contrast, no staining above the background level was observed when 3,4-dideoxy-, α-ManCer-, or β-GalCer-loaded tetramers were used (Fig. 8, which is published as supporting information on the PNAS web site).
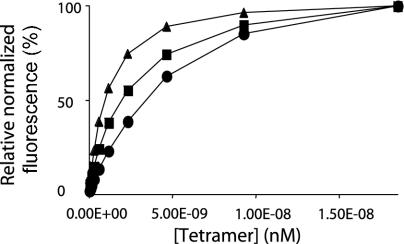
Fig. 4.
Specific CD1d tetramer staining. Binding isotherm of α-GalCer-loaded CD1d tetramer (▴), α-GlcCer-loaded CD1d tetramer (•), and 4-deoxy-loaded CD1d tetramer (▪) to the 2C12 hybridoma. Tetramer fluorescence, normalized to the surface level of TCRβ staining, is plotted against tetramer concentration. 2C12 hybridoma cells were stained for3hat room temperature with an anti-TCRβ mAb and the indicated concentrations of WT CD1d tetramers. Cells were then washed two times and analyzed by flow cytometry. One representative experiment of five is shown.
Binding of the Soluble Vα14i TCR to Glycolipid/CD1d Complexes. We used surface plasmon resonance to examine directly the binding of the Vα14i TCR to antigen plus CD1d complexes. To detect a specific binding to the less potent agonist α-ManCer at each concentration of scTCR used, it was necessary to immobilize glycolipid/CD1d complexes at a density up to 4,000 resonance units (RU). In those conditions, a specific, dose-dependent binding of the scTCR to immobilized α-ManCer/CD1d complexes was observed (Fig. 5D), as well as binding to α-GalCer-, 4-deoxy-, and α-GlcCer-loaded CD1d molecules (Fig. 5 A–C). No binding was detected when β-GalCer/CD1d complexes or 3,4-deoxy/CD1d complexes were used (data not shown).
The equilibrium dissociation constants (KD), based on TCR binding at equilibrium (Fig. 5E), ranged from 13 μM for α-ManCer to 0.35 μM for α-GalCer. The interaction between α-GalCer/CD1d complexes and the Vα14+ TCR displayed a kd of 3.87 × 10-3 s-1 and a ka value was calculated by using the relation ka = kd/KD (Table 1). The calculated value for ka was 1.10 × 104 M-1·s-1, consistent with the reported values for this interaction (15–17). Compared to α-GalCer, there was a reduction of the association rate constant (ka) by 2.3-, 3.4-, or 3.7-fold for 4-deoxy, α-GlcCer, or α-ManCer, respectively. The kd was only slightly modified when 4-deoxy/CD1d complexes were immobilized (Table 1). Indeed, it was increased from 3.87 × 10-3 s-1 to 5.38 × 10-3 s-1, which leads to a decrease of the half-life (t1/2) from 179 to 128 s. When α-GlcCer/CD1d complexes were immobilized, the kd was further increased to 12.11 × 10-3 s-1, which corresponds to t1/2 of 57 s (Table 1). Finally, when α-ManCer/CD1d complexes were immobilized, the kd was 40.21 × 10-3 s-1, leading to t1/2 of 17 s.
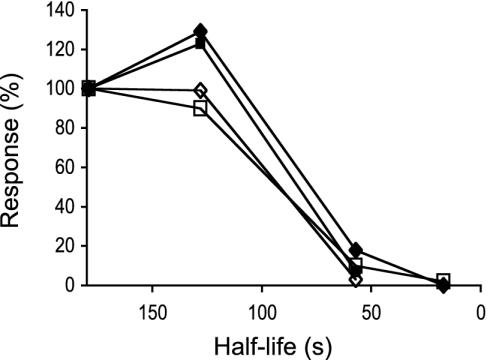
With the series of compounds tested here, we found a good correlation between the t1/2 of the TCR interaction and all of the immune assays, with no exceptional compounds (Fig. 6). The ability of α-GalCer-related glycolipids to stimulate Vα14i NKT cell hybridomas and the cytokines produced in vivo after i.p. injection were related to the t1/2 of the TCR interaction.
Fig. 6.
Correlation between t1/2 of the interaction and the quantity of cytokine produced by Vα14i NKT cells. The relative quantities of IL-4 (full symbols) and IFN-γ (open symbols) measured by ELISA 2 (♦) and 14 h (▪) after i.p. injection with 2 μg of antigen are plotted against the half-life of the interaction between the respective antigen/CD1d complexes and the NKT cell TCR.
A Distinct Binding Mechanism for Vα14i TCR Recognition. We and others have shown that binding of the Vα14i TCR to glycolipid/CD1d complexes displays almost no temperature dependence (15, 17), which also distinguishes binding by the Vα14i TCR from recognition of peptide–MHC complexes by αβ TCRs (24). In a similar approach to the one undertaken by Wu et al. (24), we calculated the variation of the free energy standard (ΔG°) from the KD and converted ka to an activation energy standard (Ea°) (see Materials and Methods). The comparison, expressed as phi (φ) values, allowed us to probe the effect of structural modifications of the antigen or CD1d on the initial TCR association. When comparing an α-GalCer-related glycolipid to α-GalCer itself, the φ value is the representation of the contribution of the α-GalCer-related glycolipid to the activation energy of association, normalized to its contribution to the free energy of binding. A φ value of 0 indicates that the contact is not formed in the transition state nor participates in the initial association, whereas contacts for which φ values are higher (up to a maximum of 1, which represents full formation in the transition state) are contacts driving the initial interaction.
We found that the interaction between α-GlcCer/CD1d and α-ManCer/CD1d complexes with the Vα14i TCR exhibited φ values of 0.52 and 0.35, respectively (Table 1). This finding suggests that the polar head group of the antigen is contacting the TCR in the initial phase of the interaction. We also compared the interaction between α-GalCer/CD1d complexes and α-GalCer/CD1dR79E complexes. CD1dR79E has a mutation at position 79 that alters the charge of an amino acid near the end of the α1 helix of mouse CD1d pointing upward toward the TCR (29, 30). We calculated the φ value and found it to be very close to 0 (Table 1), suggesting that this contact, between the CD1d α1 helix and the Vα14i TCR, is not implicated in the formation of the transition state.
Discussion
In this article we examined the biochemical basis for glycosphingolipid antigen recognition by the Vα4i TCR expressed by NKT cells. Mice contain several million Vα14i NKT cells with essentially identical specificity for α-GalCer presented by CD1d (4, 31). Vα14i NKT cells can activate the immune system through the immediate secretion of diverse cytokines, and they have been reported to be crucial for the outcome of a number of immune responses (2, 3). In addition to their numerical prevalence and immunologic importance, the availability of a set of reagents for investigating the antigen specificity of these cells makes glycosphingolipid antigen recognition by Vα14i NKT cells an attractive model system for the study of glycolipid antigen recognition.
Our findings highlight differences between glycolipid and peptide antigen recognition, with respect to the t1/2 of the TCR interaction and the relative importance of the bound antigen in formation of the initial antigen contact. Several previous results suggested that TCR recognition of glycolipids could be different, without providing direct evidence for this point. First, the diversity of antigens naturally bound to CD1d is limited (32), and CD1 molecules are not polymorphic. Second, CD1-bound antigens are not exposed along the surface of the antigen-binding groove, and the hydrophilic head group is highly exposed only near the center of the groove (33, 34). Third, many Vα14i NKT cells do not express CD4 or CD8, suggesting they may not require coreceptors (10). Fourth, previous work indicated that the Vα14i TCR has a high affinity and long t1/2 of interaction with α-GalCer plus CD1d, although this could be attributed to the extraordinary potency of this antigen (15–18). Fifth, the results from fluorescence resonance energy transfer analysis suggest the Vα14i TCR might be distributed differently on the cell surface (18).
To investigate how Vα14i NKT cell glycolipid recognition might be distinct, we used compounds related to α-GalCer. Differences in the potency of the compounds we used had already been reported (14, 22). Here, using an antigen-presenting cell free antigen presentation assay, we demonstrated a ranking of the five α-GalCer-related antigens tested similarly to the one previously observed with presentation by antigen-presenting cells. The results demonstrate that the differences in antigenic potency are caused by differences in the strength and kinetics of Vα14i TCR engagement. Thus, similar to conventional T cells (11–13), this finding indicated that t1/2 may be a good indicator of the ability to stimulate a response.
α-GlcCer was the weakest antigen to display agonist activity under most conditions. α-ManCer had been reported to have no agonist activity (14, 35, 36). Consistent with this result, we found that tetramers of α-ManCer/CD1d did not stain Vα14i NKT cells, and α-ManCer only poorly stimulated Vα14i NKT cell hybridomas and did not cause IL-4 release in vivo. It did, however, stimulate some release of IFN-γ in the blood and TCR down-regulation. IFN-γ in the serum primarily reflects the activity of NK cells activated by the stimulated Vα14i NKT cells (8), and therefore it could reflect amplification of small Vα14i NKT cell responses.
The interaction between α-ManCer/CD1d complexes and the Vα14i TCR had a t1/2 of 17 s, with 57 s for α-Glc/CD1d complexes. Thus, we conclude that among this series of compounds there is a cutoff for agonist activity, with a t1/2 of the TCR interaction somewhere between 17 and 57 s. This is significantly longer than what is required for a moderate degree of agonist activity for peptide reactive T cells, suggesting a different kinetic window for Vα14i NKT cell activation. Although the natural antigen(s) that stimulate Vα14i NKT cells could behave in a similar way, it also is possible that cytokine or other signals may help to sensitize Vα14i NKT cells, and that the integration of TCR and non-TCR signals may allow simulation by relatively weak antigens (37). Therefore, we cannot exclude the possibility that natural antigens for Vα14i NKT cells might interact differently with the Vα14i TCR.
The recognition of glycolipid antigens in the context of CD1d was reported to display almost no temperature dependence (15, 17), suggesting that the Vα14i TCR makes little accommodation when recognizing glycolipid/CD1d complexes, compared to the accommodation made by αβ TCRs recognizing peptide–MHC complexes. By analyzing the energetic parameters of the interaction, we showed that glycolipid antigen contacts with the Vα14i TCR dominated the association phase of the interaction, whereas CD1d contacts with the TCR were found to contribute to the stability of the complex. This finding supports a critical role for the antigen's most rigid moiety (the carbohydrate unit), and it also suggests that recognition of glycolipid/CD1d complexes is more likely to fit a lock-and-key model. This is further supported by the crystal structures of CD1b bound to glycolipids, where the polar head group of the antigens were found protruding toward the TCR (33, 34), although similar observations were not made in the crystal structure of the CD1a/sulfatide complex (38). Altogether, these data suggest that Vα14i NKT cells may have evolved a different way of sensing their environment. It remains to be determined whether a similar mechanism applies to the more diverse TCRs expressed by T cells reactive with mycobacterial lipid antigens.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 AI45053 and U54 GM61502 and a grant from the Human Frontiers of Science Program (to M.K.), National Institutes of Health Grants RO1AI45889 and RO1 AI48933 (to S.A.P.), Medical Research Council Grant G9901077 and Wellcome Trust Grant 072021/Z/03/Z (to G.S.B), and National Science Foundation Grant CHE-0111522 (to A.R.H.). K.J.L.H. is supported by a C. J. Martin Fellowship from the National Health and Medical Research Council of Australia (ID no. 237029). This is manuscript no. 616 from the La Jolla Institute for Allergy and Immunology.
Abbreviations: α-GalCer, α-galactosyl ceramide; α-ManCer, α-mannosyl ceramide; β-Gal-Cer, β-galactosyl ceramide; NK, natural killer; RU, resonance units; sc, single chain; TCR, T cell antigen receptor; Vα14i, invariant Vα14 rearrangement.
References
- 1.Porcelli, S. A. (1995) Adv. Immunol. 59, 1-98. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey, D. I., Hammond, K. J., Poulton, L. D., Smyth, M. J. & Baxter, A. G. (2000) Immunol. Today 21, 573-583. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg, M. & Gapin, L. (2002) Nat. Rev. Immunol. 2, 557-568. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda, J. L., Naidenko, O. V., Gapin, L., Nakayama, T., Taniguchi, M., Wang, C. R., Koezuka, Y. & Kronenberg, M. (2000) J. Exp. Med. 192, 741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond, K. J. & Kronenberg, M. (2003) Curr. Opin. Immunol. 15, 683-689. [DOI] [PubMed] [Google Scholar]
- 6.Brutkiewicz, R. R. & Sriram, V. (2002) Crit. Rev. Oncol. Hematol. 41, 287-298. [DOI] [PubMed] [Google Scholar]
- 7.Smyth, M. J. & Godfrey, D. I. (2000) Nat. Immunol. 1, 459-460. [DOI] [PubMed] [Google Scholar]
- 8.Smyth, M. J., Crowe, N. Y., Hayakawa, Y., Takeda, K., Yagita, H. & Godfrey, D. I. (2002) Curr. Opin. Immunol. 14, 165-171. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac, A., Rivera, M. N., Park, S. H. & Roark, J. H. (1997) Annu. Rev. Immunol. 15, 535-562. [DOI] [PubMed] [Google Scholar]
- 10.Porcelli, S. A. & Modlin, R. L. (1999) Annu. Rev. Immunol. 17, 297-329. [DOI] [PubMed] [Google Scholar]
- 11.Malissen, B. (2001) Nat. Immunol. 2, 196-198. [DOI] [PubMed] [Google Scholar]
- 12.Gascoigne, N. R., Alam, S. M., Lin, C. M., McGuire, M. V., Marine, S., Niederberger, N., Redpath, S., Sim, B. C., Travers, P. J., Yachi, P., et al. (2000) Immunol. Res. 21, 225-231. [DOI] [PubMed] [Google Scholar]
- 13.Davis, M. M., Boniface, J. J., Reich, Z., Lyons, D., Hampl, J., Arden, B. & Chien, Y. (1998) Annu. Rev. Immunol. 16, 523-544. [DOI] [PubMed] [Google Scholar]
- 14.Kawano, T., Cui, J., Koezuka, Y., Toura, I., Kaneko, Y., Motoki, K., Ueno, H., Nakagawa, R., Sato, H., Kondo, E., et al. (1997) Science 278, 1626-1629. [DOI] [PubMed] [Google Scholar]
- 15.Sidobre, S., Naidenko, O. V., Sim, B. C., Gascoigne, N. R., Garcia, K. C. & Kronenberg, M. (2002) J. Immunol. 169, 1340-1348. [DOI] [PubMed] [Google Scholar]
- 16.Sim, B. C., Holmberg, K., Sidobre, S., Naidenko, O., Niederberger, N., Marine, S. D., Kronenberg, M. & Gascoigne, N. R. (2003) Immunogenetics 54, 874-883. [DOI] [PubMed] [Google Scholar]
- 17.Cantu, C., III, Benlagha, K., Savage, P. B., Bendelac, A. & Teyton, L. (2003) J. Immunol. 170, 4673-4682. [DOI] [PubMed] [Google Scholar]
- 18.Stanic, A. K., Shashidharamurthy, R., Bezbradica, J. S., Matsuki, N., Yoshimura, Y., Miyake, S., Choi, E. Y., Schell, T. D., Van Kaer, L., Tevethia, S. S., et al. (2003) J. Immunol. 171, 4539-4551. [DOI] [PubMed] [Google Scholar]
- 19.Morita, M., Motoki, K., Akimoto, K., Natori, T., Sakai, T., Sawa, E., Yamaji, K., Koezuka, Y., Kobayashi, E. & Fukushima, H. (1995) J. Med. Chem. 38, 2176-2187. [DOI] [PubMed] [Google Scholar]
- 20.Sidobre, S. & Kronenberg, M. (2002) J. Immunol. Methods 268, 107-121. [DOI] [PubMed] [Google Scholar]
- 21.Hammond, K. J., Pellicci, D. G., Poulton, L. D., Naidenko, O. V., Scalzo, A. A., Baxter, A. G. & Godfrey, D. I. (2001) J. Immunol. 167, 1164-1173. [DOI] [PubMed] [Google Scholar]
- 22.Brossay, L., Naidenko, O., Burdin, N., Matsuda, J., Sakai, T. & Kronenberg, M. (1998) J. Immunol. 161, 5124-5128. [PubMed] [Google Scholar]
- 23.Burdin, N., Brossay, L., Koezuka, Y., Smiley, S. T., Grusby, M. J., Gui, M., Taniguchi, M., Hayakawa, K. & Kronenberg, M. (1998) J. Immunol. 161, 3271-3281. [PubMed] [Google Scholar]
- 24.Wu, L. C., Tuot, D. S., Lyons, D. S., Garcia, K. C. & Davis, M. M. (2002) Nature 418, 552-556. [DOI] [PubMed] [Google Scholar]
- 25.Fersht, A. R. (1997) Curr. Opin. Struct. Biol. 7, 3-9. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto, K., Miyake, S. & Yamamura, T. (2001) Nature 413, 531-534. [DOI] [PubMed] [Google Scholar]
- 27.Schmieg, J., Yang, G., Franck, R. W. & Tsuji, M. (2003) J. Exp. Med. 198, 1631-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naidenko, O. V., Maher, J. K., Ernst, W. A., Sakai, T., Modlin, R. L. & Kronenberg, M. (1999) J. Exp. Med. 190, 1069-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng, Z., Castaño, A. R., Segelke, B. W., Stura, E. A., Peterson, P. A. & Wilson, I. A. (1997) Science 277, 339-345. [DOI] [PubMed] [Google Scholar]
- 30.Burdin, N., Brossay, L., Degano, M., Iijima, H., Gui, M., Wilson, I. A. & Kronenberg, M. (2000) Proc. Natl. Acad. Sci. USA 97, 10156-10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benlagha, K. & Bendelac, A. (2000) Semin. Immunol. 12, 537-542. [DOI] [PubMed] [Google Scholar]
- 32.Joyce, S., Woods, A. S., Yewdell, J. W., Bennink, J. R., De Silva, A. D., Boesteanu, A., Balk, S. P., Cotter, R. J. & Brutkiewicz, R. R. (1998) Science 279, 1541-1544. [DOI] [PubMed] [Google Scholar]
- 33.Gadola, S. D., Zaccai, N. R., Harlos, K., Shepherd, D., Castro-Palomino, J. C., Ritter, G., Schmidt, R. R., Jones, E. Y. & Cerundolo, V. (2002) Nat. Immunol. 3, 721-726. [DOI] [PubMed] [Google Scholar]
- 34.Batuwangala, T., Shepherd, D., Gadola, S. D., Gibson, K. J., Zaccai, N. R., Fersht, A. R., Besra, G. S., Cerundolo, V. & Jones, E. Y. (2004) J. Immunol. 172, 2382-2388. [DOI] [PubMed] [Google Scholar]
- 35.Naumov, Y. N., Bahjat, K. S., Gausling, R., Abraham, R., Exley, M. A., Koezuka, Y., Balk, S. B., Strominger, J. L., Clare-Salzer, M. & Wilson, S. B. (2001) Proc. Natl. Acad. Sci. USA 98, 13838-13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saubermann, L. J., Beck, P., De Jong, Y. P., Pitman, R. S., Ryan, M. S., Kim, H. S., Exley, M., Snapper, S., Balk, S. P., Hagen, S. J., et al. (2000) Gastroenterology 119, 119-128. [DOI] [PubMed] [Google Scholar]
- 37.Brigl, M., Bry, L., Kent, S. C., Gumperz, J. E. & Brenner, M. B. (2003) Nat. Immunol. 4, 1230-1237. [DOI] [PubMed] [Google Scholar]
- 38.Zajonc, D. M., Elsliger, M. A., Teyton, L. & Wilson, I. A. (2003) Nat. Immunol. 4, 808-815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.